Abstract
The effect of probenecid (PRO) on norfloxacin (NOR) blood-brain barrier transport was investigated with rats by microdialysis. Maximum brain drug concentrations were rapidly attained, and the brain penetration factor was close to 5% in the absence and presence of PRO. In conclusion, PRO has no effect on NOR blood-brain barrier transport.
Central nervous system (CNS) side effects represent a common adverse event during fluoroquinolone (FQ) therapy (3, 7). High potential toxic risk exists with FQs exhibiting limited CNS distribution, such as norfloxacin (NOR) (2, 5, 6, 9), because of possible blood-brain barrier (BBB) permeation under various conditions, such as disease state or drug-drug interactions. Probenecid (PRO) is known as an inhibitor of anionic transport proteins, such as multidrug resistance-associated protein, organic anion transporting polypeptides, and organic anion transporters (8, 13), previously shown to interfere with the renal tubular secretion of NOR, at least in rabbits and humans (12). PRO could therefore be responsible for drug-drug interactions at the BBB by various mechanisms and was selected as a good candidate drug with potential effect on the CNS distribution of NOR, chosen as a representative FQ with limited CNS distribution.
NOR and PRO were obtained from Sigma (Saint-Quentin Fallavier, France). A NOR salt was prepared as described previously (5). Solvents, including water, were of analytical grade. Experiments were done in accordance with the Principles of Laboratory Animals Care (NIH Publication no. #85-23, revised in 1985). Male Sprague-Dawley rats (Janvier Laboratories, Le Genet-St-Isle, France) weighing 287 ± 8 g were anesthetized and equipped with catheters (2, 9) and blood microdialysis CMA/20 probes (polycarbonate; membrane length, 10 mm; cutoff, 20,000 Da; CMA Microdialysis, Phymep, Paris, France) (10, 14, 17). Anesthetized rats were placed on a stereotaxic instrument (David Kopf Instruments, Tujunga, Calif.) and a CMA/12 guide cannula was implanted into the left dorsal hippocampus (2, 9). The day of the experiment, a CMA/12 probe (polycarbonate; membrane length, 3 mm; cutoff, 20,000 Da; CMA Microdialysis, Phymep) was inserted into the dorsal hippocampus. To estimate individual in vivo recovery, a retrodialysis by drug period was done, consisting of an equilibration and a collection period. Probes were then perfused with Ringer containing NOR (100 nM for brain, 6 μM for blood) at 2 μl · min−1 during the first hour of equilibration and 0.5 μl · min−1 during the second hour. Ringer solution for blood microdialysis was from CMA (perfusion fluid T1; CMA Microdialysis, Phymep) and Ringer medium for brain microdialysis was as described previously (2, 9). After the equilibration period, four dialysates were collected for 60 min by fractions corresponding to 15-min intervals, and the mean in vivo recovery was determined for each probe and used to estimate actual unbound concentrations (9). A 2-h washout period was then performed, with probes being perfused with blank Ringer at a flow rate of 2 μl · min−1 and 0.5 μl · min−1 for the first and second hours, respectively. For a NOR CNS distribution study, a loading dose of PRO (20 mg · kg−1 or 70 μmol · kg−1 as a 1-ml intravenous (i.v.) bolus) was administered via the femoral vein catheter at the beginning of the equilibration time, followed by a constant infusion (20 mg · kg−1 · h−1 or 70 μmol kg−1 · h−1) at a flow rate of 0.5 ml · min−1 (PHD 2000 infusion pump; Harvard, France) for the PRO group (14). For the control group, rats received a sodium bicarbonate solution under similar conditions. After the washout period, an i.v. bolus dose of NOR (50 mg · kg−1 or 141 μmol · kg−1) was administrated via the femoral vein to rats of both groups. Dialysates from blood and brain were collected over a period of 8 h at a flow rate of 0.5 μl · min−1. Samples were collected at 15- and 30-min intervals during the first and second hours, respectively, and then every hour over the remaining 6 h. The NOR assay with dialysates was as described previously (2, 9). Drug concentrations in blood and brain extracellular fluid (ECF) were analyzed simultaneously by a population approach. Distribution equilibrium of NOR within the brain was supposed to be attained instantaneously (2, 9), and blood and brain ECF data were analyzed simultaneously, considering that brain ECF was part of the central compartment, with a tissue penetration factor (R) relating free brain ECF and free blood drug concentrations at any time (2). The population pharmacokinetic (PK) model after i.v. bolus administration of NOR in rat was a two-compartment model in which an interanimal variability modeled exponentially was added to the R parameter. In the exponential variance model (equation 1), Ri and Rpop are the parameters for the ith (i = 1, … n) subject and the average population estimates, respectively. ηi is a zero mean and normally distributed variable with standard deviation ω, which was estimated.
