Abstract
Pneumocystis carinii is an opportunistic fungal pathogen that causes P. carinii pneumonia (PCP) in the immunocompromised host. We investigated the role of antibody Fc-mediated function in passive prophylaxis against the development of PCP in SCID mice. By comparison of anti-mouse P. carinii immunoglobulin G1 monoclonal antibody (MAb) 4F11(G1) and its F(ab′)2 derivative in an intranasal immunoprophylaxis model, we determined that Fc-mediated function is required for maximum effect of this antibody. Comparison of efficacy of antibody prophylaxis in SCID mice depleted of complement to that in nondepleted mice demonstrated that complement fixation by MAb 4F11(G1) is also necessary for optimal effect of passively administered antibody, although residual protection was observed in complement-depleted SCID mice. The necessity of complement for optimal PCP prophylaxis by MAb 4F11(G1) suggests that complement may play a role in antibody-mediated protection against development of PCP.
Pneumocystis carinii is an opportunistic fungal pathogen that causes pneumonia in the immunocompromised host. The primary requirement for protection against P. carinii pneumonia (PCP) is normal CD4+ T-cell function (7, 17, 27), though mice and humans with B-cell deficiencies are also susceptible to PCP (16, 25), suggesting a role for antibody in protection. Although drug treatments for P. carinii pneumonia exist, poor compliance to drug treatment schedules, recurrent infections, and adverse side effects to the drugs are problems (9, 19). Therefore, investigation of host-parasite interactions which could lead to new treatment methods is worthwhile.
A growing body of evidence suggests that anti-Pneumocystis antibody therapy may be an effective means of preventing and treating PCP. Hyperimmune sera from mice immunized with P. carinii organisms resolved existing P. carinii infections in SCID mice and decreased the hyperinflammatory reaction inimmune-reconstituted mice (23, 24). An early study using an anti-mouse P. carinii antibody demonstrated partial protection against development of PCP (1). Our previous studies demonstrated that SCID mice are also protected from the development of PCP by passive prophylaxis using the anti-P. carinii immunoglobulin M (IgM) monoclonal antibody (MAb) 4F11 or its IgG1 switch variant, MAb 4F11(G1) (2). MAb 4F11(G1) recognizes multiple, similar epitopes on the surface of P. carinii isolated from mice within at least two different antigens, kexin and cDNA clone A12 (13, 29). MAb 4F11(G1) is also capable of recognizing P. carinii derived from humans, rhesus macaques, rats, and ferrets (2, 29).
In this investigation, we set out to determine the mechanism of protection against development of PCP by MAb 4F11(G1). Specifically, we wanted to determine whether protection by MAb 4F11(G1) was mediated through antibody Fc region-dependent mechanisms, such as complement fixation and/or Fc receptor-mediated phagocytosis by macrophages or neutrophils, or whether it occurred in the absence of Fc region via binding and agglutination of P. carinii organisms.
MATERIALS AND METHODS
Antibodies.
An IgG1 switch variant of IgM monoclonal antibody 4F11(G1) specific for mouse P. carinii Kex1 and cDNA clone A12 (2, 13, 29) and an anti-Haemophilus influenzae IgG1 monoclonal antibody were purified from ascites fluid by passage over a protein A-Sepharose column (Pierce, Rockford, Ill.).
Mice.
Pathogen-free SCID (C.B-Igh-1b/IcrTac-Prkdcscid) mice were obtained from Taconic Farms (Germantown, NY), housed in microisolator cages in the University of Rochester animal care facilities, and fed sterile food and water. All procedures performed were subject to University of Rochester Committee on Animal Resources approval.
Production, purification, and antigen binding analysis of F(ab′)2 fragments of 4F11(G1).
