Abstract
The isotype/subclass of immunoglobulin determines antibody function, but rather little is known about factors that direct class switching in vivo. To evaluate factors that might influence the maturation of the antibody response during infection, we conducted a seroepidemiological study of the immunoglobulin G (IgG) subclass response to four merozoite-associated antigens of Plasmodium falciparum in a mountainous region of northeastern Tanzania, where malaria endemicity declines with increasing altitudes. We found that IgG1/IgG3 class switching is independently affected by the nature of the antigen, cumulative exposure to the antigen, and the maturity of the immune system (i.e., the age of the individual). These observations provide insights into the effects of immune system maturity, the duration and intensity of antigen exposure, and inherent characteristics of individual antigens on the process of class switching in human B cells. Our data also throw light on the consequences of class switch decisions on the gradual acquisition of antimalarial immunity.
The isotype/subclass of immunoglobulin determines antibody function (e.g., complement fixation or the activation of phagocytes), and in humans, immunoglobulin G1 (IgG1) and IgG3 are important mediators of pathogen clearance. Specific combinations of cytokines and B-cell activators have been shown to induce class switching to certain isotypes or subclasses in model systems (13), but less is known about factors that direct class switching in vivo during infection. While it has long been suspected that characteristics of antigens themselves influence class switching in B cells (41, 43), and while some antigens induce characteristic patterns of Ig class switching, most notably (in humans) encapsulated bacteria (IgG2) (27, 28) and allergens and helminths (IgG4 and IgE) (20), the characteristics of antigens that induce switching to human IgG1 and IgG3 are not well described.
Numerous studies have reported that IgG subclass profiles differ among antibodies targeted to different malarial antigens, with the best example being the tendency of merozoite surface protein 2 (MSP-2) to induce very strong IgG3 responses (39, 46), in contrast to the tendency of the C terminus of MSP-1, MSP-119, to induce IgG1 or a mixed IgG1/IgG3 response (7, 18). Here we demonstrate that characteristics of antigens per se can regulate the IgG1/IgG3 class switch, in that different antigens of Plasmodium falciparum, the causative agent of the virulent form of human malaria, elicit entirely different antibody subclasses even though they are presented to the immune system at the same time and as part of the same single-celled organism (i.e., the malaria merozoite). To evaluate the effects of antigens per se, immune system maturity (age), and cumulative exposure to antigens (which varies according to the intensity of malaria transmission) on the maturation of the IgG response, we conducted a seroepidemiological study in a mountainous region of northeastern Tanzania, where malaria endemicity declines with increasing altitudes (16), affecting both the age distribution and the clinical presentation of severe malaria (38). Having compared immune responses of individuals of similar ages (and similar genetic backgrounds) living at different altitudes and those of individuals of different ages living at similar altitudes, we demonstrate that IgG1/IgG3 class switching is independently affected by the nature of the antigen, cumulative exposure to the antigen, and the maturity of the immune system (i.e., the age of the individual).
MATERIALS AND METHODS
Study site and sample collection.
The study area, study design, and local epidemiology of malaria have been described previously (16) and confirm that the malaria transmission intensity varies with altitude in a predictable manner (16). Finger-prick blood samples were collected from an age-stratified sample of approximately 250 people from each of five villages; the villages were situated at differing altitudes but selected to be as similar as possible in terms of socioeconomic status and accessibility of health care. All blood donors were healthy at the time of sampling; asymptomatic parasite carriage varied according to location and was used to classify villages according to transmission intensity. Malaria transmission intensities ranged from low (Kwadoe [altitude, 1,564 m; point prevalence of P. falciparum parasitemia, 1% in June 2002]) to moderate (Ngulu [832 m, 18% prevalence], Tamota [1,055 m, 22% prevalence], and Funta [1,240 m, 32% prevalence]) or very high (Mgila [375 m, 49% parasite prevalence]) (16). Four of the villages are situated in the West Usambara mountains and are populated by the Wasambaa ethnic group; Ngulu, in the North Pare mountains, is inhabited by members of the Wapare ethnic group. The use of bed nets and other preventive measures was tightly correlated with the malaria transmission intensity but did not vary systematically with age.
This study received ethical approval from the London School of Hygiene and Tropical Medicine and the Tanzanian National Institute for Medical Research, and informed consent was obtained from all participants.
P. falciparum antigens.
Recombinant P. falciparum merozoite surface proteins MSP-119 (Wellcome sequence) and MSP-2 (full-length 3D7 sequence) were produced in Escherichia coli as glutathione S-transferase fusion proteins as described previously (10, 19, 32). Apical membrane antigen 1 (AMA-1; 3D7 sequence), a polymorphic protein of merozoite micronemes that associates with the merozoite surface during invasion, was produced as a hexa-His-tagged fusion protein in E. coli and purified on a nickel agarose column (1). Soluble P. falciparum glycosylphosphatidylinositol (GPI), which anchors proteins—including MSP-1 and MSP-2—to the merozoite surface, was purified by high-performance liquid chromatography from a P. falciparum (FCR-3) culture, as described previously (30).
Antibody detection by indirect ELISA.
IgG and IgG subclass antibodies to recombinant proteins (46) and GPI (30) were assayed by enzyme-linked immunosorbent assays (ELISAs) as described previously. Secondary Ig detection reagents were rabbit anti-human IgG-horseradish peroxidase (Dako Ltd., High Wycombe, United Kingdom) or mouse anti-human IgG subclass antibodies (IgG1 clone NL16/HP6012, IgG2 clone GOM1/HP6008, IgG4 clone RJ4/HP6011 [all from SkyBio, Wybotson, United Kingdom], and IgG3 clone HP6050 [Serotec, Oxford, United Kingdom]) followed by rabbit anti-mouse IgG-horseradish peroxidase (Dako).
