Abstract
The protozoan parasite Entamoeba histolytica causes invasive amoebiasis characterized by amoebic dysentery and liver abscesses (ALA). The E. histolytica galactose/N-acetyl-d-galactosamine-inhibitable lectin (Gal-lectin), an immunogenic surface molecule involved in colonization and invasion, is a promising vaccine candidate against amoebiasis. Gal-lectin is known to induce Th1 cytokines in macrophages and spleen cells in vitro, and a Th1 response is thought to be protective against ALA. In this study, we report the use of cytosine guanine oligodeoxynucleotide (CpG-ODN) as adjuvant to augment Th1 responses against Gal-lectin in the gerbil model of ALA. Gerbils were vaccinated intramuscularly with the native Gal-lectin plus CpG-ODN or a paired non-CpG control GpC-ODN, and control gerbils received CpG-ODN alone. One week after the last boost gerbils were challenged intrahepatically with 106 amoebae. Gerbils receiving CpG-ODN as adjuvant with Gal-lectin were completely protected against the development of ALA, whereas 50% of gerbils receiving GpC-ODN and Gal-lectin developed ALA and 85% of controls developed ALA. Stronger lymphoproliferation in response to the Gal-lectin and higher prechallenge titers of serum Gal-lectin-specific antibodies, capable of blocking amoebic adherence, were observed when CpG-ODN was used as adjuvant. Gerbils vaccinated with CpG-ODN and Gal-lectin also had significantly higher levels of gamma interferon, interleukin-12 (IL-12), and IL-2 mRNA than controls. These data indicate that CpG-ODN can enhance the Th1 responses, which improve the protective effects of Gal-lectin. This is the first report of the use of CpG as a potent Th1 adjuvant with Gal-lectin to increase protection against ALA formation.
Entamoeba histolytica, the etiological agent of amoebic dysentery and amoebic liver abscesses (ALA), causes 100,000 deaths annually (46). Chemotherapy is currently available; however, there remains a critical need for the development of alternate approaches to control disease caused by this organism. Vaccination is a possible solution, as epidemiological studies suggest the occurrence of acquired protective immunity to E. histolytica infections (19, 20). In contrast to other protozoan parasites with complex life cycles, E. histolytica cycles only between cyst and mobile-trophozoite stages, and humans are the only relevant hosts. These features of the parasite and the supporting epidemiological data make vaccine development an attractive therapeutic addition to chemotherapy.
One of the leading candidates for an antiamoebic vaccine is galactose/N-acetyl-d-galactosamine-inhibitable lectin (Gal-lectin). Gal-lectin is a 260-kDa heterodimer surface glycoprotein which consists of heavy and light subunits linked by disulfide bonds (36, 37). The heavy subunit contains a cysteine-rich carbohydrate recognition domain, and monoclonal antibodies (Abs) directed against this region can inhibit amoebic adherence to target cells. Gal-lectin plays an important role in adherence to colonic mucins for colonization (9) and for contact-dependent killing of cells and resistance to complement attack. Apart from its biological importance, Gal-lectin is an immunodominant and immunogenic molecule. Gal-lectin induces the production of Th1 cytokines in vitro and in vivo, and cell-mediated immunity is important in controlling the formation of ALA in animal models. In vitro stimulation of macrophages with Gal-lectin induces the production of tumor necrosis factor alpha (TNF-α) (41), and splenocytes from ALA patients stimulated with Gal-lectin produce large amounts of interleukin-12 (IL-12) and gamma interferon (IFN-γ) (39). Several trials of vaccine in mice and gerbils using Gal-lectin have shown the induction of protective immunity against ALA challenge (34, 40, 43, 47). In these trials, like most vaccine research with animals, the Gal-lectin was administered with Freund's adjuvant. An alternative to Freund's adjuvant is cytosine guanine oligodeoxynucleotide (CpG-ODN), which can induce strong immune responses without the toxicity of conventional adjuvants.
Unmethylated CpG motifs are present in bacterial DNA and are recognized by the innate mammalian immune system via Toll-like receptor 9 (11). This triggers an immune reaction characterized by Th1 cytokine expression and activation of immune cells, specifically dendritic cells and B cells (3, 5, 14, 26). This activation is nonspecific but can be used to enhance immune responses to specific antigens. CpG-ODNs are Toll-like receptor 9 agonists, which are designed to mimic immunostimulatory sequences found in pathogens. These synthetic CpG motifs have been used as adjuvants in many systems either to enhance the immunogenicity of a vaccine or to skew the immune response from Th2 to Th1. In vaccine trials against bacterial infections, viral infections, and parasitic infections, CpG-ODNs have shown the ability to increase both innate immune responses and protective immunity (13, 15, 44). Current clinical trials using humans testing CpG-7909 as a vaccine adjuvant are demonstrating that CpG-ODN can safely activate antigen-specific immune responses (10). Gal-lectin has the innate ability to induce Th1 responses, and in the present study we have examined the ability of CpG-ODN in combination with the Gal-lectin antigen to safely enhance protective immunity in gerbils infected with E. histolytica.
MATERIALS AND METHODS
Native Gal-lectin.
Native Gal-lectin was purified as described previously (38). Briefly, log-phase amoeba (HM1:IMSS) grown in TYI-S-33 medium (16a) were chilled and centrifuged at 900 rpm for 5 min. The pellet was washed twice in wash buffer (75 mM Tris, 65 mM NaCl, pH 7.2), and then the final pellet was solubilized in solubilization buffer (150 mM NaCl, 50 mM Tris, pH 8.3, and 0.5% Nonidet P-40) supplemented with a cocktail of protease inhibitors. The solubilized amoebaewere microcentrifuged at 10,000 × g at 4°C for 30 min. The supernatant was kept and run through an immunoaffinity column, generously provided by B. Petri (University of Virginia, Charlottesville), consisting of protein A-purified anti-Gal-lectin monoclonal Abs (H85, 7F4, 5B8, 3F4, and 6D2) for 48 h at 4°C with a peristaltic pump. The column was washed first with solubilization buffer and then with phosphate-buffered saline (PBS), and finally the Gal-lectin was eluted with elution buffer (4 M MgCl2, 10 mM Tris, pH 7.2). The Gal-lectin was dialyzed against PBS and concentrated. Purified Gal-lectin did not contain detectable levels of endotoxin contamination, as measured by E-TOXATE assay (Sigma).
ODNs.
