Abstract
BAL8557 is the water-soluble prodrug of a novel antifungal triazole, BAL4815. BAL4815 is active against a broad spectrum of major opportunistic and pathogenic fungi, including strains that are resistant to other azoles. Cohorts of healthy male subjects received single-ascending oral (p.o.) doses of BAL8557 that were equivalent to 100, 200, or 400 mg of BAL4815 or single-ascending, 1-h constant-rate intravenous (i.v.) infusions of BAL8557 which were equivalent to 50, 100, or 200 mg of BAL4815. In each cohort, six subjects were randomly assigned to receive active drug and two subjects were assigned to receive the placebo. All doses were well tolerated, and no severe or serious adverse events occurred. Maximum plasma concentrations of BAL4815 were observed 1.5 to 3 h after p.o. drug intake or at the end of the 1-h infusion. After both routes of administration, values for maximum drug concentration observed in plasma and area under the concentration-time curve increased slightly more than proportionally to the administered dose. Mean elimination half-lives were particularly long (56 to 77 h after p.o. administration and 76 to 104 h after i.v. administration). The volume of distribution was large (155 to 292 liters after p.o. administration and 304 to 494 liters after i.v. administration) and systemic clearance was low (1.9 to 2.8 liter/h after p.o. administration and 2.8 to 5.0 liter/h after i.v. administration). Urinary recovery of BAL4815 was less than 0.4% of the infused dose. Based on the exposure data, oral bioavailability of BAL4815 is assumed to be very high. The pharmacokinetics of BAL4815 are well suited to maintaining concentrations of BAL4815 for a long period of time in the body and to enabling an effective treatment of systemic mycoses.
Systemic mycoses are difficult to diagnose, and they are often fatal (12). In general, these mycoses require systemic therapy and prolonged stays in hospitals (5, 8, 11, 16).
The rapidly growing emergence of azole-resistant fungal pathogens poses difficulties for the treatment of fungal infections in critically ill patients (8). Failure rates of currently available antifungal drugs exceed 50% for acute invasive aspergillosis and 20% to 30% for candidemia (7, 9, 17). Thus, there is an urgent need for potent azole antifungal agents which can be administered both orally and intravenously.
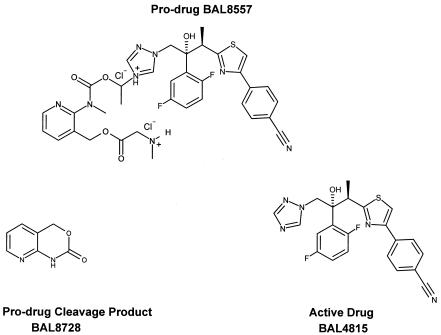
The prodrug BAL8557 (Fig. 1) is a water-soluble triazole precursor that is suitable for oral and intravenous administration. Its active moiety, BAL4815, is a potent inhibitor of ergosterol biosynthesis, resulting in the disruption of fungal membrane structure and function (18). In vitro, the active drug shows broad-spectrum activity against all major opportunistic fungi, e.g., Candida, Aspergillus, and Cryptococcus, and the true pathogenic fungi, including Histoplasma capsulatum and Blastomyces dermatitidis. The active drug also shows strong antifungal activity against fluconazole-resistant Candida albicans and is active against Zygomycetes and Absidia, Rhizopus, and Rhizomucor species. In rat models, the active drug is highly effective against systemic candidiasis and aspergillosis (2).
FIG. 1.
Structure of prodrug BAL8557, active drug BAL4815, and prodrug cleavage product BAL8728.
In vitro, BAL8557 is rapidly converted to BAL4815 (active drug) and to BAL8728 (prodrug cleavage product) in a reaction catalyzed by plasma esterases of rats, monkeys, and humans (2). In animals, the pharmacokinetics of BAL4815 are characterized by slow elimination, low plasma clearance (approximately 10% of liver blood flow), and extensive tissue distribution (volume of distribution at steady state [VSS], 3 liter/kg). In rats, excretion of 14C-labeled prodrug into feces/bile (81% of radioactivity) and urine (18% of radioactivity) is complete within 4 days after intravenous (i.v.) or oral (p.o.) administration (2). In vitro, plasma protein binding of BAL4815 was high and reached 98% in humans. Binding was linear and occurred almost exclusively to serum albumin (2).
The toxicity profile of BAL8557 in animals was consistent with that of other azoles; BAL8557 revealed no mutagenic, allergenic, phototoxic, or irritant potential.
In view of the promising broad-spectrum activity of BAL4815 in vitro and the efficacy of BAL8557 in animal models, we have investigated the safety and pharmacokinetic characteristics of BAL8557 in a single-ascending-dose clinical study.