Data management and analysis.
Data were double entered and validated in Microsoft Access and analyzed using STATA 8 (StataCorp, Austin, TX). The mean plus 3 standard deviations of optical density (OD) values for non-malaria-exposed European sera (n = 10) was used to define cutoffs for positive and negative sera. Antibody prevalence and median optical densities were compared with nonparametric (Wilcoxon rank-sum) tests. For comparisons of immunoglobulin levels among age groups and villages, ODs of positive sera were converted to midpoint titers (defined as the midpoint of the fitted sigmoid curve obtained from the titration of 30 hyperimmune reference sera; see Fig. 1); values below the cutoff were assigned an arbitrary titer of 0.1.
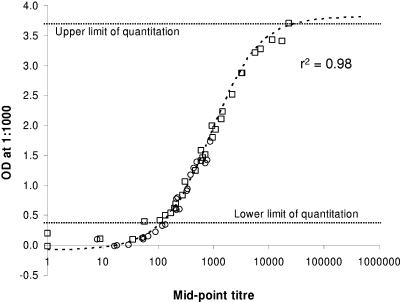
FIG. 1.
Single-point IgG1 and IgG3 OD values are valid proxies for serum IgG1 and IgG3 midpoint titers. The graph shows the relationship between OD values, measured at a dilution of 1:1,000, and titers, determined as the midpoints of the fitted sigmoids for each of 30 malaria-immune sera measuring anti-MSP-2 IgG3 (circles) and anti-AMA-1 IgG1 (squares). The unweighted least-square best fit sigmoid (dotted line) is given by the following equation: titer = 905 × [3.90/(OD + 0.074) − 1]. The r2 value shown is for the linear least-square fit of the logit-transformed data, omitting values with titers of <10.
To compare the relative abundance of IgG1 and IgG3 among age groups or villages, ratios of the ODs of IgG1/IgG3 were dichotomized into ratios of ≥1 (i.e., OD for IgG1 > OD for IgG3) or <1 (i.e., OD for IgG1 < OD for IgG3) for each serum; associations with age and parasite prevalence were then assessed by generalized linear modeling.
Sera giving OD values outside the linear range of the calibration curve were assigned OD values equal to the cutoff (for those below the linear range) or the maximum linear observation (OD = 3.7, for those above the linear range). This approach effectively overestimates low values and underestimates high values and is thus conservative in its effects.
RESULTS
Assay validation.
Previous studies had indicated that despite reports that IgG2 antibodies might be able to confer protection in certain settings (2), IgG2 and IgG4 antibodies to blood-stage malaria proteins are virtually undetectable in sera from many populations from areas of malaria endemicity (18, 46). Screening a random sample of 44 sera from Kwadoe (hypoendemic) and Mgila (hyperendemic) for IgG2 and IgG4 to MSP-2, AMA-1, and MSP-119 confirmed that this was also true for this population; the prevalence of IgG2 and IgG4 antibodies was extremely low for all three antigens (that for IgG2 ranged from 0% to 5.75% and that for IgG4 ranged from 0% to 1.15%), and thus further analyses were restricted to total IgG, IgG1, and IgG3.
Due to the large number and small volumes of samples to be tested, it was not practical to obtain end-point titers for each serum for each antigen and subclass. However, full end-point titrations against AMA-1 (IgG1) and MSP-2 (IgG3) for 30 sera showed a very close correlation between the midpoint titer for an individual serum and the OD value obtained with a single dilution (Fig. 1). Thus, OD values obtained with a single serum dilution were converted, where appropriate, to midpoint titers by using these standard curves. These titrations also demonstrated that the relationships between OD and titer were similar for both IgG1 and IgG3 antibodies (even though data were obtained using different secondary antibodies, which might have very different binding characteristics in ELISA), allowing us to make valid comparisons of the levels of the two subclasses. Thus, with the precise assay conditions employed here, a serum dilution of 1:1,000 (for the three protein antigens) or 1:100 (for anti-GPI antibodies) gave reliable indications of titers which were comparable for the two IgG subclasses.
Prevalence of IgG1 and IgG3 against malaria antigens is affected by age and intensity of malaria exposure.
The prevalence and titers of total IgG, IgG1, and IgG3 specific for the three protein antigens were determined with sera from approximately 1,000 individuals, aged 0 to 45 years, from four villages comprising an altitude transect in the West Usambara Mountains (Kwadoe, Funta, Tamota, and Mgila). The prevalence and titers of anti-GPI antibodies were determined with sera from 462 children and adults in Mgila (designated a high-transmission village) and Ngulu (designated a moderate-transmission village).
For all the antigens tested, both the prevalence (Fig. 2) and the median titers (Fig. 3) of IgG1 and IgG3 antibodies increased significantly with both age and malaria transmission intensity. AMA-1 was clearly the most immunogenic antigen, with a very high prevalence and median titers among children under 5 years in all villages except the one with the very lowest transmission intensity. MSP-2 and MSP-119 showed intermediate levels of immunogenicity, with GPI being markedly less immunogenic than any of the protein antigens (both the prevalence and median titers of anti-GPI antibodies were much lower, despite the assay being carried out at a 10-fold higher serum concentration).
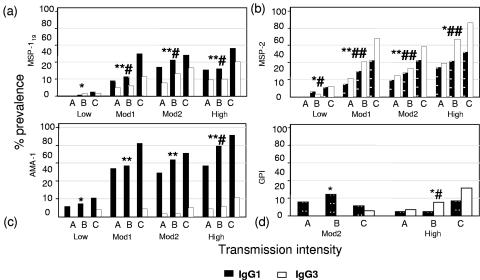
FIG. 2.