Aliquots containing 1.5 mg of purified 4F11(G1) in digest buffer (0.1 M sodium citrate, 5 mM EDTA, 1 mM cysteine, pH 6.0) were digested overnight into F(ab′)2 fragments and Fc fragments by using an immobilized Ficin chromatography column as described by the manufacturer (Pierce). Digested protein was eluted by application of 4 ml of binding buffer (0.1 M sodium citrate, 5 mM EDTA, pH 6.0) to the Ficin column, and Fc fragments and intact IgG molecules were removed by passage over a protein A affinity chromatography column. Purity of the F(ab′)2 fragments in the protein A column flowthrough was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)and silver staining. Protein concentrations of F(ab′)2 preparations were determined by bicinchoninic acid assay as described by the manufacturer (Pierce). F(ab′)2 fragments were adjusted to a concentration of 1 mg/ml and filter sterilized, and single-use aliquots were stored at −80°C until use. Antigen binding capability of F(ab′)2 fragments was determined by enzyme-linked immunosorbent assay (ELISA). Plates were coated with sonicated P. carinii-infected mouse lung homogenates diluted to an approximate protein concentration of 1 to 10 μg/ml in carbonate-bicarbonate buffer. The plates were incubated with twofold dilutions of either 4F11(G1) or 4F11(G1) F(ab′)2 fragments, starting at a concentration of 60 μg/ml in triplicate wells. A goat anti-mouse IgG Fab′-specific antibody conjugated to alkaline phosphatase was used as a secondary antibody. Control wells received secondary antibody alone. The optical density at 660 nm was determined after 20 min of color development using a 96-well plate reader (Bio-Rad, Hercules, CA).
Intranasal immunization of mice and cohousing.
Starting on day 1 of the experiment and continuing daily through day 16, groups of six to seven mice were given 50 μl of a 1-mg/ml suspension of either 4F11(G1), 4F11(G1) F(ab′)2, or anti-H. influenzae type b monoclonal antibody intranasally under light ketamine-xylazine (experiment 1) or halothane (experiment 2) anesthesia. All mice in the different treatment groups were cohoused together with three P. carinii-infected source mice to ensure equal exposure to P. carinii beginning on day 1 and ending on day 14, at which time the three treatment groups were separated and removed from the source mice. Mice were sacrificed by intraperitoneal (i.p.) injection of sodium pentobarbital on day 42 for experiment one and on day 54 for experiment 2, and lungs were removed and stored at −80°C until use. For analysis of the role of complement in MAb 4F11(G1) PCP prophylaxis, mice received either two doses of 5 U cobra venom factor (Calbiochem, La Jolla, CA) or sterile saline i.p. on day 1 of the passive prophylaxis experiment and every 5 days thereafter, ending on day 14. This method has been shown previously to deplete mice of circulating complement components (26). Half of the complement-depleted mice and half of the nondepleted mice also received daily doses of 50 μl of a 1-mg/ml suspension of MAb 4F11(G1) intranasally (i.n.), while the other half received 50 μl of sterile saline i.n. The mice were cohoused with three P. carinii-infected source mice for 14 days, at which time the source mice were removed and the mice were given three additional daily doses of antibody or saline. The mice were sacrificed 6 weeks after completion of cohousing, and their lungs were removed for determination of P. carinii burden.
Real-time PCR analysis of P. carinii kex1 gene copy number.
P. carinii burden was calculated using real-time PCR as previously described (3). Briefly, real-time PCRs used a primer/fluorogenic probe set specific for a 96-bp region of the P. carinii kex1 gene (Applied Biosystems, Foster City, CA). This gene is present as a single copy within the mouse P. carinii genome (13), allowing organism burden to be determined by comparison to a standard curve generated by using 10-fold dilutions of a known copy number of the plasmid pRSETB (Invitrogen, Carlsbad, CA) containing P. carinii kex1. Copy number was calculated from the plasmid molecular weight and concentration as determined spectrophotometrically at A260 and adjusted to give a starting copy number of 1010/ml. Lung homogenate samples were prepared by processing 150 mg P. carinii-infected mouse lung tissue per 1 ml sterile phosphate-buffered saline using a Kinematica Polytron model PT2100 electric tissue stomacher (Brinkmann, Westbury, NY). For PCRs, homogenates were subjected to three freeze-thaw cycles followed by boiling for 20 min and centrifugation to remove debris. PCRs used 2.5 μl of a 1:3 dilution of cleared supernatant as template. Real-time PCR quantification of organism burden was performed by the ABI Prism 7000 sequence detection system and its associated SDS software version 1.0 (Applied Biosystems). Organism burdens of the different treatment groups were compared using Student's t test. Observations shown to be statistically significant by t test were confirmed by a two-factor analysis of variance. The Student-Newmann-Keuls method was used for multiple pairwise comparisons.