MATERIALS AND METHODS
Study design.
This was a double-blind, placebo-controlled, randomized single-ascending-dose study, investigating three sequential dose levels for each route of administration. The study was conducted in full compliance with the principles of the Declaration of Helsinki as amended at Somerset West (1996) and according to the International Conference on Harmonization Guideline for Good Clinical Practice. Prior to study start, written informed consent was obtained from all participating volunteers. The oral part of this study was performed at the Clinical Research Center of the University Hospital in Basel, Switzerland. Prior to study start, the study protocol was approved by the Ethical Committee of Basel in Basel, Switzerland. The intravenous part of this study took place at Applied Analytical Industries GmbH & Co. in Neu-Ulm, Germany, and was approved by the Ethics Committee of the Bavarian Chamber of Physicians, Munich, Germany.
Rationale for dosage selection.
The initial doses were chosen according to conventional toxicological criteria. After repeated intravenous administrations of 10 mg/kg of body weight to rats and cynomolgus monkeys over two weeks and considering the predicted pharmacokinetics of BAL4815 in human for a 50-mg intravenous dose, Cmax (maximum drug concentration observed in plasma) values revealed a safety margin of up to 10 for systemic exposure to BAL4815 in human (based on unbound concentrations and taking into account the species differences in plasma protein binding). The corresponding safety margin based on the area under the concentration-time curve (AUC) values determined in rats and monkeys was up to 3.
After repeated oral administrations of 30 mg/kg to rats and 10 mg/kg to monkeys over 4 weeks and considering the predicted pharmacokinetic properties of BAL4815 in human for a 100-mg oral dose, Cmax values revealed a safety margin of 12 to 25 for systemic exposure to BAL4815 in human (based on unbound concentrations and taking into account the species differences in plasma protein binding). The corresponding safety margin based on the AUC values determined in rats and monkeys was 1.5 to 4.5.
Subjects and study procedures.
Healthy male subjects were sequentially enrolled and randomly assigned to receive BAL8557 prodrug (four subjects per cohort) or placebo (two subjects per cohort) p.o. or i.v. After p.o. administration, safety data were obtained from 23 subjects (20 to 45 years of age; mean age plus or minus standard deviation (SD), 26.2 ± 6.4 years; body weight, 57 to 88 kg; mean weight plus or minus SD, 73.9 ± 9.5 kg). After i.v. administration, safety data were obtained from 24 subjects (21 to 45 years of age; mean age plus or minus SD, 35.0 ± 7.4 years; body weight, 57 to 100 kg; mean weight plus or minus SD, 77.1 ± 10.4 kg).
BAL8557 doses given p.o. were 100-, 200-, or 400-mg equivalents of BAL4815 (corresponding to 180.5, 361, or 722 mg of BAL8557). Doses given i.v. were 50-, 100-, or 200-mg equivalents of BAL4815. Oral BAL8557 was given as hard gelatin capsules containing 100 mg of active drug, administered together with 200 ml tap water after an overnight fast. Intravenous BAL8557 in 200 ml saline was infused into a convenient forearm vein over a period of 60 min by using a constant-rate infusion pump. Before proceeding to the next higher dose, a full assessment was made of all relevant tolerability and safety data at the previous dose level.
Safety parameters assessed at screening, during the study, and at follow-up included gathering of medical history, physical examination, recording of vital signs, 12-lead electrocardiograms, monitoring of adverse events, performance of laboratory safety (hematology, blood chemistry, creatinine clearance, and urinalysis) and serology (hepatitis B, hepatitis C, and human immunodeficiency virus) tests, and drug abuse screening.
Pharmacokinetic sampling.
All subjects reported to the clinical unit on the evening before the day of administration and remained in the unit until their 48-h blood sample had been taken. Blood samples for pharmacokinetic assessment were collected from the cubital vein of the forearm (opposite to the arm where the catheter was placed) immediately before start of the infusion or oral drug intake and at 15, 30, 45, 60, 75, and 90 min and 2, 3, 4, 6, 8, 10, 12, 14, 16, 24, 36, 48, 60, 72, 96, 120, 144, 168, 192, 216, 240, 264, and 288 h after administration or until BAL4815 concentrations were below the limit of quantification on two subsequent days. In most cases, concentrations of active drug were quantifiable up to more than 400 h after drug administration.
