Abstract
Further to the previous finding of the rainbow trout rtCATH_1 gene, this paper describes three more cathelicidin genes found in salmonids: two in Atlantic salmon, named asCATH_1 and asCATH_2, and one in rainbow trout, named rtCATH_2. All the three new salmonid cathelicidin genes share the common characteristics of mammalian cathelicidin genes, such as consisting of four exons and possessing a highly conserved preproregion and four invariant cysteines clustered in the C-terminal region of the cathelin-like domain. The asCATH_1 gene is homologous to the rainbow trout rtCATH_1 gene, in that it possesses three repeat motifs of TGGGGGTGGC in exon IV and two cysteine residues in the predicted mature peptide, while the asCATH_2 gene and rtCATH_2 gene are homologues of each other, with 96% nucleotide identity. Salmonid cathelicidins possess the same elastase-sensitive residue, threonine, as hagfish cathelicidins and the rabbit CAP18 molecule. The cleavage site of the four salmonid cathelicidins is within a conserved amino acid motif of QKIRTRR, which is at the beginning of the sequence encoded by exon IV. Two 36-residue peptides corresponding to the core part of rtCATH_1 and rtCATH_2 were chemically synthesized and shown to exhibit potent antimicrobial activity. rtCATH_2 was expressed constitutively in gill, head kidney, intestine, skin and spleen, while the expression of rtCATH_1 was inducible in gill, head kidney, and spleen after bacterial challenge. Four cathelicidin genes have now been characterized in salmonids and two were identified in hagfish, confirming that cathelicidin genes evolved early and are likely present in all vertebrates.
Living organisms are exposed to microbial pathogens all the time. To protect themselves from infection, they produce a vast array of germ-killing antimicrobial peptides (AMPs) as one of the first lines of defense. AMPs are gene-encoded peptides that kill bacteria by disrupting their cell membrane. They normally display amphipathic and cationic properties and have a molecular mass less than 30 kDa. More than 800 AMPs have been described (www.bbcm.univ.trieste.it/∼tossi/antimic.html) in the past 25 years. Among them, a family recognized in the early 1990s (34), the cathelicidin family, is found in a variety of mammalian species and is regarded as one of the most prominent AMPs in mammals.
Cathelicidins are a group of numerous proteins that share a highly conserved preproregion containing the cathelin-like domain (24) at the N terminus but carry a substantially heterogeneous C-terminal domain that encodes the mature antimicrobial peptide. Until recently, it was thought that cathelicidins were found only in mammals. However, in 2003 two sequences from hagfish were discovered and cited as representing an “ancient ancestor of the cathelicidin gene family” (33). Mammalian species in which cathelicidin genes have been studied include humans (9), mice (7), rabbits (13), guinea pigs (18), pigs (36), cows (29), sheep (17), goats (28), and horses (27). Most species have more than one member of the cathelicidin family present, with the exact number being variable (23). The exception is in humans and mice, where only one member is present. Recently, we reported the first teleost cathelicidin gene, rtCATH_1, identified in rainbow trout (5). In view of the above, it is quite possible that more congeners of trout cathelicidins are awaiting discovery.
With the considerable increase in fish expressed sequence tags (EST) available in GenBank, it is now possible to undertake a screening for other related genes in an efficient and time-saving manner. Based on the cDNA sequence of rtCATH_1, we discovered two salmon cathelicidin cDNA clones, named Atlantic salmon cathelicidin_1 (asCATH_1) and asCATH_2, using this approach. Primers designed to match conserved regions of both the Atlantic salmon and rainbow trout molecules allowed the discovery of the second cathelicidin gene in rainbow trout, named rtCATH_2. In this study the two peptides deduced from the rtCATH_1 and the rtCATH_2 genes were chemically synthesized and their antibacterial activities were assessed. The expression of the two rainbow trout cathelicidins in different tissues was also studied, as was the elastase cleavage site, with the expression being confirmed by mass spectroscopy of the cleaved mature peptide.
MATERIALS AND METHODS
Fish rearing and sampling.
Rainbow trout (Oncorhynchus mykiss) weighing 300 to 350 g and Atlantic salmon (Salmo salar) parr weighing 40 to 60 g were obtained from local fish farms (Almond Bank, Perthshire, United Kingdom, for trout and Marine Harvest Ltd. for salmon). They were maintained and acclimatized in a recirculating freshwater system at 14°C with regular feeding. The fish were killed as required; and tissues (gill, head kidney, intestine, liver, skin, spleen) were removed, placed in TRIzol Reagent (Invitrogen, United Kingdom), and kept at −80°C until use.
Isolation of cDNA sequences.
By using the cDNA sequence of rtCATH-1 to screen the EST database (http://www.ncbi.nlm.nih.gov/), BLAST analysis revealed three related ESTs from Atlantic salmon (GenBank accession numbers BG935125, BM414188, and CB506020). Further analysis revealed that the three ESTs represented two types of cathelicidin genes, named asCATH_1 (GenBank accession number BG935125) and asCATH_2 (GenBank accession numbers BM414188 and CB506020). Primers designed to match conserved regions of both the Atlantic salmon and the rainbow trout sequences were applied to get the related sequence, rtCATH_2, from the cDNA of rainbow trout. The full-length cDNA sequences were cloned from bacterially stimulated fish by using 5′ and 3′ rapid amplification of cDNA ends (5′- and 3′-RACE) PCR approaches, as described below.
Fish were injected intraperitoneally with Aeromonas salmonicida MT423 (11) at a dose of 2 × 106 bacteria (suspended in 100 μl of phosphate-buffered saline [PBS]) per fish and killed at 24 h postinjection. Total RNA was isolated from head kidney by using the Trizol reagent (Invitrogen, United Kingdom), according to the manufacturer's recommendations. The first-strand cDNA was synthesized with Moloney murine leukemia virus reverse transcriptase RNase H Minus, Point Mutant (Promega, United Kingdom), in a reaction mixture of 20 μl containing 2 μg of total RNA and 500 ng primer APT (Table 1). The cDNA obtained was then purified and a homopolymeric dC tail was appended to the 3′ end by using terminal deoxynucleotidyl transferase (Promega) and dCTP (Bioline, United Kingdom), according to the recommendations of the manufacturer of a 5′- and 3′-RACE kit (Roche, France).