RESULTS
Preparation and testing of MAb 4F11(G1) F(ab′)2 fragments.
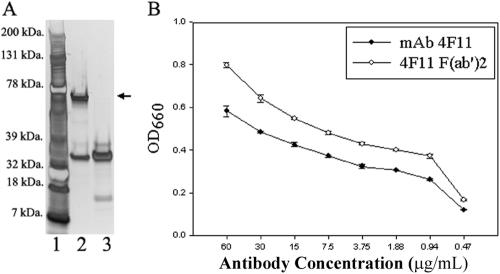
As shown in Fig. 1A, the F(ab′)2 preparation was devoid of intact heavy chain when 1 μg of preparation was analyzed by SDS-PAGE and silver staining (Fig. 1A, lane 3). An ELISA was used to confirm that MAb 4F11(G1) F(ab′)2 fragments recognized P. carinii antigens. As shown in Fig. 1B, the F(ab′)2 fragment preparation recognized P. carinii antigens at antibody concentrations as low as 0.47 μg/ml. The higher optical densities at 660 nm for the F(ab′)2 preparation compared to intact MAb 4F11(G1) are reflective of the presence of more F(ab′)2 molecules at equal protein concentrations, as F(ab′)2 molecules are approximately two-thirds the size of the intact IgG.
FIG. 1.
(A) SDS-PAGE analysis of intact MAb 4F11(G1) and 4F11(G1) F(ab′)2 fragments. Lane 1, molecular mass markers; lane 2, intact MAb 4F11(G1); lane 3, 4F11(G1) F(ab′)2 fragments. One microgram of total protein was added to each well. The arrow indicates the IgG heavy chain. (B) ELISA using soluble P. carinii antigens from infected mouse lung homogenates as target and MAb 4F11(G1) and 4F11(G1) F(ab′)2 as primary antibodies. Results represent means ± standard deviations of duplicate experiments with each condition tested in triplicate.
The Fc portion of MAb 4F11(G1) is required for protection against PCP.
To determine whether the Fc region of MAb 4F11(G1) was required for protection, we compared the efficacy to intact MAb 4F11(G1) and purified F(ab′)2 fragments of MAb 4F11(G1) in a passive immunoprophylaxis model (2). SCID mice were treated with equivalent amounts of either intact MAb 4F11(G1), F(ab′)2 fragments of MAb 4F11(G1), or an isotype-matched control anti-H. influenzae monoclonal antibody which does not cross-react with mouse P. carinii (data not shown). For these experiments mice were cohoused in colony cages with three P. carinii-infected SCID mice to serve as a source of infection. The mice then received three additional daily doses of the appropriate antibody preparation to ensure that mice that may have been protected during the cohousing period did not acquire a P. carinii infection from organisms inadvertently transferred to the new cages. On day 42 of experiment 1 or day 54 of experiment 2, the mice were sacrificed and their lungs were removed for P. carinii burden determination by quantitative real-time PCR using the single-copy kex1 gene (13) as a target. As seen in Table 1, P. carinii burden was significantly greater in the 4F11(G1) F(ab′)2-treated group (P ≤ 0.001 for experiment 1 and P = 0.005 for experiment 2) and anti-H. influenzae MAb-treated group (P ≤ 0.001 for experiments 1 and 2) compared to the intact MAb 4F11(G1)-treated group. Compared to mice treated with intact MAb 4F11(G1), mice treated with the F(ab′)2 preparation had approximately 10-fold more organisms in their lungs, despite using more molecules of F(ab′)2 antibody fragments. Mice treated with the isotype-matched control anti-H. influenzae monoclonal antibody had 13- to 14-fold greater organism burdens than mice treated with intact MAb 4F11(G1). Mice treated with the F(ab′)2 fragment had approximately half as many organisms as mice treated with the control antibody, a difference that was statistically significant only in the first experiment. These results show that MAb 4F11(G1) Fc-mediated function was required for optimal protection against PCP.