At each time point, a 5-ml blood sample was collected into Monovetten tubes containing EDTA as anticoagulant. To avoid degradation of the analytes, 10 μl of 2 M citric acid and 10 μl of 0.1 M paraoxon (plasma esterase inhibitor) were added per ml blood immediately after blood was drawn. The plasma was separated from blood cells by centrifugation (15 min at 1,500 × g; temperature, 4°C), transferred into polypropylene tubes, and stored at −70°C.
Urine samples for assessment of urinary recovery of BAL4815 were collected predose and at intervals of 0 to 6, 6 to 12, 12 to 24, 24 to 36, and 36 to 48 h after drug intake or start of infusion. Thereafter, urine was collected in 24-h intervals up to 168 h after drug administration or until BAL4815 concentrations were below the limit of quantification on two successive days. Urine samples were collected into polypropylene containers that were pretreated with 2 M citric acid.
Analytical methods.
Quantification of BAL8557, BAL4815, and BAL8728 in plasma and urine was performed by liquid chromatography coupled online with tandem mass spectrometry operating in the positive electrospray ionization and selected reaction-monitoring modes. Calibration and quality control samples were prepared in pretested blank plasma and urine samples. Day-to-day performance was controlled by analysis of quality control samples.
Sample analysis was carried out with 50 μl of plasma or with 10 μl of urine diluted with 40 μl plasma. Plasma was stabilized with 10 mM citric acid at pH 5 and 1 mM paraoxon to prevent metabolic degradation. After precipitation of proteins with acetonitrile-water (8/2, vol/vol) and centrifugation, a 100-μl aliquot of supernatant was diluted with 350 μl of 1 mM ammonium acetate (pH 5). An aliquot of each solution (10 to 30 μl) was injected into system of liquid chromatography coupled online with tandem mass spectrometry. All unknown, calibration, or quality control samples were subjected to the same procedure.
Approximately 20% of the plasma and urine samples were randomly analyzed in duplicate, and the means of both measured values were reported. The interassay precision in human plasma was ≤9.0% for BAL8557, ≤7.6% for BAL4815, and ≤8.3% for BAL8728 after oral administration and ≤10.9% for BAL8557, ≤8.6% for BAL4815, and ≤15.3% for BAL8728 after i.v. infusion. The interassay precision in human urine was ≤9.5% for BAL8557, ≤8.6% for BAL4815, and ≤7.7% for BAL8728 after oral administration and ≤8.6% for BAL4815 following i.v. infusion.
The validated lower limit of quantification in plasma was 5 ng/ml for the prodrug BAL8557 and active drug BAL4815 and 10 ng/ml for the prodrug cleavage product BAL8728. The respective values for urine samples were 25 ng/ml for BAL8557 and BAL4815 and 50 ng/ml for BAL8728.
Analysis of pharmacokinetic data.
Pharmacokinetic parameters were derived by noncompartmental analysis (6) using the computer program WinNonlin version 4.0.1 (15). As plasma profiles revealed a biphasic decline, an additional two-compartmental analysis was performed. Uniform weighting was used for noncompartmental analysis and 1/[predicted y2] was used for the two-compartmental analysis.
After oral administration, the following pharmacokinetic parameters were estimated from the plasma concentration-versus-time curves for BAL4815 by noncompartmental analysis: Cmax, Tmax (time to reach maximum drug concentration in plasma), t1/2β (apparent terminal elimination half-life), AUC0-∞ (area under the plasma concentration time curve from time point zero extrapolated to infinity), and AUClast (area under the plasma concentration time curve from time point zero up to the time point of the last quantifiable concentration). AUC was calculated according the linear trapezoidal rule. In addition, because conversion of prodrug to BAL4815 was virtually complete, CLS/F (total systemic clearance, where F is the fraction of dose available to the systemic circulation) (bioavailability) and V/F (apparent volume of distribution in the postdistributive phase) were assessed. These pharmacokinetic parameters (excluding AUClast) were also assessed by two-compartmental analysis. t1/2α was the initial elimination half-life in the distributive phase. After oral administration, a pharmacokinetic assessment of prodrug BAL8557 and prodrug cleavage product BAL8728 was not possible because ≥99% of all plasma samples were below the limit of quantification.
After constant-rate intravenous infusion, the following pharmacokinetic parameters were estimated from the plasma concentration-versus-time curves for active drug BAL4815 and for prodrug cleavage product BAL8728 by noncompartmental analysis: Cmax, Tmax, AUC0-∞, AUClast, t1/2β, CLS (estimated by dose/AUC0-∞), and VSS (estimated by [dose × AUMC/AUC2] − [dose × T/2AUC], where AUMC is area under the first moment of the concentration-time curve). These pharmacokinetic parameters (including t1/2α, but excluding AUClast) were also assessed for BAL4815 by two-compartmental analysis. After intravenous infusion, noncompartmental pharmacokinetic assessment of prodrug BAL8557 included only AUClast, Cmax, and Tmax, as plasma concentrations of prodrug were below the limit of quantification in most samples.
