Abstract
Three groups of six monkeys (Aotus nancymae) each were inoculated intragastrically with increasing doses of Campylobacter jejuni. Infection resulted in fecal colonization (100% of monkeys), dose-related diarrhea, and robust immune responses. Colonization duration and diarrhea rate were reduced upon secondary challenge. A. nancymae may be useful for studying anti-Campylobacter vaccine efficacy.
Campylobacter jejuni is among the leading causes of bacterial diarrhea in the United States and second only to enterotoxigenic Escherichia coli as a cause of traveler's diarrhea throughout the world (9, 12, 19). In developing countries, Campylobacter enteritis incidence rates as high as 0.4 episode per child per year have been reported (8, 9, 19, 30). C. jejuni or Campylobacter coli organisms were isolated from approximately 50% of stool samples obtained from military personnel exhibiting diarrhea while deployed in Thailand (16, 18, 20).
A major impediment to the preclinical evaluation of candidate Campylobacter vaccines is the absence of a suitable animal diarrheal disease model. Different animal models have been used for Campylobacter research, including mice (3, 6, 29, 31), rabbits (7), ferrets (4, 11), hamsters (14), chicks (26), piglets (1), dogs (21), and Old World monkeys (i.e., Macaca nemestrina, Macaca mulatta, and Macaca fascicularis) (10, 24, 25). Animal models in which Campylobacter spp. produce diarrhea are limited by availability (10, 24, 25), the need for surgical or chemical manipulations (7, 29), the lack of immunologic reagents (4, 11), and the young age of the animals (1, 26).
Old World monkeys exhibit limited Campylobacter-related clinical signs and pathology, potentially due to previous Campylobacter exposure (10, 24, 25). New World monkeys are at lower risk of spreading pathogens throughout their colony than Old World monkeys due to differences in housing practices (indoor versus outdoor breeding) (22, 23, 28). For this reason, we sought to develop an animal model for Campylobacter disease using the New World monkey Aotus nancymae.
A. nancymae primates (of the captive-born IVITA/PAHO strain) were purchased from the Instituto Veterinario de Investigaciones Tropicales y de Altura (IVITA), University of San Marcos, Peru. Monkeys (25 male, 13 female; average age, 43 ± 19 months; weight, 1 to 1.5 kg) were randomly selected from the primate pool at the Naval Medical Research Center Detachment, Lima, Peru. The experimental monkeys had never been used for Campylobacter research, were stool culture negative for Campylobacter (before initial inoculation and subsequent challenge), and had a baseline immunoglobulin G (IgG) or IgA titer of less than 200 or 20, respectively, against a glycine extract (GE) (3 μg/ml) of whole Campylobacter organisms, as measured by enzyme-linked immunosorbent assay (ELISA) (2, 17).
C. jejuni strain 81-176 (5, 15) was used, and inocula were prepared as described by Black et al. (5) except that Mueller-Hinton soft agar was used to obtain highly motile C. jejuni organisms that were pooled and plated on brucella agar (BA). The bacterial lawn was harvested from each BA plate in ice-cold phosphate-buffered saline (PBS), and the concentration of organisms was adjusted photometrically with PBS to the target dose in 5 ml. The actual dose was determined by serial dilution and plating on BA.
To buffer gastric contents, the monkeys were injected intramuscularly with ranitidine (1.5 mg/kg of body weight; Zantac; GlaxoSmithKline) 90 min prior to C. jejuni inoculation. Thirty minutes prior to C. jejuni inoculation, the monkeys were anesthetized with ketamine hydrochloride (4 to 5 mg/kg; Ketalar; Park-Davis) plus diazepam (0.2 mg/kg; Valium; Roche) and then administered 5.0 ml CeraVacx I (CeraProducts, Jessup, MD) intragastrically (i.g.). CeraVacx and C. jejuni preparations were administered through a 5 French/Charrière (1.7-mm), 16-in. (41-cm) feeding tube inserted orally.
The veterinarian examined the monkeys twice daily for 21 days after inoculation for physical signs of disease. Weight and temperature were measured during anesthesia for blood draws. A technician examined the cage pans twice daily for 7 days prior to and 21 days after inoculation for the presence of diarrheal stools. To prevent cross-contamination, the PBS control monkeys were separated from the infected monkeys in cages on opposite sides of the room. It was therefore not possible for the technicians scoring the diarrhea to be blinded. The stools were cultured daily for 21 days to assess C. jejuni excretion and were examined for 7 days for fecal leukocytes and occult blood. The experimental animals did not exhibit more physical signs of disease, fecal leukocytes, or occult blood than at baseline. SAS v8.2 software (The SAS Institute, Cary, NC) was used for statistical analyses, and tests were interpreted in a two-tailed fashion, with P values of <0.05 being significant.