TABLE 1.
Effects of antibody prophylaxis on mouse P. carinii burden
| Expt no. | Treatment | P. carinii burdena | n |
|---|---|---|---|
| 1 | MAb 4F11(G1) | (1.90 ± 1.15) × 104b | 7 |
| 4F11(G1) F(ab′)2 | (1.78 ± 0.75) × 105c | 6 | |
| Anti-H. influenzae | (3.27 ± 0.97) × 105 | 7 | |
| 2 | MAb 4F11(G1) | (1.18 ± 1.14) × 104d | 7 |
| 4F11(G1) F(ab′)2 | (1.96 ± 1.40) × 105 | 6 | |
| Anti-H. influenzae | (3.95 ± 1.84) × 105 | 6 |
Results represent means ± standard deviations of duplicate analyses of boiled lung homogenates from each mouse quantified by comparison to a standard curve generated from known quantities of plasmid-encoded mouse P. carinii kex1.
P ≤ 0.05 compared to isotype-matched control anti-H. influenzae antibody treatment group; P ≤ 0.001 compared to 4F11(G1) F(ab′)2 treatment group.
P = 0.01 compared to anti-H. influenzae antibody treatment group.
P = 0.005 compared to 4F11(G1) F(ab′)2 treatment group; P ≤ 0.001 compared to anti-H. influenzae antibody treatment group.
Complement is essential for optimal MAb 4F11(G1) PCP prophylaxis.
Complement activation by IgG1 is Fc dependent. To determine whether complement was required for passive protection by MAb 4F11(G1), we repeated the passive prophylaxis experiments using cobra venom factor to deplete complement. Half of the complement-depleted mice and half of the nondepleted mice also received daily doses of 50 μl of a 1-mg/ml suspension of MAb 4F11(G1) i.n., while the other half received 50 μl of sterile saline i.n. The mice were cohoused with three P. carinii-infected source mice for 14 days, at which time the source mice were removed and the mice were given three additional daily doses of antibody or saline. The mice were sacrificed 6 weeks after completion of cohousing, and their lungs were removed for determination of P. carinii burden by quantitative real-time PCR. While complement depletion had no effect on organism burden in mice that received intranasal saline compared to nondepleted mice treated with intranasal saline, complement depletion was shown to decrease the amount of protection provided by MAb 4F11(G1) (Table 2). Complement-sufficient mice treated with MAb 4F11(G1) had a 22-fold reduction in organism burden compared to saline-treated mice (P ≤ 0.01). Complement-depleted mice treated with MAb 4F11(G1) had only a fourfold reduction in organism burden compared to saline-treated mice (P ≤ 0.01), significantly lower than the amount of protection in nondepleted mice (P ≤ 0.01). The differences in organism burden were corroborated by blinded microscopic quantification of silver-stained P. carinii cysts in individual lung homogenate samples (data not shown). The results of these experiments suggest that complement is required for optimal protection against the development of PCP by MAb 4F11(G1).
TABLE 2.
Effects of complement depletion on MAb 4F11(G1) PCP prophylaxis
| Treatment | P. carinii burdena | n |
|---|---|---|
| MAb 4F11(G1) i.n., saline i.p. | (1.06 ± 0.34) × 105b,c | 10 |
| MAb 4F11(G1) i.n., CVF i.p. | (5.38 ± 1.76) × 105b | 9 |
| Saline i.n., CVF i.p. | (2.36 ± 0.74) × 106 | 9 |
| Saline i.n., saline i.p. | (2.35 ± 1.43) × 106 | 10 |
Results represent means ± standard deviations of duplicate analyses of boiled lung homogenates from each mouse quantified by comparison to a standard curve generated from known quantities of plasmid-encoded mouse P. carinii kex1.