The ODNs used in this study were CpG-ODN 2006 (TCG TCG TTT TGT CGT TTT GTC GTT) and paired non-CpG control GpC-ODN 2137 (TGC TGC TTT TGT GCT TTT GTG CTT). These ODNs have nuclease-resistant phosphorothioate backbones and sequences known to be immunostimulatory in many species, including humans. All ODNs were purchased from Coley Pharmaceutical Group (Kanata, Canada).
Vaccinations and challenge infections.
Male gerbils (Meriones unguiculatus) 6 to 9 weeks old (Charles River, St. Constant, Canada) were injected intramuscularly in the hind legs with either 50 μg CpG-ODN 2006 only, 50 μg CpG-ODN 2006 and 10 μg Gal-lectin, or 50 μg GpC-ODN 2137 and 10 μg Gal-lectin in 100 μl PBS. Gerbils received identical booster injections at 14 and 28 days after the initial injection. At day 35, gerbils were anesthetized and challenged via intrahepatic injection of 106 log-phase E. histolytica trophozoites (HM1:IMSS) into the left liver lobe as previously described (8). Gerbils were sacrificed postchallenge (2, 5, 15, 20), and their spleens and sera were collected. Livers were removed, and ALA weight relative to total liver weight was measured. All protocols in this study were carried out with the approval of the McGill University Animal Care Committee.
Immunoblotting.
Sera from vaccinated gerbils were tested for the presence of anti-Gal-lectin Abs. Electrophoresis was performed on the native Gal-lectin in a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, and products were transferred onto a nitrocellulose membrane. Membranes were probed with either a 1:1,000 dilution of pooled sera from control or vaccinated gerbils or 1G7 antilectin monoclonal Ab. The blots were incubated with 1:10,000-diluted horseradish peroxidase (HRP)-conjugated anti-gerbil immunoglobulin G (IgG; Immunology Consultants Laboratory Inc.) or 1:3,000-diluted HRP-conjugated anti-mouse IgG (Amersham) and developed by enhanced chemiluminescence (Amersham).
ELISA.
A total serum IgG enzyme-linked immunosorbent assay (ELISA) was performed on pooled sera from vaccinated or control gerbils. One hundred nanograms of native Gal-lectin per well was used to coat NUNC-Immuno Maxisorp 96-well plates (Falcon) in 50 μl carbonate buffer (pH 9.5). Plates were blocked overnight at 4°C in blocking buffer (PBS-1% bovine serum albumin, 0.1% Tween 20) and then washed three times in wash buffer (PBS-0.1% Tween 20). One hundred microliters of pooled sera (1:100) was added and serially diluted 1:1 in blocking buffer and then incubated at 37°C for 1 h. The plates were washed three times and incubated as above with 100 μl of HRP-conjugated anti-gerbil IgG Ab (1:10,000). After the plates were washed, 100 μl of 3,3′,5,5′-tetramethylbenzidine (Sigma)-HRP substrate was added to each well, and the assay was developed for 20 min. The reaction was stopped with 50 μl 2 M H2SO4 and absorbance read at 450 nm in a Microplate Autoreader (Mandel Scientific Company Ltd./Bio-tek Instruments).
Adherence assay.
The ability of serum Abs to inhibit amoebic adherence to target cells was determined by a previously described adherence assay (29). Briefly, log-phase E. histolytica trophozoites were washed in M199 (Gibco) supplemented with 5.7 mM cysteine, 0.5% bovine serum albumin, and 25 mM HEPES (pH 6.8). Amoebae (105/ml) resuspended in M199 were preincubated for 1 h at 4°C with a 1:100 serum dilution from gerbils receiving CpG only and gerbils receiving CpG and Gal-lectin or GpC and Gal-lectin and human ALA patient serum as positive control. During the preincubation, Chinese hamster ovary (CHO) cells grown in Ham's F-12 medium (Gibco) supplemented with 24 mM HEPES, 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin were trypsinized and resuspended at a cell density of 2.0 × 105/ml. Amoebae (105) were mixed with 1 ml of CHO cell suspension and centrifuged at 900 × g for 5 min. The pellets were incubated at 4°C for 2 h undisturbed. Seventy-five percent of the supernatant was decanted, and the pellets were resuspended by gentle vortexing. Amoebic adherence was determined by light microscopy by counting the amoebae with three or more CHO cells attached (positive rosette) out of 100 random amoebae.
Immunofluorescence.
Log-phase amoebae were washed and resuspended in M199 (supplemented as above) at 1.0 × 106 amoebae/ml. Parasites were incubated for 1 h on ice with the following: M199 alone, 1G7 monoclonal Ab (1:50), or prechallenge serum from vaccinated (CpG plus Gal-lectin) and control (CpG or GpC plus Gal-lectin) gerbils (1:100). Amoebae were washed three times in fresh M199 at 150 × g and incubated with either rabbit anti-rat IgG fluorescein isothiocyanate (FITC)-conjugated Ab (Sigma) or goat anti-mouse IgG FITC-conjugated Ab (1:200) for 1 h on ice. After thorough washing, the amoebae were pelleted at 150 × g and mounted onto glass slides with Vectashield mounting medium. Slides were kept at 4°C until analysis through a Nikon Eclipse800 epifluorescence stereomicroscope. Images were collected with a 40× oil immersion lens.
Lymphoproliferation assay.
Single-cell suspensions were prepared from the immunized and control gerbil spleens by grinding the organs through a 40-μm cell strainer (Falcon) in RPMI 1640 (Gibco) supplemented with 10 mM HEPES, 50 μM 2-mercaptoethanol, 2 mM glutamine, 5% fetal bovine serum, and 5 mg/ml penicillin-streptomycin sulfate. Red blood cells were lysed in red blood cell lysing buffer (Sigma), and the remaining cells were suspended at 2.5 × 106 cells/ml in complete RPMI 1640. One hundred microliters of cell suspension was added to each well of a 96-well culture plate (Falcon), and an equal volume of stimulus was added to the wells in triplicate. Stimuli for this experiment included complete RPMI 1640 (no stimulus), Gal-lectin (10 μg/ml), soluble amoebic proteins (SAP; 50 μg/ml), or concanavalin A (ConA; 2.5 μg/ml). Cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 60 to 72 h, and 1 μCi [methyl-3H]thymidine (Amersham) was added to each well in 25 μl of RPMI 1640 for the last 18 h. Labeled cells were harvested onto glass fiber filters using an automated cell harvester (Filtermate harvester; Perkin-Elmer), and incorporated radioactivity was measured by scintillation counting (Trilux Counter LKB; Wallac, Pharmacia). Lymphoproliferation responses are expressed as stimulation indices: the ratio of cpm counts of cells receiving antigen to the cpm counts of cells without antigen.