Age- and exposure-dependent variation in prevalence of IgG1 and IgG3 antibodies to malarial merozoite-associated antigens. The prevalence (%) of sera giving an OD of more than the mean + 3 standard deviations of the ODs for nonexposed European sera for IgG1 (▪) or IgG3 (□) antibodies to MSP-119 (a), MSP-2 (b), AMA-1 (c), and GPI (d) is shown. Data are arranged by increasing age (grouped as follows: A, 0 to 4 years; B, 5 to 14 years; and C, 15 to 45 years) and by increasing malaria prevalence (low, 1%; Mod1, 22%; Mod2, 32%; and high, 49%); residents of only two villages (moderate [18%] and high [49%] prevalence) were screened for anti-GPI antibodies. Differences in antibody prevalence with age were assessed using nonparametric (Wilcoxon rank-sum) tests and are indicated for IgG1 (* and **) and IgG3 (# and ##). * or #, P ≤ 0.05; ** or ##, P ≤ 0.005.
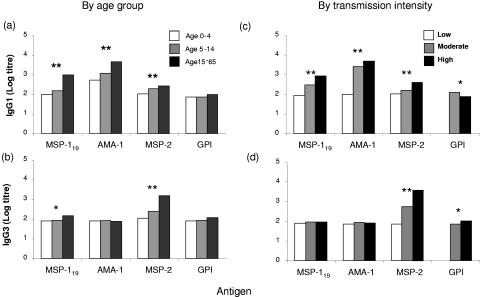
FIG. 3.
Age- and exposure-dependent variation in titers of IgG1 and IgG3 antibodies to malarial merozoite-associated antigens. Median log titers for IgG1 (a and c) and IgG3 (b and d) antibody binding to MSP-119, AMA-1, MSP-2, and GPI by increasing age (a and b) and by increasing malaria intensity (low, 1%; moderate, 18 to 32%; high, 49%) (c and d) are shown. Differences in titers were assessed using nonparametric (Wilcoxon rank-sum) tests and are indicated as follows: *, P ≤ 0.05; **, P ≤ 0.005.
The predominant IgG subclass varied for different antigens. For AMA-1, antibodies were entirely of the IgG1 subclass, whereas for MSP-2 IgG3 antibodies were more prevalent and median titers were significantly higher than those for IgG1. Both IgG1 and IgG3 antibodies were detected for MSP-119, but titers of IgG1 were significantly higher than those of IgG3. For anti-GPI antibodies, IgG1 predominated in the moderate-transmission village and IgG3 predominated in the high-transmission village. Differences in antibody subclasses between antigens were most marked for the moderate- and high-transmission villages and the oldest age group, suggesting that antibody responses continue to evolve with repeated exposure to infection.
Increasing polarization of IgG subclass responses with age.
To examine the relationship between age and IgG subclass, data from all villages were combined, the median ODs for IgG1 and IgG3 were plotted by age for each antigen (Fig. 4), and IgG1/IgG3 ratios were calculated for each age group.
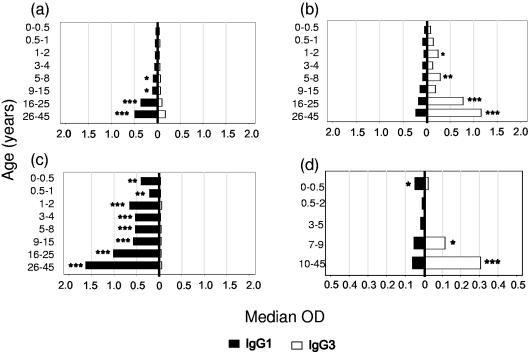
FIG. 4.
Increasing polarization of IgG subclass responses to malaria antigens with increasing age. Data from all villages were combined, and the median ODs for IgG1 (▪) and IgG3 (□) were plotted by age group. (a) Recombinant MSP-119 (rMSP-119); (b) rMSP-2; (c) rAMA-1; (d) GPI. Differences in median ODs were assessed using nonparametric (Wilcoxon rank-sum) tests. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005.
For MSP-119, median ODs for IgG1 and IgG3 were similar among young children, but from the age of 5 years onwards IgG1 antibodies were present at significantly higher levels than IgG3 antibodies (Fig. 4a). Antibodies to AMA-1 were exclusively of the IgG1 subclass in individuals of all ages (Fig. 4c). For MSP-2, IgG3 antibodies began to predominate over IgG1 from the age of 2 years, but the dominance of IgG3 over IgG1 was not fully established until after the age of 15 years (Fig. 4b); the proportion of sera with IgG1 ODs greater than the IgG3 ODs (IgG1/IgG3 OD ratio of ≥1) decreased steadily between successive age groups (odds ratio [OR] = 0.62; 95% confidence interval [CI], 0.50 to 0.77; P < 0.001), indicating a gradual skewing of the response towards IgG3. Anti-GPI antibody concentrations, while much lower than those against protein antigens, followed a pattern similar to that seen for anti-MSP-2 responses, with a mixed IgG1/IgG3 response in children being replaced by a predominantly IgG3 response in adults (Fig. 4d). Thus, the proportion of sera with IgG1 ODs greater than the IgG3 ODs (IgG1/IgG3 OD ratio of ≥1) for GPI decreased significantly between successive age groups (OR = 0.80; 95% CI, 0.63 to 1.00; P = 0.05).
These data suggest that the default pathway for IgG class switching in children is towards a mixed IgG1/IgG3 response but that certain antigens (e.g., AMA-1) can override this to induce highly polarized IgG1 responses in young children. In contrast, the propensity of some antigens to induce IgG3 only becomes apparent in older individuals.
Increasing polarization of IgG subclass responses with increasing exposure to malaria.