Dose-escalating study.
Three groups of six monkeys each were inoculated i.g. with escalating doses of C. jejuni (Table 1). In addition, three procedural controls per experimental group were given PBS i.g. to ensure that the procedures did not cause stress on the monkeys and artificially increase diarrhea rates. One (11.1%) of nine controls in the dose-escalating study had diarrhea but for only 2 days. None of the 12 total controls had a serum IgG or IgA response, whereas two (16.7%) had a transient fecal IgA response, with a mean peak increase (n-fold) in titer of 9.2 ± 0.8 at day 14 that returned to baseline at day 21. In a previous study, 109 to 1011 CFU of the nonpathogenic E. coli strain HS did not cause diarrhea in this model (13).
TABLE 1.
Diarrhea rates, diarrhea duration, and C. jejuni excretion in A. nancymae monkeys inoculated with escalating doses of C. jejuni strain 81-176 and then challenged with 7 × 1011 CFU of the homologous strain
| Experimental group | No. of monkeys | Dose (CFU) | Inoculation with C. jejuni
|
Challenge with 7 × 1011 CFU of C. jejuni
|
||||
|---|---|---|---|---|---|---|---|---|
| No. (%) of monkeys with a diarrhea episodea | Mean no. (range) of days with diarrheab | Mean no. (range) of excretion daysc | No. (%) of monkeys with a diarrhea episode | Mean no. (range) of days with diarrhea | Mean no. (range) of excretion days | |||
| Low dose | 6 | 8 × 108 | 1 (17) | 5.0 | 13.8 (7-18) | NDf | ||
| Medium dose | 6 | 6 × 1010 | 4 (67) | 4.5 (3-7) | 10.5 (4-19) | 2 (33) | 3.5 (2-5) | 4.3 (4-5) |
| High dose | 6 | 5 × 1012 | 5 (83) | 6.8 (2-14) | 12.7 (2-19) | 2 (33) | 12.5 (12-13) | 4.0 (1-6) |
| Challenge controlsd | 12 | NAe | 8 (67) | 4.4 (2-8) | 15.8 (6-20) | |||
An episode of diarrhea was defined as passing at least one loose-to-watery stool on at least two consecutive days during the observation period.
The duration of diarrhea was defined as the number of days between the first day of a diarrhea episode and the last day of diarrhea, followed by two consecutive diarrhea-free days.
“Excretion days”indicates the number of days between the first day of colonization and the last day that C. jejuni was isolated from the stool.
Naïve animals inoculated in parallel with challenge animals in the medium- and high-dose groups.
NA, not applicable.
ND, not done.
All animals in all three experimental groups were colonized with C. jejuni and excreted organisms for an average of 12.3 days; no significant difference was observed between groups (Table 1). Colonization was defined as at least two consecutive days of Campylobacter being isolated from the stool, beginning on or after the second day following inoculation; as such, the fecal shedding of the challenge inoculum that was likely on day 1 was excluded from the analysis.
The diarrhea rate, but not the duration, was dose dependent, being higher in the medium- and high-dose groups (which had similar diarrhea rates) than in the low-dose group (Table 1) (risk ratio, 4.50; P = 0.043, Fisher's exact test). One log fewer C. jejuni organisms was required to cause diarrhea in A. nancymae than was reported for M. nemestrina, where 3 × 1011 CFU were required, although a dose-ranging study was not performed (24). In the human challenge model, doses of 106 to 109 CFU of C. jejuni resulted in diarrhea in 41% to 60% of volunteers, but no dose effect was observed (5). Our findings are similar to what occurs during C. jejuni infection in humans, who experience diarrhea symptoms for 3 to 5 days and can be colonized for several weeks (27).
The majority of monkeys inoculated with C. jejuni (including the challenge-positive control group [see below]) had anti-GE serum IgG (25 of 30 monkeys; 83.3%), serum IgA (26 of 30; 89.7%), and fecal IgA (26 of 29; 89.7%) responses, as measured by ELISA. The magnitude (mean peak increases [n-fold]) of the immune response to Campylobacter inoculation was robust (Table 2). Groups were not statistically different with regard to the numbers of responders or the mean peak increases (n-fold). There was, however, a significant correlation between peak serum IgG and IgA titers and between peak serum IgG or IgA and fecal IgA titers (P < 0.0001; Wilcoxon rank sum).
TABLE 2.