Real-time PCR.
Total RNA was isolated (RNeasy minikit; QIAGEN) from gerbil spleens or mesenteric lymph nodes (MLNs). During RNA purification, samples were treated with DNase (QIAGEN) to remove residual genomic DNA contamination. Total RNA (2.5 μg) from each sample was reverse transcribed with the Omniscript reverse transcription kit (QIAGEN) using random hexamer primers. The resulting cDNA was subsequently diluted 1:100 and used for real-time PCR with the Rotor-Gene 3000 system (Corbett Research). Amplification of cytokine cDNA was carried out with gerbil-specific primers and Taqman probes generated by Applied Biosystems, as listed in Table 1. All amplifications were carried out with PE Biosystems Universal PCR Master Mix and the following cycling parameters: 50°C for 2 min, 95°C for 10 min, and then 55 cycles of 95°C for 15 s and 60°C for 60 s. Quantitative analysis was conducted by the comparative cycle threshold method normalized to 18S rRNA as an internal control (4). Cytokine expression levels are represented as increases in expression over expression levels for naive gerbils receiving no treatment. All quantitative PCR analyses were carried out in triplicate.
TABLE 1.
Gerbil-specific primers and Taqman probes for real-time PCR
| Gene product | Sequence(s) (5′-3′) of:
|
|
|---|---|---|
| Primers (sense, antisense) | Probea | |
| 18S rRNA | GGCTTAATTTGACAACACGGGAAAC, CACGGAATCGAGAAAGAGCTATCAA | CTCACCCCGGCCCGGACA |
| IL-12p40 | GCTGGTCAATATACCTGCCACAAA, GGAGCAGCAGACGGAAGTG | CTGAGGGTCTGGTCTCC |
| IL-4 | ATAGCAACGAAGAACACCACAGA, GCGGAGCACCCTGGTA | TCTGCAGAGGAGTCCCTT |
| IL-2 | GCTCCTGAGAGGGATCAACAATTAC, GCCTTCCTCGGCATGTAAAATTTAA | AAACTCCCCATGCTGCTCA |
| IFN-γ | CAGAGCAAAGCTATCAATGAACTTGT, CGACTCCTTTTCCGCTTCCTTAG | CTGTCACCCAGAGTCACC |
Probes for Taqman real-time PCR were labeled with 6-carboxy fluorescein at the 5′ terminus and a nonfluorescent quencher at the 3′ terminus.
Statistical method.
All animal experiments were repeated at least twice with similar results. Results are expressed as means ± standard errors of the means (SEM) of triplicate experiments. Data were analyzed using one-way analysis of variance or paired-sample t tests. Significance was set at a P value of <0.05 for all tests.
RESULTS
CpG-ODN increases Gal-lectin-specific serum Ab levels.
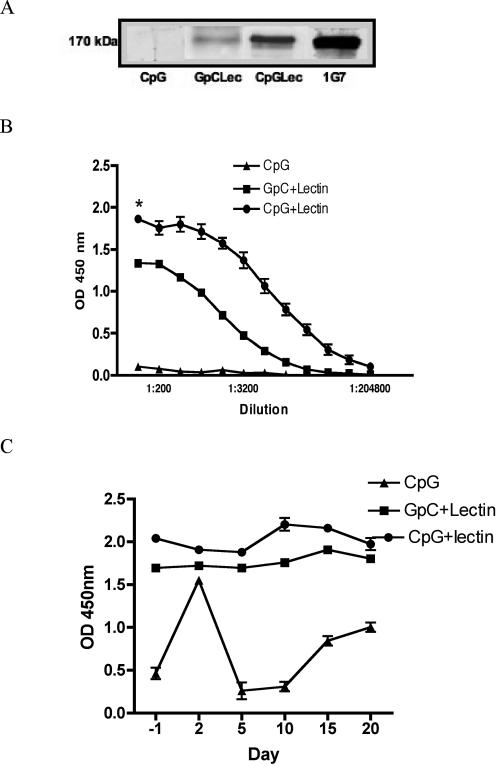
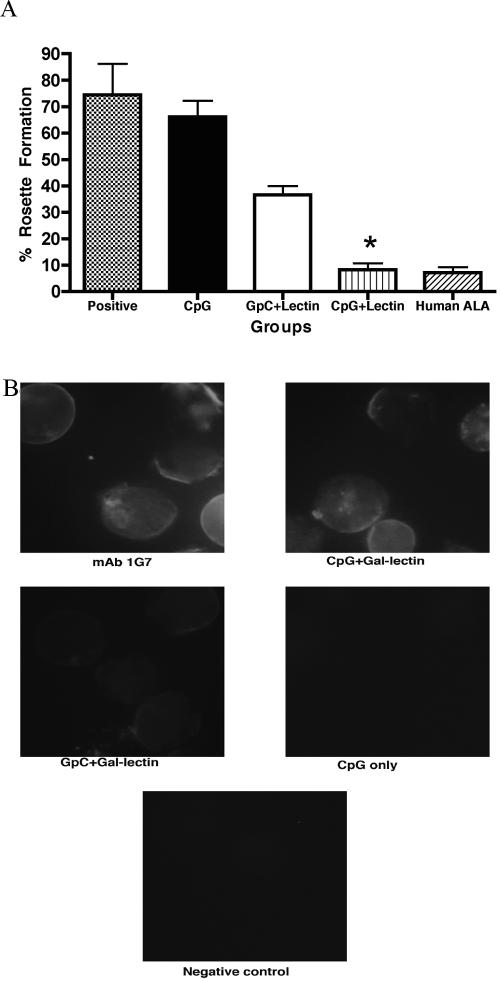
Since CpG-ODN 2006 is known to have a direct effect on B cells, the level of Gal-lectin-specific serum Abs was determined. Prechallenge serum from gerbils immunized with CpG only had no detectable levels of Gal-lectin Abs, while both groups receiving Gal-lectin in the vaccine had detectable Abs as seen in an immunoblot against the native protein (Fig. 1A). CpG-ODN significantly enhanced the Ab response to Gal-lectin compared to the GpC-ODN control. In a total-serum IgG ELISA, gerbils vaccinated with CpG plus Gal-lectin had higher Gal-lectin-specific Ab titers (P = 0.004) than gerbils receiving control GpC plus Gal-lectin (Fig. 1B). Ab levels in the CpG-plus-Gal-lectin group were high and titrated out at a 1:200,000 dilution. These Gal-lectin-specific Ab levels remained high in the groups receiving Gal-lectin even after challenge infection, whereas the Ab levels in the CpG control group showed an increase at 2 days postinfection (p.i.) and then a marked decrease at 5 days p.i., followed by a slow increase in Ab titers with time (Fig. 1C). In an in vitro model of the disease, serum from ALA in humans or monoclonal Abs against Gal-lectin inhibited amoebic adherence. To determine if the prechallenge anti-Gal-lectin Abs were capable of blocking amoebic adherence to target cells, we performed a CHO cell adherence assay with all sera at 1:100 dilution. As shown in Fig. 2A, immunization with CpG plus Gal-lectin inhibited amoebic adherence by 92% ± 3%, which was comparable to the inhibition seen with serum derived from patients with ALA. While CpG controls did not significantly inhibit adherence, the GpC-plus-Gal-lectin group inhibited amoebic adherence by 64%.