P ≤ 0.01 compared to i.n. and i.p. saline control, and P ≤ 0.01 compared to saline i.n. and cobra venom factor (CVF) i.p. control.
P ≤ 0.01 compared to MAb 4F11(G1) i.n. and CVF i.p.
DISCUSSION
Despite the many recent advances in our understanding of host defense against P. carinii, we still do not understand the final mechanism of host-mediated destruction of this organism. These studies were undertaken to add to our understanding of how humoral immune factors might be involved in the control of P. carinii. We have shown that the Fc region of MAb 4F11 is essential for optimal effect of the antibody in PCP prophylaxis. Prophylaxis against PCP using F(ab′)2 fragments of MAb 4F11 was approximately 90% less effective than prophylaxis with the intact molecule. The Fc regions of IgG1 molecules are recognized by Fc receptors on the surface of phagocytes which, upon binding IgG-opsonized organisms, leads to phagocytosis. Enhanced phagocytosis by alveolar macrophages may be the potential mechanism by which MAb 4F11 protects against development of PCP.
In addition to Fc receptor-mediated phagocytosis, antibody can also enhance phagocytosis through activation of the complement cascade and deposition of complement component C3b on the surface of the pathogen. Since complement activation by antibody is also Fc dependent, we decided to test the requirement for complement in MAb 4F11(G1) prophylaxis. Mice treated with cobra venom factor during MAb 4F11(G1) prophylaxis had significantly greater P. carinii burdens than mice with intact complement systems, suggesting that complement is required for an optimal effect of MAb 4F11(G1) in protecting against the development of PCP. This was somewhat surprising, given the low relative ability of IgG1 to fix complement compared to other antibody isotypes. However, under conditions of high epitope density, mouse IgG1 exhibits enhanced ability to fix complement (20). In addition, IgG1 mediates rejection of xenografts (30). This effect of IgG1 was only demonstrable in the presence of complement, NK cells, and functional Fc receptor activity on the NK cells. Thus, in addition to traditional interactions with phagocytic cells, IgG1 may fix complement as a means of increasing the interaction of NK cells with their target. Whether this function of IgG1 is confined to graft rejection or can also be implicated in control of infection is unknown. It should be noted, however, that NK cells have direct antifungal activity (15, 18, 22). Nonetheless, our results suggest that complement fixation by MAb 4F11(G1) plays a role in protection against P. carinii.
Our earlier study showed that an IgM isotype variant of MAb 4F11(G1) was also capable of preventing PCP in SCID mice (2). Since there are no known receptors for IgM on phagocytes and IgM is a highly efficient activator of complement, it is possible that the protective effects of IgM MAb 4F11 are through complement activation. Interestingly, stimulation of the respiratory burst by phagocytes after phagocytosis of P. carinii is greatest in the presence of the combination of both antibody and complement (8, 10-12), and complement fixation enhances in vitro phagocytosis of P. carinii compared to unopsonized P. carinii (28). It is possible that opsonization by complement through the classical pathway led to enhanced PCP prophylaxis in our experiment. It has not been demonstrated that the membrane attack complexes formed by C6 to C9 of the complement system are capable of causing P. carinii lysis, though it is possible that complement-mediated lysis of P. carinii trophozoites may have occurred in our experiment. Although complement was necessary for optimal prophylaxis in our model system, C3 knockout mice are not susceptible to PCP (14), suggesting that complement is not required for protection against PCP in mice with functioning CD4+ T cells.
Complement has also been shown to be essential for antibody-mediated protection against the fungal pathogen Candida albicans (6). In C. albicans, complement fixation has been linked to epitope specificity, location, and density (4, 5). MAb 4F11(G1) recognizes multiple copies of similar epitopes that are uniformly distributed on the surface of the organism (13, 29), an attribute that likely contributes to protection by this antibody.
