Abstract
Microsporidia form environmentally resistant spores that are critical for their host-to-host transmission and persistence in the environment. The spore walls of these organisms are composed of two layers, the exospore and the endospore. Two spore wall proteins (SWP1 and SWP2) have been previously identified in members of the Encephalitozoonidae family. These proteins localize to the exospore. The endospore is known to contain chitin, and a putative glycosylphosphatidylinositol (GPI)-anchored chitin deacetylase has been localized to the plasmalemma-endospore interface. Using proteomic techniques, we have identified a new spore wall protein (SWP3) that is located in the endospore. The gene for this protein is located on chromosome 1 and corresponds to the open reading frame ECU01_1270. SWP3 is predicted to have a signal peptide and to be GPI anchored. Consistent with these modifications, two-dimensional electrophoresis demonstrated that SWP3 has an acidic pI and a molecular mass of <20 kDa. By immunoelectron microscopy, this protein was found on the cell surface during sporogony and in the endospore in mature spores. SWP3 has several potential O-glycosylation sites, and it is possible that it is a mannosylated protein like the major polar tube protein (PTP1).
Microsporidia are eukaryotic obligate intracellular spore-forming parasites in the phylum Microsporidia (34, 35). They are ubiquitous in the animal kingdom, with over a thousand species parasitizing a wide range of invertebrate and vertebrate hosts, including humans (44). Microsporidia were first recognized as human pathogens over 75 years ago, but prior to the AIDS epidemic there were less than a dozen reports of human microsporidiosis (44). Since the recognition that Enterocytozoon bieneusi causes diarrhea in patients with AIDS (11), many infections with different species of microsporidia have been reported from all over the world. Microsporidia are now also recognized as etiologic agents of infections in organ transplant recipients, patients being treated with immunosuppressive drugs, and immunocompetent patients (28, 30, 33, 39, 44). Although the phylum Microsporidia consists of nearly 150 genera, only 7 genera (Enterocytozoon, Encephalitozoon [including Septata], Pleistophora, Trachipleistophora, Vittaforma, Brachiola, and Nosema) as well as a few unclassified microsporidia (e.g., Microsporidium) have been described as pathogens in humans (14, 44). Microsporidia have been found in municipal water supplies, tertiary sewage effluent, and groundwater (12). It is likely that many human infections with microsporidia are of zoonotic origin (10).
Recent phylogenetic data suggest that microsporidia are related to the fungi (22, 42) and that they have a mitochondrial relic organelle, the mitosome (43). The fungal origin of microsporidia has a significant impact on how we interpret their unusual characteristics, as these no longer represent ancestral features but instead are indicative of the highly derived nature of these intracellular parasites. Members of the microsporidian family Encephalitozoonidae have very small genomes (2.9, 2.6, and 2.3 Mb for Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis, respectively) (2, 44), and the entire genome of Encephalitozoon cuniculi has been obtained (21).
The microsporidian life cycle consists of a proliferative phase, the spore production phase (sporogony), and the mature spore, or infective, phase. The unicellular spore has a resistant spore wall with a uninucleate or binucleate sporoplasm and an extrusion apparatus consisting of a single polar tube with an anterior attachment complex, which is characteristic of the phylum (37, 44). Spores range in size from 1 to 12 μm. The spore coat consists of an electron-dense, proteinaceous exospore, an electron-lucent endospore composed of chitin and protein, and an inner membrane, or plasmalemma (37, 44). The invasion apparatus consists of a long polar tube which is divided into the following two regions: the anterior straight portion, which is surrounded by a lamellar polaroplast and attached to the inside of the anterior end of the spore by an anchoring disk, and the posterior coiled region, which forms from 4 to approximately 30 coils around the sporoplasm in the spore, depending on the species (37, 44). When triggered by appropriate stimuli, the polar tube rapidly discharges from the anterior pole of the spore, forming a hollow tube that remains attached to the anterior end of the spore (17), and the sporoplasm flows through the tube, appearing as a droplet at its distal end.
In the past few years, three different proteins, polar tube protein 1 (PTP1), PTP2, and PTP3 (8, 9, 24-26, 31), have been identified as being components of the polar tube. We had previously developed a purification protocol for polar tubes and spore walls that involved glass bead disruption of spores followed by detergent and urea washes. Both the polar tube and the spore wall remained intact following these washes; however, when this material was treated with reducing agents (e.g., dithiothreitol [DTT]), the polar tube was solubilized, leaving a resistant spore wall. This solubilized material clearly contained polar tube proteins and, most likely, spore wall components that were solubilized by reducing agents. Using high-performance liquid chromatography (HPLC), we demonstrated that PTP1 could be purified from this DTT-solubilized polar tube preparation. The availability of the E. cuniculi genome has made a proteomic approach feasible for the characterization of components in this DTT-solubilized polar tube protein preparation, using both mass spectrometry and two-dimensional isoelectric focusing-polyacrylamide gel electrophoresis (2D-IEF-PAGE). In performing this characterization, we have identified that the open reading frame ECU01_1270 encodes a spore wall protein (SWP3) that localizes to the endospore and plasmalemma of Encephalitozoon cuniculi.
(Portions of this work were part of the Ph.D. thesis of Yanji Xu [45a].)
MATERIALS AND METHODS
Cell culture.
Encephalitozoon cuniculi was cultivated in RK13 cells (CCL37; American Type Culture Collection, Manassas, Va.). Infected RK13 cells were maintained in continuous culture in minimum essential medium (MEM) supplemented with 7% heat-inactivated fetal calf serum and 1% penicillin-streptomycin-amphotericin B (Invitrogen, Carlsbad, CA). Cultures were subpassaged every 3 weeks as previously described (24). Seven to 10 days after the infection of nearly confluent RK13 cells in T75 flasks, spores were harvested from the medium. Supernatants from infected flasks containing microsporidian spores were collected twice weekly and replaced with fresh medium, and the free spores of E. cuniculi were purified from the collected cell culture medium by centrifugation at 2,500 × g. These spores were washed three times in phosphate-buffered saline (PBS), using 20 ml of PBS for each wash, and then filtered through a 12.0-μm and then a 3.0-μm Nuclepore filter. Spore concentrations were determined by counting spores using a Neubauer hemocytometer.
Preparation of DNA.
Spores were treated with 0.2% sodium dodecyl sulfate (SDS) for 20 min, washed with PBS, and resuspended in proteinase K buffer (10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 150 mM NaCl, 0.4% SDS) (23). Purified spores were then disrupted using acid-washed 500-μm glass beads in a Mini-Bead beater (Biospec Products Inc., Bartlesville, OK). Proteinase K was added to the disrupted spores, and the solution was incubated for 15 min at 65°C. DNAs were then prepared by phenol-chloroform extraction followed by ethanol precipitation. Purified microsporidian DNA was dissolved in Tris-EDTA (TE) buffer and stored at −20°C.
Preparation of DTT-solubilized polar tube proteins.
Purified spores were centrifuged at 2,000 × g and resuspended in PBS at a concentration of approximately 109 per ml of PBS. Spores (5 × 108 to 7 × 109) were disrupted with glass beads, sequentially extracted with 1% SDS five times and 9 M urea once, and then solubilized in 2% DTT as previously published (23, 24, 26).
