Abstract
Escherichia coli 83972 is a clinical asymptomatia bacteriuric isolate that is able to colonize the human urinary bladder without inducing an immune response. Here we demonstrate that one of the mechanisms by which this strain has become attenuated is through the mutation of its genes encoding type 1 and P fimbriae.
Urinary tract infections (UTI) are among the most common infectious diseases of humans and a major cause of morbidity and mortality. Acute pyelonephritis and asymptomatic bacteriuria (ABU) represent the two extremes of UTI. Acute pyelonephritis is a severe systemic infection caused by uropathogenic Escherichia coli (UPEC) (6, 7, 29, 31). ABU, on the other hand, is an asymptomatic carrier state in which patients may carry >105 CFU/ml of a single E. coli strain for years without provoking a host response. In early studies, this was explained by a lack of virulence genes; however, molecular epidemiology has shown that >60% of ABU strains carry virulence genes but fail to express the phenotype (18, 19).
The ability of UPEC to cause symptomatic UTI is enhanced by adhesins, including type 1 and P fimbriae (11, 17). P fimbriae enhance the establishment of bacteriuria and activate the innate immune response in animal models and in the human urinary tract (2, 20, 21, 33, 35, 36). Binding is mediated by the PapG adhesin, which is located at the tips of the fimbriae and which recognizes the α-d-galatopyranosyl-(1-4)-β-d-galactopyranoside receptor epitope in the globoseries of glycolipids (3, 12, 13). Type 1 fimbriae enhance colonization, induce host responses in the murine UTI model, and promote biofilm formation and invasion (4, 14, 16, 23). Receptor binding is also mediated by an adhesin located at the tips of the fimbriae (FimH) that binds to α-d-mannosylated proteins, such as uroplakins, which are abundant in the bladder (32). In this study, we characterized the type 1- and P-encoding fimbrial genes from the prototypical ABU strain E. coli 83972. The strain is a clinical isolate capable of long-term bladder colonization (1). It was isolated from a patient with ABU who had carried it for 3 years, and it has been used in colonization studies as a prophylactic agent to prevent UTI in humans (2, 8, 30, 34, 35).
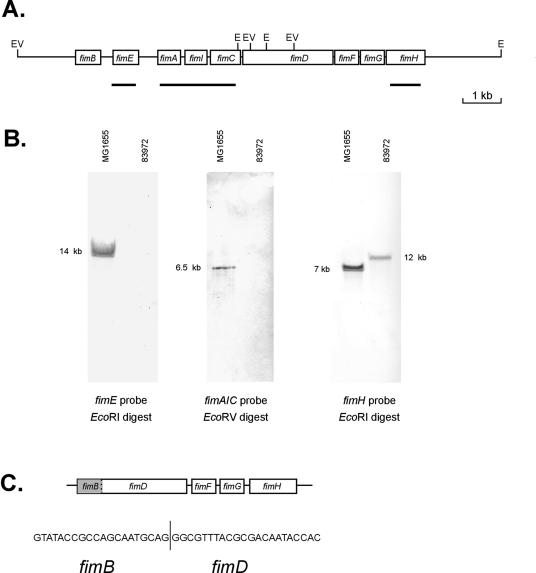
E. coli 83972 does not express a detectable type 1 fimbrial phenotype when recovered from the urinary tract or after in vitro subculture (35). However, previous genetic analysis of the strain revealed that it contains the genes for type 1 fimbriae (9). To examine the E. coli 83972 type 1 fimbria-encoding genes in more detail, we performed a series of Southern hybridizations with probes spanning different regions within the MG1655 type 1 fimbrial gene cluster. A positive hybridization signal was obtained with a fimH gene probe but not with fimE or fimAIC probes (Fig. 1). Subsequent PCR amplification and sequencing of the fim cluster from E. coli 83972 revealed a 4.25-kb deletion affecting all genes except those encoding the minor components fimF, fimG, and fimH (Fig. 1). The chromosomally located fimH gene was expressed as a functional product, since the transformation of E. coli 83972 with plasmid pPKL114 (containing fimBEAICDFG) induced a mannose-sensitive agglutination of yeast cells (Table 1). Sequencing of the fimH gene from E. coli 83972 revealed the following changes relative to FimH from E. coli K-12: V48A, G87S, N91S, and S99N.
FIG. 1.
