Abstract
The antimicrobial properties and the elution characteristics of gentamicin-vancomycin-loaded hip spacers were studied in vivo and in vitro. Vancomycin elution was greater than gentamicin elution. The antibiotic concentrations in vivo were less than those in vitro. Not dependent on implantation duration, growth inhibition by spacers in vitro was observed for 2 weeks. The reason for protracted wound healing cannot be insufficient antibiotic release.
An innovative method, with numerous advantages, to optimize the two-stage treatment protocol in hip joint infections is the implantation of a standardized, antibiotic-loaded polymethylmethacrylate (PMMA) spacer. This technique provides the possibility of adding antibiotics for eradication of infection, ensuring the functional and stable condition of the joint and the prevention of soft tissue shortening (3, 8).
The exact interval during implantation in which a sufficient antibiotic elution is preserved with regard to antimicrobial properties is still unknown. The aim of our study was to measure the antibiotic release by PMMA-gentamicin-vancomycin-loaded hip spacers during the first postoperative days and to evaluate their efficiency regarding antibiotic elution and growth inhibition of Staphylococcus epidermidis after explantation in vitro.
Ten hip spacers (containing 80 g of PMMA, 1 g of gentamicin, and 4 g of vancomycin [Refobacin-Palacos from Merck, Darmstadt, Germany; Vanco-cell from Cell-Pharm, Hannover, Germany]) were studied. The spacers (head diameter, 50 mm; stem length, 10 cm; surface area, 13,300 mm2) had been produced by a mold (3).
All tests were carried out with Staphylococcus epidermidis strain DSM 3269 due to its general role and frequency in hip joint infections. As a culture medium, we used tryptic soy broth in blood agar plates (Becton Dickinson, Heidelberg, Germany).
A fluorescence polarization immunoassay (Abbot, Wiesbaden,Germany) was used to determine the released concentrations of gentamicin and vancomycin (3, 4).
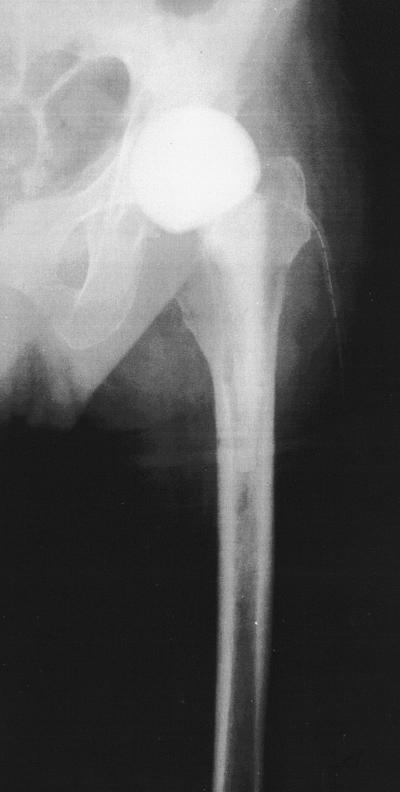
The spacers' release characteristics were determined by in vivo measurements (wound secretion from the Redon drainage) in 10 patients. Over the first postoperative days a wound secretion specimen was taken from the Redon drainage bottle every 24 h via the drainages placed directly at the head of the spacers (Fig. 1) (11). The drainage system was changed daily.
FIG. 1.
Spacer in situ. A wound secretion specimen was taken from the Redon drainage bottle every 24 h via the drainages placed directly at the head of the spacer.
After infection sanitation, the spacers were explanted. Their further antibiotic release and the resulting bacterial growth inhibition were analyzed as follows (3).
The explanted PMMA spacers were cleaned with distilled water under sterile conditions. The spacers were placed in cylindrical glass containers (diameter, 55 mm; height, 190 mm) into which 230 ml of tryptic soy broth was poured. Then the cylinders were placed on a vibrating plate at 37°C. One milliliter of a culture suspension of the S. epidermidis strain (4 × 106 CFU/ml) was then pipetted into the containers. The suspension was then incubated aerobically, at 37°C, for 24 h. (Every day, fresh suspensions were prepared for the following day.) The spacers were then removed from the cylinders and placed, for about 5 min, in an alcohol bath in order to eliminate any possibility of contamination by bacteria other than the bacterium being tested. The spacers were then removed from the bath, washed thoroughly with distilled water, and placed into fresh aliquots of tryptic soy broth, and 1 ml of a suspension was repipetted out for assaying.