Two-dimensional gel electrophoresis.
A Bio-Rad protein IEF cell system (Bio-Rad, Hercules, CA) was used for 2-D electrophoresis. Ten to 30 μl of protein (DTT-solubilized PTPs) was mixed with rehydration buffer {8 to 9.8 M urea, 1 to 4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 15 to 100 mM DTT, 0 to 0.2% [wt/vol] Bio-Lytes, 0.001% bromophenol blue} to a final volume of 125 μl. This rehydration buffer with a protein sample was placed in a channel in the focusing tray, and then a 7-cm immobilized pH gradient strip (pH 3 to 10) was placed on this buffer and run at 50 V for 12 to 16 h for rehydration. Once the immobilized pH gradient strip was rehydrated, it was isoelectrically focused using the preset Bio-Rad protein IEF program. After isoelectric focusing, the strip was equilibrated for 15 min with equilibration buffer I (6 M urea, 2% SDS, 0.375 M Tris-HCl, pH 8.8, 20% glycerol, 1.3 mM DTT) followed by 15 min with equilibration buffer II (6 M urea, 2% SDS, 0.375 M Tris-HCl, pH 8.8, 20% glycerol, 135 mM iodoacetamide). The strip was then placed on the top of a 10% polyacrylamide slab gel for SDS-PAGE at 100 V for 1.5 h. After electrophoresis, the gel was either stained with Coomassie blue for protein identification and mass mapping or transferred to a nitrocellulose membrane at 100 V for 1.5 to 2 h for use in immunoblot analysis.
Mass spectrometry.
Solubilized E. cuniculi PTPs were run in a 2D SDS-PAGE gel and stained with Coomassie blue. Selected bands were excised from the 2D gel and subjected to reduction with 10 mM dithiothreitol and alkylation with 55 mM iodoacetamide, followed by in-gel trypsin digestion (43a). The digest was purified and concentrated using a C18 ZipTip (Millipore Corp., Bedford, MA). The tryptic peptides were eluted with 1.5 μl of 10-mg/ml α-cyano-4-hydroxycinnamic acid in 50% acetonitrile-water (vol/vol) containing 0.1% trifluoroacetic acid. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry was performed on an Applied Biosystems Voyager-DE STR biospectrometry workstation (Foster City, CA) equipped with a 2.0-m flight tube and a 337-nm nitrogen laser. The system was operated in the reflectron mode with an accelerating voltage of 20 kV using delayed extraction. A total of 150 scans were summed to obtain the spectrum. Database searches were performed using the Profound algorithm (http://129.85.19.192/profound_bin/WebProFound.exe) and the Encephalitozoon cuniculi genome database (http://www.ncbi.nlm.nih.gov/mapview/map_search.cgi?taxid=6035). Profound calculates the probability that a candidate in a database search is the protein being analyzed as a Z score (http://129.85.19.192/profound/zscore.pdf).
A tryptic digest of the solubilized E. cuniculi PTP mixture was also analyzed by high-performance liquid chromatography (HPLC) coupled with tandem mass spectrometry (MS/MS). LC/MS/MS analysis was performed using a Dionex LC Packings Switchos microcolumn switching unit and a Dionex LC Packings UltiMate nano-HPLC system (Sunnyvale, CA) linked to an Applied Biosystems Qstar Pulsar I hybrid quadrupole time-of-flight mass spectrometer (Foster City, CA) equipped with a micro-ion-spray device. The sample was loaded onto a 300-μm (inner diameter) by 5-mm reversed-phase precolumn and desalted before being backflushed onto an analytical column. Solvent A was 2% acetonitrile containing 0.1% formic acid, and solvent B was 80% acetonitrile containing 0.1% formic acid. Separation was achieved on a nano-LC column (75 μm inner diameter × 150 mm) packed with Vydac C18 (5-μm particles) at a flow rate of 200 nl/min, with a gradient from 2% to 42% solvent B in 30 min followed by a 5-min gradient from 42% to 90% solvent B. MS/MS data were searched using Mascot (Matrix Science Inc., Boston, Mass.) against eukaryotes in the NCBInr database, with a peptide mass tolerance of 0.3 Da and a fragment mass tolerance of 0.8 Da.
In silico analysis.
Protean and SeqMan (Lasergene; DNASTAR Inc., Madison, WI) were used for characterization of the open reading frames of identified proteins and for predictions of pI and molecular mass. Protein localization and posttranslational modifications were predicted using the following bioinformatic programs available on the Internet: PSORT (http://www.psort.org/), NetOglyc (http://www.cbs.dtu.dk/services/NetOGlyc/), NetNglyc (http://www.cbs.dtu.dk/services/NetNGlyc/), the glycosylphosphatidylinositol (GPI) modification site predictor (big-PI predictor; http://mendel.imp.univie.ac.at/sat/gpi/gpi_server.html), DGPI http://129.194.185.165/dgpi/index_en.html), and SignalP (http://www.cbs.dtu.dk/services/SignalP/). Homologs were identified using BLAST (protein- protein BLAST [http://www.ncbi.nlm.nih.gov/BLAST/]).
Construction and expression of glutathione S-transferase (GST) fusion proteins.
Oligonucleotide primers Ec6-forward (5′-CGGAATTCATGGTTAGAAGGAGCTG), containing an EcoRI restriction site (underlined), and Ec6-reverse (5′-CCGCTCGAGTTACATCACAATTGAGAAC), containing an XhoI restriction site (underlined) to facilitate cloning, were designed based on the predicted open reading frame ECU01_1270. The gene was amplified using Perkin-Elmer GeneAmp PCR system 2400 (Perkin-Elmer Corporation, Foster City, CA), using a program of 95°C for 4 min followed by 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 3 min and then a final step of 72°C for 7 min. Each PCR mixture contained 1 μg of E. cuniculi DNA, 1.5 mM MgCl2, 1× PCR buffer minus Mg2+, a 0.2 mM concentration of each deoxynucleoside triphosphate, 100 ng of each primer, and 2.5 units of Platinum Taq DNA polymerase. The PCR products were digested with EcoRI and XhoI and ligated into pGEX 4T-1 (Pharmacia Biotech, Piscataway, NJ) that had been digested with EcoRI and XhoI. Recombinant vectors were sequenced on an ABI Prism model 377 DNA sequencer (Perkin-Elmer Corporation, Foster City, CA), using a pGEX 3′ sequencing primer flanking the insertion site as well as additional 18- to 20-bp oligomers selected using Oligo primer analysis software (NBI, Plymouth, MN) based on the observed sequence data. Sequence data sets were assembled using the SeqMan program of the LaserGene software package (DNASTAR, Madison, WI). DNA and protein homology was examined with BLAST (http://www.ncbi.nlm.nih.gov/BLAST/), protein motifs were determined with PROSITE (GCG, version 8.0; GCG, Madison, WI), and phylogenetic analysis was performed using MegAlign (DNASTAR, Madison, WI).