In the absence of complement, partial protection against development of PCP by MAb 4F11(G1) is dependent on another antibody Fc region-mediated function, and antigen recognition alone is insufficient for protection by this antibody. Antibody opsonization of P. carinii enhanced in vitro phagocytosis and stimulation of the respiratory burst by neutrophils and macrophages (10, 11) and enhanced tumor necrosis factor alpha production by alveolar macrophages (21). We have determined that MAb 4F11(G1) opsonization of P. carinii leads to enhanced phagocytosis by the THP-1 monocyte line in vitro (data not shown), suggesting that Fc receptor-mediated phagocytosis of MAb 4F11(G1)-opsonized P. carinii may account for the fourfold reduction in organism burden in the absence of complement shown in Table 2. It would be interesting to see whether MAb 4F11 could provide passive protection in mice that are unable to phagocytose IgG1-opsonized pathogens, such as knockouts in the common gamma chain of Fcγ receptors. It has been shown that the common gamma chain knockout strain does not succumb to P. carinii pneumonia, though this mouse strain does show a delay in clearance of the pathogen (14); therefore, these mice would have to be crossed with SCID mice to render them susceptible to infection by P. carinii.
This study has provided insight into how anti-P. carinii antibodies act to prevent P. carinii infection. By understanding the mechanism by which MAb 4F11(G1) provides protection against development of PCP, we may be able to design more effective PCP prophylaxis in humans.
Acknowledgments
We thank Margaret Chovaniec, Stephanie Campbell, and Victoria Houseknecht for technical assistance. We also thank Ann Harmsen at Montana State University for performing P. carinii cyst counts.
This work was supported by NIH grants RO1 AI23302 and NIAID 5T32AI07362.
Editor: A. Casadevall
REFERENCES
- 1.Gigliotti, F., and W. T. Hughes. 1988. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J. Clin. Investig. 81:1666-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gigliotti, F., C. G. Haidaris, T. W. Wright, and A. G. Harmsen. 2002. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infect. Immun. 70:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gigliotti, F., A. G. Harmsen, and T. W. Wright. 2003. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect. Immun. 71:3852-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han, Y., and J. E. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han, Y., T. Kanbe, R. Cherniak, and J. E. Cutler. 1997. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 65:4100-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han, Y., T. R. Kozel, M. X. Zhang, R. S. MacGill, M. C. Carroll, and J. E. Cutler. 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J. Immunol. 167:1550-1557. [DOI] [PubMed] [Google Scholar]
- 7.Harmsen, A. G., and M. Stankiewicz. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 172:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hidalgo, H. A., R. J. Helmke, V. F. German, and J. A. Mangos. 1992. Pneumocystis carinii induces an oxidative burst in alveolar macrophages. Infect. Immun. 60:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacs, J. A., V. J. Gill, S. Meshnick, and H. Masur. 2001. New insights into transmission, diagnosis, and drug treatment of Pneumocystis carinii pneumonia. JAMA 286:2450-2460. [DOI] [PubMed] [Google Scholar]
- 10.Laursen, A. L., B. Moller, J. Rungby, C. M. Petersen, and P. L. Andersen. 1994. Pneumocystis carinii-induced activation of the respiratory burst in human monocytes and macrophages. Clin. Exp. Immunol. 98:196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laursen, A. L., N. Obel, J. Rungby, and P. L. Andersen. 1993. Phagocytosis and stimulation of the respiratory burst in neutrophils by Pneumocystis carinii. J. Infect. Dis. 168:1466-1471. [DOI] [PubMed] [Google Scholar]
- 12.Laursen, A. L., N. S. Obel, U. Holmskov, J. C. Jensenius, M. Aliouat el, and P. L. Andersen. 2003. Activation of the respiratory burst by Pneumocystis carinii. Efficiency of different antibody isotypes, complement, lung surfactant protein D, and mannan-binding lectin. APMIS 111:405-415. [DOI] [PubMed] [Google Scholar]