Urinary excretion was calculated from urine concentrations of BAL4815 and urine volumes that were collected during the specified sampling intervals.
Statistical evaluation.
To test the dose proportionality of exposure, a one-way analysis of variance (ANOVA) was applied to the logarithmically transformed and dose-normalized values of AUC0-∞ and Cmax values of BAL4815. The linear dependency of the variables VSS, CLS, V/F, CLS/F, and t1/2 on dose was tested by comparing the slope of a fitted regression line to zero. The mean values of the dose groups were estimated together with 95% confidence intervals. All tests were performed at a level of α equals 0.05. The P values of the ANOVA were interpreted in only an exploratory sense.
RESULTS
Subjects.
Due to an error in drug packaging and labeling, 11 subjects (rather than 4) were dosed in the 100-mg p.o. cohort, with 6 subjects receiving BAL8557 and 5 subjects receiving placebo. In the 200-mg p.o. cohort, six subjects received BAL8557 and two subjects received the placebo. In the 400-mg p.o. cohort, three subjects received BAL8557 and one subject received the placebo. This deviation from the protocol in the p.o. cohorts may have led to greater variability in the 400-mg dosing group, due to the smaller numbers of subjects analyzed, but is not believed to compromise the overall objectives of the study. Subjects in the i.v. dosing cohorts received BAL8557 and placebo as planned.
After p.o. administration, safety data were obtained from all 23 participating subjects and all 15 subjects who received active drug were evaluable for pharmacokinetics. After i.v. administration, safety data were obtained from all 24 participating subjects and pharmacokinetics were assessable from all 18 subjects who had received active drug.
Pharmacokinetics of BAL4815 after oral administration.
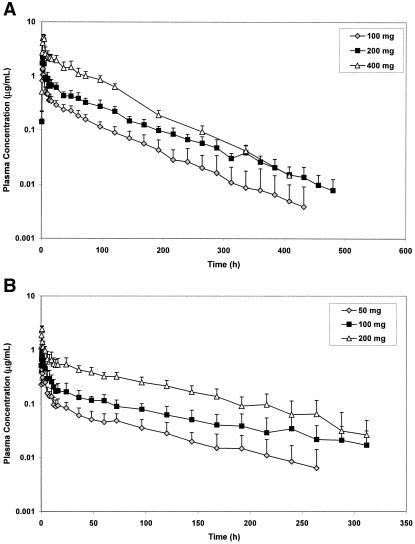
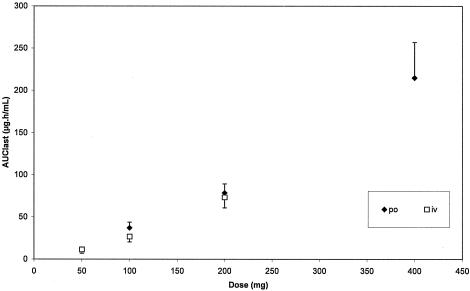
The mean (plus or minus SD) plasma concentration-time profiles of BAL4815 after p.o. administration of BAL8557 are presented in Fig. 2A. Plasma concentrations of BAL4815 increased rapidly after administration of BAL8557 and reached a maximum at 1.8 to 3 h after drug intake. Thereafter, plasma levels declined biphasically and remained detectable for more than 16 days postdose. Pharmacokinetic parameters of BAL4815 assessed after single oral administration of BAL8557 are presented in Table 1. After administrations of BAL8557, which were equivalent to 100 mg, 200 mg, and 400 mg of BAL4815, AUC values increased slightly more than proportionally to the dose (Fig. 3), indicating a moderate deviation from dose linearity. Mean elimination half-lives estimated in the terminal elimination phase (t1/2β) were particularly long (56 to 77 h), and half-lives in the distributive phase (t1/2α) reached 1.7 to 2.1 h. Total systemic clearance (CLS/F) was low (1.91 to 2.80 liter/h), and the volume of distribution in the postdistributive phase (VZ/F) was large (155 to 292 liters), with no marked differences between dose groups. The pharmacokinetic parameters of BAL4815 showed low intersubject variability. For example, the coefficient of variation (CV) ranged from 3.8% to 17.3% for Cmax and from 13.8% to 19.5% for AUC0-∞.
FIG. 2.