FIG. 1.
(A) Immunoblot with immune or control gerbil serum (1:1,000) against purified Gal-lectin. Gal-lectin-specific serum IgG levels from immunized gerbils recognize the 170-kDa band. Data shown are representative of three independent experiments. (B) Gal-lectin-specific serum IgG levels from immunized gerbils (n = 10 per group). ELISAs were performed on prechallenge serum at a starting dilution of 1:100. Points represent total IgG titers ± SEM of data from four experiments. An asterisk indicates a significant difference between groups at the starting dilution. OD, optical density. (C) ELISA OD results from gerbil serum IgG (1:100) collected on days 2, 5, 10, and 15 postchallenge (n = 3 per group per time point) and treated as above. There was a significant difference in day 2 Ab titers within the CpG group compared to prechallenge titers (P < 0.05).
FIG. 2.
(A) Inhibition of amoebic adherence to target CHO cells by immune gerbil serum. Amoebae were preincubated with 1:100-diluted immune or control serum, and subsequent amoebic adherence to CHO cells was determined by adherence assay. CpG-plus-Gal-lectin immune serum significantly inhibited amoebic adherence. An asterisk indicates a significant decrease in amoebic adherence compared to CpG or GpC-plus-Gal-lectin immunization (P < 0.05). Data shown are from triplicates of three independent experiments. Bars represent the percentages of amoebic adherence to target cells. (B) Immunofluorescence analysis of anti-Gal-lectin serum Abs on the amoebic surface. Amoebae were incubated with immune or control gerbil sera (1:100), monoclonal Ab (mAb) 1G7, or secondary FITC-conjugated Ab only (negative control). Images were collected at 40× oil immersion.
As predicted by the adherence assay, we found that CpG-plus-Gal-lectin immunization generated immune sera which had higher titers of adherence-inhibiting and surface binding antibodies than GpC-plus-Gal-lectin immunization (Fig. 2B). Immunofluorescence analysis using prechallenge immune or control gerbil serum revealed that the combination of CpG-ODNwith Gal-lectin, and not either component alone, resulted in strong positive Ab binding to the Gal-lectin on the trophozoite surface. The amoebae alone and CpG-only sera had no detectable fluorescence signal. The group receiving GpC plus Gal-lectin had weak fluorescence, whereas the CpG-plus-Gal-lectin sera had strong fluorescence comparable to that of the positive-control monoclonal Ab 1G7.
CpG-ODN increases Gal-lectin-specific cellular responses.
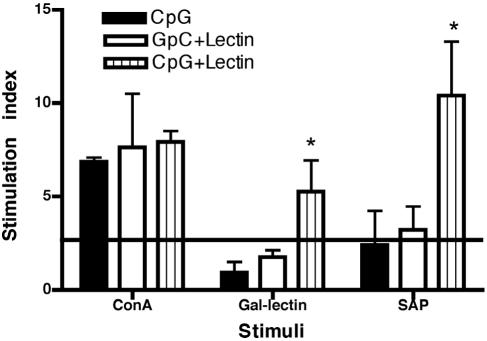
To determine if vaccination with CpG plus Gal-lectin could increase Gal-lectin-specific cellular responses, we measured spleen cell proliferation upon stimulation with the native antigen and SAP. As expected, gerbils vaccinated with CpG plus Gal-lectin demonstrated strong proliferation in response to both the native Gal-lectin and SAP (Fig. 3). This proliferation was significantly stronger (P = 0.03) than that in gerbils receiving GpC plus lectin; however, this last group still responded to the Gal-lectin to a greater degree than the group receiving control CpG alone. All groups exhibited strong proliferation in response to the T-cell mitogen ConA, demonstrating comparable levels of T-cell viability. The higher proliferative response observed with SAP was probably a result of the higher concentration of Gal-lectin in the preparation compared to the known amount plated in the Gal-lectin wells. This experiment indicates that CpG-ODN can augment Gal-lectin-specific cellular immune responses in vitro.
FIG. 3.
Lymphoproliferation of gerbil splenocytes in response to Gal-lectin stimulation. Immunized or control gerbil spleens (n = 11 per group) were collected prior to challenge infection, and cells were restimulated in vitro for 72 h with either ConA (2.5 μg), Gal-lectin (10 μg), or 50 μg of SAP. Proliferation is represented as a stimulation index (cpm of cells with antigen/cpm of cells without antigen). A stimulation index above 2.5 is considered significant, as indicated by an asterisk.
CpG-ODN adjuvant increases the protective effects of Gal-lectin.