TABLE 1.
Primers used for cloning and sequencing of Atlantic salmon asCATH_1 and asCATH_2 and rainbow trout rtCATH_2
| Use and gene | Primer | Nucleotide sequence (5′→3′) |
|---|---|---|
| cDNA cloning | ||
| RACE | APT | CCAGACTCGTGGCTGATGCA(T)16Va |
| AP | CCAGACTCGTGGCTGATGCA | |
| RAP | TGCCACGCGTCGACTAGTAC(G)16 | |
| UAP | TGCCACGCGTCGACTAGTAC | |
| asCATH_1 | asCATH_1_F0 | GAATCAGACATGAAGATGAA |
| asCATH_1_F1 | TGGCAGCATTCGTTGTCGTG | |
| asCATH_1_F2 | GATTTATCACAGATCAACGTGA | |
| asCATH_1_R0 | TTATGACATTTCTTTGATAG | |
| asCATH_1_R1 | TCCATCACGTTGATCTGTGA | |
| asCATH_1_R2 | CCTCAGGCGACCAATTAAGG | |
| asCATH_2 | asCATH_2_F0 | ATCAGACATGAAGATGAAGGC |
| asCATH_2_F1 | ATGCCAGTACTGTGCACT | |
| asCATH_2_F2 | TCCAAGGGGGGTTGGAGAGG | |
| asCATH_2_R0 | GTTGCATGTTCAATCATTAACC | |
| asCATH_2_R1 | ACCTTGGTCTCTTCCGCTAGC | |
| asCATH_2_R2 | CCTCTCCAACCCCCCTTGGA | |
| rtCATH_2 | as_rt_F1 | TGAATACAGAGGATGTGGACCA |
| as_rt_F2 | CCATGCCCTCTGAAGAAAAATG | |
| rtCATH_1_F4 | CTGACCTGTCTACATTTGTTTG | |
| rtCATH_1_R1 | GCGATTTCCATCACTGTGATCTCT | |
| rtCATH_2_F0 | ATCAGACATGAAGATGAAGGC | |
| rtCATH_2_R0 | CTCAAGGTTTCATGTTCACCCA | |
| rtCATH_2_R1 | TGTTCGATGCAGGGAGAGTT | |
| rtCATH_2_R2 | CCACTCCCAGGACGACCTCT | |
| Genomic DNA cloning | ||
| asCATH_1 | asCATH_1_F0 | GAATCAGACATGAAGATGAA |
| asCATH_1_R0 | TTATGACATTTCTTTGATAG | |
| asCATH_2 | asCATH_2_F0 | ATCAGACATGAAGATGAAGGC |
| asCATH_2_R0 | GTTGCATGTTCAATCATTAACC | |
| rtCATH_2 | rtCATH_2_F0 | ATCAGACATGAAGATGAAGGC |
| rtCATH_2_R0 | CTCAAGGTTTCATGTTCACCCA | |
| Expression analysis | ||
| rtCATH_1 | rtCATH_1_F4 | CTGACCTGTCTACATTTGTTTG |
| rtCATH_1_R1 | GCGATTTCCATCACTGTGATCTCT | |
| rtCATH_2 | rtCATH_2_F1 | ATTGGCAACACCCTCAACACT |
| rtCATH_2_R0 | CTCAAGGTTTCATGTTCACCCA |
V, A, C, or G.
Rapid amplification of the cDNA 3′ ends (3′-RACE) was performed with 0.5 μl DNA polymerase mix (BioTaq [5 U/μl; Bioline]):Pfu [3 U/μl; Promega] = (1:1) in 50-μl reaction mixtures containing the following components: 5 μl reaction buffer (10×; Bioline), 1.5 μl MgCl2 (50 mM; Bioline), 1 μl deoxynucleoside triphosphate mix (10 mM each; Bioline), 2 μl forward primers (asCATH_1_F1 and asCATH_2_F1 for asCATH_1 and asCATH_2, respectively) and reverse primer (AP) (10 μM each), and 1 μl of the dC-tailed cDNA template described above. The PCR products were further amplified by a second PCR with primer AP and nested primers, primers asCATH_1_F2 and asCATH_2_F2 for asCATH_1 and asCATH_2, respectively. The cycling protocol was 1 cycle of 2 min at 94°C and 30 cycles of 20 s at 94°C, 20 s at 60°C, and 45 s at 72°C, with a final extension step of 8 min at 72°C. The amplified products were analyzed on a 1.5% agarose gel containing ethidium bromide (100 ng/ml).
Rapid amplification of the cDNA 5′ ends (5′-RACE) was performed by using the forward adaptor primer RAP; reverse gene-specific primers asCATH_1_R1 and asCATH_2_R1 for asCATH_1 and asCATH_2, respectively; and the dC-tailed cDNA template. The PCR products were further amplified by a second PCR with primer UAP and nested, gene specific primers asCATH_1_R2 and asCATH_2_R2 for asCATH_1 and asCATH_2, respectively.
To find the sequence of rtCATH_2, two forward primers (as_rt_F1 and as_rt_F2) were designed to cover conserved regions of both asCATH_1 and rtCATH_1. Clones from the nested PCR with forward primers as_rt_F1 and reverse primer AP were screened by use of a pair of primers specific to rtCATH_1 (primers rtCATH_1_F4 and rtCATH_1_R1). Clones that did not show a fragment amplified by primers rtCATH_1_F4 and rtCATH_1_R1 were analyzed and revealed the second rainbow trout cathelicidin sequence, named rtCATH_2.
5′-RACE was performed to get 5′ end of rtCATH_2 by using the forward adaptor primer RAP and reverse gene-specific primer rtCATH_2_R1, followed by a nested PCR with the forward adaptor primer UAP and reverse gene-specific primer rtCATH_2_R2.
The cDNA sequences of asCATH_1, asCATH_2, and rtCATH_2, including the whole open reading frames, were confirmed by PCR with the primers designed to be specific for the 5′ untranslated regions (5′-UTRs) (primers asCATH_1_F0, asCATH_2_F0, and rtCATH_2_F0) and 3′-UTRs (primers asCATH_1_R0, asCATH_2_R0, and rtCATH_2_R0). All of the PCR products described above were cloned and sequenced as described below.
Isolation of genomic sequences.