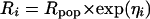 |
(1) |
To characterize the residual variability, an additive random error (equation 2) was used for unbound blood concentrations (Yobs, plasma), whereas a proportional random error (equation 3) was selected for ECF brain concentrations (Yobs, brain),
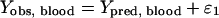 |
(2) |
 |
(3) |
with Ypred, blood and Ypred, brain, the predicted concentrations in blood and brain, respectively, and ɛ1 and ɛ2, the zero-mean normally distributed variables with standard deviations σ1 and σ2 for blood and brain concentrations, respectively. Population parameters were estimated with the software NONMEM (version V) using the first-order conditional estimate with interaction method (1). Goodness of fit was checked by visual inspection of residuals and predictions. Estimates of population blood PK parameters are given with the relative standard error (RSE) of estimation expressed as a percentage. A Wald test was used to compare parameter estimates within the two groups (11). The results were confirmed by likelihood ratio tests coupled with a randomization procedure to correct the nominal P value because of the limited number of animals (15).
Nine rats were included in this study, four in the control group and five in the PRO-treated group. The mean recovery by loss of NOR estimated from four consecutive samples for each animal ranged from 4.5% ± 0.6% to 14.5% ± 4.9% in brain and from 24.4% ± 8.7% to 58.8% ± 4.0% in blood. One rat of each group presented uncompleted data (blood or brain data only). They were not excluded, but instead, a population pharmacokinetic approach was chosen for data analysis. Concentration-time profiles of unbound NOR in rat blood and brain ECF showed that NOR distribution equilibrium in brain was attained instantaneously in the absence of PRO, as demonstrated previously (2, 9), and in the presence of PRO, as illustrated with typical rats (Fig. 1). Pharmacokinetic population parameters are displayed in Table 1. RSE of estimation obtained from NONMEM were quite low, and no particular trend was observed in checking residuals. The population PK model was suitable to predict concentrations in each animal and a good correlation was observed between individual predicted and observed concentrations (Fig. 2). No significant difference was observed within groups for population estimates of clearance as well as volume of distribution at steady state according to Wald individual tests (Table 1) and consistent with the conclusions from model building using the likelihood ratio test (results not shown). Although the limited sample size in this study limits the power to detect small differences, this result suggests that PRO has no major effect on NOR pharmacokinetics, consistent with the fact that NOR renal clearance represents only 15% on average of total clearance, with limited if any tubular secretion (4). The brain penetration factor R was in the order of 5% in the absence of PRO, consistent with previously reported values (2, 9) and apparently not altered by the presence of PRO (Table 1). However, drug-drug interactions are dose dependent, and therefore, an interaction could have been observed at higher doses. Yet the PRO dose was the same as those previously used by others to demonstrate significant interactions with zidovudine (16) and morphine-3-glucuronide (17) BBB transport. The NOR dose was not too high in order to avoid CNS side effects but corresponded to 3,000 mg · 60 kg−1, which is severalfold higher than the usual dose (400 mg) used in clinical practice. Furthermore, peak blood levels in the order of 50 to 100 μM (15 to 30 μg · ml−1) were also much higher than the usual value (1 to 2 μg · ml−1) encountered in clinical practice.
FIG. 1.
Observed (•) and predicted free concentrations of NOR versus time in brain ECF (dashed lines) and blood (solid lines) from representative rats of the control (a) and PRO-treated (b) groups.
TABLE 1.
Population PK parameter estimates of mean population time course of NOR after i.v. bolus administration of 50 mg · kg−1 (141 μmol · kg−1) to rats
| Parameter or categorya | Estimated value for group, mean (RSE [%])b
|
|||||
|---|---|---|---|---|---|---|
| Without probenecid
|
With probenecid
|
|||||
| Mean population | Interanimal variability | Residual variability | Mean population | Interanimal variability | Residual variability | |
| CL (ml · min−1 · kg−1) | 7.9 (15.8) | 10.0 (5.5) | ||||
| VSS (ml · kg−1) | 2,260 (13.4) | 2,310 (2.5) | ||||
| R (%) | 4.2 (14.7) | 36.7 (58.1) | 5.3 (36) | 69.9 (51.9) | ||
| Blood data (μM) | 11.9 (7.1)b | 6.2 (4.6)b | ||||
| Brain data (%) | 24.4 (30.1) | 25.6 (13.9) | ||||
CL, clearance; VSS, volume of distribution at steady state.
Statistically different (Wald test).
FIG. 2.
Individual predictions versus actual concentrations (in log) by population approach after NOR intravenous bolus administration of 50 mg · kg of body weight−1 (141 μM · kg−1) in rats. (a) In blood for the control (•) and for PRO groups (○) and (b) in brain for the control (•) and for PRO groups (○). Straight lines are lines of identity.
In conclusion, PRO has virtually no effect, if any, on NOR BBB transport, at least in this experimental setting, and although there is a theoretical risk, no experimental data have been provided yet to support an increased CNS toxicity of FQs in the presence of coadministered drugs interfering with their BBB transport.