The tests were carried out until bacterial growth was measured and the smear test showed the same bacterium as the one pipetted. In case of contamination, the particular spacers were not evaluated.
Bacterial growth was determined by a photometric technique (STA-SAR II; Gilford Instrument Laboratories Inc., Oberlin, Ohio) at a wavelength of 546 nm. After calibration, bacterial growth was defined at an extinction coefficient (E) of >0.05. Should the extinction exceed E > 0.05, the antibiotic release would be described as “insufficient” due to the inability to inhibit bacterial growth (Fig. 2).
FIG. 2.
Evaluation of bacterial growth inhibition in vitro by gentamicin-vancyomcin-impregnated hip spacers. On the left side, the antibiotic elution is still sufficient (the tryptic soy broth is clear). On the right side, the turbidity of the tryptic soy broth indicates bacterial growth with insufficient antibiotic release from the spacer.
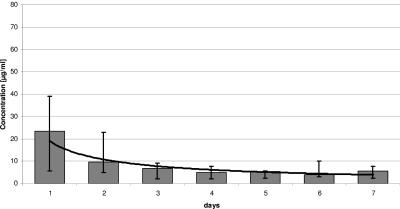
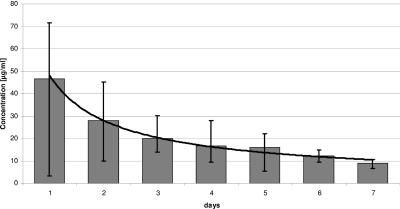
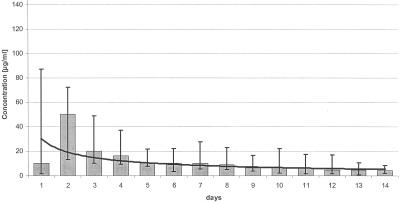
With the software program Microsoft Excel, we assayed concentration-time curves for both antimicrobial agents. The vancomycin elution was quantitatively higher than that of gentamicin in all patients (Fig. 3 and 4). The elution of both antibiotics was high at the beginning and then declined and reached the serum's therapy recommendation of 2 to 5 μg/ml after 2 to 3 days. The extrapolated concentration-time curves showed power functions, so that subtherapeutic antibiotic levels can be expected for vancomycin on the 17th postoperative day and for gentamicin on the 14th postoperative day.
FIG. 3.
Release of gentamicin in the first postoperative week.
FIG. 4.
Release of vancomycin in the first postoperative week.
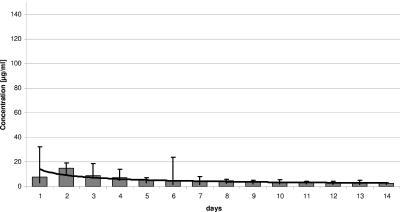
The comparison between amounts of vancomycin and gentamicin release in vitro (Fig. 5 and 6) shows that—in relation to the initial amount—the quantity of vancomycin elution was higher than that of gentamicin, whereas the percentage of the gentamicin elution was higher than that of vancomycin (Table 1). The antibiotic elutions reached their peak values within the first 2 or 3 days, declined constantly over the following 10 to 12 days, and then fell under the therapeutic serum concentration values. All explanted spacers were able to inhibit bacterial growth for at least 2 weeks (14 to 30 days), independently of their implantation period (Table 1).
FIG. 5.
Release of gentamicin in vitro after spacer explantation.
FIG. 6.
Release of vancomycin in vitro after spacer explantation.
TABLE 1.