To examine the relationship between malaria exposure and IgG subclass responses to the different malaria antigens, data for individuals in each village were combined, the median ODs for IgG1 and IgG3 were plotted by malaria endemicity for each antigen (Fig. 5), and mean IgG1/IgG3 ratios were calculated for each village. For the three protein antigens, mixed IgG1 and IgG3 responses were seen for the villages with low malaria prevalence, whereas highly polarized responses were seen for the villages with high malaria prevalence (Fig. 5a to c), indicating that there is a threshold of antigen exposure that is required to fully polarize the antibody response. This threshold is reached at lower antigen exposure (i.e., fully evident in moderate-transmission villages) for the most immunogenic antigen, AMA-1 (Fig. 5c). The polarization of antibodies towards the IgG3 subclass was evident only in high-transmission villages for MSP-2 and GPI (Fig. 5b and d).
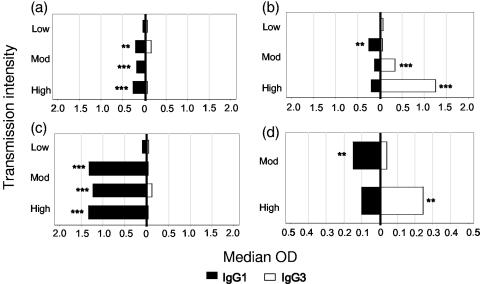
FIG. 5.
Increasing polarization of IgG subclass responses with increasing malaria transmission (parasite prevalence). Data from all age groups were combined, and the median ODs for IgG1 (▪) and IgG3 (□) were plotted by transmission intensity (parasite prevalence). (a) rMSP-119; (b) rMSP-2; (c) rAMA-1; (d) GPI. Differences in median ODs were assessed using nonparametric (Wilcoxon rank-sum) tests. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005.
IgG1/IgG3 ratios varied significantly with exposure for all antigens. The proportion of sera with IgG1 ODs greater than the IgG3 ODs (IgG1/IgG3 ratio of ≥1) was significantly greater for the high-prevalence village than for other villages for AMA-1 (OR = 1.99; 95% CI, 1.81 to 2.16; P < 0.001) and for MSP-119 (OR = 1.40; 95% CI, 1.20 to 1.63; P < 0.001), but the proportions of sera with IgG1 ODs greater than the IgG3 ODs for MSP-2 (OR = 0.67; 95% CI, 0.58 to 0.77; P < 0.001) and for GPI (OR = 0.87; 95% CI, 0.83 to 0.91; P < 0.001) were significantly lower for the high-prevalence village than for the other villages.
Separating the effects of age and intensity of malaria exposure on IgG class switching.
Both age and malaria endemicity appear to affect IgG subclass switching, but in populations in areas where malaria is endemic, cumulative exposure to malaria antigens is a function of both the intensity of malaria transmission and the duration of exposure (i.e., age). Logistic modeling was therefore used to separate the effects of age per se from the effects of cumulative antigen exposure. After allowing for the effect of malaria transmission intensity, the proportion of sera with IgG1 ODs greater than the IgG3 ODs (IgG1/IgG3 OD ratio of ≥1) showed no significant association between age groups for anti-MSP-119 (OR = 1.04; 95% CI, 0.85 to 1.28; P = 0.70), anti-AMA-1 (OR = 1.27; 95% CI, 0.72 to 2.25; P = 0.41), or anti-GPI (OR = 0.89; 95% CI, 0.70 to 1.14; P = 0.35). After allowing for the effects of age, the proportions of sera with IgG1 ODs greater than the IgG3 ODs (IgG1/IgG3 OD ratio of ≥1) remained significantly associated with high malaria transmission intensity for anti-MSP-119 (OR = 1.40; 95% CI, 1.20 to 1.63; P < 0.001), anti-AMA-1 (OR = 1.99; 95% CI, 1.20 to 1.63; P < 0.001), and anti-GPI (OR = 0.87; 95% CI, 0.83 to 0.91; P < 0.001). Thus, gradual polarization of the response towards IgG1 antibodies for MSP-119 and AMA-1 and towards IgG3 antibodies for GPI seems to be dependent on cumulative antigen exposure.
In contrast, for anti-MSP-2 antibodies, IgG1/IgG3 ratios were independently associated with both the age group (OR = 0.60; 95% CI, 0.48 to 0.75; P < 0.001), after allowing for the effects of malaria prevalence, and high parasite prevalence (OR = 0.67; 95% CI, 0.58 to 0.77; P < 0.001), after allowing for the effects of age.
Independent regulation of IgG1 and IgG3 responses to different malaria antigens.
The data presented thus far are population averages which may obscure important differences between individuals in terms of their propensity to produce antibodies to different antigens and of different subclasses. To determine whether IgG1 to MSP-119 or AMA-1 and IgG3 to MSP-2 could be detected within a single serum, we compared these parameters in the sera of individual donors. We reasoned that if IgG3 responses to MSP-2 were regulated entirely independently of IgG1 responses to MSP-119 or AMA-1, then 50% of sera that gave an OD above the median for MSP-2-specific IgG3 should also give an OD above the median for MSP-119- or AMA-1-specific IgG1, i.e., 25% of sera would give an OD above the median for both MSP-2-specific IgG3 and MSP-119- or AMA-1-specific IgG1 (double positive). Conversely, if making an IgG1 response to MSP-119 or AMA-1 inhibited the simultaneous secretion of IgG3 to MSP-2, then significantly less than 25% of sera would give ODs above the median for both. In fact, in both cases the proportion of “double positive” sera was significantly higher than expected (Table 1). Thus, while we cannot entirely rule out some degree of competition or cross-inhibition between responses to different antigens, these data do tend to suggest that there is no major cross-inhibition of IgG1 and IgG3 production within an individual.
TABLE 1.