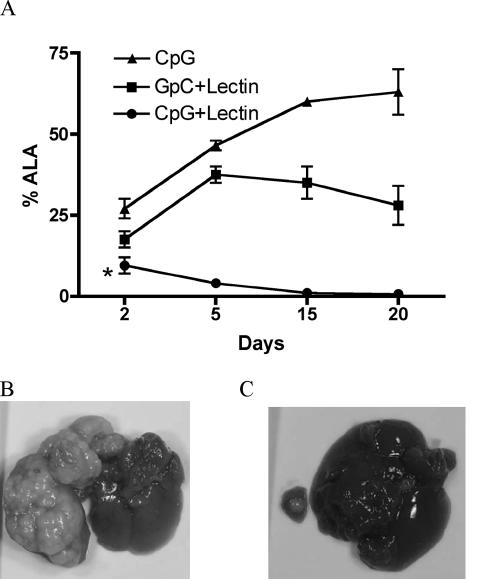
In this vaccination study our strategy was to use immunostimulatory CpG-ODN or control GpC-ODN as adjuvant with a known protective antigen, the E. histolytica Gal-lectin. One week after the last immunization, gerbils were anesthetized and intrahepatically challenged with 106 E. histolytica trophozoites, and animals were sacrificed at days 2, 5, 15, or 20 p.i. As shown in Table 2, CpG-plus-Gal-lectin treatment protected (100%) gerbils from ALA formation, as determined by necropsy at day 20 p.i. Gal-lectin with GpC-ODN had a vaccine efficacy of 50%, whereas 85% of CpG-treated gerbils developed ALA. Amoebic liver abscess was detectable in all groups as early as 2 days p.i. At this early time point the ALAs were already smaller in the CpG-plus-Gal-lectin group compared to controls. Figure 4A demonstrates the progressive development of ALA at various times after challenge infection. As shown, gerbils receiving CpG plus Gal-lectin were able to clear the infection as early as 5 days p.i. This clearance continued until the abscesses were no longer detected or, if they were detected, there were no viable trophozoites. In contrast, the CpG-treated group developed larger abscesses with time while the group treated with GpC plus Gal-lectin demonstrated a slight decrease in ALA size over time (Fig. 4A). Intrahepatic challenge of trophozoites into gerbils treated with CpG resulted in the formation of very large abscesses, sometimes reaching 13 g (Fig. 4B). ALA size relative to total liver weight in this CpG control group was significantly larger (60% versus 20%; P = 0.04) than that observed in PBS-treated controls (data not shown). The abscesses in this group were also larger than those excised from the GpC-plus-Gal-lectin group, indicating a CpG effect rather than an ODN effect.
TABLE 2.
Prevention of ALA in gerbils by vaccination
| Immunization group | No. of gerbils with ALA/total no. of gerbils in expt:
|
Presence of viable parasitesa | % Efficacyb | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| CpG | 6/8 | 9/9 | 9/11 | + | ND |
| GpC + Gal-lectin | 4/6 | 4/10 | 5/11 | + | 50 |
| CpG + Gal-lectin | 0/11 | 0/11 | 0/11 | − | 100* |
ALA contents were aspirated and viable trophozoites determined by cultured in vitro. +, viable parasites present; −, viable parasites absent.
All gerbils were sacrificed at day 20 postinfection to determine vaccine efficacy. ND, not determined. An asterisk indicates a significant difference between groups (P < 0.05).
FIG. 4.
(A) Progression of ALA formation. Gerbils were sacrificed (n = 3 per group per time point) at days 2, 5, 15, and 20 postchallenge, and their livers were examined for ALA formation. Abscess sizes are represented as percentages of total liver weight. The asterisk indicates significantly smaller ALA in the CpG-plus-Gal-lectin group compared to the other groups at all time points (P < 0.05). (B and C) Liver pathology of CpG-treated (B) and CpG-plus-Gal-lectin-treated (C)gerbils 20 days after intrahepatic challenge infection with E. histolytica trophozoites. Control CpG gerbils developed large multilobed abcesses, whereas CpG-plus-Gal-lectin-immunized gerbils showed small abscesses or none at all. ALA pictures are representative of all challenge infection experiments.
Protection against ALA is associated with increased production of Th1 cytokine mRNA.
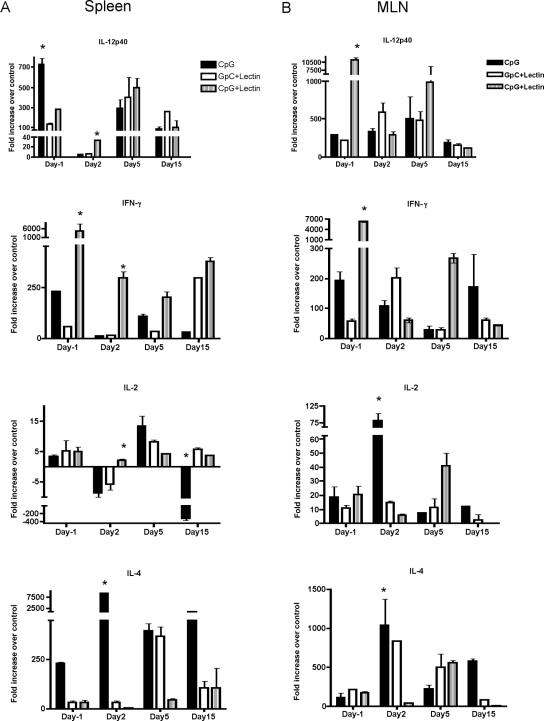
To analyze the spleen and MLN cytokine profile at different times postinfection, real-time Taqman PCR analysis was performed. Cytokine mRNA levels were quantified from necropsied gerbils and compared to mRNA levels from naive untreated, uninfected gerbils. Prechallenge (day 1) spleen samples from the CpG-plus-Gal-lectin group showed elevated levels of IFN-γ (4,500-fold), whereas the CpG control group showed elevated levels (724-fold) of IL-12p40 (Fig. 5A). The GpC-plus-Gal-lectin-treated group did not express elevated levels of any cytokine examined compared to the other groups at the prechallenge time point. At 2 days p.i., mRNA levels for all Th1 cytokines (IFN-γ, IL-12p40, and IL-2) were low in all groups. However, at this early time point, protected gerbils still had significantly higher levels of IFN-γ, IL-12p40, and IL-2: 295-, 32-, and 2.1-fold increases, respectively (P = 0.005). The CpG-treated group showed a 9,000-fold increase in IL-4 mRNA at day 2 postchallenge. The level of IL-4 in CpG-treated gerbils remained elevated during the course of the infection, while the level of IL-2 markedly decreased (−300-fold; P = 0.0014). MLN samples from the CpG-plus-Gal-lectin-treated gerbils also showed increases in Th1 cytokine mRNA compared to controls; however the expression levels and kinetics were different (Fig. 5B). In the CpG-treated gerbils, the MLN samples again showed the highest increase in IL-4 mRNA, but also an increase in IL-2 mRNA, at day 2 postchallenge. However, this IL-2 expression decreased throughout the course of the infection while IL-4 levels remained elevated. The GpC-plus-Gal-lectin control, unlike the CpG-plus-Gal-lectin group, did not express high IFN-γ cytokine mRNA levels either in the spleen or MLNs. Comparing abscessed and nonabscessed gerbils within an experimental group at day 20 demonstrated that IL-4 was more elevated in abscessed animals within each group compared to protected gerbils (32-fold increase; data not shown). At this time point IFN-γ was also more elevated in abscessed gerbils (10-fold increase), as they had an active infection (data not shown).
FIG. 5.
