Abstract
Zygomycetes are emerging opportunistic molds resistant to most conventional antifungals. We evaluated the in vitro activity of lovastatin (LOV), a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, against seven clinical isolates of Zygomycetes by using standard microdilution methods in three different media, disk diffusion testing, and viability dye staining. To further study the in vivo efficacy of LOV against zygomycetes, we developed a Drosophila melanogaster model of zygomycosis. In different experiments, groups of Toll-deficient (Tl) flies fed LOV-containing food were subsequently injected with two representative Zygomycetes isolates (Mucor and Rhizopus spp.). Finally, we examined the effects of LOV on voriconazole (VRC) activity against zygomycetes in vitro by checkerboard dilution, Epsilometer test-based methods, and bis-(1,3-dibutylbarbituric acid) trimethine oxonol staining and in vivo in Tl flies fed food containing LOV plus VRC and infected with zygomycetes. LOV exhibited significant, medium, and strain-independent fungicidal activity against all Zygomycetes isolates in vitro by all testing methods (MIC50, 48.0 μg/ml; 50% minimal fungicidal concentration, 56.0 μg/ml; 50% effective concentration, 29.4 μg/ml [6.6 to 38.9 μg/ml]). Tl flies fed LOV-containing food and infected with Mucor had a significantly better 6-day survival rate than did infected Tl flies fed regular food (P = 0.0005). LOV displayed in vitro synergy with VRC against all Zygomycetes isolates (fractional inhibitory concentration index, 0.104 to 0.290) by all methods used. LOV also displayed synergy with VRC in the Drosophila model of zygomycosis (P < 0.01). LOV is significantly active against zygomycetes and synergizes with triazoles inherently resistant to them, such as VRC. The clinical significance of these findings needs to be further explored.
Fungi of the class Zygomycetes, order Mucorales, have been increasingly reported to cause opportunistic infections in a variety of immunocompromised hosts (11, 28). Rhizopus species cause more than 70% of Zygomycetes infections, whereas Mucor, Absidia, Rhizomucor, and Cunninghamella species are less frequently encountered pathogens (11, 16, 28). Importantly, zygomycosis has a particularly unfavorable outcome (11, 16, 28) because of delayed diagnosis as well as the inherent resistance of zygomycetes against most conventional antifungal agents (6, 11, 28, 30). Besides amphotericin B (AMB), only the investigational triazole posaconazole has shown promising activity against zygomycetes (6, 11, 28, 30, 32). Therefore, there is a dire need for development of novel treatment strategies against zygomycosis.
Lovastatin (LOV) is a statin that acts by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase, which is the rate-limiting enzyme of the mevalonate pathway (26). LOV as well as other statins has activity against a variety of pathogens both in vitro and in vivo (12, 13, 31). Importantly, statins have been shown to have antifungal activity in vitro against both the nonpathogenic yeast Saccharomyces cerevisiae and the pathogenic yeasts Candida spp. and Cryptococcus neoformans (5, 22). In a recent study, LOV induced apoptosis-like cell death in a Mucor racemosus isolate at relatively high concentrations (29). Moreover, statins have been reported to exhibit synergistic interactions with azoles against Candida, even against azole-resistant Candida isolates (5).
In the present study, we evaluated the activity of LOV against a range of clinically important Zygomycetes spp. in vitro by using independent susceptibility methods. We also tested in a Drosophila melanogaster model of zygomycosis the efficacy of LOV in vivo against two representative Zygomycetes isolates (21). We further evaluated whether LOV and voriconazole (VRC), a triazole with an inherent lack of activity against Zygomycetes spp. (6, 11, 30), have synergistic effects in vitro and in vivo (Drosophila model) against zygomycetes. Both these pilot in vitro and in vivo studies against zygomycetes demonstrated that LOV has significant, broad-spectrum fungicidal activity and synergy with VRC.
MATERIALS AND METHODS
Zygomycetes isolates.
We used four Rhizopus isolates (two Rhizopus homothallicus isolates and two Rhizopus oryzae isolates), two Mucor circinelloides isolates, and one Cunninghamella bertholletiae isolate collected from patients with cancer who had zygomycosis. We confirmed genus identification of the Zygomycetes isolates as described previously (33). Candida parapsilosis strain ATCC 22019 was used for quality control purposes in all in vitro experiments.
Drug stock solutions.
LOV (mevilonin) was kindly provided by Merck (Merck, Sharp, and Dohme Research Laboratories, Rahway, N.J.). LOV was hydrolyzed in active open acid form as described previously (9), and a stock solution (4 mg/ml in a 10% alcohol sterile solution) was stored at −20°C until use. Additionally, AMB deoxycholate (Sigma Chemical Co., St. Louis, Mo.), itraconazole (ITC) (Janssen Pharmaceutica, Titusville, N.J.), VRC (Pfizer, Inc., New York, N.Y.), and caspofungin (CAS) (Merck, Rahway, N.J.) were obtained in assay powder form. Drug stock solutions for each antifungal agent (1,280 μg/ml) were prepared in 100% dimethyl sulfoxide (for AMB and ITC) or double-distilled water (for all other agents).