Simultaneous production of IgG1 and IgG3 by B cells with differing malaria antigen specificitiesa
| Parameter | No. (%) of sera with indicated MSP-119-specific IgG1 OD
|
No. (%) of sera with indicated AMA-1-specific IgG1 OD
|
||
|---|---|---|---|---|
| Less than median | More than median | Less than median | More than median | |
| MSP-2-specific IgG3 with OD < median | 341 (35.2) | 136 (15.8) | 318 (33.4) | 162 (17.5) |
| MSP-2-specific IgG3 with OD > median | 120 (14.8) | 306 (34.2) | 152 (16.5) | 296 (32.5) |
| χ2 value | 14.1 | 13.5 | ||
| P | <0.001 | <0.001 | ||
IgG1 responses to MSP-119 or AMA-1 and IgG3 responses to MSP-2 were compared with sera from individual donors. If IgG3 responses to MSP-2 are regulated independently of IgG1 responses to MSP-119 or AMA-1, then 25% of sera should give ODs above the median for both MSP-2-specific IgG3 and MSP-119- or AMA-1-specific IgG1. Conversely, if making an IgG1 response to MSP-119- or AMA-1-specific IgG1 inhibited the simultaneous generation of IgG3 responses to MSP-2, then significantly less than 25% of sera would give ODs above the median for both. The data indicate that IgG1 and IgG3 occur together significantly more often than expected, suggesting that there is no cross-inhibition of IgG1 and IgG3 production.
DISCUSSION
Antibody subclasses crucially affect pathogen clearance, but rather little is known of antigen-specific factors that drive Ig class switching during infection. In humans, both IgG1 and IgG3 have high affinities for Fc receptors, fix complement, and are preferentially induced during Th1-type responses, but IgG3 appears to be a more efficient mediator of FcR cross-linking and phagocyte activation (8, 22). Consistent with this, IgG3 antibodies to various blood-stage antigens are particularly effective mediators of antibody-dependent cellular inhibition of malaria parasite growth in vitro (5) and have been repeatedly associated with the acquisition of clinical immunity to malaria (12, 14, 29, 42, 45, 48). These data make a strong case for trying to engineer antimalarial vaccines to induce high titers of IgG3 antibodies, but this will only be feasible when we have a better understanding of the requirements for induction of IgG3 class switching in human B cells.
The objective here was thus to explore factors that might influence IgG class switching during the process of acquisition of protective antimalarial immunity. The apparently low prevalence of IgG2 and IgG4 antibodies to malaria proteins is in accordance with many previous reports and is not surprising, although it is possible that the anti-IgG2 and anti-IgG4 monoclonal antibodies used may not efficiently recognize immunoglobulin allotypes that are prevalent in this study population. A systematic evaluation of the utility of different subclass reagents for different African and Asian populations would be very valuable. In contrast, IgG1 and IgG3 antibodies were readily detected in individuals from all the study villages, but the relative proportions of these two subclasses differed for different P. falciparum antigens even though all the antigens studied are part of the same stage of the life cycle (the mature merozoite) and are presented to the immune system at the same time (i.e., during schizont rupture and erythrocyte invasion during blood-stage malaria infection). MSP-119 and AMA-1 preferentially induce class switching to IgG1, with the degree of polarization of the response being influenced by the intensity of antigen exposure, whereas MSP-2, and to a lesser extent, GPI preferentially induce class switching to IgG3, with the degree of polarization being influenced by both the intensity of antigen exposure and the age of the individual. These data suggest, firstly, that inherent characteristics of antigens have a major influence on the molecular events leading to class switching, and secondly, that young children may be relatively defective in the ability to produce IgG3. The gradual polarization of the antibody subclass response also implies an ongoing influence of antigen on the maturing immune response.
We do not believe that these IgG subclass patterns can be explained by genetic or other environmental effects. Individuals from four of the five villages came from the same ethnic group (which is clearly culturally and linguistically defined), and antibody responses from residents of the fifth village were exactly as expected given its transmission intensity. The prevalence of human immunodeficiency virus, hepatitis B virus, or hookworm (the commonest helminth infection in the area) is not known, or expected, to vary systematically with altitude, and although bed net use varied with transmission intensity, it did not vary systematically with age. In any event, for extraneous host or environmental factors to explain differences in IgG1/IgG3 ratios, they would have to act differently on immune responses to different antigens, which seems rather unlikely.
In vitro experiments with human and murine B cells have identified cytokines which selectively induce the synthesis of particular Ig classes and subclasses by stimulating DNA rearrangement and selective transcription of CH genes (23, 24, 41, 43); for example, interleukin-10 (IL-10) induces the secretion of IgG1 and IgG3 from human secretory IgD+ B cells in vitro (9). However, most of these studies used T-cell-independent B-cell activators such as bacterial lipopolysaccharide and anti-IgD conjugated to dextran supplemented with exogenous cytokines. Of the studies that have evaluated the role of T-cell-dependent antigens in inducing specific class switch events in disease settings, perhaps the best examples are the IL-4-driven secretion of helminth-specific IgE and IgG4 (26) and IL-10/transforming growth factor β1-driven IgA and/or IL-10/IL-2-driven IgG1 or IgG3 responses to Schistosoma hematobium antigens (4); in all cases, antibody secretion was induced in vitro from class-switched memory B cells that had been primed by infection in vivo and reflected the predominant antibody classes in serum from the same individuals.