Immune responses of naïve A. nancymae monkeys to inoculation with escalating doses of C. jejuni 81-176
| Group | No. of monkeys | Dose (CFU) | No. (%) with an IgG responsea | Mean peak fold increase in IgGb | No. (%) with a serum IgA response | Mean peak fold increase in serum IgA | No. (%) with a fecal IgA response | Mean peak fold increase in fecal IgA |
|---|---|---|---|---|---|---|---|---|
| Low dose | 6 | 8 × 108 | 4 (66.7) | 53 ± 56 | 5 (83) | 62 ± 61 | 4c (80) | 54 ± 91 |
| Medium dose | 6 | 6 × 1010 | 5 (83) | 64 ± 108 | 5 (83) | 35 ± 28 | 6 (100) | 37 ± 46 |
| High dose | 6 | 5 × 1012 | 6 (100) | 29 ± 48 | 6 (100) | 26 ± 22 | 6 (100) | 53 ± 36 |
| Challenge controlsd | 12 | 7 × 1011 | 10 (83) | 109 ± 161 | 10 (83) | 49 ± 36 | 10 (83) | 85 ± 115 |
Response is defined as a fourfold or greater increase in titer from baseline against Campylobacter glycine extract antigen.
Mean peak increases (n-fold) calculated from responders only ± standard deviations.
Only five low-dose animals were evaluable for fecal IgA due to insufficient stool for performing baseline analysis.
Naïve A. nancymae monkeys inoculated in parallel with challenge animals in medium- and high-dose groups.
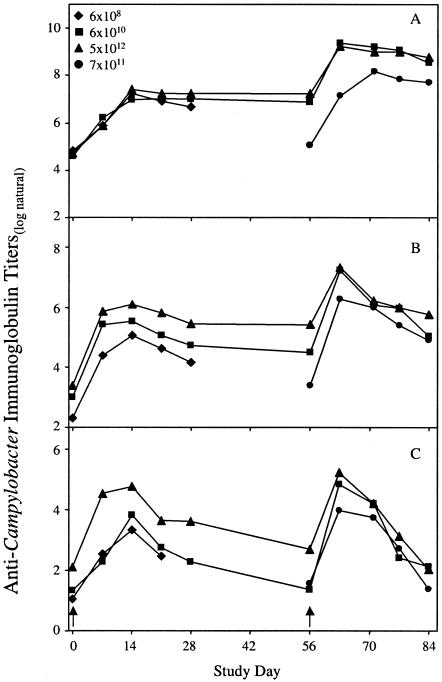
The kinetics of the serum IgG, serum IgA, and fecal IgA responses is shown in Fig. 1. There was no association between the kinetics of the immune response and the inoculation dose or between the magnitude of immune responses and the rates of colonization or diarrhea or the duration of excretion. Although serum IgG (Fig. 1A) and IgA (Fig. 1B) titers declined steadily after day 14, they did not return to baseline levels for any group of monkeys up to day 28. Before returning to the pool, all monkeys were treated orally with erythromycin (20 mg/kg) (Eritromicina Genfar, Colombia) twice a day for 10 days to ensure that the monkeys were no longer carrying C. jejuni.
FIG. 1.
Kinetics of the serum IgG (A) and IgA (B) and fecal IgA (C) responses against Campylobacter GE antigen in A. nancymae monkeys inoculated with escalating doses of C. jejuni and then challenged 56 days later with 7 × 1011 CFU of the homologous strain. Peak immune responses were observed on day 14 in the majority of animals. Arrow indicates dates of challenge of the medium- and high-dose groups, as well as of the naïve positive-control group.
Challenge study.
Repeated Campylobacter infections in humans are believed to result in immunity over time (5, 12). Fifty-six days after initial inoculation, monkeys in the medium- and high-dose groups were challenged with 7 × 1011 CFU of C. jejuni to determine whether prior exposure would protect the animals from infection or disease. Two groups of six naïve monkeys (challenge controls; n = 12) were inoculated as positive controls. Although all animals were colonized with C. jejuni, the duration of excretion was significantly shortened in animals that had been previously exposed to C. jejuni (Table 1) (P = 0.007, Wilcoxon signed-rank sum).
Since the diarrhea rates following initial inoculation between the medium- and high-dose groups were not statistically different, the two groups were combined for analysis. When this was done, 9 (75%) of 12 monkeys had diarrhea following the initial inoculation, compared with 4 (33%) of 12 following challenge (Table 1); this difference was significant (risk ratio, 0.44; P = 0.05, Fisher's exact test). Challenged monkeys had a lower rate of diarrhea than naïve controls (33% versus 66%) (Table 1), but this difference was not significant. Two monkeys from the high-dose group that had diarrhea upon challenge appeared to have a longer course of diarrhea than other groups; their diarrhea lasted for a longer period than they shed bacteria. Due to the small number of animals, it is difficult to speculate why this occurred. Future studies using more animals should help to address this issue. The finding that previous C. jejuni infection partially protected A. nancymae from homologous challenge is consistent with what occurs during natural human infection and with findings using other animal models, including humans (5) and M. nemestrina (24).