After verifying the DNA sequence, the recombinant plasmid pGEX-ECU01_1270 was transformed into Escherichia coli DH5α, and GST-ECU01_1270 fusion proteins were expressed. A single colony of E. coli containing the pGEX-ECU01_1270 plasmid was used to inoculate 10 ml of LB medium for 6 to 9 h at 37°C with vigorous shaking, followed by 1:100 dilution in fresh prewash LB medium at 30°C with shaking until the A600 reached 1.0. After the addition of 100 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to induce protein expression for an additional 6 h, the cells were spun down and resuspended in PBS. The cells were then lysed by sonication in PBS-Triton X-100 and centrifuged at 12,000 rpm for 10 min at 4°C, and the supernatant was placed onto a glutathione-Sepharose 4B (Pharmacia Biotech, Piscataway, NJ) column. After being washed with 10 column volumes of PBS, the GST-ECU01_1270 fusion protein was eluted using glutathione elution buffer (Pharmacia, Piscataway, NJ).
Antibody production and immunoblotting.
Three mice were immunized intradermally (at the base of the tail) with 0.25 μg of GST-ECU01_1270 fusion protein mixed with Hunter's Titermax adjuvant (1:1 [vol/vol]) and then boosted at 6 weeks. At 12 weeks, sera were obtained from all three mice and pooled. Polyclonal mouse-anti-GST-ECU01_1270 serum and an anti-GST monoclonal antibody (a gift of Peter Davies, Albert Einstein College of Medicine) were used for immunoblot studies employing the E. cuniculi DTT preparation and lysates from E. coli expressing GST-Ec6. For immunoblot analysis, proteins were transferred to a nitrocellulose membrane after 1D or 2D polyacrylamide gel electrophoresis. Membranes were blocked with 10 ml of BLOTTO (5% fat-free dried milk powder in PBS containing 0.1% Tween 20) solution for 1 h at room temperature and then incubated with 1:5,000 to 1:20,000 diluted mouse anti-GST-ECU01_1270 or mouse monoclonal anti-GST in BLOTTO for 1 h at room temperature. Membranes were washed twice in PBS containing 0.1% Tween 20 and incubated with a 1:5,000 diluted goat anti-mouse alkaline phosphatase-conjugated antibody, and reactivity was detected using disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricycle[3.3.1.13,7]decan}-4-yl)phenyl phosphate as a chemiluminescent reagent (Western Light; Tropix, Bedford, MA) (25).
Immunofluorescence assay.
A slide culture chamber was used to culture E. cuniculi in RK13 cells for 3 days. Slides were fixed with 2% formaldehyde in PBS for 30 min, washed with PBS three times, and then blocked with 1% bovine serum albumin (BSA) overnight at 4°C. After blocking of the slides, an antibody (1:200 diluted mouse anti-GST-ECU01_1270 fusion protein polyclonal antibody, 1:100 diluted rabbit anti-recombinant E. hellem PTP1 [anti-EhPTP1] as a positive control, or 1:50 diluted mouse anti-GST monoclonal antibody as a negative control in 1% BSA) was then added to different slide wells. Slides were incubated at 37°C in a moist chamber for 90 min, washed with PBS, incubated with a fluorescein-conjugated goat affinity-purified antibody to mouse immunoglobulin G (IgG; whole molecule) (Organon Teknika Corp., West Chester, PA) or a fluorescein-conjugated goat IgG fraction specific to rabbit immunoglobulins (IgG, IgA, and IgM) (Organon Teknika Corp.) in 1% BSA, washed with PBS, and mounted with PBS containing 2.5% DABCO [1,4-diazabicyclo (2,2,2)octane; Sigma, St. Louis, MO]. Slides were observed and photographed using an inverted Nikon Diaphot microscope equipped to detect fluorescence.
Electron microscopy.
Rabbit kidney cells (RK13) were grown to 70 to 80% confluence in flasks, using the previously described culture medium and conditions. The culture medium was removed, and 1 ml of 1 × 107/ml E. cuniculi spores in medium was added to each cell culture flask for 1 h. After this initial exposure, 4 ml of additional fresh culture medium was added to each flask. The exposed cultures were maintained for 14 days, with medium changes every 3 to 4 days. On the 14th day, the medium was removed, and 1 ml of trypsin-EDTA was added to the cells for 3 to 4 min with rocking to release the cells from the substrate. An additional 4 to 5 ml of complete medium was added to each flask. The medium with cells was removed from each flask, added to four 1.5-ml microcentrifuge tubes, and centrifuged in an Eppendorf 5414 centrifuge for 2 minutes at 12,000 rpm. The supernatant was removed from the tubes, leaving a pellet of cells that were then fixed for immunogold electron microscopy with 2.0% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, for 1 hour. The fixed pellets were rinsed several times in buffer, dehydrated with graded ethanols, and infiltrated with LR White resin overnight. The resin was polymerized at room temperature by exposure to a 254-nm UV light source for 8 to 12 h. Thin sections of the LR White blocks were placed on 300-mesh nickel grids coated with Formvar and carbon. Grids were incubated in blocking buffer (1% bovine serum albumin [Sigma fraction V], 0.02% sodium azide in PBS, pH 7.35, with 5% goat serum) at 4°C for 12 h, followed by incubation with a 1:50 dilution of primary antiserum for 1 h at 25°C, rinsed in PBS, and then incubated with a 1:25 dilution of goat anti-mouse IgG conjugated to 12-nm colloidal gold (Jackson ImmunoResearch, West Grove, PA) at 25°C for 1 h (24). Grids were rinsed with PBS, dried, strained with uranyl acetate or lead citrate, and then examined and photographed with a Phillips/FEI Tecnai 12 transmission electron microscope at the Rutgers University at Newark Electron Microscopy Facility (23).
RESULTS
Analysis of DTT-solubilized polar tube preparation from Encephalitozoon cuniculi.
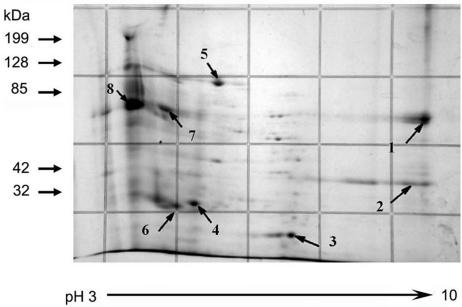
We previously developed a reversed-phase HPLC method to purify PTP1 from a DTT-solubilized polar tube preparation (23, 24, 26). Using this method, four peaks could be identified in a DTT-solubilized polar tube preparation of Glugea americanus. Similar peaks were seen for E. cuniculi, and for G. americanus these peaks corresponded to the major bands of 43, 34, 27, and 22 kDa visualized in a Coomassie blue-stained SDS-PAGE gel (23, 24, 26). The DTT-solubilized polar tube protein preparation from E. cuniculi was examined using 2D electrophoresis and demonstrated many minor and major protein spots (Fig. 1). Eight major spots (Ec1 through Ec8) were selected for analysis by mass spectrometry. By immunoblotting using a rabbit polyclonal antibody to E. hellem PTP1 (anti-EhPTP1), which cross-reacts with E. cuniculi PTP1 (EcPTP1) (25), Ec8 was identified as EcPTP1 (data not shown). Since the predicted peptides of EcPTP1 from trypsin digestion were either too small or too large for mass mapping analysis, reliable mass spectrometry confirmation of this spot was not possible. Ec7 was identified as being similar to chitin synthase 1 (ECU01_1390); however, the observed pI was not consistent with the predicted pI of this protein. Ec1 was predicted (Z = 1.11) to be translation elongation factor 1 alpha (ECU04_1100). Given the acidic charge of PTP1, we believe that translation elongation factor 1 alpha binds to PTP1 when the spores are disrupted in the purification process. Ec5 was predicted (Z = 1.14) to be an HSP70-related protein (ECU2_0100). This chaperone may also bind to proteins when spores are disrupted during the purification process. Ec2, Ec3, Ec4, and Ec6 were predicted to be hypothetical proteins in the E. cuniculi genome (ECU07_0530, ECU09_1290, ECU10_1620, and ECU01_1270, respectively). Of these proteins, ECU01_1270 had a Z value of 0.88 (Fig. 2).