Taqman real-time PCR analysis of spleen (A) and mesenteric lymph node (B) cytokine gene expression (n = 3 per group per time point). Gene expression was normalized to 18S rRNA and represented as the increase over normal nontreated gerbil mRNA. There was substantially higher IFN-γ levels in the protected gerbils 2 days after challenge infection, whereas IL-4 was more prevalent in CpG control gerbils at this time point. An asterisk represents a significantly higher increase in the CpG-plus-Gal-lectin group compared to other groups (P < 0.05). Data represent means ± SEM of results from three independent PCRs.
DISCUSSION
Effective vaccine therapy requires the identification of a protective antigen and a potent vaccine adjuvant. For several reasons, the E. histolytica Gal-lectin is an attractive vaccine candidate as it is not only an important molecule involved in parasite colonization and invasion but also an immunodominant antigen recognized by sera from amoebiasis patients throughout the world (35). CpG-ODN is a novel adjuvant which has been shown to activate innate immune cells and enhance the vaccine efficacy of protective antigens (1, 25). In this study, we have shown that CpG-ODN adjuvant with native Gal-lectin enhances IFN-γ production and protects gerbils from ALA. The enhanced effect can be attributed to the combination of the protective antigen and this potent Th1 adjuvant, as controls with either component alone did not show comparable levels of protection.
The enhanced immune responses to the Gal-lectin with CpG-ODN adjuvant were characterized by increased IgG Ab production, T-cell proliferation, and, more importantly, production of Th1 cytokines. As a component of our vaccination studies, we examined prechallenge Ab titers and their ability to inhibit amoebic functions mediated by Gal-lectin. We used an in vitro adherence assay to determine the blocking effects of serum Abs. This assay revealed the ability of CpG-plus-Gal-lectin immunization to increase the production of Abs capable of blocking the carbohydrate recognition domain on the Gal-lectin molecule, required for amoebic adherence to Gal/GalNAcresidues on mucins or target cells. The immunofluorescence images confirmed the observed adherence inhibition, revealing serum Abs strongly binding the Gal-lectin on the surface of the parasite only in the group receiving adjuvant and amoebic antigen. Although Abs could clearly play a role in preventing amoebic adherence for colonization or cell killing, it is apparent that other immune mechanisms are involved in protection against disease. In fact, postchallenge Ab titers were not significantly different between both groups that had received Gal-lectin, yet the two groups were not equally protected. The highest level of protection was demonstrated in the CpG-plus-Gal-lectin group, which had significantly higher IL-12, IL-2, and IFN-γ mRNA levels 2 days postchallenge than control groups. The elevated levels of IFN-γ mRNA detected in the protected group, both prechallenge and at day 2, demonstrate the importance of this cytokine in parasite clearance. The results from gerbils sacrificed at day 20 demonstrated that IL-4 and IFN-γ were more elevated in abscessed animals within each group compared to protected gerbils (data not shown). We did not measure abscessed versus nonabscessed mRNA at day 2 postinfection, which would have determined the protective effects of IFN-γ within groups. Therefore we correlate IFN-γ with protection between groups at this point and not within groups. In a previous study with the SCID mouse model of amoebiasis, where the IFN-γ receptor gene was disrupted, mice had greater susceptibility to ALA formation than control SCID mice and developed significantly larger abscesses (42). IFN-γ-primed macrophages readily kill trophozoites in vitro (16), and cell-mediated immune responses have been observed in patients with ALA. In fact, humans with ALA demonstrate amoebicidal IFN-γ production (39); however, correlations of cell-mediated responses with immunity in humans are limited. It has been demonstrated in vitro that Gal-lectin activates IFN-γ-primed macrophages for amoebicidal activity via nitric oxide (41). This report indicates that CpG-ODN in combination with Gal-lectin safely confers protection mediated by IFN-γ, which is consistent with previous studies.
The kinetics of cytokine induction in the abscessed gerbils was marked by elevated levels of IL-4, which down-regulate the protective Th1 effects on macrophages. This reduction in macrophage activation could lead to increased parasite numbers and disease progression. The CpG control group also showed greatly reduced IL-2 mRNA levels at days 15 to 20 postinfection, considered the peak infection period in the gerbil model. This corresponds to previous reports that E. histolytica-infected gerbil serum induces a transient T-cell suppression by inhibiting IL-2 production (6). Furthermore, CpG-treated gerbils developed larger abscesses than those excised from PBS-treated or GpC-plus-Gal-lectin treated gerbils (data not shown). This exacerbation of ALA could be due in part to the innate ability of CpG-ODN to activate innate immune cells and induce cytokine secretion, which could recruit immune cells, which the amoebae subsequently kill. Furthermore, in the absence of a Gal-lectin-specific immune response, the amoebae are able to proliferate and ALA formation goes uncontrolled. Although a previous report indicated that CpG-ODN administration can protect against bacterial challenge infections (e.g., reference 15), this adjuvant alone could not protect gerbils from ALA and in fact led to disease exacerbation. Unmethylated CpG DNA, which is present in bacterial and viral genomes, is recognized by innate immune cells via Toll-like receptor 9. There have been reports that CpG-ODN administration alone can protect mice from subsequent lethal challenge with bacteria sharing similar unmethylated CpG DNA patterns (17, 24, 27). This is because the immune system recognizes these CpG motifs as foreign DNA and initiates innate responses. CpG suppression is common in vertebrate genomes; however, E. histolytica is a protozoan parasite with a significant underrepresentation of CpG in its genome compared to other protists (23). In fact an E. histolytica 5-cytosine DNA methyltransferase has been identified, and inhibition of its activity has been shown to impair parasite virulence in vivo (18). Therefore administration of CpG-ODN alone could not confer protection, as it does not sensitize the host to E. histolytica DNA. This underlines the importance of the Gal-lectin as a protective antigen in directing the proper T helper response to combat this parasitic attack.