Genomic DNA was isolated from the livers of the salmon and the trout by using a genomic DNA isolation kit (QIAGEN, United Kingdom). The resultant DNA pellets were dissolved in sterile H2O to a concentration of 0.25 μg/μl. The genomic sequences of asCATH_1, asCATH_2, and rtCATH_2 were obtained by PCR, performed under the conditions described above but with 2 μl of genomic DNA used as template, with primers designed to be specific for the 5′-UTRs (primers asCATH_1_F0, asCATH_2_F0, and rtCATH_2_F0, respectively) and 3′-UTRs (primers asCATH_1_R0, asCATH_2_R0, and rtCATH_2_R0, respectively). The products obtained were cloned and sequenced as described below.
Cloning, sequencing, and sequence analysis.
All products obtained by PCR were directly cloned into the pGEM-T Easy vector (Promega). Plasmid DNA was isolated from bacterial colonies that carried an appropriately sized insert by using the Qiaprep spin miniprep kit (QIAGEN). Three randomly selected clones representing each product were sequenced by the dideoxy chain-termination method on an automated DNA sequencer (ABI 377; Applied Biosystems) with vector- or gene-specific primers. The nucleotide sequences were translated into amino acid sequences by using DNA Tools software. Signal peptides were predicted by using SignalP (20). Multiple-sequence alignments were generated by using the CLUSTAL W program, version 1.8 (32). A phylogenetic tree of the cathelicidin amino acid sequences was constructed based on the principle of parsimony by the PROTPARS program in PHYLIP and tested for reliability by using 1,000 bootstrap replications. The tree was drawn by use of the Treeview program (version 1.6.6) (22).
Tissue expression of the rtCATH_1 and rtCATH_2 genes.
Northern blot analysis was used to compare the expression of the rtCATH_1 and rtCATH_2 genes in normal and infection situations. Fish were injected intraperitoneally with A. salmonicida MT423 at a dose of 2 × 106 bacteria (suspended in 100 μl PBS) per fish. Three fish were killed separately at 8 and 24 h postinjection. Tissues were sampled from gill, head kidney, intestine, liver, skin, and spleen. Total RNA was extracted from all tissues by using Trizol (Invitrogen), and the RNAs were pooled together by different tissues. Twenty micrograms of RNA was loaded onto each lane of a 1.1% agarose gel. The 18S rRNA was visualized and photographed to assess the quality of the RNA and to ensure the equal loading of RNA for each sample. Following electrophoresis at 100 V for 3 h in 1× morpholinepropanesulfonic acid buffer and transfer to an N+ nylon filter (Amersham), the membrane was baked at 80°C for 1 h. To prepare the probes specific for the mRNAs of rtCATH_1 and rtCATH_2, the region of rtCATH_1 cDNA between primers rtCATH_1_F4 and rtCATH_1_R1 and the region of rtCATH_2 cDNA between primers rtCATH_2_F1 and rtCATH_2_R0 were amplified by wax-mediated hot-start PCR, labeled with [32P]dCTP by using the Megaprime radiolabeling kit (Amersham, United Kingdom), and hybridized to RNA. After stringent washing of the membrane, the membrane was exposed to X-ray film (Kodak) at −70°C for 72 h. The relative levels of mRNA were quantified by densitometric scanning of the exposed film by using a UVP gel-imaging system and UVP Gelworks ID advanced software.
Cleavage site.
To study the cleavage sites for the generation of mature peptides of salmonid cathelicidins, the recombinant protein of pro-rtCATH_1 with a six-His tag at the C terminus, named prtCATH_1-6His, was prepared. The cDNA of rtCATH_1 was amplified by PCR, sequenced, and cloned into the pGEM-T Easy vector (Promega). By using the sequenced plasmid DNA as the template, the cDNA fragment encoding the proregion of rtCATH_1 (prtCATH_1), from Gln23 to the C terminus, was again amplified by PCR and an ATG translation start codon was added. The expression vectors pQE70 (QIAGEN) containing a six-His tag at the C terminus were used to produce the recombinant proteins. For ligation into the pQE70 vector, restriction enzyme sites for SphI and BglII were added at the 5′ and 3′ ends, respectively, by PCR. The resultant expression plasmid was named pQE70-rtCATH_1.
To express the recombinant prtCATH_1 protein, plasmid pQE70-rtCATH_1 was transformed into Escherichia coli JM109 cells (Promega). A single colony containing the target plasmid was cultured in 5 ml of Luria-Bertani (LB) medium (Sigma) containing ampicillin (100 μg/ml) at 37°C with shaking (300 rpm) overnight, prior to culture in 250 ml LB medium containing ampicillin for 4 h, and was then incubated for 2 h with the addition of isopropyl β-d-1-thiogalactopyranoside (final concentration, 0.5 mM). The E. coli cells were then harvested by centrifugation at 4°C, lysed in phosphate buffer (100 mM NaH2PO4, 10 mM Tris-Cl, pH 8.0) containing 8 M urea, and loaded onto a nickel-nitrilotriacetic acid column (QIAGEN). Purification was conducted according to QIAGEN′s protocols. Briefly, the column containing the bacterial lysate was washed several times with the buffer described above at pH 6.3 to remove proteins without a six-His tag. The recombinant protein was then eluted with the phosphate buffer at pH 5.9 and 4.5, respectively. The protein size, purity, and concentration of the flowthrough fractions were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and UVP image analysis. The resultant protein generated from plasmid pQE70-rtCATH_1 was named prtCATH_1-6His.
Fifty nanograms of human neutrophil elastase (Elastin Products Company, Inc.) in Tris buffer (2 M Tris, 0.1 M sodium acetate, 0.01% bovine serum albumin, pH 7.4) was incubated with 5 μg of pQE70_prtCATH_1 at 20°C for 2 h. The reaction was stopped by adding an equal volume of 5% acetic acid. The peptide fragments generated from elastase cleavage were separated on a 4 to 12% polyacrylamide gel. Western blotting was performed with a Westernbreeze chemiluminescent immunodetection system (Invitrogen) and the monoclonal anti-six-His (at the C terminus) antibody (Invitrogen), according to the manufacturer's instructions. The mature peptide of rtCATH_1 with the six-His tag at the C terminus was recovered from the gel, and the molecular mass was determined by matrix-assisted laser desorption ionization (MALDI) mass spectroscopy at the Proteome Facility, University of Aberdeen, Aberdeen, United Kingdom.