Culture medium.
The Clinical and Laboratory Standards Institute (CLSI) M38-A standard medium RPMI 1640 (Sigma Chemical Co.) was buffered with 0.165 M 3-(N-morpholino) propanesulfonic acid (MOPS) to pH 7 according to the manufacturer's instructions. Two other media were used to test the in vitro activity of LOV against the Zygomycetes isolates: RPMI medium plus 2% glucose (RPMI-2) and a yeast nitrogen base (YNB) medium (Difco, Detroit, Mich.).
CLSI susceptibility testing.
The MICs of each antifungal agent and LOV against all of the Zygomycetes isolates were determined according to CLSI guidelines (M38-A document [27]). Standardized inoculum suspensions were prepared from 5- to 7-day-old cultures grown on potato dextrose agar slants, filtered twice through sterile syringes filled with glass wool, and adjusted with a hemacytometer to a concentration of 1 × 106 to 5 × 106 conidia/ml in sterile water. Conidial suspensions were further diluted 1:50 in RPMI medium, and 100 μl was dissolved in each well of a 96-well flat-bottomed microtitration plate (Corning, Inc., Corning, N.Y.) containing 100 μl of a serial twofold dilution of each antifungal agent. The final concentrations of the tested drugs ranged from 0.03 to 16.00 μg/ml for AMB, ITC, and VRC; 0.06 to 32.00 μg/ml for CAS; and 2.00 to 64.00 μg/ml for LOV. The MICs of LOV and each antifungal agent were determined at 24 h as the lowest drug concentration at which there was complete inhibition of growth. The minimal effective concentration of CAS was defined as the lowest drug concentration that resulted in the formation of aberrantly growing hyphal tips (3). All of the isolates were tested in triplicate on different days.
The minimum fungicidal concentration (MFC) of each antifungal agent was determined as described previously (8). Briefly, 20-μl suspensions from each well that showed complete inhibition of growth (100%) and from the last positive well (showing growth similar to that in the control well) were subcultured onto YNB plates prepared according to the manufacturer's instructions. The MFC was defined at 24 h as the lowest drug concentration at which fewer than three colonies were observed, which corresponded with a killing activity of approximately ≥99.9%. MFC determinations were done in triplicate on different days.
Disk diffusion susceptibility testing.
We performed disk diffusion susceptibility testing on RPMI agar plates previously prepared by using standardized methods (20). Two hundred microliters of a standardized suspension of conidia (106 conidia/ml) of each Zygomycetes isolate was plated. After the plates were allowed to dry, a sterile one-quarter-inch paper disk (Schleicher and Schuell, Keene, N.H.) was placed on the agar surface and inoculated with 125 μl of LOV (4 mg/ml), producing a final LOV concentration in each plate of 20 μg/ml. Plates were incubated at 35°C, and the radius of the zone of inhibition was measured at 24 h by using a micrometer. AMB (final concentration, 10 μg/ml) was used as a control. Three independent experiments were performed at different time points.
XTT colorimetric assay.
We performed the 2,3-bis[2-methyloxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-5-carboxanilide] (XTT) colorimetric formazan reduction assay by using the method developed by Meletiadis et al. (25). We initially determined the relationship of formazan production to fungal inoculum by incubating standardized conidial suspensions of each isolate (102 to 105 conidia/ml) in RPMI medium for 12 h at 35°C. After 10 h of incubation, 50 μl of an XTT solution (1 mg/ml) containing 125 μg of menadione (Sigma Chemical Co.) was added to each well, and the tray was incubated for an additional 2 h. Formazan absorbance in each well was read at 492 nm and 690 nm (plate absorbance) with the use of a microplate spectrophotometer (Powerwave X; Biotech Instruments, Winooski, Vt.).
We performed XTT-based microdilution studies with CLSI microtiter plates prepared as described above. To evaluate the interaction of LOV with VRC against Zygomycetes, we performed two-dimensional (eight-by-eight) checkerboard studies into microtiter plates as previously described (19). The final concentrations of the drugs ranged from 2.00 to 64.00 μg/ml for LOV and 0.5 to 64.00 μg/ml for VRC. Wells were subsequently inoculated with 100 μl of a standardized conidial suspension of each Zygomycetes isolate (final concentration, 0.4 × 104 to 5.0 × 104 conidia/ml), and trays were incubated for 22 h at 35°C. Next, 50 μl of the XTT solution was added to each well, the tray was incubated for an additional 2 h, and formazan absorbance was determined as described above. The fractional inhibitory concentration (FIC) of each drug was calculated by dividing the MIC of the drug when used in combination by its MIC when used alone. FIC values then were added together to define the interaction of the combination (19). Synergy was defined as an FIC of ≤0.5, whereas antagonism was defined as an FIC of >4; off-scale MICs were raised to the next highest MIC. Control wells containing medium alone were included in each experiment. All experiments were performed in triplicate.