Previous vaccination studies with Gal-lectin have used Freund's adjuvant (complete or incomplete) and shown high protection levels characterized by cell-mediated responses against the parasite (34, 40). Freund's adjuvant cannot be used in humans due to its toxicity (45), and therefore it is important to devise alternative vaccine formulations for potential use in humans. Several groups have tested CpG-ODN as a vaccine adjuvant against parasitic infections, such as leishmaniasis, and have shown promising results in animal models (7, 12, 22). The degree of protection depends on the immunogenicity of the selected vaccine antigen, but all trials demonstrated the induction of Th1 responses. Currently in clinical trials is CpG 7909 (“Promune”) as a vaccine adjuvant (10). These ODNs have been shown to be safe in humans even at high doses, although adjuvant properties are observed at μg/kg doses (28). In the present study we tested the ability of an adjuvant which could be administered to humans to augment the protective effects of an immunogenic E. histolytica protein. We have demonstrated that CpG-ODN can enhance the protective immune responses to Gal-lectin and prevent ALA formation in the gerbil model. The major drawback in amoebiasis research is the lack of an adequate animal model for the disease. No animal has been able to reproduce the typical lesions of intestinal amoebiasis. The Mongolian gerbil is the best model thus far, as it can be used to mimic both intestinal and hepatic amoebiasis (8). E. histolytica is an intestinal parasite, and the development of a mucosal vaccine could preclude parasite colonization and invasion. Protection against parasite colonization in humans has been correlated with mucosal antilectin IgA antibodies (19). Other groups have successfully developed mucosal Gal-lectin vaccines using cholera toxin B adjuvant or attenuated Salmonella strains for antigen delivery in the mouse model of intestinal amoebiasis (21, 30, 31). CpG-ODN has also proven to be effective as a mucosal vaccine adjuvant, increasing both systemic and mucosal responses to the selected antigens (2, 32, 33). On the basis of these results, it would be possible to design a Gal-lectin mucosal vaccine using CpG-ODN as adjuvant to induce both mucosal and systemic Th1 responses capable to blocking parasite colonization, thus preventing intestinal amoebiasis in mice. Development of a vaccine using recombinant portions of Gal-lectin and CpG-ODN could augment the efficacy of protective regions on the Gal-lectin. This would be more practical since it would not require the purified antigen from parasite cultures. Future work should test the mucosal vaccine efficacy of CpG-ODN with Gal-lectin, providing insight on the possibility of this agent as a choice for human adjuvant in an amoebiasis vaccine.
Acknowledgments
This research was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada. C. Ivory is a recipient of a Ph.D. scholarship from the Institute of Parasitology of McGill University.
We thank Bill Petri from the University of Virginia for providing the immunoaffinity column for Gal-lectin purification.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Agrawal, S., and E. R. Kandimalla. 2002. Medicinal chemistry and therapeutic potential of CpG DNA. Trends Mol. Med. 8:114-121. [DOI] [PubMed] [Google Scholar]
- 2.Alignani, D., B. Maletto, M. Liscovsky, A. Ropolo, G. Moron, and M. C. Pistoresi-Palencia. 2005. Orally administered OVA/CpG-ODN induces specific mucosal and systemic immune response in young and aged mice. J. Leukoc. Biol. 77:898-905. [DOI] [PubMed] [Google Scholar]
- 3.Askew, D., R. S. Chu, A. M. Krieg, and C. V. Harding. 2000. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J. Immunol. 165:6889-6895. [DOI] [PubMed] [Google Scholar]
- 4.Bas, A., G. Forsberg, S. Hammarström, and M.-L. Hammarström. 2004. Utility of the housekeeping genes 18srRNA, β-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand. J. Immunol. 59:566-573. [DOI] [PubMed] [Google Scholar]
- 5.Behboudi, S., D. Chao, P. Klenerman, and J. Austyn. 2000. The effects of DNA containing CpG motif on dendritic cells. Immunology 99:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell, D., D. Gaucher, and K. Chadee. 1999. Serum from Entamoeba histolytica-infected gerbils selectively suppresses T cell proliferation by inhibiting interleukin-2 production. J. Infect. Dis. 179:1495-1501. [DOI] [PubMed] [Google Scholar]
- 7.Cervi, L., J. Borgonovo, M. Egea, L. Chiapello, and D. Masih. 2004. Immunization of rats against Fasciola hepatica using crude antigens conjugated with Freund's adjuvant or oligodeoxynucleotides. Vet. Immunol. Immunopathol. 97:97-104. [DOI] [PubMed] [Google Scholar]
- 8.Chadee, K., and E. Meerovitch. 1984. The pathogenesis of experimentally induced amebic liver abscess in the gerbil (Meriones unguiculatus). Am. J. Pathol. 117:71-80. [PMC free article] [PubMed] [Google Scholar]
- 9.Chadee, K., W. A. Petri, Jr., D. J. Innes, and J. I. Ravdin. 1987. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J. Clin. Investig. 80:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, C. L., H. L. Davis, M. L. Morris, S. M. Efler, A. M. Krieg, Y. Li, C. Laframboise, M. J. Al Adhami, Y. Khaliq, I. Seguin, and D. W. Cameron. 2004. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine 22:3136-3143. [DOI] [PubMed] [Google Scholar]
- 11.Cornelie, S., J. Hoebeke, A. M. Schacht, B. Bertin, J. Vicogne, M. Capron, and G. Riveau. 2004. Direct evidence that toll-like receptor 9 (TLR9) functionally binds plasmid DNA by specific cytosine-phosphate-guanine motif recognition. J. Biol. Chem. 279:15124-15129. [DOI] [PubMed] [Google Scholar]
- 12.Corral, R. S., and P. B. Petray. 2000. CpG DNA as a Th1-promoting adjuvant in immunization against Trypanosoma cruzi. Vaccine 19:234-242. [DOI] [PubMed] [Google Scholar]
- 13.Davis, H. L., R. Weeratna, T. J. Waldschmidt, L. Tygrett, J. Schorr, and A. M. Krieg. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160:870-876. [PubMed] [Google Scholar]
- 14.Decker, T., F. Schneller, M. Kronschnabl, T. Dechow, G. B. Lipford, H. Wagner, and C. Peschel. 2000. Immunostimulatory CpG-oligonucleotides induce functional high affinity IL-2 receptors on B-CLL cells: costimulation with IL-2 results in a highly immunogenic phenotype. Exp. Hematol. 28:558-568. [DOI] [PubMed] [Google Scholar]