Peptide synthesis.
Two peptides, referred to as rtCATH_1(R146-P181) and rtCATH_2(R143-I178), were chemically synthesized at the Proteome Facility, University of Aberdeen. rtCATH_1(R146-P181) corresponded to the fragment from residues 146 to 181 (RRSKVRICSRGKNCVSRPGVGSIIGRPGGGSLIGRP) of the sequence and has a molecular weight of 3,734.4 and a molecular charge of +9. rtCATH_2(R143-I178) corresponded to the fragment from residues 143 to 178 (RRGKDSGGPKMGRKNSKGGWRGRPGSGSRPGFGSGI) of the sequence and has a molecular weight of 3,687.1 and a molecular charge of +9. To compare the activities of rainbow trout peptides directly with those of a mammalian cathelicidin, the bovine cyclic dodecapeptide (amino acid sequence RLCRIVVIRVCR) (25) was also synthesized. The peptides were stored at −20°C until they were dissolved in 10 ml sterile 0.15 M PBS (pH 7.2; Gibco). Stock solutions (10 mM) were stored in 0.5-ml aliquots at −80°C until they were required.
Bacteria and culture conditions.
The reference strain Escherichia coli NCIMB12210 (ATCC 25922) was obtained from NCIMB Ltd., Aberdeen, United Kingdom. Nine field isolates of fish pathogens were kindly provided by Tony Ellis, FRS Marine Laboratory, Aberdeen, United Kingdom. A. salmonicida strain MT004 and A-layer-positive, virulent strain MT423 (11) were isolated from rainbow trout. Photobacterium damselae subsp. piscicida MT1415 was a virulent capsulated strain isolated from sea bass (Dicentrarchus labrax), and EPOY8803II was an avirulent noncapsulated strain isolated from red grouper (Epinephelly akaara) (15). Vibrio anguillarum serogroup O1 MT1741 (4), serogroup O2 MT1742 (3), and Yersinia ruckeri MT252 were isolated from rainbow trout. Lactococcus garvieae noncapsulated strains MT2055 and MT2059 and capsulated strains MT2290 and MT2291 were originally isolated from yellowtail and rainbow trout, respectively (2).
E. coli was maintained in LB medium at 37°C. The aquatic strains were maintained in Trypticase soy broth at 22°C. All the strains were transferred to Mueller-Hinton broth (MHB; Sigma) and cultured to optimal concentrations before the antimicrobial assays.
Antimicrobial assays.
The MIC and minimal bactericidal concentration (MBC) determination methods of Steinberg et al. (31) were modified to determine the bacteriostatic and bactericidal activities, respectively. The MIC was obtained by using a modified twofold broth microdilution method (19), since high concentrations of cationic peptides tended to precipitate in MHB and led to inaccurate dilution of the peptides in sequential wells. Briefly, serial twofold dilutions of peptides were prepared in 0.2% bovine serum albumin-0.01% acetic acid buffer in tubes. Eighty microliters of each concentration was added to each corresponding well of a 96-well microtiter plate. The bacteria were incubated to mid-logarithmic phase and diluted in MHB to give a final inoculum concentration of 105 CFU/ml. A suspension of 20 μl of bacteria was added to each well of a 96-well plate, and the plate was incubated for 48 h at 22°C. Visual verification of microbial sedimentation as well as the absorbance reading (600 nm) confirmed the MIC. Five microliters of the contents from each well showing no visible growth was plated out on a MH agar plate, and the MBC was determined as the lowest concentration of protein that prevented any residual colony formation after incubation at 28°C for 2 days. Experiments were performed with three replicates of each assay.
Nucleotide sequence accession numbers.
The sequence data presented in this study have been deposited in GenBank, as follows: asCATH_1 cDNA, GenBank accession number AY728057; asCATH_2 cDNA, GenBank accession number AY360357; rtCATH_2 cDNA, GenBank accession number AY360356; asCATH_1 genomic DNA, GenBank accession number AY728901; asCATH_2 genomic DNA, GenBank accession number AY542961; and rtCATH_2 genomic DNA, GenBank accession number AY542963.
RESULTS
Cloning and features of salmonid cathelicidin sequences.
The three full-length nucleotide sequences and the deduced amino acid sequences, obtained from the genomic DNA and the overlapping 5′ and 3′ cDNA ends, are shown in Fig. 1. The predicted polypeptides in the preprosequences display the characteristic features of cathelicidins and show high similarity (Fig. 2). Analysis of the N-terminal region predicts a cleavage site for signal peptides at residue Ser22-Gln23 in the sequence shown in Fig. 1A and at residue Gly22-Gln23 in the sequences shown in Fig. 1B and Fig. 1C. The putative 22-residue signal peptide is followed by a cathelin-like proregion that conserves the four cysteine residues present in all the congeners characterized thus far.
FIG. 1.
Nucleotide and deduced amino acid sequences of the precursors of asCATH_1 (A), asCATH_2 (B), and rtCATH_2 (C). Numbering is on the left for nucleotides and on the right for amino acids. Exons are shown in uppercase letter, and introns are shown in lowercase letters. The predicted translation of the exon-coding regions is given. The putative mature antimicrobial sequences are underlined. The arrows show the respective putative cleavage site for signal peptidase and elastase. Asterisks mark the stop codons. Polyadenylation signals are underlined.
FIG. 2.
Multiple-sequence alignment of the predicted translation of rainbow trout cathelicidins rtCATH_1 and rtCATH_2 and Atlantic salmon cathelicidins asCATH_1 and asCATH_2. Identical residues (indicated by asterisks) and similar residues (indicated by periods or colons) identified by the CLUSTAL program are indicated. The conserved motifs encoded by exon IV in each sequence are boxed. The cysteines involved in disulfide bond formation are in boldface text and are indicated with a percent sign. The predicted mature peptides are in italics, and the signal peptide is indicated by a bar above the alignment.