FIG. 1.
Two-dimensional electrophoresis of DTT-solubilized polar tube protein preparation. The DTT-solubilized polar tube preparation was subjected to IEF using the Bio-Rad IEF system followed by polyacrylamide gel electrophoresis. The gel was stained with Coomassie blue. The arrows point to eight spots (Ec1 through Ec8) selected for mass spectrometry (mass mapping). Ec8 in the immunoblot corresponds to PTP1. Ec6 corresponds to ECU01_1270. In addition to the eight selected spots, many other protein spots can be seen in this DTT-solubilized polar tube preparation.
FIG. 2.
Amino acid sequence of ECU01_1270 (i.e., SWP3). The signal sequence (first 17 amino acids) is indicated in bold. The predicted omega cleavage site (serine 193) is shaded, and the GPI anchor signal sequence is underlined. There is a KS-rich region in the C-terminal region of this protein. The GenBank accession number for this protein is CAD25000.
An examination of the DTT-solubilized polar tube preparation from E. cuniculi using LC/MS/MS demonstrated the presence of at least 30 potential proteins from the E. cuniculi genome in this preparation. As expected, PTP2 was identified in this mixture. Due to the glycosylation of PTP1 and its paucity of trypsin sites, this protein does not yield fragments that are useful for mass spectrometry, and no fragments were identified by LC/MS/MS. In addition, it was clear that some components of the DTT-solubilized polar tube preparation came from the spore wall, as peptides from spore wall protein 1 (SWP1) were present. Both translation elongation factor 1 alpha (17 peptides) and the heat shock-related 70-kDa protein (11 peptides) were found in the analysis. The majority of the identified peptides were from hypothetical proteins of unknown function; however, several of the peptides mapped to ECU01_1270 (e.g., Ec6). We therefore sought to determine the subcellular localization of ECU01_1270.
In silico analysis of ECU01_1270.
The ECU01_1270 open reading frame is 663 bp long, encoding a protein of 221 amino acids with a calculated pI of 8.4 and a molecular weight of 22,506. There is a predicted 17-amino-acid signal sequence (Fig. 2, bold text), resulting in a mature protein with a predicted pI of 5.9 and a molecular weight of 20,539. This is consistent with the location of this protein (e.g., Ec6) on 2D-IEF-PAGE gels. By PSORT (http://www.psort.org/), ECU01_1270 is predicted to be an extracellular (e.g., cell wall) protein. The enzymes for N-linked glycosylation do not appear to be present in the E. cuniculi genome, but those for O-linked glycosylation (including mannosylation) are clearly present. According to NetOglyc (http://www.cbs.dtu.dk) and NetNglyc (http://www.cbs.dtu.dk), no N-glycosylation sites are predicted in ECU01_1270; however, 24 O-glycosylation sites are predicted, with the majority being in the central region of this protein. There is a very basic C-terminal KS-rich domain (Fig. 2). Interestingly, this protein is predicted to have a GPI anchor by both DGPI and the big-PI predictor (GPI modification site predictor [http://mendel.imp.univie.ac.at/sat/gpi/gpi_server.html]), with the omega cleavage site being at the serine at position 193 (Fig. 2, highlighted serine residue in the basic KS domain). The presence of such GPI-anchored proteins has not been experimentally determined with microsporidia, but enzymes such as GlcNAc-Pi that are associated with GPI anchor formation are clearly present in the E. cuniculi genome (21, 36).
Construction and expression of an ECU01_1270-GST fusion protein.
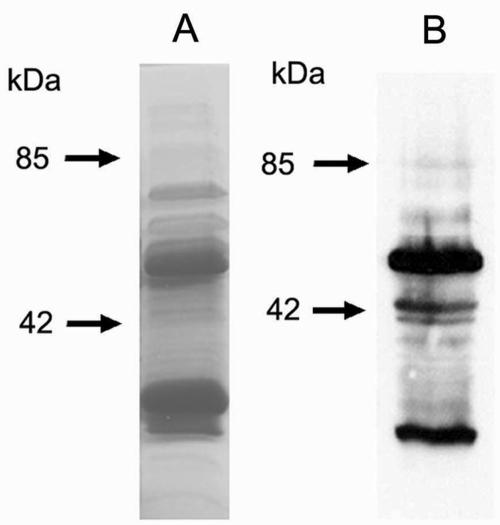
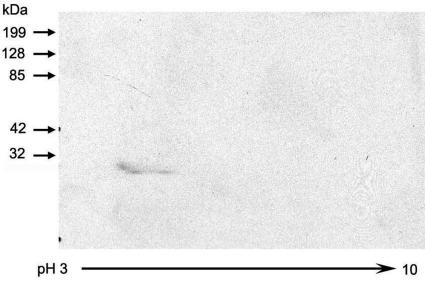
Two oligonucleotide primers corresponding to the entire open reading frame of ECU01_1270, one with an EcoRI restriction site and one with an XhoI restriction site, were used for PCR to amplify ECU01_1270 from the E. cuniculi genome. PCR-amplified ECU01_1270 was ligated into EcoRI- and XhoI-digested pGEX 4T-1 vector (with an N-terminal GST tag) to generate pGEX-ECU01_1270. The recombinant protein was expressed in E. coli and isolated by affinity chromatography using a glutathione-Sepharose 4B column. As demonstrated in Fig. 3 by both Coomassie-stained SDS-PAGE and immunoblotting using an anti-GST monoclonal antibody, the ECU01_1270-GST fusion protein was evident at ∼51 kDa, and free GST was present at ∼29 kDa. The fusion protein was cut out of a preparative SDS-PAGE gel, and three mice were immunized with the purified fusion protein. An immunoblot of a 2D-IEF-PAGE gel containing the DTT-solubilized E. cuniculi preparation clearly demonstrated reactivity of the antiserum to ECU01_1270 with the area corresponding to Ec6 (Fig. 4).
FIG. 3.
Purification of recombinant ECU01_1270. (A) Coomassie blue-stained SDS-PAGE gel containing recombinant ECU01_1270 protein after elution from a glutathione-Sepharose 4B column. Bands for the full-length recombinant protein (∼51 kDa) and free GST (∼29 kDa) are evident. The presence of free GST may be due to the use of the full-length reading frame of ECU01_1270, including the N-terminal signal sequence. (B) Immunoblot of SDS-PAGE gel of recombinant ECU01_1270 protein, using a monoclonal antibody against the GST label. The full-length (∼51 kDa) recombinant protein and free GST (∼29 kDa) reacted with the anti-GST monoclonal antibody.
FIG. 4.