Antimicrobial and pharmacokinetic properties of antibiotic-loaded hip spacers after 14 days in vitro
| Spacer | Implantation period (wk) | Duration of bacterial growth inhibition (days) | Release of antibiotic in μg (% release) after 14 days
|
|
|---|---|---|---|---|
| Gentamicin | Vancomycin | |||
| 1 | 12 | 14 | 18,563.3 (1.85) | 47,467.4 (1.19) |
| 2 | 8 | 14 | 23,857.9 (2.38) | 39,652.0 (0.99) |
| 3 | 12 | 15 | 24,628.2 (2.46) | 75,497.0 (1.89) |
| 4 | 14 | 15 | 27,641.4 (2.76) | 28,363.6 (0.71) |
| 5 | 3 | 30 | 25,467.9 (2.54) | 75,039.8 (1.88) |
| 6 | 7 | 15 | 21,279.6 (2.12) | 52,053.6 (1.30) |
| 7 | 14 | 16 | 28,474.0 (2.84) | 64,209.1 (1.60) |
| 8 | 13 | 16 | 16,162.1 (1.61) | 32,211.5 (0.81) |
| 9 | 8 | 14 | 15,288.1 (1.52) | 24,244.3 (0.61) |
| 10 | 4 | 18 | 15,336.4 (1.53) | 54,726.2 (1.09) |
Despite the quantities of antibiotic already released in vivo during the implantation period, the spacers' antibiotic elution measured over the same intervals was higher in vitro than in vivo.
The optimum antibiotic proportion in the spacers' PMMA with respect to elution characteristics is still unknown. Depending on the bacterium's resistance, bone cement is frequently impregnated with an aminoglycoside and a glycopeptide (3, 5, 9, 11, 13). The combination of these two antibiotics is intended to avoid emergence of resistance and profits from the already known synergism of these substances regarding their elution from the PMMA (3, 11). For the impregnation of antibiotic-loaded spacers, many authors recommend an antibiotic proportion of 3 to 6% (5, 6, 13), whereby the use of a gentamicin-vancomycin combination requires a higher proportion of the latter due to its worse release kinetics (10, 13). We can confirm these results from the literature with our own tests. The relative proportion of the vancomycin release from the bone cement was lower, although its initial quantity was four times higher than that of gentamicin.
Adams et al. (2) showed in an animal experiment that 4 weeks after the implantation of a defined quantity of antibiotic-loaded PMMA beads, the antibiotic concentrations in the surrounding granulation tissue were higher than those in the serum liquid flowing around the beads. Possibly, the initially high antibiotic elution causes a quick saturation of the surrounding tissue in vivo. The diffusion gradient that supported the antibiotic elution at the beginning (7) was decreasing when the tissue saturation was increasing. This could lead to a severe time-dependent reduction of the antibiotic release from the spacer, thus confirming our extrapolated functions. A high local tissue saturation may also explain the low serum antibiotic levels measured after implantation of antibiotic-releasing PMMA chains or collagen sponges (12, 14).
In contrast to that finding, the diffusion gradient in vitro is permanently high. Since the culture medium was changed daily during the in vitro studies, the permanently existing concentration differences between spacer surface and culture medium were causing a new, high, and longer lasting antibiotic elution (7).
We were not able to observe any relationship between the duration of the implantation period and the spacers' antibiotic elution measured in vitro. Spacer no. 1, for example, had different elution quantities from spacer no. 3, despite the same implantation period. An in vitro study of explanted spacers by Bertazonni et al. (4) showed, in accordance with our studies, there was no relationship between the implantation period of the spacers and the antibiotic quantities released in vitro. A possible explanation for this might be that if all spacers are provided with the same PMMA, cement additions, and implantation duration, anatomic and physiological differences like local blood flow and the tissue's pH value (15) may have an influence on the pharmacokinetic reaction of spacers, causing different antibiotic elution quantities.
Due to the sufficient antibiotic elution measured in vivo, complications in postoperative protracted wound healing during the early postoperative period are not caused by low, subtherapeutic, local antibiotic concentrations. According to the literature, prostheses are being reimplanted after an average of 6 to 8 weeks (8-9, 11, 13). Nevertheless, shorter or longer periods are also recommended (1, 10). Our tests show that—independently of their implantation period—spacers still contain sufficient antibiotic quantities and are able to release them in vitro, as proven by a significant bacterial growth inhibition. Hence, for the clinical practice, it could be stated that there can be a longer period of time between septic removal and reimplantation of a prosthesis without any risk of persistence of infection or reinfection.