The 207-amino-acid (aa) cathelicidin sequence of Atlantic salmon (Fig. 1A) has a calculated mass of 22.9 kDa and a pI of 9.82. The nucleotide sequence in exon IV possesses three repeat motifs of TGGGGGTGGC. The signal peptide is identical to the signal peptide of rtCATH_1, and the putative mature peptide has two cysteine residues, also as in rtCATH_1. Analysis of the sequence confirms that this is the Atlantic salmon homologue of rtCATH_1, with 92% nucleotide identity and similar repeat motifs. Hence, it was named asCATH_1.
The 202-aa cathelicidin sequence of Atlantic salmon (Fig. 1B) has a calculated mass of 21.9 kDa and a pI of 9.36. It possesses a longer exon III and a shorter exon IV relative to their lengths in asCATH_1 and rtCATH_1 and has neither a repeat motif nor cysteine residues in the mature peptide region. Analysis of the sequence suggests that this is a second cathelicidin gene sequence in Atlantic salmon and was named asCATH_2.
The 208-aa cathelicidin of rainbow trout (Fig. 1C) has a calculated mass of 22.7 kDa and a pI of 8.96. The sequence also has no repeat motif in exon IV or cysteine residues in the mature peptide. The signal peptide of this sequence is identical to the signal peptide of the sequence of asCATH_2. Analysis of the sequence reveals that this is the rainbow trout homologue of the Atlantic salmon asCATH_2 gene, with 96% nucleotide identity. It was named rtCATH_2.
Multiple-sequence alignment (Fig. 2) of the four salmonid cathelicidin preproproteins shows high amino acid conservation in the sequence encoded by exons I, II, and III. The amino acid sequence encoded by exon IV is very diverse, with the exception of a 7-amino-acid identical motif of QKIRTRR at the beginning of the sequence.
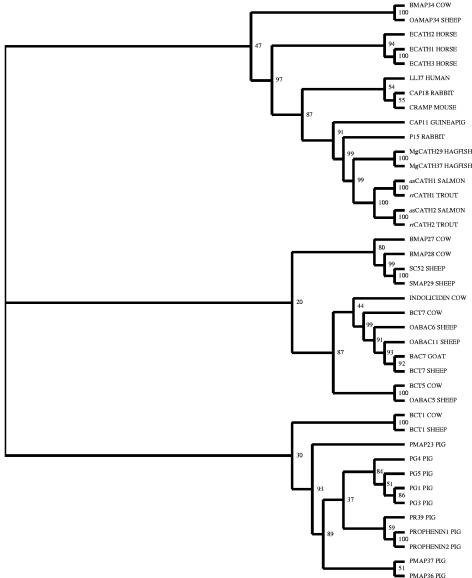
Phylogenetic analysis (Fig. 3) groups the salmonid cathelicidins together, with hagfish cathelicidins as the nearest group. Rabbit p15s and guinea pig CAP11 are next closest, with mouse CRAMP, rabbit CAP18, human LL37, horse ECATH1-3, sheep OAMAP34, and bovine BMAP34 being in the same clade but farther away. Other mammalian cathelicidins, primarily from ungulates, formed two clades that were more distantly related.
FIG. 3.
Unrooted phylogram showing the phylogenetic relationships between known cathelicidin genes that have been sequenced within the vertebrates. Phylogeny was estimated by using the amino acid sequences of precursors of cathelicidins with the neighbor-joining method. The resulting tree was bootstrapped 1,000 times, and the bootstrap values are shown as percentages.
Differential tissue expression of rtCATH_1 and rtCATH_2.
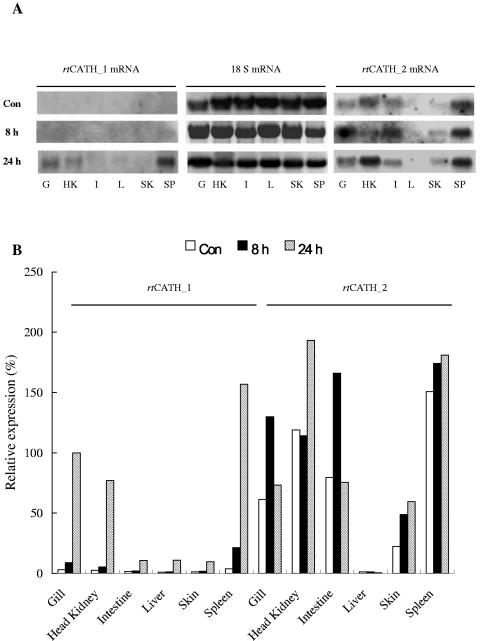
Northern blot analysis showed that rtCATH_1 and rtCATH_2 exhibited differential expression (Fig. 4). The level of rtCATH_1 expression in samples from healthy fish was not detectable but was markedly increased after A. salmonicida MT423 challenge in gill, head kidney, and spleen. In contrast to rtCATH_1, rtCATH_2 showed constitutive expression in gill, head kidney, intestine, skin, and spleen samples from healthy fish. Expression levels increased further after bacterial stimulation, especially in the gill, head kidney, and intestine. Interestingly, no mRNA of either rtCATH_1 or rtCATH_2 could be detected in the liver samples of untreated or bacterially stimulated fish.
FIG. 4.
Northern blot analysis of rtCATH_1 and rtCATH_2 mRNA expression: exposed film (A) and densitometric reading of the film (B). The mRNA levels were normalized by the 18S RNA level and expressed as a percentage of the mRNA level in the gill of a fish challenged for 24 h (100%). Con, control fish; 8 h, fish injected intraperitoneally intraperitoneally with 2 × 106 CFU of Aeromonas salmonicida MT423 for 8 h; 24 h, fish injected intraperitoneally with 2 × 106 CFU of A. salmonicida for 24 h; G, gill; HK, head kidney; I, intestine; L, liver; SK, skin; SP, spleen.
Cleavage site.