Immunoblot of 2D-IEF-PAGE of DTT-solubilized protein preparation. The mouse polyclonal antiserum to recombinant ECU01_1270 localizes to the protein spot Ec6 identified previously by 2D-IEF-PAGE (see Fig. 1).
Expression of ECU01_1270 in E. cuniculi spores in tissue culture.
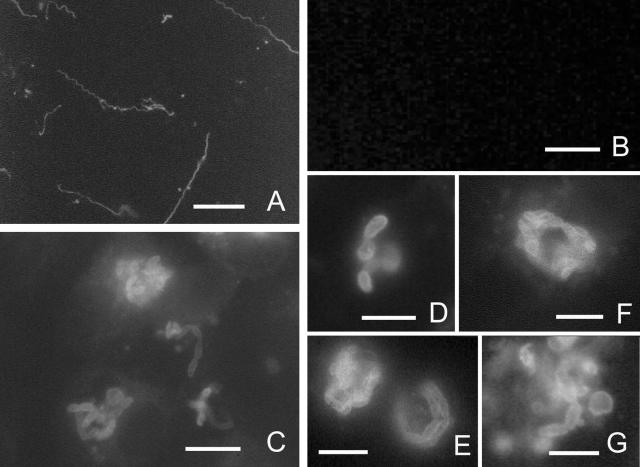
The antiserum to ECU01_1270 was used in an immunofluorescence assay with E. cuniculi cultured in RK13 cells (Fig. 5). As controls, rabbit anti-rEhPTP1 (which cross-reacts with E. cuniculi PTP1) and an anti-GST monoclonal antibody were used. Rabbit anti-rEhPTP1 clearly reacted with E. cuniculi polar tubes (Fig. 5A), and no fluorescence was seen with anti-GST (Fig. 5B). Mouse anti-ECU01_1270 was demonstrated to bind to the E. cuniculi spores, including developing stages at the edge of a parasitophorous vacuole (Fig. 5C through G). The fluorescence pattern is consistent with this protein being located in the spore wall of E. cuniculi. Mouse anti-ECU01_1270 did not react with RK13 cells.
FIG. 5.
Immunofluorescence microscopy of Encephalitozoon cuniculi in tissue culture. (A) Anti-EhPTP1 serum (1:100) demonstrating staining of E. cuniculi polar tubes. Bar, 10 μm. (B) There is no reactivity of anti-GST serum (1:50) with this organism. Bar, 10 μm. (C) Photomicrograph (×400) of the reactivity of anti-recombinant ECU01_1270 serum with E. cuniculi. This serum reacts with the spore wall and also stains some elongated proliferating forms at the edge of the vacuole (consistent with staining sporont membranes). Bar, 10 μm. (D) Photomicrograph (×1,000) of the reactivity of anti-recombinant ECU01_1270 serum with mature spores, demonstrating staining of the spore wall. (E to G) Photomicrograph (×1,000) of RK13 cells containing proliferating stages of E. cuniculi staining with anti-recombinant ECU01_1270 serum. Bars, 5 μm.
Ultrastructural localization and developmental expression of ECU01_1270.
Immunogold electron microscopy was used to locate ECU01_1270 in E. cuniculi during development. Electron microscopic examination of both the control (no ECU01_1270 antibody) and experimental (ECU01_1270 antibody present) sections showed comparable numbers of infected cells and parasites at different stages of parasite development. The cytoplasm of approximately 60% of the rabbit kidney cells examined contained at least one phagosome-like parasitophorous vesicle (vacuole). This morphological entity is a characteristic of the genus Encephalitozoon and aids in the initial identification of infected host cells. Early E. cuniculi infections appear as a small vesicle or vacuole in the host cell cytoplasm (Fig. 6A). Uninucleate proliferative parasite cells have a relatively simple cytoplasm covered by a typical “thin” plasmalemma (Fig. 6A) and are tightly abutted to the interfacial envelope (the inner periphery of the parasitophorous vacuole). As the parasite cells increase in number, the vacuole increases in size, and more mature parasite cells are observed in the vacuolar space. The maturity of the parasite stages can be assessed by the thickening of the plasmalemma. Parasites in the sporogonic stage of development (early sporonts) have a number of irregular surface thickenings on the plasmalemma, giving the membrane a scalloped appearance (Fig. 6A). The sporonts are generally clustered in the center of the vacuole, while the proliferative-stage parasites tend to remain abutted to the vacuole interface. The sporont surface coat eventually becomes uniformly thick (Fig. 6A), and the sporont eventually transitions into a sporoblast. The sporoblast is an irregularly shaped dense cell with a uniformly thick surface coat and a cytoplasm that contains portions of the developing polar tube, a Golgi complex, and other organelles (Fig. 6B). Sporoblasts metamorphose into spores that contain a dense cytoplasm, a polar tube-anchoring disk complex, and a single nucleus (Fig. 6C). The surface of the spore plasmalemma is abutted by a relatively thick electron-lucent endospore that is in turn covered by an electron-dense exospore coat (Fig. 6C). The host cell cytoplasm and the proliferative and sporogonic E. cuniculi parasites in the control sections were devoid of any gold particles (Fig. 6A, B, and C).
FIG.6.
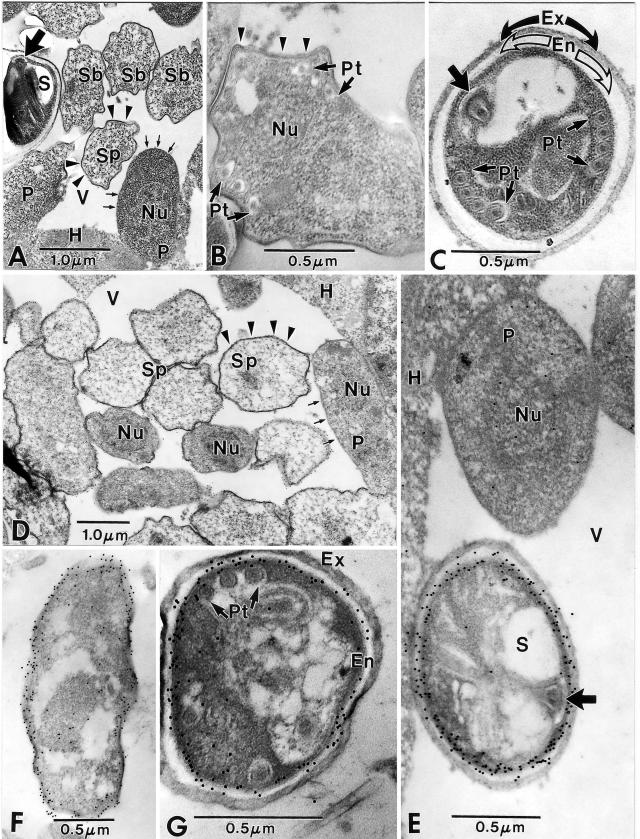
Immunoelectron microscopy of Encephalitozoon cuniculi using anti-ECU01_1270. (A to C) Control sections of Encephalitozoon cuniculi-infected rabbit kidney cells. (A) Low-magnification image of a portion of host cell cytoplasm (H) containing a parasitophorous vacuole (V) with proliferative parasite cells (P) abutted to the edge of the host-parasite interface. These cells have a simple cytoplasm, are uninucleate (Nu), and have a typical “thin” plasmalemmal surface (short arrows). The sporonts (Sp) and sporoblasts (Sb) have a “thick” dense surface coat (arrowheads) and a more complex and dense cytoplasm. The spore (S) contains a polar tube, and the anterior anchoring disk (broad arrow) is present. (B) Sporoblast containing portions of the developing polar tube (Pt), a single nucleus (Nu), and a “thick” surface coat (arrowheads). Note the irregular shape of the cell and the more complex cytoplasm. (C) Spore demonstrating groups of four cross sections of the polar tube (Pt) and its anterior anchoring disk (broad arrow). The sporoplasm is enclosed by a wide electron-lucent endospore (En), which is in turn covered by a dense, thick exospore (Ex). Note the absence of gold particles in all three images (A, B, and C). (D to G) Sections of Encephalitozoon cuniculi-infected rabbit kidney cells incubated with anti- ECU01_1270. (D) Portion of a parasitophorous vacuole (V) in the host cell cytoplasm (H) with proliferative parasite cells (P) abutted to the edge of the host-parasite interface. These cells are uninucleate (Nu) and have a typical “thin” plasmalemmal surface (short arrows). The sporonts (Sp) have a “thick” dense surface coat (arrowheads) and a more complex and dense cytoplasm. Colloidal gold particles (12 nm) are dispersed throughout the cytoplasm of the proliferative cells, while the sporogonic cells tend to have more gold localized near their plasmalemmal surfaces. There are a few gold particles at the host cell-parasite interface, but the vacuolar space (V) is free of gold. (E) High-magnification image of a portion of the host cell cytoplasm (H) and a parasitophorous vacuole (V) containing a proliferative parasite cell (P) abutted to the edge of the host-parasite interface. This uninucleate (Nu) cell has several gold particles in its cytoplasm and in the vicinity of the nucleus. The spore (S) with an anchoring disk (broad arrow) next to the proliferative cell has extensive quantities of gold distributed almost exclusively along the periphery of the plasmalemma-endospore interface. The host cell cytoplasm has a few scattered gold particles at the interface with the vacuolar space. (F) Sporont with extensive gold particles distributed along the periphery of the cell and abutting the plasmalemma and the overlying “thick” surface coat that is present during the transition to sporogony. The cytoplasm still has some diffuse gold particles in it. (G) Typical late sporoblast/early spore containing several cross sections of the polar tube and a dense sporoplasm enclosed by a well-defined plasmalemma abutted to a wide electron-lucent endospore (En) that is in turn covered by the dense exospore (Ex). A few gold particles are present in the sporoplasm, but the vast majority of them are distributed at the endospore-plasmalemma interface.