DiBAC morbidity staining of Zygomycetes isolates.
Staining with the fluorescent dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC) was performed as described previously by Bowman et al. (3). Briefly, conidia from representative Zygomycetes isolates (one each of R. oryzae, M. circinelloides, and C. bertholletiae) were suspended in RPMI medium at a final concentration of 0.4 × 104 to 5.0 × 104 conidia/ml and incubated for 12 h at 35°C to generate hyphae. Aliquots of the hyphae were then mixed with each drug to produce the desired final concentration of LOV alone (4× 50% effective concentration [EC50]), VRC alone (2 μg/ml), VRC in combination with LOV (2 and 4 μg/ml, respectively), and AMB (2 μg/ml). After incubation at 35°C for an additional 6 h, hyphae were washed twice in MOPS (pH 7) buffer solution. DiBAC stain (final concentration, 2 μg/ml) was added accordingly, and samples were incubated for 1 h at room temperature in the dark with shaking (3). Samples were then washed twice again with MOPS, pH 7, and resuspended for photomicrography. Photomicrographs of the hyphae were taken under a triple-band fluorescent microscope (Olympus BX-51; Olympus, Melville, N.Y.) as described previously (3).
Study of in vitro synergy of LOV and VRC by agar dilution and Epsilometer testing methods.
To evaluate the effects of LOV on VRC activity against zygomycetes, we used a strategy combining the Epsilometer test (Etest; AB Biodisk, Solna, Sweden) and agar dilution susceptibility methods as described previously (15). We performed Etest susceptibility testing of VRC against each Zygomycetes isolate in RPMI plates containing a standard noninhibitory concentration of LOV (4 μg/ml) (7). As controls, we determined VRC MICs against Zygomycetes by using the Etest method on regular RPMI plates (without LOV). We read the VRC MIC at 24 h as the drug concentration at the point where the growth ellipse intersected the strip (7) and, accordingly, statistically compared the changes in VRC MICs (15). All MIC determinations were carried out in triplicate on different days; the median 24-h MICs were reported.
Drosophila infection model.
In different experiments, we infected Toll-deficient flies (Tl flies) (2- to 4-day-old female flies, 30 per experimental group) with two representative Zygomycetes isolates (M. circinelloides 424760 and R. oryzae 557969). We injected the thoraxes of Tl flies with a thin sterile needle that had been dipped into a concentrated solution (108 conidia/ml) of each Zygomycetes isolate, as described previously (17, 20). After injection, we housed the flies at 29°C and transferred them to fresh vials every 2 days. We assessed survival daily until day 6 after injection. We performed each experiment at least in triplicate on different days.
Drug protection experiments.
For assessment of LOV protection against lethal infection by each Zygomycetes isolate, different groups of Tl flies (30 per experimental group) were housed in empty vials for 6 to 8 h to starve and then transferred into vials containing LOV-mixed fly food (10 mg/ml) as described previously (2, 20). After 48 h, each group of Tl flies was infected with the corresponding Zygomycetes isolate by injection and transferred daily into fresh LOV-containing vials at 29°C for 6 days. Flies that were starved for 6 to 8 h, transferred to vials containing regular fly food (without LOV), infected, and maintained in regular vials were used as controls. LOV protection was assessed daily until day 6 after infection. For the combination drug experiments, vials containing VRC alone (1 mg/ml), LOV alone (10 mg/ml), or VRC plus LOV (1 and 10 mg/ml, respectively) were prepared as described previously (20). Each experiment was performed at least in triplicate on different days.
Statistical analysis.
Median MIC and MFC values were calculated based on experiments performed in triplicate. The Mann-Whitney U test or Kruskal-Wallis one-way analysis of variance with Dunn's test was used when appropriate to assess significant differences in the corresponding MICs and MFCs. Survival curves were plotted by using Kaplan-Meier analysis, and differences in survival rates between the groups were analyzed by using the log rank test. A four-parameter logistic regression model (Hill equation) was fitted to XTT reduction assay data to determine EC50 values and the steepness of inhibitory dose-response curves (Hill slope) with the use of a curve-fitting software program (Prism 4; GraphPad Software, Inc., San Diego, Calif.). P values of less than 0.05 were considered statistically significant.