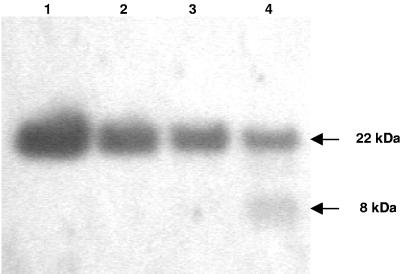
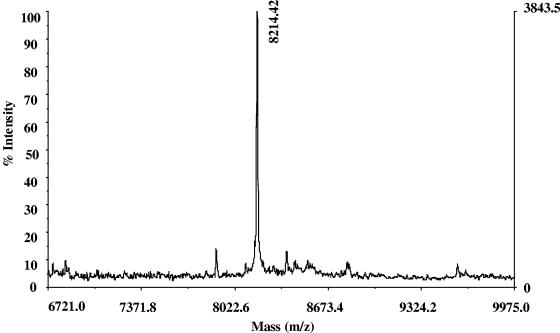
The cleavage sites for the mature peptides of salmonid cathelicidins were studied by using a full-length recombinant protein and Western blot analysis and were confirmed by mass spectroscopic analysis. Western blotting showed a clear time-dependent proteolytic cleavage effect on the recombinant pro-rtCATH_1 protein by elastase treatment (Fig. 5). Only one of the two fragments generated is visualized, together with the uncut pro-rtCATH_1, as the N-terminal fragment does not contain the six-His tag. The C-terminal peptide generated by elastase cleavage had a molecular mass of approximately 8 kDa. The precise molecular mass of this fragment was determined by MALDI mass spectroscopy and was found to be 8,214.42 Da (Fig. 6). The calculated mass of the amino acid sequence starting from Arg146 of the rtCATH_1 precursor and ending with the six-His tag was 8,214.08 Da, which was in agreement with that obtained from the mass spectroscopic analysis. This result confirmed that the elastase cleavage site for the mature C-terminal rtCATH_1 peptide is at residue Thr145-Arg146.
FIG. 5.
Time-dependent cleavage of prtCATH_1 by elastase. Shown is a Western blot of a 4 to 12% polyacrylamide gel after incubation with an anti-six-His tag antibody. Five micrograms of prtCATH_1-6His was incubated with 5 μl Tris buffer (lane 1) or elastase (50 ng in 5 μl Tris buffer) for 10 min (lane 2), 60 min (lane 3), and 120 min (lane 4). The reactions were stopped by acidification with an equal volume of 5% acetic acid, and the samples were run on the gel.
FIG. 6.
Mass spectroscopic analysis of the mature peptide of rtCATH_1. The mature peptide was generated from elastase digestion of the recombinant rtCATH_1 proprotein with a six-His tag at the C terminus and was recovered from the 4 to 12% polyacrylamide gel after separation. The mass of the peptide was determined by MALDI mass spectrometry to be 8,214.42 Da.
Antimicrobial assays.
The effects of the synthesized peptides against a panel of bacteria were evaluated as the minimal concentration capable of inhibiting visible microbial growth (MIC). Peptides derived from the rtCATH_1 and the rtCATH_2 genes displayed various antibacterial activities (Table 2). Against gram-negative bacteria, the rtCATH_1(R146-P181) peptide had the strongest activity against V. anguillarum strain MT1741, while the rtCATH_2(R143-I178) peptide had the strongest activity against P. damselae subsp. piscicida MT1415. When these values are compared with the MBCs, it is clear that the rainbow trout cathelicidins are bacteriostatic rather than bactericidal, for the MBCs are all higher than the MICs for all the gram-positive bacteria tested (Table 2). Neither the rtCATH_1(R146-P181) peptide nor the rtCATH_2(R143-I178) peptide had significant bactericidal activity against the gram-positive bacterium (L. garvieae) tested, but they had potent inhibitory effects, particularly on the capsulated strains of this species. In general, the bovine cyclic dodecapeptide was less potent than the fish peptides.
TABLE 2.
Antimicrobial activities of rtCATH_1(R146-P181), rtCATH_2(R143-I178) and bovine cyclic dodecapeptide (Bovdode)
| Organism and strain | Relevant strain or phenotype | MIC/MBC (μM)
|
||
|---|---|---|---|---|
| Bovdode | rtCATH_1(1-36) | rtCATH_2(1-36) | ||
| Gram negative | ||||
| Escherichia coli NCIMB 12210 | ATCC 25922 | 5/10 | 1/20 | 2.5/5 |
| Aeromonas salmonicida MT004 | A-layer negative | 16/64 | 5/40 | 1/20 |
| A. salmonicida MT423 | A-layer positive | >64/>64 | 10/>64 | 5/16 |
| Photobacterium damselae MT1415 | Capsulated | 40/40 | 1/2.5 | 0.5/2.5 |
| P. damselae EPOY 8803 II | Noncapsulated | 2/5 | 2.5/5 | 1/8 |
| Vibrio anguillarum MT1741 | Serogroup O1 | 10/40 | 0.5/20 | 2.5/20 |
| V. anguillarum MT1742 | Serogroup O2 | 2.5/10 | 2/64 | 4/>64 |
| Yersinia ruckeri MT252 | 16/40 | 8/40 | 8/40 | |
| Gram positive | ||||
| Lactococcus garvieae MT2055 | Noncapsulated | 6/50 | 4/>64 | 8/>64 |
| L. garvieae MT2059 | Noncapsulated | 3/50 | 2/>64 | 4/>64 |
| L. garvieae MT2290 | Capsulated | 1.5/25 | 0.2/>64 | 0.2/>64 |
| L. garvieae MT2291 | Capsulated | 0.8/50 | 0.1/>64 | 0.1/>64 |
DISCUSSION
Six cathelicidin genes have now been identified in fish: two in hagfish (33), two in rainbow trout (5), and two in Atlantic salmon. The fish cathelicidins share several characteristics in common with their mammalian congeners (1). Both mammalian and fish cathelicidin genes contain four exons and three introns. The first three exons encode the conserved preproregion, including the signal peptide and cathelin-like domain, while the cleavage site and the varied antimicrobial domain are encoded by the fourth exon. Four invariant cysteines clustered in the C-terminal region of the cathelin-like domain are arranged to form two disulfide bonds, which may impose structural constraints on the molecule (23). In comparison with mammalian cathelicidins, fish cathelicidins have a shorter signal peptide (22 to 26 aa in fish versus 29 to 30 aa in mammals) and a longer cathelin-like domain (115 to 127 aa in fish versus 94 to 114 aa in mammals).