An examination of the sections incubated with ECU01_1270 antibody revealed all of the stages of development described above. Unlike the control sections (no ECU01_1270 antibody), there was some light staining with gold in the cytoplasm of parasites in the early proliferative stages and at the periphery of the vacuole (Fig. 6D and E), indicating the presence of ECU01_1270 protein. The scant number of gold particles and their diffuse distribution indicate low levels of the protein. The sporonts with “thickened” plasmalemmas had some gold particles in their cytoplasm and a noticeable concentration of gold along their thickened plasmalemmal surfaces (Fig. 6D and F). Although the cytoplasm of sporoblasts has very few gold particles, their plasmalemmas and overlying endospores contain very intense concentrations of gold (Fig. 6G). This change in gold quantity and localization from the cytoplasm during the proliferative and early sporogonic stages to the cell surface and endospore is well illustrated by comparison of these earlier stages with the spores (Fig. 6E). Overall, as demonstrated in Fig. 5, anti-ECU01_1270 clearly reacted with the spore wall, suggesting that this gene encodes a spore wall protein (which we now call SWP3) and that SWP3 is localized to the endospore.
DISCUSSION
In the microsporidia, spore wall formation is initiated early in sporogony by the deposition of electron-dense material at the plasma membrane and continues while the organisms differentiate into sporoblasts and finally into mature spores (6). The rigid spore wall protects the sporoplasm of mature spores against environmental stress and permits long-term survival in the environment after their release from host cells. The spore wall also prevents the sporoplasm from expanding when the internal pressure necessary for extrusion of the polar tube is generated (17). It has been possible to distinguish subcompartments within the spore wall by using polyclonal antisera against partially purified microsporidial proteins. A 30-kDa antigen was found to be located on the outer spore wall, while a 33-kDa protein was found in a region close to the plasma membrane (7). In addition, several monoclonal antibodies have been reported to recognize spore wall antigens (1, 29, 38). Ultrastructural studies of the genus Encephalitozoon using transmission electron microscopy, freeze fracture, and deep etching demonstrated that the exospore is very complex and consists of the following three layers: an outer spiny layer, an intermediate electron-lucent lamina, and an inner fibrous layer (3). The endospore is observed as a space crossed by bridges connecting the exospore to the plasma membrane. Chitin, a major component of the endospore, appears to be a component of the fibrils forming the bridges across the endospore and is involved in the fibrillar system of the exospore (3, 13, 37), as confirmed by an immunohistochemical study with E. intestinalis (32).
In the current study, using proteomic techniques combined with immunolocalization by light and electron microscopy, we have identified that the open reading frame ECU01_1270 in the E. cuniculi genome encodes a new spore wall protein that localizes to the endospore and endospore-plasmalemma interface. A 51-kDa spore wall protein, SWP1, localized to the exospore (4) has been identified in E. cuniculi (4) and E. intestinalis (18). The corresponding gene, swp1, has been identified in E. cuniculi (4), E. hellem (4), and E. intestinalis (18). SWP1 is absent from meronts and is first seen in early sporonts at a time when organisms translocate from the periphery to the center of the parasitophorous vacuole (4). A second spore wall protein (SWP2), a 150-kDa glycoprotein found on mature spores, has been identified in E. intestinalis (18), but the gene is not present in the E. cuniculi genome (21). It appears that both cysteine residues and the N-terminal signal sequences are conserved among SWP1 and -2, suggesting that these proteins may have similar secondary structures and functions (4, 18). Sequence analysis demonstrates that ECU01_1270 is distinct from the previously reported spore wall proteins (SWP1 and SWP2) (4, 18), and thus we have designated this protein spore wall protein 3 (SWP3).
SWP3 does not have homology, by BLAST, with any other proteins in GenBank (including cell wall proteins of fungi and bacteria). In addition, we were unable to find a homolog in the Antonospora (Nosema) locustae genome sequence survey (http://www.botany.ubc.ca/keeling/AntonosporaGSS.html); however, this genome is not yet complete. This protein is predicted by PSORT to be a secreted cell wall protein, which is consistent with its immunolocalization to the juncture of the endospore and the plasmalemma. By scanning electron microscopy, the spore coat and polar tubes are seen after glass bead-disrupted spores are washed with SDS and urea (23, 24, 26). After SDS and urea extraction, SWP3 was still present in the polar tube preparation, suggesting that SWP3 is integrated into the spore wall and not just a component of the plasma membrane (20). The prediction of a GPI anchor on this protein is consistent with its localization to the endospore-plasmalemma interface and is similar to GPI-anchored cell wall proteins described for several fungi (27). In addition, the sequence of amino acids upstream of the omega cleavage site (serine 193) and the presence of a polyserine region also suggest that ECU01_1270 would be incorporated into the spore wall (15, 16). An E. cuniculi chitin deacetylase has also been found to have a predicted GPI anchor and localizes to the endospore-plasmalemma interface (5), consistent with the presence of chitin in the endospore, as seen in fungal cell walls (3, 13, 32, 37).