Malaria antigens tend to induce either IgG1 or IgG3. Our observation that MSP-119 induces a mixed IgG1/IgG3 response which then polarizes, with increasing exposure to infection, to IgG1 is consistent with previous reports (11, 15, 18, 34), as is our observation that MSP-2 and GPI preferentially induce IgG3 antibodies (6, 29, 39, 46). Polarized IgG1 responses are also reported for Pf155/RESA (17), crude schizont lysate (33), RAP-1 (44), and MSP-6 (50), while IgG3 responses are seen for a polymorphic C-terminal region (block 2) of MSP-1 (11), MSP-3 (11, 31), MSP-4 (51), and MSP-7 (50). A crucial role for antigens per se in driving these patterns of subclass switching has been confirmed experimentally both in vitro with human B cells (21) and in a mouse model where different recombinant P. falciparum antigens—expressed with the same fusion protein tag and administered with the same adjuvant—induced very different IgG subclasses (47). This raises the important question of whether there are intrinsic features of individual malarial antigens which influence their interactions with antigen-presenting cells, T cells, or B cells, leading to the preferential induction of different IgG subclasses. Reviewing the data for different malaria antigens, there is a striking correlation between the presence of amino acid sequences that are simultaneously polymorphic and repetitive in the antigens that induce predominantly IgG3 (MSP-1 block 2, MSP-2, MSP-3, MSP-7, and indeed, circumsporozoite protein) (25) and the absence of such polymorphic repeats in the IgG1-inducing antigens (MSP-119, AMA-1, RAP-1, MSP-5, and MSP-6). MSP-4, which is polymorphic but lacks repetitive sequences, induces a mixed IgG1/IgG3 response with some predominance of IgG3 (51), whereas Pf155/RESA, which contains extensive repeats yet is conserved, induces an IgG1 or mixed IgG1/IgG3 response, with evidence that IgG3 increases with age (3, 17). In contrast, other characteristics of the same antigens (e.g., posttranslational processing, the presence or absence of a GPI anchor, being membrane bound or secreted, or having complex disulfide-bonded tertiary structures) do not correlate with the antibody subclass, suggesting that antigen presentation of membrane-bound versus secreted proteins by macrophages or follicular dendritic cells, respectively, as suggested by Cavanagh et al. (11), presentation of disulfide-bonded proteins to helper T cells by the de novo rather than the recycling major histocompatibility complex class II pathway (35), and specific induction of IgG3 by glycolipid anchor molecules (6) are all unlikely to explain differential class switching.
On the other hand, the delivery of a T-cell-independent signal to B cells by cross-linking of the B-cell receptor by polyepitopic proteins (36, 49) combined with modified costimulatory signals from memory T cells triggered by polymorphic variants of the priming peptide (altered peptide ligands [APL]) (40) might well favor class switching to IgG3. In particular, alterations in the concentrations of IL-10 and/or gamma interferon produced by APL-restimulated memory T cells may influence the IgG1/IgG3 balance, as shown for human antibody responses to schistosomiasis (37) and, in a model system, murine IgG subclass responses to P. falciparum MSP-2 (47). Shifts in helper T-cell phenotype with increasing age and increasing malaria exposure as a result of encountering APLs during successive infections with genetically distinct malaria parasites might also explain the age and exposure dependence of the IgG3 response reported here.
In summary, we have shown that different malaria antigens induce very different IgG subclass profiles, even though they are apparently presented to the immune system under identical conditions. Intrinsic characteristics of the malaria antigens themselves are thus likely to influence IgG subclass switching, and this is explained most parsimoniously in terms of interactions between antigens and helper T cells. Further work is needed to elucidate these interactions and to exploit them for rational vaccine design; we propose that malaria may prove to be a useful model system for such studies.
Acknowledgments
This study was conducted within the Joint Malaria Programme, a collaboration between the National Institute for Medical Research (NIMR), Kilimanjaro Christian Medical College (KCMC), the London School of Hygiene and Tropical Medicine (LSHTM), and the Centre for Medical Parasitology, University of Copenhagen (CMP). We thank A. Kitua (NIMR), B. M. Greenwood (LSHTM), J. Shao (KCMC), and I. Bygbjerg (CMP) for their support and advice. We also thank C. Jones, F. Lazier, M. Mosha, B. P. Mmbando, members of the JMP community studies team, F. Magogo, E. Lyatuu, J. Akida, and laboratory staff at KCMC and NIMR Amani. The assistance and support of regional medical officers Mwengee and Olomi are gratefully acknowledged. Finally, we thank A. Holder (NIMR, United Kingdom) for providing the MSP-119-glutathione S-transferase-transformed E. coli clones and R. Anders and V. Murphy (La Trobe University, Australia) for providing recombinant AMA-1.
We have no conflicts of interest to declare.