A, Mean (plus or minus SD) plasma profiles of BAL4815 after oral administration of BAL8557 (equivalent to 100 mg, 200 mg, and 400 mg BAL4815). B, Mean (plus or minus SD) plasma profiles of BAL4815 after intravenous infusion of BAL8557 (equivalent to 50 mg, 100 mg, and 200 mg BAL4815).
TABLE 1.
Pharmacokinetic parameters of BAL4815 estimated after single oral administration or intravenous infusion of prodrug BAL8557a
| Parameter | Doseb
|
|||||
|---|---|---|---|---|---|---|
| Oral administration
|
Intravenous infusion
|
|||||
| 100 mg (n = 6) | 200 mg (n = 6) | 400 mg (n = 3) | 50 mg (n = 6) | 100 mg (n = 6) | 200 mg (n = 6) | |
| AUC0-∞ (μg·h/ml) | 37.0 (±6.75) | 78.5 (±10.8) | 215 (±42.0) | 11.3 (±4.43) | 26.6 (±6.25) | 73.2 (±12.4) |
| AUClast (μg·h/ml) | 36.5 (±6.70) | 77.7 (±11.0) | 214 (±41.9) | 10.5 (±4.04) | 24.9 (±5.92) | 72.2 (±12.1) |
| Cmax (μg/ml) | 1.45 (±0.177) | 2.59 (±0.449) | 5.57 (±0.212) | 0.446 (±0.076) | 1.03 (±0.184) | 2.47 (±0.374) |
| Tmax (h) | 2.0 (1.6-2.0) | 1.8 (1.5-3.0) | 3.0 (2.1-3.0) | 0.75 (0.5-1.0) | 1.0 (0.75-1.0) | 1.0 (0.75-1.0) |
| t1/2αc (h) | 1.70 (±0.34) | 2.05 (±0.53) | 2.06 (±0.59) | 1.57 (±0.52) | 1.53 (±0.59) | 0.42 (±0.31) |
| t1/2β (h) | 63.1 (±21.7) | 77.1 (±12.8) | 56.0 (±2.49) | 76.2 (±32.0) | 104 (±56.7) | 80.4 (±33.0) |
| Vd (liters) | 248 (±80.4) | 292 (±80.8) | 155 (±39.2) | 444 (±175) | 494 (±280) | 304 (±86.6) |
| CLSd (liters/h) | 2.80 (±0.548) | 2.59 (±0.363) | 1.91 (±0.418) | 5.03 (±1.99) | 3.96 (±1.04) | 2.80 (±0.519) |
Results are from a noncompartmental analysis except where indicated. Values are presented as arithmetic means plus or minus SD; median and range are listed for Tmax.
Dose corresponds to mg equivalents of BAL4815.
Obtained by two-compartmental analysis.
V corresponds to VZ/F after oral administration and to VSS after intravenous infusion; CLS corresponds to CLS/F after oral administration.
FIG. 3.
Mean (plus or minus SD) AUClast values of BAL4815 versus dose after single oral or intravenous infusion of prodrug BAL8557.
Pharmacokinetics of BAL4815 after intravenous infusion.
Figure 2B shows the mean (plus or minus SD) plasma concentration-time profiles of BAL4815 that were obtained after the 1-h constant-rate intravenous infusion of BAL8557. Plasma concentrations of BAL4815 reached a maximum at 0.75 to 1 h after start of infusion and declined subsequently in a biphasic manner. Plasma concentrations were detectable for more than 10 days after infusion. After infusions of BAL8557, which were equivalent to 50 mg, 100 mg, and 200 mg of BAL4815, AUC values increased somewhat more than dose proportionally (Fig. 2B), indicating a moderate deviation from dose linearity; Cmax values also appeared to increase somewhat more than dose proportionally. Mean elimination half-lives estimated in the terminal elimination phase (t1/2β) were long (76 to 104 h), and the half-lives in the distributive phase (t1/2α) were 0.42 to 1.6 h. Total systemic clearance was low (2.80 to 5.03 liter/h) and the volume of distribution at steady state was large (304 and 494 liters). The pharmacokinetic parameters of BAL4815 showed a low to moderate intersubject variability (CV was 15.1% to 17.9% for Cmax and 16.9% to 39.0% for AUC0-∞).
After both routes of administration, there was a good agreement between data assessed by noncompartmental and two-compartmental analyses.
Dose proportionality and effect of dose on pharmacokinetic parameters of BAL4815.
The statistical assessment of dose proportionality of AUC0-∞ and Cmax was based on the comparison of the dose-normalized exposure values between dose groups using a one-way ANOVA. After oral administration, a significant dose effect was observed for AUC (P = 0.028), but for Cmax (P = 0.39) there was no significant dose effect and dose proportionality can be assumed for this parameter. After intravenous infusion, there was a significant dose effect for AUC (P = 0.021) and for Cmax (P = 0.016).