REFERENCES
- 1.Beal, S. L., and L. B. Sheiner. 1992. (ed.) NONMEM users' guides-part VII. NONMEM Project Group, University of California, San Francisco, Calif.
- 2.Chenel, M., S. Marchand, A. Dupuis, I. Lamarche, J. Paquereau, C. Pariat, and W. Couet. 2004. Simultaneous central nervous system distribution and pharmacokinetic/pharmacodynamic modeling of the electroencephalogram effect of norfloxacin administered at a convulsant dose in rats. Br. J. Pharmacol. 142:323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christ, W. 1990. Central nervous system toxicity of quinolones: human and animal findings. J. Antimicrob. Chemother. 26:219-225. [DOI] [PubMed] [Google Scholar]
- 4.Davis, J. D., L. Aarons, and J. B. Houston. 1995. Effect of norfloxacin on theophyllin disposition: a comparison with other fluoroquinolones. Pharm. Res. 12:257-262. [DOI] [PubMed] [Google Scholar]
- 5.Delon, A., S. Bouquet, F. Huguet, V. Brunet, P. Courtois, and W. Couet. 1999. Pharmacokinetic-pharmacodynamic contributions to the convulsant activity of fluoroquinolones in rats. Antimicrob. Agents Chemother. 43:1511-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delon, A., F. Huguet, P. Courtois, J. M. Vierfond, S. Bouquet, and W. Couet. 1997. Pharmacokinetic-pharmacodynamic contributions to the convulsant activity of pefloxacin and norfloxacin in rats. J. Pharmacol. Exp. Ther. 280:983-987. [PubMed] [Google Scholar]
- 7.De Sarro, A., and G. De Sarro. 2001. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects. Curr. Med. Chem. 8:371-384. [DOI] [PubMed] [Google Scholar]
- 8.Foote, E. F., and C. E. Halstenson. 1998. Effects of probenecid and cimetidine on renal disposition of ofloxacin in rats. Antimicrob. Agents Chemother. 42:456-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchand, S., M. Chenel, I. Lamarche, C. Pariat, and W. Couet. 2003. Dose ranging pharmacokinetics and brain distribution of norfloxacin using microdialysis in rats. J. Pharm. Sci. 92:2467-2474. [DOI] [PubMed] [Google Scholar]
- 10.Marchand, S., C. Dahyot, I. Lamarche, O. Mimoz, and W. Couet. 2005. Microdialysis study of imipenem distribution in skeletal muscle and lung extracellular fluids of noninfected rats. Antimicrob. Agents Chemother. 49:2356-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panhard, X., and F. Mentre. 2005. Evaluation by simulation of tests based on non-linear mixed-effects models in pharmacokinetic interaction and bioequivalence cross-over trials. Stat. Med. 24:1509-1524. [DOI] [PubMed] [Google Scholar]
- 12.Shimada, J., T. Yamaji, Y. Ueda, H. Uchida, H. Kusajima, and T. Irikura. 1983. Mechanism of renal excretion of AM-715, a new quinolonecarboxylic acid derivative, in rabbits, dogs, and humans. Antimicrob. Agents Chemother. 23:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiyama, D., H. Kushuara, Y. Shitara, T. Abe, P. T. Meier, T. Sekine, H. Endou, H. Suzuki, and Y. Sugiyama. 2001. Characterization of the efflux transport of 17β-estradiol-D-17β-glucuronide from the brain across the blood-brain-barrier. J. Pharmacol. Exp. Ther. 298:316-322. [PubMed] [Google Scholar]
- 14.Tunblad, K., E. N. Jonsson, and M. Hammarlund-Udenaes. 2003. Morphine blood-brain barrier transport is influenced by probenecid co-administration. Pharm. Res. 20:618-623. [DOI] [PubMed] [Google Scholar]
- 15.Wahlby, U., E. N. Jonsson, and M. O. Karlsson. 2001. Assessment of actual significance levels for covariate effects in NONMEM. J. Pharmacokinet. Pharmacodyn. 28:231-252. [DOI] [PubMed] [Google Scholar]
- 16.Wong, S. L., K. Van Belle, and R. J. Sawchuck. 1993. Distributional transport kinetics of zidovudine between plasma and brain extracellular fluid/cerebrospinal fluid in the rabbit: investigation of the inhibitory effect of probenecid using microdialysis. J. Pharmacol. Exp. Ther. 264:899-909. [PubMed] [Google Scholar]
- 17.Xie, R., M. R. Bouw, and M. Hammarlund-Udenaes. 2000. Modelling of the blood-brain barrier transport of morphine-3-glucuronide studied using microdialysis in the rat: involvement of probenecid-sensitive transport. Br. J. Pharmacol. 131:1784-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]