Processing of cathelicidin precursors to mature antimicrobial peptides is mediated by elastase in most of the cathelicidins identified so far, although exceptions exist, as seen with human LL-37, where proteinase 3 cleaves the molecule (30), and rabbit p15s, where no cleavage site for elastase is present and the molecule is secreted in an unprocessed form (14). In the elastase-processed cathelicidins, the elastase-sensitive residue is typically a valine or an alanine positioned near the beginning of exon IV (8). However, in some more distantly related congeners, such as rabbit CAP18 (13) and the hagfish molecules MgCath29 and MgCath37 (33), the elastase cleavage site is at a threonine. In this study salmonid cathelicidins have been shown to possess the same elastase-sensitive residue as hagfish cathelicidins and rabbit CAP18. The paper published by Chang et al. (5) predicted that the cutting site would be at Val150-Arg151, since valine was a more common cleavage site than threonine. This prediction is clearly incorrect, and the mass spectroscopic analysis used here has given a result much more precise than that obtained by the methods used in that study (i.e., electrophoresis and Western blotting analysis). That Thr145-Arg146 is the cleavage site of rtCATH_1 was also suggested by the absolutely conserved motif of QKIRTRR, seen in all the salmonid sequences at the beginning of the amino acid sequence encoded by exon IV (Fig. 2). Thus, it is highly likely that all these salmonid cathelicidins have the same elastase cleavage site. That rabbit CAP18 is in the same clade and is one of the more closely related mammalian cathelicidins is also an interesting finding in this regard.
Since two cathelicidin genes exist in rainbow trout and Atlantic salmon, the relative activities and levels of expression of the two molecules were also studied. Thirty-six amino acid peptides of both molecules were synthesized and tested against a range of bacterial species, and both had a net charge of +9. Relative to the findings in the study of Chang et al. (5), the rtCATH_1 peptide began and finished 5 amino acids upstream of the previously studied peptide and had a charge of +9 rather than +6. Both rtCATH-1 and rtCATH_2 peptides had potent antibacterial activities, with similar MICs, although rtCATH_1 was relatively more active against E. coli 12210 and V. anguillarum MT1741 and rtCATH_2 was relatively more active against A. salmonicida MT004 and P. damselae EPOY 8803II. Both peptides were less active against the A-layer-positive strain of A. salmonicida tested than they were against the A-layer-negative strain, as seen previously (5). Also, both peptides were less active against the noncapsulated strains of L. garvieae than they were against the capsulated strains. While the latter result may seem counterintuitive, in fact, the role of the capsule is as a physical barrier that prevents phagocytosis by host leukocytes (16) or hides cell surface antigens (21). The capsule is a loose gelatinous mass with negative charges (12) that cannot prevent peptides from passing through it and, indeed, could potentially attract positively charged molecules such as AMPs. If this were the case, then the differences in susceptibilities to the trout cathelicidins would be explained.
Perhaps the more striking difference between the two peptides was in their relative levels of expression, where rtCATH_2 showed constitutive expression in a range of tissues, including the main mucosal sites (gills, skin, intestine), in contrast to rtCATH_1, where no constitutive expression was apparent. The mucous layer forms an initial line of defense that protects epithelial tissues against microbial, mechanical, and chemical insults. The production of antimicrobial peptides in mucus is often continuous, as seen in healthy epithelial cells of the urogenital tract and kidney in humans, which constitutively express human β-defensin-1 (HBD-1) at a low level (26). However, rather than being expressed continuously, some defensin genes are induced on treatment with proinflammatory cytokines, lipopolysaccharide, or bacteria, as seen with HBD-2, which is strongly induced in the skin, foreskin, lungs, and trachea when keratinocytes contact gram-negative bacteria (10). Interestingly, the expression of the two rainbow trout cathelicidins, rtCATH_1 and rtCATH_2, is similar to those of HBD-2 and HBD-1, respectively. rtCATH_2 showed constitutive expression in gill, head kidney, intestine, skin, and spleen samples from healthy fish, while the expression of rtCATH_1 was not detectable. However, after challenge, the levels of rtCATH_1 mRNA were markedly increased in gill, head kidney, and spleen, although no clear expression of rtCATH_1 in intestine and skin was detectable even then. Such differences in expression presumably relate to the promoters of these genes, and it will be interesting to analyze the 5′ flanking regions of both genes for regulatory elements. Curiously, neither gene showed expression in the liver. This may relate to the presence of other antimicrobial peptides that are known to be produced there in fish, such as hepcidins (6) and LEAP-2 (35).
In conclusion, fish clearly possess a repertoire of antimicrobial peptides that can be induced upon bacterial infection. Whether these molecules can have therapeutic application and what mechanisms control their expression can now be studied.
Acknowledgments
This work was supported financially by EWOS Innovation.
Thanks also go to the Taiwanese government for financial assistance to C.-I.C. and the Royal Society China-United Kingdom Joint Project (reference no. 13255) for financial assistance to Y.-A.Z.
REFERENCES
- 1.Bals, R., and J. M. Wilson. 2003. Cathelicidins—a family of multifunctional antimicrobial peptides. Cell Mol. Life Sci. 60:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, A. C., C. Guyot, B. G. Hanse, K. Mackenzie, M. T. Horn, and A. E. Ellis. 2002. Resistance to serum killing may contribute to differences in the abilities of capsulate and non-capsulated isolates of Lactococcus garvieae to cause disease in rainbow trout (Oncorhynchus mykiss L.). Fish Shellfish Immunol. 12:155-168. [DOI] [PubMed] [Google Scholar]
- 3.Boesen, H. T., M. H. Larsen, J. L. Larsen, and A. E. Ellis. 2001. In vitro interactions between rainbow trout (Oncorhynchus mykiss) macrophages and Vibrio anguillarum serogroup O2a. Fish Shellfish Immunol. 11:415-431. [DOI] [PubMed] [Google Scholar]
- 4.Boesen, H. T., K. Pedersen, J. L. Larsen, C. Koch, and A. E. Ellis. 1999. Vibrio anguillarum resistance to rainbow trout (Oncorhynchus mykiss) serum: role of O-antigen structure of lipopolysaccharide. Infect. Immun. 67:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, C. I., O. Pleguezuelos, Y. A. Zhang, J. Zou, and C. J. Secombes. 2005. Identification of a novel cathelicidin gene in rainbow trout Oncorhynchus mykiss. Infect. Immun. 73:5053-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas, S. E., A. Patrzykat, J. Pytyck, and J. W. Gallant. 2003. Identification, structure and differential expression of novel pleurocidins clustered on the genome of the winter flounder, Pseudopleuronectes americanus (Walbaum). Eur. J. Biochem. 270:3720-3730. [DOI] [PubMed] [Google Scholar]
- 7.Gallo, R. L., K. J. Kim, M. Bernfield, C. A. Kozak, M. Zanetti, L. Merluzzi, and R. Gennaro. 1997. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272:13088-13093. [DOI] [PubMed] [Google Scholar]
- 8.Gennaro, R., and M. Zanetti. 2000. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers 55:31-49. [DOI] [PubMed] [Google Scholar]
- 9.Gudmundsson, G. H., B. Agerberth, J. Odeberg, T. Bergman, B. Olsson, and R. Salcedo. 1996. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238:325-332. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, R. E. W., and M. G. Scott. 2000. Antimicrobial peptides in animal defences. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry, M. A., and C. J. Secombes. 2000. The A-layer influences the susceptibility of Aeromonas salmonicida to antibacterial peptides. Fish Shellfish Immunol. 10:637-642. [DOI] [PubMed] [Google Scholar]
- 12.Jann, K., and B. Jann. 2001. Capsular polysaccharides, p. 47-67. In M. Sussman (ed.), Molecular medical microbiology. Academic Press, Inc., San Diego, Calif.