The dependency of variables VSS, VZ/F, CLS, CLS/F, and t1/2 on dose was tested by comparing the slope of a fitted regression line to zero. After intravenous infusion, there was a significant slope for CLS (Pr = 0.0098), indicating a linear dependency on dose. For VSS and t1/2, no significant slope was obtained for the independent variable dose (Pr = 0.1688 and Pr = 0.9533, respectively). Similar results were obtained after oral administration: a significant slope and hence linear dependency on dose was found for CLS/F (Pr = 0.0143), and no linear dependency on dose was seen for VZ/F (Pr = 0.1337) and t1/2 (Pr = 0.5859).
Urinary recovery of BAL4815.
After oral administration, urinary recovery of BAL4815 was negligible and amounted to only 0.02 to 0.04% of the administered dose in the various dose groups. Mean maximum urinary concentrations reached 0.020 μg/ml in the 100-mg dose group, 0.045 μg/ml in the 200-mg dose group, and 0.092 μg/ml in the 400-mg treatment group. After intravenous infusion, urinary excretion of BAL4815 reached 0.06 to 0.38% of the administered dose. Mean maximum urinary concentrations amounted to 0.132 μg/ml after infusion of 50 mg, 0.289 μg/ml after infusion of 100 mg, and 0.890 μg/ml after infusion of 200 mg.
Pharmacokinetics of prodrug BAL8557.
After oral administration, plasma concentrations of prodrug BAL8557 were quantifiable in only 1% of all samples and ranged from 0.004 μg/ml to 0.006 μg/ml. Because concentrations of BAL8557 were very low or not quantifiable, pharmacokinetic parameters could not be assessed. No prodrug was detected in urine samples.
After the 1-h constant-rate intravenous infusion, plasma concentrations of BAL8557 were quantifiable up to 1 h after start of infusion in the lowest dose group and up to 2 h in the highest dose group. Maximum concentrations were reached in all three dose groups before the end of infusion (Table 2), and Cmax and AUClast values increased more than proportionally to the dose. AUClast values of prodrug BAL8557 reached on average (plus or minus SD) 0.31% ± 0.22% of the corresponding AUClast values of active drug BAL4815, and Cmax values of BAL8557 amounted to 11.7% ± 9.9% of those determined for BAL4815. Intersubject variability of the pharmacokinetic parameters of BAL8557 was moderate to high (CV values were 22% to 88% for Cmax and 31% to 79% for AUClast). Due to insufficient data points, elimination half-lives could not be determined for the prodrug BAL8557.
TABLE 2.
Pharmacokinetic parameters of prodrug BAL8557 estimated after single intravenous infusions of BAL8557a
| Parameter | Dose
|
||
|---|---|---|---|
| 50 mg | 100 mg | 200 mg | |
| AUClast (μg·h/ml) | 0.017 (±0.005) | 0.092 (±0.073) | 0.279 (±0.145) |
| Cmax (μg/ml) | 0.031 (±0.007) | 0.121 (±0.107) | 0.351 (±0.179) |
| Tmax (h) | 0.50 (0.25-1.0) | 0.38 (0.25-0.75) | 0.75 (0.25-0.75) |
Infusion doses were equivalent to 50, 100, and 200 mg of BAL4815. Results shown are from six subjects. Values are presented as arithmetic means plus or minus SD; median and range are listed for Tmax.
Pharmacokinetics of prodrug cleavage product BAL8728.
After oral administration, plasma concentrations of BAL8728 were detectable in less than 1% of all samples and therefore pharmacokinetic parameters were not evaluable. BAL8728 concentrations in plasma ranged from 0.010 μg/ml to 0.024 μg/ml, and BAL8728 was not detectable in urine. After intravenous infusion, plasma concentrations of BAL8728 were quantifiable up to 3 to 4 h after start of infusion. Maximum concentrations of BAL8728 were reached shortly before or at the end of the 1-h infusion (Table 3). Cmax and AUClast values increased more than proportionally to the dose. AUClast values of prodrug cleavage product BAL8728 amounted to 0.98% ± 0.31% of the corresponding AUClast values of active drug, and Cmax values of BAL8728 amounted to 21.5% ± 6.4% of those determined for BAL4815. The elimination half-lives were short and ranged between 0.69 and 1.01 h. Intersubject variability of the pharmacokinetic parameters of BAL8728 was low to moderate (CV values were 9.9% to 33% for Cmax and 6.4% to 25% for AUC0-∞).