- 13.Lee, L. H., F. Gigliotti, T. W. Wright, P. J. Simpson-Haidaris, G. A. Weinberg, and C. G. Haidaris. 2000. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene 242:141-150. [DOI] [PubMed] [Google Scholar]
- 14.Lund, F. E., K. Schuer, M. Hollifield, T. D. Randall, and B. A. Garvy. 2003. Clearance of Pneumocystis carinii in mice is dependent on B cells but not on P. carinii-specific antibody. J. Immunol. 171:1423-1430. [DOI] [PubMed] [Google Scholar]
- 15.Ma, L. L., C. L. Wang, G. G. Neely, S. Epelman, A. M. Krensky, and C. H. Mody. 2004. NK cells use perforin rather than granulysin for anticryptococcalactivity. J. Immunol. 173:3357-3365. [DOI] [PubMed] [Google Scholar]
- 16.Marcotte, H., D. Levesque, K. Delanay, A. Bourgeault, R. de la Durantaye, S. Brochu, and M. C. Lavoie. 1996. Pneumocystis carinii infection in transgenic B cell-deficient mice. J. Infect. Dis. 173:1034-1037. [DOI] [PubMed] [Google Scholar]
- 17.Masur, H., M. A. Michelis, J. B. Greene, I. Onorato, R. A. Stouwe, R. S. Holzman, G. Wormser, L. Brettman, M. Lange, H. W. Murray, and S. Cunningham-Rundles. 1981. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N. Engl. J. Med. 305:1431-1438. [DOI] [PubMed] [Google Scholar]
- 18.Mathews, H. L., and L. Witek-Janusek. 1998. Antifungal activity of nterleukin-2-activated natural killer (NK1.1+) lymphocytes against Candida albicans. J. Med. Microbiol. 47:1007-1014. [DOI] [PubMed] [Google Scholar]
- 19.Morris, A., J. D. Lundgren, H. Masur, P. D. Walzer, D. L. Hanson, T. Frederick, L. Huang, C. B. Beard, and J. E. Kaplan. 2004. Current epidemiology of Pneumocystis pneumonia. Emerg. Infect. Dis. 10:1713-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada, M., K. Takahashi, and S. Utsumi. 1983. Conditions which favor C1-fixation by mouse IgG1. Mol. Immunol. 20:279-285. [DOI] [PubMed] [Google Scholar]
- 21.Neese, L. W., J. E. Standing, E. J. Olson, M. Castro, and A. H. Limper. 1994. Vitronectin, fibronectin, and gp120 antibody enhance macrophage release of TNF-alpha in response to Pneumocystis carinii. J. Immunol. 152:4549-4556. [PubMed] [Google Scholar]
- 22.Petkus, A. F., and L. L. Baum. 1987. Natural killer cell inhibition of young spherules and endospores of Coccidioides immitis. J. Immunol. 139:3107-3111. [PubMed] [Google Scholar]
- 23.Roths, J. B., and C. L. Sidman. 1992. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J. Clin. Investig. 90:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roths, J. B., and C. L. Sidman. 1993. Single and combined humoral and cell-mediated immunotherapy of Pneumocystis carinii pneumonia in immunodeficient scid mice. Infect. Immun. 61:1641-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepkowitz, K. A. 2002. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin. Infect. Dis. 34:1098-1107. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro, S., D. O. Beenhouwer, M. Feldmesser, C. Taborda, A. Casadevall, and M. D. Scharff. 2002. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect. Immun. 70:2598-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shellito, J., V. V. Suzara, W. Blumenfeld, J. M. Beck, H. J. Steger, and T. H. Ermak. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Investig. 85:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor, M. B., M. Phillips, and C. S. Easmon. 1992. Opsonophagocytosis of Pneumocystis carinii. J. Med. Microbiol. 36:223-228. [DOI] [PubMed] [Google Scholar]
- 29.Wells, J., F. Gigliotti, P. J. Simpson-Haidaris, and C. G. Haidaris. 2004. Epitope mapping of a protective monoclonal antibody against Pneumocystis carinii with shared reactivity to Streptococcus pneumoniae surface antigen PspA. Infect. Immun. 72:1548-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin, D., H. Zeng, L. Ma, J. Shen, H. Xu., G. W. Byrne, and A. S. Chong. 2004. Cutting edge: NK cells mediate IgG1-dependent hyperacute rejection of xenografts. J. Immunol. 172:7235-7238. [DOI] [PubMed] [Google Scholar]