- 13.Larrick, J. W., J. G. Morgan, I. Palings, M. Hirata, and M. H. Yen. 1991. Complementary DNA sequence of rabbit CAP18—a unique lipopolysaccharide binding protein. Biochem. Biophys. Res. Commun. 179:170-175. [DOI] [PubMed] [Google Scholar]
- 14.Levy, O., J. Weiss, K. Zarember, C. E. Ooi, and P. Elsbach. 1993. Antibacterial 15-kDa protein isoforms (p15s) are members of a novel family of leukocyte proteins. J. Biol. Chem. 268:6058-6063. [PubMed] [Google Scholar]
- 15.Lopez-Doriga, M. V., A. C. Barnes, N. M. dos Santos, and A. E. Ellis. 2000. Invasion of fish epithelial cells by Photobacterium damselae subsp. piscicida: evidence for receptor specificity, and effect of capsule and serum. Microbiology 146(Pt 1):21-30. [DOI] [PubMed] [Google Scholar]
- 16.Madigan, M. T., J. M. Martinko, and J. Parker. 2003. Essentials of immunology, p. 755-786. In M. T. Madigan, J. M. Martinko, and J. Parker (ed.), Biology of microorganisms, 10th ed. Pearson Education, Inc., Upper Saddle River, N.J.
- 17.Mahoney, M. M., A. Y. Lee, D. J. Brezinski-Caliguri, and K. M. Huttner. 1995. Molecular analysis of the sheep cathelin family reveals a novel antimicrobial peptide. FEBS Lett. 377:519-522. [DOI] [PubMed] [Google Scholar]
- 18.Nagaoka, I., Y. Tsutsumi-Ishii, S. Yomogida, and T. Yamashita. 1997. Isolation of cDNA encoding guinea pig neutrophil cationic antibacterial polypeptide of 11 kDa (CAP11) and evaluation of CAP11 mRNA expression during neutrophil maturation. J. Biol. Chem. 272:22742-22750. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1993. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard M7-A3. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 20.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 21.Ørskov, I., F. Ørskov, B. Jann, and K. Jann. 1977. Serology, chemistry and genetics of O and K antigens of Escherichia coli. Bacteriol. Rev. 41:667-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 23.Ramanathan, B., E. G. Davis, C. R. Ross, and F. Blecha. 2002. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 4:361-372. [DOI] [PubMed] [Google Scholar]
- 24.Ritonja, A., M. Kopitar, R. Jerala, and V. Turk. 1989. Primary structure of a new cysteine proteinase inhibitor from pig leucocytes. FEBS Lett. 255:211-214. [DOI] [PubMed] [Google Scholar]
- 25.Romeo, D., B. Skerlavaj, M. Bolognesi, and R. Gennaro. 1988. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J. Biol. Chem. 263:9573-9575. [PubMed] [Google Scholar]
- 26.Schroder, J. M. 1999. Epithelial antimicrobial peptides: innate local host response elements. Cell Mol. Life Sci. 56:32-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scocchi, M., D. Bontempo, S. Boscolo, L. Tomasinsig, E. Giulotto, and M. Zanetti. 1999. Novel cathelicidins in horse leukocytes(1). FEBS Lett. 457:459-464. [DOI] [PubMed] [Google Scholar]
- 28.Shamova, O., K. A. Brogden, C. Zhao, T. Nguyen, V. N. Kokryakov, and R. I. Lehrer. 1999. Purification and properties of proline-rich antimicrobial peptides from sheep and goat leukocytes. Infect. Immun. 67:4106-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skerlavaj, B., R. Gennaro, L. Bagella, L. Merluzzi, A. Risso, and M. Zanetti. 1996. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 271:28375-28381. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen, O. E., P. Follin, A. H. Johnsen, J. Calafat, G. S. Tjabringa, P. S. Hiemstra, and N. Borregaard. 2001. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97:3951-3959. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. C. Kung, J. F. Ho, F.-C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uzzell, T., E. D. Stolzenberg, A. E. Shinnar, and M. Zasloff. 2003. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides 24:1655-1667. [DOI] [PubMed] [Google Scholar]
- 34.Zanetti, M., G. Del Sal, P. Storici, C. Schneider, and D. Romeo. 1993. The cDNA of the neutrophil antibiotic Bac5 predicts a pro-sequence homologous to a cysteine proteinase inhibitor that is common to other neutrophil antibiotics. J. Biol. Chem. 268:522-526. [PubMed] [Google Scholar]
- 35.Zhang, Y. A., J. Zou, C. I. Chang, and C. J. Secombes. 2004. Discovery and characterization of two types of liver-expressed antimicrobial peptide 2 (LEAP-2) genes in rainbow trout. Vet. Immunol. Immunopathol. 101:259-269. [DOI] [PubMed] [Google Scholar]
- 36.Zhao, C., T. Ganz, and R. I. Lehrer. 1995. The structure of porcine protegrin genes. FEBS Lett. 368:197-202. [DOI] [PubMed] [Google Scholar]