The identification of ECU01_1270 from a mass mapping study of DTT-solubilized E. cuniculi PTPs was most likely a consequence of the similar solubility properties of these proteins, as both of these proteins appear to be detergent resistant and soluble in DTT. There are hints that the spore surface, besides providing mechanical protection, is involved in the initiation of polar tube extrusion and that modifications of the spore wall architecture occur during this activation (40, 41). Electron microscopy has demonstrated that at the time of spore activation, the outer spore envelopes of both Spraguea lophii and Thelohania sp. are disassembled (40, 41). For example, using antikeratin antibodies, it has been demonstrated that the outer spore wall of Thelohania sp. consists in part of keratin-like proteins that form 10-nm intermediate filaments, which become phosphorylated and disassemble during spore activation (41). Therefore, another possible explanation for the presence of SWP3 in the DTT-solubilized polar tube preparation is that there is an interaction of the endospore and polar tube proteins during germination.
We previously published techniques that permit the purification of spore coat proteins as well as polar tube proteins (23-26). With the publication of the E. cuniculi genome, this has permitted us and other investigators to initiate proteomic studies on the composition of these structures (36). PTP1 has been identified to be modified by posttranslational glycosylation involving mannosylation (45), and some of the components of the spore wall appear to be similarly modified (L. M. Weiss, unpublished observation). SWP3 contains many O-glycosylation sites and may be a mannosylated protein. In several yeast species, mannosylated proteins are found in the cell wall and have a GPI anchor that is used in the process of targeting these proteins to cell wall glucans (27). Posttranslational glycosylation in the microsporidia may be important in adherence of the spore wall to mucin or to host cells during passage of the spores in the gastrointestinal tract, thereby facilitating invasion. For example, it has been demonstrated that exogenous glycosaminoglycans can decrease the adherence of spores to a host cell monolayer (19).
The environmentally resistant spores formed by the microsporidia are critical for the transmission of these organisms and their persistence in the environment. The spore walls of these organisms are composed of two layers, the exospore and the endospore. Two spore wall proteins (SWP1 and SWP2) localized to the exospore have been previously identified in members of the family Encephalitozoonidae, and in this paper we report the identification of a new spore wall protein (SWP3) that localizes to the endospore. The immune response to microsporidia is often directed at the polar tube and spore wall, as evidenced by studies of sera from infected patients (44). Studies of the spore wall and its composition should help to define new antigens for immunoprotection studies and new diagnostic reagents. In addition, given the importance of the spore wall in the functioning of the invasion apparatus, studies of its composition should also improve our understanding of the process of germination in these ubiquitous pathogens.
Acknowledgments
This work was supported by grant AI37188 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Beckers, P. J., G. J. Derks, T. Gool, F. J. Rietveld, and R. W. Sauerwein. 1996. Encephalocytozoon intestinalis-specific monoclonal antibodies for laboratory diagnosis of microsporidiosis. J. Clin. Microbiol. 34:282-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biderre, C., A. Mathis, P. Deplazes, R. Weber, G. Metenier, and C. P. Vivares. 1999. Molecular karyotype diversity in the microsporidian Encephalitozoon cuniculi. Parasitology 118:439-445. [DOI] [PubMed] [Google Scholar]
- 3.Bigliardi, E., M. G. Selmi, P. Lupetti, S. Corona, S. Gatti, M. Scaglia, and L. Sacchi. 1996. Microsporidian spore wall: ultrastructural findings on Encephalitozoon hellem exospore. J. Eukaryot. Microbiol. 43:181-186. [DOI] [PubMed] [Google Scholar]
- 4.Bohne, W., D. J. Ferguson, K. Kohler, and U. Gross. 2000. Developmental expression of a tandemly repeated, glycine- and serine-rich spore wall protein in the microsporidian pathogen Encephalitozoon cuniculi. Infect. Immun. 68:2268-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosson, D., L. Kuhn, G. Prensier, C. P. Vivares, and C. Texier. 2005. The putative chitin deacetylase of Encephalitozoon cuniculi: a surface protein implicated in microsporidian spore-wall formation. FEMS Microbiol. Lett. 247:81-90. [DOI] [PubMed] [Google Scholar]
- 6.Canning, E. U., and J. Lom. 1986. The microsporidia of vertebrates. Academic Press, London, England.
- 7.Delbac, F., F. Duffieux, D. David, G. Metenier, and C. P. Vivares. 1998. Immunocytochemical identification of spore proteins in two microsporidia, with emphasis on extrusion apparatus. J. Eukaryot. Microbiol. 45:224-231. [DOI] [PubMed] [Google Scholar]
- 8.Delbac, F., I. Peuvel, G. Metenier, E. Peyretaillade, and C. P. Vivares. 2001. Microsporidian invasion apparatus: identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species. Infect. Immun. 69:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delbac, F., P. Peyret, G. Metenier, D. David, A. Danchin, and C. P. Vivares. 1998. On proteins of the microsporidian invasive apparatus: complete sequence of a polar tube protein of Encephalitozoon cuniculi. Mol. Microbiol. 29:825-834. [DOI] [PubMed] [Google Scholar]
- 10.Deplazes, P., A. Mathis, and R. Weber. 2000. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 6:236-260. [DOI] [PubMed] [Google Scholar]
- 11.Desportes, I., Y. Le Charpentier, A. Galian, F. B. Bernard, B. Cochand-Priollet, A. Lavergne, P. Ravisse, and R. Modigliani. 1985. Occurrence of a new microsporidian: Enterocytozoon bieneusi n. g., n. sp., in the enterocytes of a human patient with AIDS. J. Protozool. 32:250-254. [DOI] [PubMed] [Google Scholar]
- 12.Dowd, S. E., C. P. Gerba, and I. L. Pepper. 1998. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl. Environ. Microbiol. 64:3332-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson, B., and R. Blanquet. 1969. The occurrence of chitin in the spore wall of Glugea weissenbergi. J. Invertebr. Pathol. 14:358-364. [DOI] [PubMed] [Google Scholar]
- 14.Franzen, C., and A. Muller. 2001. Microsporidiosis: human diseases and diagnosis. Microbes Infect. 3:389-400. [DOI] [PubMed] [Google Scholar]
- 15.Frieman, M. B., and B. P. Cormack. 2004. Multiple sequence signals determine the distribution of glycosylphosphatidylinositol proteins between the plasma membrane and cell wall in Saccharomyces cerevisiae. Microbiology 150:3105-3114. [DOI] [PubMed] [Google Scholar]