Surprisingly, the magnitudes of the immune response following initial inoculation did not correlate with the reduction of diarrhea or the duration of excretion observed after challenge, potentially due to the small number of animals used. Titers in the medium- and high-dose groups were still slightly above baseline values when measured on day 56. Challenges of both groups resulted in a large boost in immune responses (Fig. 1). Serum and fecal immune responses were not significantly different between the medium- and high-dose groups following challenge. Twenty-eight days after challenge (day 84), anti-GE IgG titers remained high (Fig. 1A), while IgA titers returned to day 56 levels (Fig. 1B and C).
The results presented herein show that A. nancymae can be reproducibly infected with C. jejuni and that colonization and diarrhea rates from infection are similar to those reported in the human challenge model. Aotus monkeys born in captivity with a known clinical history and no previous exposure to Campylobacter are available. They are safer to handle (no herpes B virus or filoviruses), are smaller and thus require less space, and are not as expensive to acquire and maintain as Old World monkeys. Given the availability of A. nancymae monkeys that have a low incidence of gastrointestinal disease but are susceptible to diarrhea as adults without significant manipulation, this model may prove excellent for studies of the protective efficacy of Campylobacter vaccine candidates.
Acknowledgments
This work was funded by the Military Infectious Diseases Research Program under work unit 6000 RAD1 DA3 A0308.
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the U.S. Departments of the Navy or Army, the U.S. Department of Defense, the U.S. Government, or any other organization listed.
The experiments reported herein were conducted in compliance with the Animal Welfare Act and according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (18a) (protocol NRD-297).
Editor: V. J. DiRita
REFERENCES
- 1.Babakhani, F. K., G. A. Bradley, and L. A. Joens. 1993. Newborn piglet model for campylobacteriosis. Infect. Immun. 61:3466-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baqar, S., A. L. Bourgeois, P. J. Schultheiss, R. L. Walker, D. M. Rollins, R. L. Haberberger, and O. R. Pavlovskis. 1995. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in nonhuman primates. Vaccine 13:22-28. [DOI] [PubMed] [Google Scholar]
- 3.Baqar, S., A. L. Bourgeois, L. A. Applebee, A. S. Mourad, M. T. Kleinosky, Z. Mohran, and J. R. Murphy. 1996. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect. Immun. 64:4933-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, J. A., and D. D. Manning. 1990. A domestic ferret model of immunity to Campylobacter jejuni-induced enteric disease. Infect. Immun. 58:1848-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J., D. J. Duncan, G. H. Warren, and W.-L. L. Wang. 1983. Experimental Campylobacter jejuni infection of adult mice. Infect. Immun. 39:908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell, M. B., R. I. Walker, S. D. Stewart, and J. E. Rogers. 1983. Simple adult rabbit model for Campylobacter jejuni enteritis. Infect. Immun. 42:1176-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calva, J. J., G. M. Ruiz-Palacios, A. B. Lopez-Vidal, A. Ramos, and R. Bojalil. 1988. Cohort study of intestinal infection with Campylobacter in Mexican children. Lancet i:503-506. [DOI] [PubMed] [Google Scholar]
- 9.Dupont, H. L., and F. M. Kahn. 1994. Travelers' diarrhea: epidemiology, microbiology, prevention, and therapy. J. Travel Med. 1:84-93. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgeorge, R. B., A. Baskerville, and K. P. Lander. 1981. Experimental infection of Rhesus monkeys with a human strain of Campylobacter jejuni. J. Hyg. 86:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox, J. G., J. I. Ackerman, N. Taylor, M. Claps, and J. C. Murphy. 1987. Campylobacter jejuni infection in the ferret: an animal model of human campylobacteriosis. Am. J. Vet. Res. 48:85-90. [PubMed] [Google Scholar]
- 12.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 13.Hall, E. R., F. Cassels, F. Jones, N. Diaz-Mayoral, G. Caoili, M. Wolf, D. Scott, and S. Savarino. 2003. Development of nonhuman primate animal models for enterotoxigenic Escherichia coli (ETEC) diarrhea and vaccine testing, abstr. D-173, p. 233. Abstr. 103rd Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, D.C.