- 15.Deng, J. C., T. A. Moore, M. W. Newstead, X. Zeng, A. M. Krieg, and T. J. Standiford. 2004. CpG oligodeoxynucleotides stimulate protective innate immunity against pulmonary Klebsiella infection. J. Immunol. 173:5148-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denis, M., and K. Chadee. 1989. Cytokine activation of murine macrophages for in vitro killing of Entamoeba histolytica trophozoites. Infect. Immun. 57:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Diamond, L. S., D. R. Harlow, and C. Cunnick. 1978. A new medium for axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 17.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 18.Fisher, O., R. Siman-Tov, and S. Ankri. 2004. Characterization of cytosine methylated regions and 5-cytosine DNA methyltransferase (Ehmeth) in the protozoan parasite Entamoeba histolytica. Nucleic Acids Res. 32:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haque, R., I. M. Ali, R. B. Sack, B. M. Farr, G. Ramakrishnan, and W. A. Petri, Jr. 2001. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787-1793. [DOI] [PubMed] [Google Scholar]
- 20.Haque, R., P. Duggal, I. M. Ali, M. B. Hossain, D. Mondal, R. B. Sack, B. M. Farr, T. H. Beaty, and W. A. Petri, Jr. 2002. Innate and acquired resistance to amebiasis in Bangladeshi children. J. Infect. Dis. 186:547-552. [DOI] [PubMed] [Google Scholar]
- 21.Houpt, E., L. Barroso, L. Lockhart, R. Wright, C. Cramer, D. Lyerly, and W. A. Petri. 2004. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine 22:611-617. [DOI] [PubMed] [Google Scholar]
- 22.Jones, T. R., N. Obaldia III, R. A. Gramzinski, Y. Charoenvit, N. Kolodny, S. Kitov, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 1999. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in Aotus monkeys. Vaccine 17:3065-3071. [DOI] [PubMed] [Google Scholar]
- 23.Karlin, S., I. Ladunga, and B. E. Blaisdell. 1994. Heterogeneity of genomes: measures and values. Proc. Natl. Acad. Sci. USA 91:12837-12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinman, D. M., J. Conover, and C. Coban. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67:5658-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieg, A. M. 1999. CpG DNA: a novel immunomodulator. Trends Microbiol. 7:64-65. [DOI] [PubMed] [Google Scholar]
- 26.Krieg, A. M. 2000. Immune effects and mechanisms of action of CpG motifs. Vaccine 19:618-622. [DOI] [PubMed] [Google Scholar]
- 27.Krieg, A. M., L. Love-Homan, A. K. Yi, and J. T. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428-2434. [PubMed] [Google Scholar]
- 28.Krieg, A. M., S. M. Efler, M. Wittpoth, M. J. Al Adhami, and H. L. Davis. 2004. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J. Immunother. 27:460-471. [DOI] [PubMed] [Google Scholar]
- 29.Li, E., A. Becker, and S. L. Stanley, Jr. 1989. Chinese hamster ovary cells deficient in N-acetylglucosaminyltransferase I activity are resistant to Entamoeba histolytica-mediated cytotoxicity. Infect. Immun. 57:8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotter, H., H. Russmann, J. Heesemann, and E. Tannich. 2004. Oral vaccination with recombinant Yersinia enterocolitica expressing hybrid type III proteins protects gerbils from amebic liver abscess. Infect. Immun. 72:7318-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann, B. J., B. V. Burkholder, and L. A. Lockhart. 1997. Protection in a gerbil model of amebiasis by oral immunization with Salmonella expressing the galactose/N-acetyl D-galactosamine inhibitable lectin of Entamoeba histolytica. Vaccine 15:659-663. [DOI] [PubMed] [Google Scholar]
- 32.McCluskie, M. J., R. D. Weeratna, and H. L. Davis. 2000. Intranasal immunization of mice with CpG DNA induces strong systemic and mucosal responses that are influenced by other mucosal adjuvants and antigen distribution. Mol. Med. 6:867-877. [PMC free article] [PubMed] [Google Scholar]
- 33.Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16:1216-1224. [DOI] [PubMed] [Google Scholar]
- 34.Petri, W. A., Jr., and J. I. Ravdin. 1991. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect. Immun. 59:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petri, W. A., Jr., J. Broman, G. Healy, T. Quinn, and J. I. Ravdin. 1989. Antigenic stability and immunodominance of the Gal/GalNAc adherence lectin of Entamoeba histolytica. Am. J. Med. Sci. 297:163-165. [DOI] [PubMed] [Google Scholar]
- 36.Petri, W. A., Jr., M. D. Chapman, T. Snodgrass, B. J. Mann, J. Broman, and J. I. Ravdin. 1989. Subunit structure of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J. Biol. Chem. 264:3007-3012. [PubMed] [Google Scholar]
- 37.Petri, W. A., Jr., R. D. Smith, P. H. Schlesinger, C. F. Murphy, and J. I. Ravdin. 1987. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J. Clin. Investig. 80:1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravdin, J. I., C. F. Murphy, R. A. Salata, R. L. Guerrant, and E. L. Hewlett. 1985. N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. I. Partial purification and relation to amoebic virulence in vitro. J. Infect. Dis. 151:804-815. [DOI] [PubMed] [Google Scholar]
- 39.Schain, D. C., R. A. Salata, and J. I. Ravdin. 1992. Human T-lymphocyte proliferation, lymphokine production, and amebicidal activity elicited by the galactose-inhibitable adherence protein of Entamoeba histolytica. Infect. Immun. 60:2143-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schain, D. C., R. A. Salata, and J. I. Ravdin. 1995. Development of amebicidal cell-mediated immunity in gerbils (Meriones unguiculatus) immunized with the galactose-inhibitable adherence lectin of Entamoeba histolytica. J. Parasitol. 81:563-568. [PubMed] [Google Scholar]
- 41.Séguin, R., B. J. Mann, K. Keller, and K. Chadee. 1997. The tumor necrosis factor alpha-stimulating region of galactose-inhibitable lectin of Entamoeba histolytica activates gamma interferon-primed macrophages for amebicidal activity mediated by nitric oxide. Infect. Immun. 65:2522-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seydel, K. B., S. J. Smith, and S. L. Stanley, Jr. 2000. Innate immunity to amebic liver abscess is dependent on gamma interferon and nitric oxide in a murine model of disease. Infect. Immun. 68:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soong, C. J., K. C. Kain, M. Abd-Alla, T. F. Jackson, and J. I. Ravdin. 1995. A recombinant cysteine-rich section of the Entamoeba histolytica galactose-inhibitable lectin is efficacious as a subunit vaccine in the gerbil model of amebic liver abscess. J. Infect. Dis. 171:645-651. [DOI] [PubMed] [Google Scholar]
- 44.Stacey, K. J., and J. M. Blackwell. 1999. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect Immun. 67:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weeratna, R. D., M. J. McCluskie, Y. Xu, and H. L. Davis. 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 18:1755-1762. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. 1997. Amoebiasis. Wkly. Epidemiol. Rec. 72:97-99. [Google Scholar]
- 47.Zhang, T., and S. L. Stanley, Jr. 1994. Protection of gerbils from amebic liver abscess by immunization with a recombinant protein derived from the 170-kilodalton surface adhesin of Entamoeba histolytica. Infect. Immun. 62:2605-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]