(A) Physical map of the fim gene cluster from E. coli MG1655 indicating the arrangement of the genes, the position of relevant restriction enzyme sites (E, EcoRI; EV, EcoRV), and the region covered by each of the probes. (B) Southern blot analysis of total genomic DNA from E. coli MG1655 and 83972 digested to completion with either EcoRI or EcoRV and probed with the fimE gene, the fimA, fimB, and fimC genes, and the fimH gene (as indicated). The sizes of the hybridizing fragments are indicated in kilobase pairs. (C) Structure of the fim locus in E. coli 83972 indicating the precise start and end points of the deletion between fimB and fimD.
TABLE 1.
Agglutination profile of LB agar-grown E. coli 83972 containing plasmid-borne encoded fimbrial genes
| Plasmid | Agglutination ofa:
|
|
|---|---|---|
| Yeast cells | Human type A RBC | |
| None | − | − |
| pPKL4 (all fim genes) | + | − |
| pPKL114 (all fim genes except fimH) | + | − |
| pPAP5 (all pap genes from J96) | − | + |
| pDD3 (all pap genes except papG) | − | − |
| pDD4 (papG only) | − | + |
−, negative; +, positive.
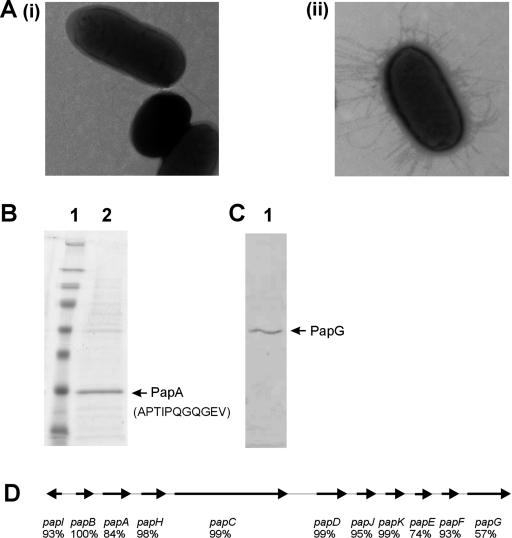
E. coli 83972 reportedly contains pap gene sequences (9) but has never been shown to express P fimbriae. However, when we grew 83972 on a solid medium and examined the cells by transmission electron microscopy, we observed that the majority produced fimbriae (Fig. 2A). Interestingly, very few cells produced fimbriae when grown as liquid cultures. Purification and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the fimbriae revealed a prominent 18-kDa protein that was confirmed by N-terminal amino acid sequencing to be the PapA major subunit (Fig. 2B). E. coli 83972 cells expressing these P fimbriae did not hemagglutinate human red blood cells (RBCs), bind to human uroepithelial cells, or bind to Galα1-4Galβ-containing glycolipids (data not shown). Thus, strain 83972 produces P fimbriae that are unable to bind to any known receptor targets.
FIG. 2.
(A) Transmission electron micrographs of E. coli 83972 grown (i) in LB broth and (ii) on LB agar. Almost all cells harvested after growth on agar produced P fimbriae, while less than 5% of cells grown as liquid cultures produced any fimbriae. This may explain previous observations that E. coli 83972 does not produce fimbriae. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of purified fimbrial proteins from E. coli 83972 (lane 2). The N-terminal amino acid sequence of the PapA fimbrial protein is indicated in brackets. Molecular size markers (lane 1) are 250, 98, 64, 50, 36, 30, 16, and 6 kDa. (C) Corresponding Western blot analysis of the same purified fimbrial preparation using a PapG-specific polyclonal antiserum indicating the expression of the PapG adhesin. (D) Physical map of the pap gene cluster from E. coli 83972 indicating the arrangement of the genes and the amino acid identity of each of the predicted proteins with the respective protein from E. coli CFT073. The predicted translational product of the papA gene is identical to the first 10 residues identified by N-terminal sequencing. PapA belongs to the F14 allele group, while PapG belongs to the class III allele group (nucleotide accession number, DQ010312).