TABLE 3.
Pharmacokinetic parameters of prodrug cleavage product BAL8728 estimated after single intravenous infusions of BAL8557a
| Parameter | Dose
|
||
|---|---|---|---|
| 50 mg | 100 mg | 200 mg | |
| AUC0-∞ (μg·h/ml) | 0.118b (±0.008) | 0.250 (±0.063) | 0.681 (±0.113) |
| AUClast (μg·h/ml) | 0.094 (±0.010) | 0.234 (±0.065) | 0.658 (±0.114) |
| Cmax (μg/ml) | 0.081 (±0.008) | 0.209 (±0.068) | 0.615 (±0.140) |
| Tmax (h) | 1.0 (1.0-1.0) | 0.88 (0.75-1.0) | 1.0 (0.75-1.0) |
| t1/2 (h) | 1.01b (±0.43) | 0.69 (±0.14) | 0.83 (±0.15) |
Infusion doses were equivalent to 50, 100, and 200 mg of BAL4815. Results shown are from six subjects except where indicated. Values are presented as arithmetic means plus or minus SD; median and range are listed for Tmax.
n = 4 (t1/2 was not assessable for two subjects).
Safety.
BAL8557 was well tolerated and no severe or serious adverse events occurred. No electrocardiogram abnormalities and no trends or clinically significant changes in laboratory parameters or vital signs were reported. The frequency of adverse events did not appear to be related to the administered dose. In the placebo groups (containing a total of 14 subjects), one episode each of mild headache, mild herpes, mild loose stools, and moderate costal contusion was reported. After oral administration of BAL8557 (n = 15 subjects), mild upper abdominal pain, moderate conjunctivitis, moderate diarrhea, mild pharyngitis, moderate influenza-like illness, moderate nausea, and moderate dizziness (one event each) were experienced. After i.v. administration of BAL8557 (n = 18 subjects), three mild headaches and one event each of mild bowel movement and upper abdominal pain occurred.
DISCUSSION
This study was designed to evaluate the pharmacokinetics of active drug BAL4815 after oral and intravenous administration of its prodrug BAL8557 and to investigate the safety and tolerability of dosing. The assessment of the pharmacokinetics of prodrug BAL8557 and prodrug cleavage product BAL8728 was a secondary objective, but the rapid rates of prodrug conversion and clearance of cleavage product did not permit a kinetic evaluation of these compounds after oral administration. As conversion of prodrug to active drug was virtually complete (>98%, based on AUC), estimation of clearance and volume of distribution of BAL4815 seemed to be justified.
In vitro, prodrug was rapidly converted in human plasma to an intermediate. This reaction proceeded with a half-life of less than 1 min (14). Therefore, the rapid cleavage of prodrug in this study and hence low concentrations of BAL8557 were expected.
The pharmacokinetics of BAL4815 assessed by noncompartmental and two-compartmental analyses were in good agreement and consistent with the predicted data from in vitro and in vivo preclinical pharmacokinetic studies of various animal species, which characterized BAL4815 as a compound with low clearance, high volume of distribution, long elimination half-life, and good oral bioavailability (63% in rats and 87% in monkeys) (2).
The low clearance of BAL4815 and its good tissue penetration permit the maintenance of therapeutically effective concentrations in the body for a long period of time.
As confirmed by a one-way ANOVA, strict dose-proportionality of BAL4815 could be assumed for Cmax after oral administration, but exposure was more than dose proportional according to Cmax after infusion and AUC after oral and i.v. administration. Deviation from dose proportionality was moderate and occurred predominantly at the highest doses (Table 1,Fig. 3). The more-than-dose-proportional increase in AUC and Cmax might be explained by partial saturation of metabolic clearance of this compound, since moderate linear dependency on dose was found for systemic clearance. As expected, no dose dependency was found for volume of distribution or elimination half-life.
A comparison of the AUC and Cmax values of BAL4815 that were obtained after intravenous infusion of BAL8557 with those that were obtained after oral administration points to an excellent oral bioavailability of BAL4815, a feature that allows oral and i.v. routes to be used interchangeably. The relatively high plasma levels of BAL4815 observed after oral administration might be explained by presystemic metabolism of prodrug to active drug. This hypothesis is supported by the finding that plasma concentrations of prodrug BAL8557 and of prodrug cleavage product BAL8728 were low and detectable only in very few samples after oral administration, indicating that conversion of prodrug to active drug is virtually complete.