This study was funded by the UK Medical Research Council and the LSHTM Gates Malaria Partnership (GMP; Bill and Melinda Gates Foundation). J.E.T. is a GMP-funded doctoral student, and C.J.D. is supported by a Wellcome Trust training fellowship.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anders, R., P. Crewther, S. Edwards, M. Margetts, M. Matthew, B. Pollock, and D. Pye. 1998. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. [DOI] [PubMed] [Google Scholar]
- 2.Aucan, C., Y. Traore, F. Tall, B. Nacro, T. Traore-Leroux, F. Fumoux, and P. Rihet. 2000. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect. Immun. 68:1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck, H.-P., I. Felger, B. Genton, N. Alexander, F. Al-Yaman, R. F. Anders, and M. Alpers. 1995. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect. Immun. 63:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beniguel, L., T. O. Diallo, F. Remoue, D. L. Williams, F. Cognasse, N. Charrier-Mze, A. A. N′Diaye, R. Perraut, M. Capron, G. Riveau, and O. Garraud. 2003. Differential production in vitro of antigen specific IgG1, IgG3 and IgA: a study in Schistosoma haematobium infected individuals. Parasite Immunol. 25:39-44. [DOI] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutlis, C. S., P. K. Fagan, D. C. Gowda, M. Lagog, C. S. Mgone, M. J. Bockarie, and N. M. Anstey. 2003. Immunoglobulin G (IgG) responses to Plasmodium falciparum glycosylphosphatidylinositols are short-lived and predominantly of the IgG3 subclass. J. Infect. Dis. 187:862-865. [DOI] [PubMed] [Google Scholar]
- 7.Branch, O. H., A. J. Oloo, B. L. Nahlen, D. Kaslow, and A. A. Lal. 2000. Anti-merozoite surface protein-1 19-kDa IgG in mother-infant pairs naturally exposed to Plasmodium falciparum: subclass analysis with age, exposure to asexual parasitemia, and protection against malaria. V. The Asembo Bay Cohort Project. J. Infect. Dis. 181:1746-1752. [DOI] [PubMed] [Google Scholar]
- 8.Bredius, R. G., C. A. Fijen, M. De Haas, E. J. Kuijper, R. S. Weening, J. G. Van de Winkel, and T. A. Out. 1994. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology 83:624-630. [PMC free article] [PubMed] [Google Scholar]
- 9.Briere, F., C. Servet-Delprat, J.-M. Bridon, J.-M. Saint-Remy, and J. Banchereau. 1994. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J. Exp. Med. 179:757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burghaus, P. A., and A. A. Holder. 1994. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol. Biochem. Parasitol. 64:165-169. [DOI] [PubMed] [Google Scholar]
- 11.Cavanagh, D. R., C. Dobano, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. A. Khalil, T. G. Theander, D. E. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 69:1207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanagh, D. R., D. Dodoo, L. Hviid, J. A. Kurtzhals, T. G. Theander, B. D. Akanmori, S. Polley, D. J. Conway, K. Koram, and J. S. McBride. 2004. Antibodies to the N-terminal block 2 of Plasmodium falciparum merozoite surface protein 1 are associated with protection against clinical malaria. Infect. Immun. 72:6492-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffman, R. L., D. A. Lebman, and P. Rothman. 1993. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54:229-270. [DOI] [PubMed] [Google Scholar]
- 14.Conway, D., D. Cavanagh, K. Tanabe, C. Roper, Z. Mikes, N. Sakihama, K. Bojang, P. Kremsner, D. Arnot, B. Greenwood, and J. McBride. 2000. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 6:689-692. [DOI] [PubMed] [Google Scholar]
- 15.Diallo, T. O., A. Spiegel, A. Diouf, R. Perraut, D. C. Kaslow, and O. Garraud. 2001. Short report: IgG1/IgG3 antibody responses to various analogs of recombinant ypfmsp119—a study in immune adults living in areas of Plasmodium falciparum transmission. Am. J. Trop. Med. Hyg. 64:204-206. [DOI] [PubMed] [Google Scholar]
- 16.Drakeley, C., I. Carneiro, H. Reyburn, R. Malima, J. Lusingu, J. Cox, T. G. Theander, W. M. M. Nkya, M. Lemnge, and E. Riley. 2005. Altitude dependent and altitude independent variations in Plasmodium falciparum transmission in North-eastern Tanzania. J. Infect. Dis. 191:1589-1598. [DOI] [PubMed] [Google Scholar]
- 17.Dubois, B., P. Deloron, P. Astagneau, C. Chougnet, and J. P. Lepers. 1993. Isotypic analysis of Plasmodium falciparum-specific antibodies and their relation to protection in Madagascar. Infect. Immun. 61:4498-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. S. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63:456-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franks, S., L. Baton, K. Tetteh, E. Tongren, D. Dewin, B. D. Akanmori, K. A. Koram, L. Ranford-Cartwright, and E. M. Riley. 2003. Genetic diversity and antigenic polymorphism in Plasmodium falciparum: extensive serological cross-reactivity between allelic variants of merozoite surface protein 2. Infect. Immun. 71:3485-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garraud, O., C. Nkenfou, J. E. Bradley, F. B. Perler, and T. B. Nutman. 1995. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J. Immunol. 155:1316-1325. [PubMed] [Google Scholar]
- 21.Garraud, O., R. Perraut, A. Diouf, W. S. Nambei, A. Tall, A. Spiegel, S. Longacre, D. C. Kaslow, H. Jouin, D. Mattei, G. M. Engler, T. B. Nutman, E. M. Riley, and O. Mercereau-Puijalon. 2002. Regulation of antigen-specific immunoglobulin G subclasses in response to conserved and polymorphic Plasmodium falciparum antigens in an in vitro model. Infect. Immun. 70:2820-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadley, A. G., and B. M. Kumpel. 1989. Phagocytosis by human monocytes of red cells sensitized with monoclonal IgG1 and IgG3 anti-D. Vox Sang. 57:150-151. [DOI] [PubMed] [Google Scholar]
- 23.Honjo, T., K. Kinoshita, and M. Muramatsu. 2002. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu. Rev. Immunol. 20:165-196. [DOI] [PubMed] [Google Scholar]
- 24.Isakson, P. C., E. Pure, E. S. Vitetta, and P. H. Krammer. 1982. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J. Exp. Med. 155:734-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John, C. C., J. S. Zickafoose, P. O. Sumba, C. L. King, and J. W. Kazura. 2003. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect. Immun. 71:4320-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King, C. L., C. C. Low, and T. B. Nutman. 1993. IgE production in human helminth infection. Reciprocal interrelationship between IL-4 and IFN-gamma. J. Immunol. 150:1873-1880. [PubMed] [Google Scholar]