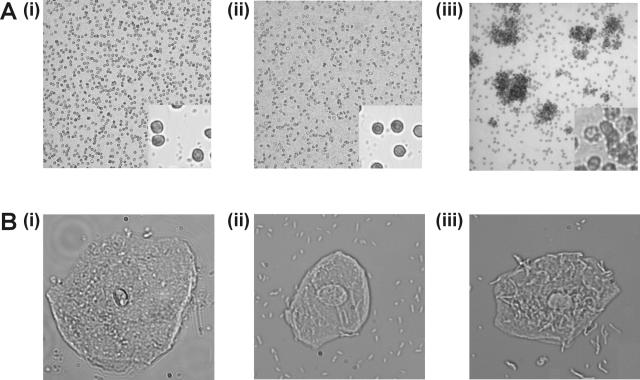
The pap gene cluster of E. coli 83972 was amplified by PCR and sequenced. A comparison of the amino acid sequence deduced from each gene with the equivalent genes from UPEC CFT073 revealed that the greatest divergence occurred in papA, papE, and papG (Fig. 2E). The function of the 83972 PapG adhesin was assessed by complementation with the following plasmids: (i) pDD3 (all pap genes from UPEC J96 except papG) and (ii) pDD4 (papG from UPEC J96). Only E. coli 83972 (pDD4) cells readily agglutinated RBCs and bound to human uroepithelial cells (Fig. 3A and B; Table 1). Thus, the recognition of receptor targets by the P fimbriae of E. coli 83972 can be complemented in trans by a plasmid carrying a functional papG gene. Western blot analysis of fimbrial proteins using PapG-specific polyclonal antisera revealed that PapG is expressed and suggests that it is incorporated into the fimbrial structure (Fig. 2C). This interpretation is supported by the fact that E. coli 83972 produces fimbriae of normal length and morphology (previous studies of fimbrial biogenesis have demonstrated that disruption of the adhesin-encoding gene results in the synthesis of organelles of aberrant length and morphology) (24). It is possible that the lack of function of PapG may be associated with minor amino acid changes in the protein sequence. In this respect, all but one of the residues predicted to contribute to the PapG binding site (5) are conserved in the E. coli 83972 PapG sequence (Fig. 4). By analogy with the FimH adhesin (22, 25), variations that alter the conformational stability of the protein loops that carry the receptor-interacting residues may also account for its lack of function.
FIG. 3.
Agglutination of human type A RBC (A) and binding to human uroepithelial cells (B) by (i) E. coli 83972, (ii) E. coli 83972 (pDD3; contains all pap genes from UPEC strain J96 except papG), and (iii) E. coli 83972 (pDD4; papG gene from J96). Functional activity was observed only in the presence of the plasmid containing the papG gene from E. coli J96. Shown are the binding phenotypes under low (10×) (A) and high (63×) (B) magnifications.
FIG. 4.
Alignment of the amino acid sequence deduced from the nucleotide sequence of the E. coli 83972 papG gene with the closely related sequence of the uropathogenic E. coli 536 prfG gene. Residues identical to those of the E. coli 83972 papG gene are indicated by dots; gaps introduced into the alignment are indicated by dashed lines. The residues conserved between PapG I, II, and III alleles are shaded.
E. coli 83972 was carried by a young girl for 3 years without any symptoms. Whether the strain had already lost the ability to express P and type 1 fimbriae previously during passage through another host or did so in this particular girl is unclear. However, several lines of evidence support the notion that the ancestor of E. coli 83972 was a pyelonephritic UPEC strain: (i) the FimH allele of 83972 contains minor amino acid variations that are consistent with those of previously characterized pyelonephritis strains (26-28); (ii) the strain is able to express P fimbriae, albeit an apparently nonadhesive type; (iii) multilocus sequence typing of 83972 shows that it belongs to the B2 clonal group (http://www.mlst.net/) and this group contains E. coli strains associated with pyelonephritis and other extraintestinal invasive clinical syndromes such as bacteremia, prostatitis, and meningitis; and (iv) the strain possesses the F14 PapA allele, which has been associated with other virulence factors, including S and F1C fimbriae, hemolysin, and cytotoxic necrotizing factor 1 from E. coli B2 strains (10). Genes of nonfunctional products tend to erode over time through accumulation of mutations, and there are many instances where genome shrinkage has been associated with bacterial lifestyle transition (15). In E. coli 83972, the two primary adhesive organelles associated with uropathogenesis have been inactivated by adaptive mutations as a trade-off with the host.