Voriconazole, another broad-spectrum antifungal azole, is pharmacokinetically characterized by a high oral bioavailability (96%), a large volume of distribution (4.6 liter/kg), and elimination through hepatic metabolism. Due to saturation of its metabolism, voriconazole exhibits nonlinear pharmacokinetics. A more-than-proportional increase in exposure is observed after intravenous or oral doses. Because of the nonlinear pharmacokinetics, the apparent terminal half-life of voriconazole is dose dependent (4). Like BAL4815, the antifungal triazole ravuconazole is characterized by a long elimination half-life (76 to 202 h) and high protein binding (98%) (1, 10). Shorter elimination half-lives than those found for BAL4815 were determined for itraconazole (21 h) (3) and fluconazole (30 h) (13). Itraconazole revealed saturable elimination characteristics and resembles voriconazole in this respect (3).
Conclusion.
BAL4815 is characterized by a low clearance, a large volume of distribution, and a particularly long elimination half-life. Concentrations of active drug will therefore be maintained for an extended time in the body and should enable an effective treatment of mycoses, including systemic candidiasis and aspergillosis. Based on the available systemic exposure data, oral bioavailability of BAL4815 is assumed to be very high.
REFERENCES
- 1.Barone, J. A., J. G. Koh, R. H. Bierman, J. L. Colaizzi, K. A. Swanson, M. C. Gaffar, B. L. Moskovitz, W. Mechlinski, and V. Van de Velde. 1993. Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers. Antimicrob. Agents Chemother. 37:778-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basilea Pharmaceutica. Data on file.
- 3.Bello, A., R. Russo, D. Grasela, and I. Salahudeen. 2003. Pharmacokinetics of intravenous BMS-379224 (pro-drug of ravuconazole) in healthy male subjects. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1567.
- 4.FDA Antiviral Drugs Advisory Committee. 2002. Voriconazole. Briefing document. [Online.] http://www.fda.gov/cder/foi/nda/2002/21-266_VFEND _biopharmr.pdf.
- 5.Garber, G. 2001. An overview of fungal infections. Drugs 61(Suppl. 1):1-12. [DOI] [PubMed] [Google Scholar]
- 6.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, p. 445-449, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 7.Granier, F. 2000. Invasive fungal infections. Epidemiology and therapies. Presse Med. 29:2051-2056. [PubMed] [Google Scholar]
- 8.Hossain, M. A., and M. A. Ghannoum. 2000. New investigational antifungal agents for treating fungal infections. Expert Opin. Investig. Drugs 9:1797-1813. [DOI] [PubMed] [Google Scholar]
- 9.Karthaus, M., and O. A. Cornely. 2005. Recent developments in the management of invasive fungal infections in patients with hematological malignancies. Ann. Hematol. 84:207-216. [DOI] [PubMed] [Google Scholar]
- 10.Marino, M. R., V. Mummanei, J. Norton, O. W. Hadjilambris, and P. Pierce. 2001. Ravuconazole exposure-response relationship in HIV+ patients with oropharyngeal candidiasis. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1622.
- 11.Meis, J. F., and P. E. Verweij. 2001. Current management of fungal infections. Drugs 61(Suppl. 1):13-25. [DOI] [PubMed] [Google Scholar]
- 12.Richardson, M., and D. Warnock. 1997. Fungal infection: diagnosis and management, 2nd ed. Blackwell Science, Ltd., Oxford, England.
- 13.Ripa, S., L. Ferrante, and M. Prenna. 1993. Pharmacokinetics of fluconazole in normal volunteers. Chemotherapy 39:6-12. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt-Hoffmann, A. H., B. Roos, M. Heep, C. Beglinger, and E. Weidekamm. 2003. Pharmacokinetics of BAL4815, a new azole antifungal,after administration of single ascending oral doses of its pro-drug BAL8557. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A1-1568.
- 15.Scientific Consulting, Inc. 2002. Professional WinNonlin, version 4.0.1. Scientific Consulting, Inc., Mountain View, Calif.
- 16.USPHS/IDSA Prevention of Opportunistic Infections Working Group. 1999. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 30:29-65. [PubMed] [Google Scholar]
- 17.Wong-Beringer, A., R. A. Jacobs, and B. J. Guglielmo. 1998. Lipids formulations of amphotericin B: clinical efficacy and toxicities. Clin. Infect. Dis. 27:603-618. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki, T., T. T. Sukaguchi, Y. Ono, Y. Satoh, T. Fujii, Y. Inagaki, S. Ichihara, J. Ohwada, I. Umeda, K. Kobayashi, N. Shimma, and M. Arisawa. 2002. RO0098557, a novel water soluble azole prodrug for parenteral and oral administration (II) prodrug principle and broad spectrum antifungal. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-821.