- 27.Lane, P. J., D. Gray, S. Oldfield, and I. C. MacLennan. 1986. Differences in the recruitment of virgin B cells into antibody responses to thymus-dependent and thymus-independent type-2 antigens. Eur. J. Immunol. 16:1569-1575. [DOI] [PubMed] [Google Scholar]
- 28.Lane, P. J., and I. C. MacLennan. 1986. Impaired IgG2 anti-pneumococcal antibody responses in patients with recurrent infection and normal IgG2 levels but no IgA. Clin. Exp. Immunol. 65:427-433. [PMC free article] [PubMed] [Google Scholar]
- 29.Metzger, W. G., D. M. Okenu, D. R. Cavanagh, J. V. Robinson, K. A. Bojang, H. A. Weiss, J. S. McBride, B. M. Greenwood, and D. J. Conway. 2003. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol. 25:307-312. [DOI] [PubMed] [Google Scholar]
- 30.Naik, R. S., O. H. Branch, A. S. Woods, M. Vijaykumar, D. J. Perkins, B. L. Nahlen, A. A. Lal, R. J. Cotter, C. E. Costello, C. F. Ockenhouse, E. A. Davidson, and D. C. Gowda. 2000. Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J. Exp. Med. 192:1563-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M.-C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 32.Okech, B. A., P. H. Corran, J. Todd, A. Joynson-Hicks, C. Uthaipibull, T. G. Egwang, A. A. Holder, and E. M. Riley. 2004. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect. Immun. 72:1557-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perraut, R., M. Guillotte, I. Drame, B. Diouf, J. F. Molez, A. Tall, J. F. Trape, O. Mercereau-Puijalon, A. Spiegel, and O. Garraud. 2002. Evaluation of anti-Plasmodium falciparum antibodies in Senegalese adults using different types of crude extracts from various strains of parasite. Microbes Infect. 4:31-35. [DOI] [PubMed] [Google Scholar]
- 34.Polley, S. D., K. K. Tetteh, D. R. Cavanagh, R. J. Pearce, J. M. Lloyd, K. A. Bojang, D. M. Okenu, B. M. Greenwood, J. S. McBride, and D. J. Conway. 2003. Repeat sequences in block 2 of Plasmodium falciparum merozoite surface protein 1 are targets of antibodies associated with protection from malaria. Infect. Immun. 71:1833-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quin, S. J., E. M. Seixas, C. A. Cross, M. Berg, V. Lindo, B. Stockinger, and J. Langhorne. 2001. Low CD4(+) T cell responses to the C-terminal region of the malaria merozoite surface protein-1 may be attributed to processing within distinct MHC class II pathways. Eur. J. Immunol. 31:72-81. [DOI] [PubMed] [Google Scholar]
- 36.Rehe, G. T., I. M. Katona, M. Brunswick, L. M. Wahl, C. H. June, and J. J. Mond. 1990. Activation of human B lymphocytes by nanogram concentrations of anti-IgM-dextran conjugates. Eur. J. Immunol. 20:1837-1842. [DOI] [PubMed] [Google Scholar]
- 37.Remoue, F., D. To Van, A. M. Schacht, M. Picquet, O. Garraud, J. Vercruysse, A. Ly, A. Capron, and G. Riveau. 2001. Gender-dependent specific immune response during chronic human Schistosomiasis haematobia. Clin. Exp. Immunol. 124:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyburn, H., R. Mbatia, C. Drakeley, J. Bruce, I. Carneiro, R. Olomi, J. Cox, W. M. Nkya, M. Lemnge, B. M. Greenwood, and E. M. Riley. 2005. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 293:1461-1470. [DOI] [PubMed] [Google Scholar]
- 39.Rzepczyk, C. M., K. Hale, N. Woodroffe, A. Bobogare, P. Csurhes, A. Ishii, and A. Ferrante. 1997. Humoral immune responses of Solomon Islanders to the merozoite surface antigen 2 of Plasmodium falciparum show pronounced skewing towards antibodies of the immunoglobulin G3 subclass. Infect. Immun. 65:1098-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloan-Lancaster, J., and P. M. Allen. 1996. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 14:1-27. [DOI] [PubMed] [Google Scholar]
- 41.Snapper, C. M., and F. D. Finkelman. 1999. Immunoglobulin class switching, p. 831-861. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 42.Soe, S., M. Theisen, C. Roussilhon, K. S. Aye, and P. Druilhe. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stavnezer, J. 1996. Immunoglobulin class switching. Curr. Opin. Immunol. 8:199-205. [DOI] [PubMed] [Google Scholar]
- 44.Stowers, A., D. Taylor, N. Prescott, Q. Cheng, J. Cooper, and A. Saul. 1997. Assessment of the humoral immune response against Plasmodium falciparum rhoptry-associated proteins 1 and 2. Infect. Immun. 65:2329-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor, R. R., S. J. Allen, B. M. Greenwood, and E. Riley. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58:406-413. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, R. R., D. B. Smith, V. J. Robinson, J. S. McBride, and E. M. Riley. 1995. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the IgG3 subclass. Infect. Immun. 63:4382-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tongren, J. E., P. Corran, W. Jarra, J. Langhorne, and E. Riley. 2005. Epitope-specific regulation of immunoglobulin class switching in mice immunized with malarial merozoite surface proteins. Infect. Immun., 73:8119-8129. [DOI] [PMC free article] [PubMed]
- 48.Topolska, A. E., T. L. Richie, D. H. Nhan, and R. L. Coppel. 2004. Associations between responses to the rhoptry-associated membrane antigen of Plasmodium falciparum and immunity to malaria infection. Infect. Immun. 72:3325-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vos, Q., A. Lees, Z. Q. Wu, C. M. Snapper, and J. J. Mond. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176:154-170. [DOI] [PubMed] [Google Scholar]
- 50.Wang, L., L. Crouch, T. L. Richie, D. H. Nhan, and R. L. Coppel. 2003. Naturally acquired antibody responses to the components of the Plasmodium falciparum merozoite surface protein 1 complex. Parasite Immunol. 25:403-412. [DOI] [PubMed] [Google Scholar]
- 51.Wang, L., T. L. Richie, A. Stowers, D. H. Nhan, and R. L. Coppel. 2001. Naturally acquired antibody responses to Plasmodium falciparum merozoite surface protein 4 in a population living in an area of endemicity in Vietnam. Infect. Immun. 69:4390-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]