The characterization of the fim- and pap-encoding genes in this study illustrates an important issue with regard to the current molecular knowledge of E. coli 83972. Previous studies demonstrating the presence of these genes in E. coli 83972 failed to correlate with its phenotypic characteristics (9, 33). Here we have shown for the first time that E. coli 83972 contains only some of the type 1 fimbrial genes and is not capable of producing these organelles. The finding that fimH is functional and constitutively expressed may explain a previous report that identified a clone capable of mannose-sensitive hemagglutination from a recombinant cosmid library derived from 83972 (9). In the case of P fimbriae, these organelles are expressed, but their function remains unknown since they do not bind to defined receptor targets. This study sheds new light on how E. coli 83972 has adapted to grow in the human bladder. The strain has lost the ability to express functional P and type 1 fimbriae and is thus able to persist in this environment without triggering a host immune response. In contrast to organisms that have acquired genes for pathogenesis, E. coli 83972 is an example of an organism that has adapted to commensalism through gene loss and mutation.
Acknowledgments
We thank Birthe Jul Jorgensen, Göran Bergsten, Jannick Jacobsen, and Rick Webb for expert technical assistance.
This work was supported by grants from the Australian National Health and Medical Research Council (401714), the University of Queensland, the Danish Medical Research Council (22-03-0462), and the Danish Research Agency (2052-03-0013).
Editor: V. J. DiRita
REFERENCES
- 1.Andersson, P., I. Engberg, G. Lidin-Janson, K. Lincoln, R. Hull, S. Hull, and C. Svanborg. 1991. Persistence of Escherichia coli bacteriuria is not determined by bacterial adherence. Infect. Immun. 59:2915-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsten, G., M. Samuelsson, B. Wullt, I. Leijonhufvud, H. Fischer, and C. Svanborg. 2004. PapG-dependent adherence breaks mucosal inertia and triggers the innate host response. J. Infect. Dis. 189:1734-1742. [DOI] [PubMed] [Google Scholar]
- 3.Bock, K., M. E. Breimer, A. Brignole, G. C. Hansson, K. A. Karlsson, G. Larson, H. Leffler, B. E. Samuelsson, N. Stromberg, C. S. Eden, et al. 1985. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1→4Gal-containing glycosphingolipids. J. Biol. Chem. 260:8545-8551. [PubMed] [Google Scholar]
- 4.Connell, H., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodson, K. W., J. S. Pinkner, T. Rose, G. Magnusson, S. J. Hultgren, and G. Waksman. 2001. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105:733-743. [DOI] [PubMed] [Google Scholar]
- 6.Eden, C. S., L. A. Hanson, U. Jodal, U. Lindberg, and A. S. Akerlund. 1976. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet i:490-492. [PubMed] [Google Scholar]
- 7.Funfstuck, R., H. Tschape, G. Stein, H. Kunath, M. Bergner, and G. Wessel. 1986. Virulence properties of Escherichia coli strains in patients with chronic pyelonephritis. Infection 14:145-150. [DOI] [PubMed] [Google Scholar]
- 8.Hull, R., D. Rudy, W. Donovan, C. Svanborg, I. Wieser, C. Stewart, and R. Darouiche. 2000. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J. Urol. 163:872-877. [PubMed] [Google Scholar]
- 9.Hull, R. A., D. C. Rudy, W. H. Donovan, I. E. Wieser, C. Stewart, and R. O. Darouiche. 1999. Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infect. Immun. 67:429-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, J. R., P. Delavari, M. Kuskowski, and A. L. Stell. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183:78-88. [DOI] [PubMed] [Google Scholar]
- 11.Klemm, P., and M. A. Schembri. 2000. Bacterial adhesins: function and structure. Int. J. Med. Microbiol. 290:27-35. [DOI] [PubMed] [Google Scholar]
- 12.Leffler, H., and C. Svanborg-Edén. 1981. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect. Immun. 34:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund, B., F. Lindberg, B. I. Marklund, and S. Normark. 1987. The PapG protein is the alpha-d-galactopyranosyl-(1→4)-beta-d-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 84:5898-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran, N. A. 2002. Microbial minimalism: genome reduction in bacterial pathogens. Cell 108:583-586. [DOI] [PubMed] [Google Scholar]