REFERENCES
- 1.Abendschein, W. 1992. Salvage of infected total hip replacement: use of antibiotic/PMMA-spacer. Orthopedics 15:228-229. [DOI] [PubMed] [Google Scholar]
- 2.Adams, K., L. Couch, G. Cierny, J. Calhoun, and J. T. Mader. 1992. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin. Orthop. 278:244-252. [PubMed] [Google Scholar]
- 3.Anagnostakos, K., J. Kelm, T. Regitz, E. Schmitt, and W. Jung. 2005. In vitro evaluation of antibiotic release from and bacteria growth inhibition by antibiotic-loaded acrylic bone cement spacers. J. Biomed. Mater. Res. Part B Appl. Biomater. 72:373-378. [DOI] [PubMed] [Google Scholar]
- 4.Bertazzoni Minelli, E., A. Benini, B. Magnan, and P. Bartolozzi. 2004. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J. Antimicrob. Chemother. 53:329-334. [DOI] [PubMed] [Google Scholar]
- 5.Duncan, C. P., and C. Beauchamp. 1993. A temporary antibiotic-loaded joint replacement system for management of complex infections involving the hip. Orthop. Clin. N. Am. 24:751-759. [PubMed] [Google Scholar]
- 6.Haddad, F. S., B. A. Masri, D. S. Garbuz, and C. P. Duncan. 1999. The treatment of the infected hip replacement: the complex case. Clin. Orthop. 369:144-156. [DOI] [PubMed] [Google Scholar]
- 7.Hansen, A. D., R. T. Trousdale, and D. R. Osmon. 1995. Patient outcome with reinfection following reimplantation for infected total knee arthroplasty. Clin. Orthop. 321:55-67. [PubMed] [Google Scholar]
- 8.Hsieh, P. H., C. H. Shih, Y. H. Chang, M. S. Lee, H. N. Shih, and W. E. Yang. 2004. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J. Bone Joint Surg. 86-A:1989-1997. [PubMed] [Google Scholar]
- 9.Koo, K.-H., J. W. Yang, S.-H. Cho, H.-R. Song, H.-B. Park, Y.-C. Ha, J.-D. Chang, S.-Y. Kim, and Y.-H. Kim. 2001. Impregnation of vancomycin, gentamicin and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J. Arthroplasty 16:882-892. [DOI] [PubMed] [Google Scholar]
- 10.Leunig, M., E. Chosa, M. Speck, and R. Ganz. 1998. A cement spacer for two-stage revision of infected implants of the hip-joint. Int. Orthop. 22:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masri, B. A., C. P. Duncan, and C. P. Beauchamp. 1998. Long-term elution of antibiotics from bone-cement. J. Arthroplasty 13:331-338. [DOI] [PubMed] [Google Scholar]
- 12.Salvati, E. A., J. J. Callaghan, B. D. Brause, R. F. Klein, and R. D. Small. 1986. Reimplantation in infection: elution of gentamicin from cement and beads. Clin. Orthop. 207:83-93. [PubMed] [Google Scholar]
- 13.Takahira, N., M. Itoman, K. Higashi, K. Uchiyama, M. Miyabe, and K. Naruse. 2003. Treatment outcome of two-stage revision total hip arthroplasty for infected hip arthroplasty using antibiotic-impregnated cement spacer. J. Orthop. Sci. 8:26-31. [DOI] [PubMed] [Google Scholar]
- 14.Wahlig, H., E. Dingeldein, R. Bergmann, and K. Reuss. 1978. The release of gentamicin from polymethylmethacrylate beads. An experimental and pharmacokinetic study. J. Bone Joint Surg. 60-B:270-275. [DOI] [PubMed] [Google Scholar]
- 15.Wiesmann, E. 1986. Medizinische Mikrobiologie, p.102. Thieme Verlag, Stuttgart, Germany.