- 16.Frieman, M. B., and B. P. Cormack. 2003. The omega-site sequence of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae can determine distribution between the membrane and the cell wall. Mol. Microbiol. 50:883-896. [DOI] [PubMed] [Google Scholar]
- 17.Frixione, E., L. Ruiz, M. Santillan, L. V. de Vargas, J. M. Tejero, and A. H. Undeen. 1992. Dynamics of polar filament discharge and sporoplasm expulsion by microsporidian spores. Cell Motil. Cytoskel. 22:38-50. [Google Scholar]
- 18.Hayman, J. R., S. F. Hayes, J. Amon, and T. E. Nash. 2001. Developmental expression of two spore wall proteins during maturation of the microsporidian Encephalitozoon intestinalis. Infect. Immun. 69:7057-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayman, J. R., T. R. Southern, and T. E. Nash. 2005. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect. Immun. 73:841-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josic, D., M. K. Brown, F. Huang, H. Callanan, M. Rucevic, A. Nicoletti, J. Clifton, and D. C. Hixson. 2005. Use of selective extraction and fast chromatographic separation combined with electrophoretic methods for mapping of membrane proteins. Electrophoresis 26:2809-2822. [DOI] [PubMed] [Google Scholar]
- 21.Katinka, M. D., S. Duprat, E. Cornillot, G. Metenier, F. Thomarat, G. Prensier, V. Barbe, E. Peyretaillade, P. Brottier, P. Wincker, F. Delbac, H. El Alaoui, P. Peyret, W. Saurin, M. Gouy, J. Weissenbach, and C. P. Vivares. 2001. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450-453. [DOI] [PubMed] [Google Scholar]
- 22.Keeling, P. J. 2003. Congruent evidence from alpha-tubulin and beta-tubulin gene phylogenies for a zygomycete origin of microsporidia. Fungal Genet. Biol. 38:298-309. [DOI] [PubMed] [Google Scholar]
- 23.Keohane, E., P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1994. The identification and characterization of a polar tube reactive monoclonal antibody. J. Eukaryot. Microbiol. 41:48S. [PubMed] [Google Scholar]
- 24.Keohane, E. M., G. A. Orr, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Witter, and L. M. Weiss. 1996. Purification and characterization of a microsporidian polar tube protein. Mol. Biochem. Parasitol. 79:255-259. [DOI] [PubMed] [Google Scholar]
- 25.Keohane, E. M., G. A. Orr, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1999. Polar tube proteins of microsporidia of the family Encephalitozoonidae. J. Eukaryot. Microbiol. 46:1-5. [DOI] [PubMed] [Google Scholar]
- 26.Keohane, E. M., G. A. Orr, H. S. Zhang, P. M. Takvorian, A. Cali, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1998. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol. Biochem. Parasitol. 94:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klis, F. M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239-256. [DOI] [PubMed] [Google Scholar]
- 28.Lewis, N. L., I. C. Francis, G. S. Hawkins, and M. T. Coroneo. 2003. Bilateral microsporidial keratoconjunctivitis in an immunocompetent non-contact lens wearer. Cornea 22:374-376. [DOI] [PubMed] [Google Scholar]
- 29.Lujan, H. D., J. T. Conrad, C. G. Clark, M. C. Touz, F. Delbac, C. P. Vivares, and T. E. Nash. 1998. Detection of microsporidia spore-specific antigens by monoclonal antibodies. Hybridoma 17:237-243. (Erratum, 17: 581.) [DOI] [PubMed] [Google Scholar]
- 30.Metge, S., J. T. Van Nhieu, D. Dahmane, P. Grimbert, F. Foulet, C. Sarfati, and S. Bretagne. 2000. A case of Enterocytozoon bieneusi infection in an HIV-negative renal transplant recipient. Eur. J. Clin. Microbiol. Infect. Dis. 19:221-223. [DOI] [PubMed] [Google Scholar]
- 31.Peuvel, I., P. Peyret, G. Metenier, C. P. Vivares, and F. Delbac. 2002. The microsporidian polar tube: evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi. Mol. Biochem. Parasitol. 122:69-80. [DOI] [PubMed] [Google Scholar]
- 32.Prigneau, O., A. Achbarou, N. Bouladoux, D. Mazier, and I. Desportes-Livage. 2000. Identification of proteins in Encephalitozoon intestinalis, a microsporidian pathogen of immunocompromised humans: an immunoblotting and immunocytochemical study. J. Eukaryot. Microbiol. 47:48-56. [DOI] [PubMed] [Google Scholar]
- 33.Raynaud, L., F. Delbac, V. Broussolle, M. Rabodonirina, V. Girault, M. Wallon, G. Cozon, C. P. Vivares, and F. Peyron. 1998. Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J. Clin. Microbiol. 36:37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sprague, V. 1977. Systematics of the microsporidia. Plenum Press, New York, N.Y.
- 35.Sprague, V. V., and J. J. Becnel. 1998. Note on the name-author-date combination for the taxon Microsporidies Balbiani, 1882, when ranked as a phylum. J. Invertebr. Pathol. 71:91-94. [DOI] [PubMed] [Google Scholar]
- 36.Texier, C., D. Brosson, H. El Alaoui, G. Metenier, and C. P. Vivares. 2005. Post-genomics of microsporidia, with emphasis on a model of minimal eukaryotic proteome: a review. Folia Parasitol. (Prague) 52:15-22. [DOI] [PubMed] [Google Scholar]
- 37.Vavra, J. 1976. Structure of the microsporidia, p. 1-85. In L. A. Bulla, Jr., and T. C. Cheng (ed.), Comparative pathobiology, vol. 1. Plenum Press, New York, N.Y. [Google Scholar]
- 38.Visvesvara, G. S., G. J. Leitch, A. J. da Silva, G. P. Croppo, H. Moura, S. Wallace, S. B. Slemenda, D. A. Schwartz, D. Moss, R. T. Bryan, et al. 1994. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J. Clin. Microbiol. 32:2760-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber, R., and R. T. Bryan. 1994. Microsporidial infections in immunodeficient and immunocompetent patients. Clin. Infect. Dis. 19:517-521. [DOI] [PubMed] [Google Scholar]
- 40.Weidner, E. 1992. Cytoskeletal proteins expressed by microsporidian parasites. Subcell. Biochem. 18:385-399. [DOI] [PubMed] [Google Scholar]
- 41.Weidner, E., and S. K. Halonen. 1993. Microsporidian spore envelope keratins phosphorylate and disassemble during spore activation. J. Eukaryot. Microbiol. 40:783-788. [Google Scholar]
- 42.Weiss, L. M., T. D. Edlind, C. R. Vossbrinck, and T. Hashimoto. 1999. Microsporidian molecular phylogeny: the fungal connection. J. Eukaryot. Microbiol. 46:17S-18S. [PubMed] [Google Scholar]
- 43.Williams, B. A., R. P. Hirt, J. M. Lucocq, and T. M. Embley. 2002. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature 418:865-869. [DOI] [PubMed] [Google Scholar]
- 43a.Wilm, M., A. Shevchenko, T. Houthaeve, S. Breit, L. Schweigerer, T. Fotsis, and M. Mann. 1996. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 379:466-469. [DOI] [PubMed]
- 44.Wittner, M., and L. M. Weiss. 1999. The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 45.Xu, Y., P. M. Takvorian, A. Cali, G. Orr, and L. M. Weiss. 2004. Glycosylation of the major polar tube protein of Encephalitozoon hellem, a microsporidian parasite that infects humans. Infect. Immun. 72:6341-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Xu, Y. 2004. An investigation of microsporidian polar tube. Ph.D. thesis. Albert Einstein College of Medicine, Bronx, N.Y.