- 16.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 17.Oelschlaeger, T. A., U. Dobrindt, and J. Hacker. 2002. Virulence factors of uropathogens. Curr. Opin. Urol. 12:33-38. [DOI] [PubMed] [Google Scholar]
- 18.Plos, K., T. Carter, S. Hull, R. Hull, and C. Svanborg Eden. 1990. Frequency and organization of pap homologous DNA in relation to clinical origin of uropathogenic Escherichia coli. J. Infect. Dis. 161:518-524. [DOI] [PubMed] [Google Scholar]
- 19.Plos, K., H. Connell, U. Jodal, B. I. Marklund, S. Marild, B. Wettergren, and C. Svanborg. 1995. Intestinal carriage of P fimbriated Escherichia coli and the susceptibility to urinary tract infection in young children. J. Infect. Dis. 171:625-631. [DOI] [PubMed] [Google Scholar]
- 20.Roberts, J. A., M. B. Kaack, G. Baskin, M. R. Chapman, D. A. Hunstad, J. S. Pinkner, and S. J. Hultgren. 2004. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J. Urol. 171:1682-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts, J. A., B. I. Marklund, D. Ilver, D. Haslam, M. B. Kaack, G. Baskin, M. Louis, R. Mollby, J. Winberg, and S. Normark. 1994. The Gal(alpha 1→4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl. Acad. Sci. USA 91:11889-11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schembri, M. A., K. Kjaergaard, E. V. Sokurenko, and P. Klemm. 2001. Molecular characterization of the Escherichia coli FimH adhesin. J. Infect. Dis. 183(Suppl. 1):S28-S31. [DOI] [PubMed] [Google Scholar]
- 23.Schembri, M. A., and P. Klemm. 2001. Biofilm formation in a hydrodynamic environment by novel FimH variants and ramifications for virulence. Infect. Immun. 69:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schembri, M. A., L. Pallesen, H. Connell, D. L. Hasty, and P. Klemm. 1996. Linker insertion analysis of the FimH adhesin of type 1 fimbriae in an Escherichia coli fimH-null background. FEMS Microbiol. Lett. 137:257-263. [DOI] [PubMed] [Google Scholar]
- 25.Schembri, M. A., E. V. Sokurenko, and P. Klemm. 2000. Functional flexibility of the FimH adhesin: insights from a random mutant library. Infect. Immun. 68:2638-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokurenko, E. V., H. S. Courtney, J. Maslow, A. Siitonen, and D. L. Hasty. 1995. Quantitative differences in adhesiveness of type 1 fimbriated Escherichia coli due to structural differences in fimH genes. J. Bacteriol. 177:3680-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokurenko, E. V., H. S. Courtney, D. E. Ohman, P. Klemm, and D. L. Hasty. 1994. FimH family of type 1 fimbrial adhesins: functional heterogeneity due to minor sequence variations among fimH genes. J. Bacteriol. 176:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokurenko, E. V., M. Feldgarden, E. Trintchina, S. J. Weissman, S. Avagyan, S. Chattopadhyay, J. R. Johnson, and D. E. Dykhuizen. 2004. Selection footprint in the FimH adhesin shows pathoadaptive niche differentiation in Escherichia coli. Mol. Biol. Evol. 21:1373-1383. [DOI] [PubMed] [Google Scholar]
- 29.Stenqvist, K., T. Sandberg, G. Lidin-Janson, F. Orskov, I. Orskov, and C. Svanborg-Eden. 1987. Virulence factors of Escherichia coli in urinary isolates from pregnant women. J. Infect. Dis. 156:870-877. [DOI] [PubMed] [Google Scholar]
- 30.Trautner, B. W., R. A. Hull, and R. O. Darouiche. 2003. Escherichia coli 83972 inhibits catheter adherence by a broad spectrum of uropathogens. Urology 61:1059-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, X. R., T. T. Sun, and J. J. Medina. 1996. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl. Acad. Sci. USA 93:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wullt, B. 2003. The role of P fimbriae for Escherichia coli establishment and mucosal inflammation in the human urinary tract. Int. J. Antimicrob. Agents. 21:605-621. [DOI] [PubMed] [Google Scholar]
- 34.Wullt, B., G. Bergsten, H. Connell, P. Rollano, N. Gebratsedik, L. Hang, and C. Svanborg. 2001. P-fimbriae trigger mucosal responses to Escherichia coli in the human urinary tract. Cell. Microbiol. 3:255-264. [DOI] [PubMed] [Google Scholar]
- 35.Wullt, B., G. Bergsten, H. Connell, P. Rollano, N. Gebretsadik, R. Hull, and C. Svanborg. 2000. P fimbriae enhance the early establishment of Escherichia coli in the human urinary tract. Mol. Microbiol. 38:456-464. [DOI] [PubMed] [Google Scholar]
- 36.Wullt, B., G. Bergsten, M. Samuelsson, and C. Svanborg. 2002. The role of P fimbriae for Escherichia coli establishment and mucosal inflammation in the human urinary tract. Int. J. Antimicrob. Agents. 19:522-538. [DOI] [PubMed] [Google Scholar]