- 14.Humphrey, C. D., D. M. Montag, and F. E. Pittman. 1985. Experimental infection of hamsters with Campylobacter jejuni. J. Infect. Dis. 151:485-493. [DOI] [PubMed] [Google Scholar]
- 15.Korlath, J. A., M. T. Osterholm, L. A. Judy, J. C. Forfang, and R. A. Robinson. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 152:592-596. [DOI] [PubMed] [Google Scholar]
- 16.Kuschner, R. A., A. F. Trofa, R. J. Thomas, C. W. Hoge, C. Pitarangsi, S. Amato, R. P. Olafson, P. Echeverria, J. C. Sadoff, and D. N. Taylor. 1995. Use of azithromycin for the treatment of Campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin. Infect. Dis. 21:536-541. [DOI] [PubMed] [Google Scholar]
- 17.Logan, S. M., and T. J. Trust. 1982. Outer membrane characteristics of Campylobacter jejuni. Infect. Immun. 38:898-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy, G. S., Jr., P. Echeverria, L. R. Jackson, M. K. Arness, C. LeBron, and C. Pitarangsi. 1996. Ciprofloxacin- and azithromycin-resistant Campylobacter causing traveler's diarrhea in U.S. troops deployed to Thailand in 1994. Clin. Infect. Dis. 22:868-869. [DOI] [PubMed] [Google Scholar]
- 18a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 19.Oberhelman, R. A., and D. N. Taylor. 2000. Campylobacter infections in developing countries, p. 139-153. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 20.Petruccelli, B. P., G. S. Murphy, J. L. Sanchez, S. Walz, R. DeFraites, J. Gelnett, R. L. Haberberger, P. Echeverria, and D. N. Taylor. 1992. Treatment of traveler's diarrhea with ciprofloxacin and loperamide. J. Infect. Dis. 165:557-560. [DOI] [PubMed] [Google Scholar]
- 21.Prescott, J. F., I. K. Barker, K. I. Manninen, and O. P. Miniats. 1981. Campylobacter jejuni colitis in gnotobiotic dogs. Can. J. Comp. Med. 45:377-383. [PMC free article] [PubMed] [Google Scholar]
- 22.Russell, R. G., S. L. Rosenkranz, L. A. Lee, H. Howard, R. F. DiGiacomo, M. A. Bronsdon, G. A. Blakley, C. C. Tsai, and W. R. Morton. 1987. Epidemiology and etiology of diarrhea in colony-born Macaca nemestrina. Lab. Anim. Sci. 37:309-316. [PubMed] [Google Scholar]
- 23.Russell, R. G., L. Krugner, C. C. Tsai, and R. Ekstrom. 1988. Prevalence of Campylobacter in infant, juvenile and adult laboratory primates. Lab. Anim. Sci. 38:711-714. [PubMed] [Google Scholar]
- 24.Russell, R. G., M. J. Blaser, J. I. Sarmiento, and J. Fox. 1989. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect. Immun. 57:1438-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell, R. G., M. O'Donnoghue, D. C. Blake, Jr., J. Zulty, and L. J. DeTolla. 1993. Early colonic damage and invasion of Campylobacter jejuni in experimentally challenged infant Macaca mulatta. J. Infect. Dis. 168:210-215. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal, S. C., K. M. N. Islam, P. K. B. Neogy, M. Islam, P. Speelman, and M. I. Huq. 1984. Campylobacter jejuni diarrhea model in infant chickens. Infect. Immun. 43:931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott, D. A. 1997. Vaccines against Campylobacter jejuni. J. Infect. Dis. 176(Suppl. 2):S183-S188. [DOI] [PubMed] [Google Scholar]
- 28.Sestak, K., C. K. Merritt, J. Borda, E. Saylor, S. R. Schwamberger, F. Cogswell, E. S. Didier, P. J. Didier, G. Plauche, R. P. Bohm, P. P. Aye, P. Alexa, R. L. Ward, and A. A. Lackner. 2003. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect. Immun. 71:4079-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanfield, J. T., B. A. McCardell, and J. M. Madden. 1987. Campylobacter diarrhea in an adult mouse model. Microb. Pathog. 3:155-165. [DOI] [PubMed] [Google Scholar]
- 30.Taylor, D. N., P. Echeverria, C. Pitarangsi, J. Seriwatana, L. Bodhidatta, and M. J. Blaser. 1988. Influence of strain characteristics and immunity on the epidemiology of Campylobacter infections in Thailand. J. Clin. Microbiol. 26:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuckovic, D., M. Abram, and M. Doric. 1998. Primary Campylobacter jejuni infection in different mice strains. Microb. Pathog. 24:263-268. [DOI] [PubMed] [Google Scholar]