RESULTS
LOV has significant in vitro activity against Zygomycetes isolates.
The MICs and MFCs for AMB, ITC, VRC, and CAS are listed in Table 1. AMB exhibited fungicidal activity against all of the Zygomycetes isolates tested, as the AMB MICs and MFCs (MIC50, 0.5 μg/ml; MFC50, 1 μg/ml) were equal or differed by less than 1 serial dilution in every case. By comparison, only ITC (MIC50, 4 μg/ml; MFC50, 8 μg/ml) demonstrated limited fungistatic efficacy against some Zygomycetes isolates, whereas neither VRC (MIC50, 8 μg/ml; MFC50, 16 μg/ml) nor CAS (MIC50, 32 μg/ml; MFC50, 32 μg/ml) had any activity at the concentrations tested.
TABLE 1.
Susceptibilities of the seven clinical isolates of Zygomycetes spp. to the antifungal agents tested in RPMI mediuma
| Isolate | MIC/MFC (μg/ml)
|
|||
|---|---|---|---|---|
| AMB | ITC | VRC | CAS | |
| C. bertholletiae 506313 | 1.0/2.0 | 2/4 | 8/>16 | >32/32 |
| R. homothallicus 541783 | 0.5/1.0 | 4/4 | 8/>16 | >32/32 |
| R. oryzae 518749 | 0.5/1.0 | 4/8 | 16/>16 | >32/32 |
| R. homothallicus 529120 | 0.5/1.0 | 2/16 | 8/>16 | >32/32 |
| R. oryzae 557969 | 0.5/0.5 | 2/8 | 8/>16 | >32/32 |
| M. circinelloides 424760 | 0.5/1.0 | 4/16 | 8/>16 | >32/32 |
| M. circinelloides 488128 | 0.5/0.5 | 4/16 | 8/>16 | >32/32 |
CLSI broth microdilution method M-38A.
The MICs and MFCs of LOV against Zygomycetes isolates in RPMI, RPMI-2, and YNB media are shown in Table 2. LOV exhibited fungicidal activity against all of the isolates (MIC50, 48 μg/ml [range, 32 to 56 μg/ml]; MFC50, 56 μg/ml [range, 48 to 64 μg/ml]) in all media tested. Although there were no significant interspecies differences, there was a trend towards improved activity of LOV against Mucor species.
TABLE 2.
MICs and MFCs of LOV against the seven clinical isolates of Zygomycetes spp. in RPMI, RPMI-2, and YNB mediaa
| Isolate | Result for LOV (μg/ml [±95% CIb])
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| RPMI
|
RPMI-2
|
YNB
|
|||||||
| MIC/MFC | EC50 | Hill slope | MIC/MFC | EC50 | Hill slope | MIC/MFC | EC50 | Hill slope | |
| C. bertholletiae 506313 | 40/48 | 33.9 ± 6.6 | −2.3 ± 1.1 | 32/32 | 7.3 ± 0.9 | −4.9 ± 7.2 | 32/40 | 28.6 ± 5.4 | −6.4 ± 7.0 |
| R. homothallicus 541783 | 56/56 | 38.9 ± 7.2 | −4.7 ± 3.7 | 56/56 | 55.8 ± 52.0 | −1.5 ± 2.2 | 48/64 | 30.6 ± 27.2 | −1.2 ± 2.3 |
| R. oryzae 518749 | 48/48 | 27.1 ± 13 | −3.3 ± 5.9 | 40/48 | 20.4 ± 12.5 | −1.3 ± 1.6 | 32/40 | 19.2 ± 10.3 | −1.5 ± 1.0 |
| R. homothallicus 529120 | 48/56 | 20.2 ± 3.1 | −4.0 ± 2.4 | 56/56 | 17.7 ± 2.6 | −4.3 ± 3.0 | 40/64 | 19.4 ± 4.0 | −1.9 ± 0.9 |
| R. oryzae 557969 | 56/64 | 32.9 ± 2.4 | −5.5 ± 1.9 | 48/64 | 35.9 ± 11.6 | −1.2 ± 0.7 | 56/64 | 11.3 ± 9.7 | −0.8 ± 0.7 |
| M. circinelloides 424760 | 32/32 | 6.6 ± 2.5 | −1.2 ± 0.6 | 16/24 | 6.2 ± 2.5 | −1.8 ± 1.5 | 24/24 | 9.8 ± 3.5 | −2.4 ± 2.3 |
| M. circinelloides 488128 | 56/56 | 29.4 ± 4.4 | −3.8 ± 2.3 | 48/48 | 27.0 ± 3.0 | −4.4 ± 1.8 | 40/40 | 24.7 ± 6 | −2.4 ± 1.3 |
CLSI broth microdilution method M-38A.
CI, confidence interval.
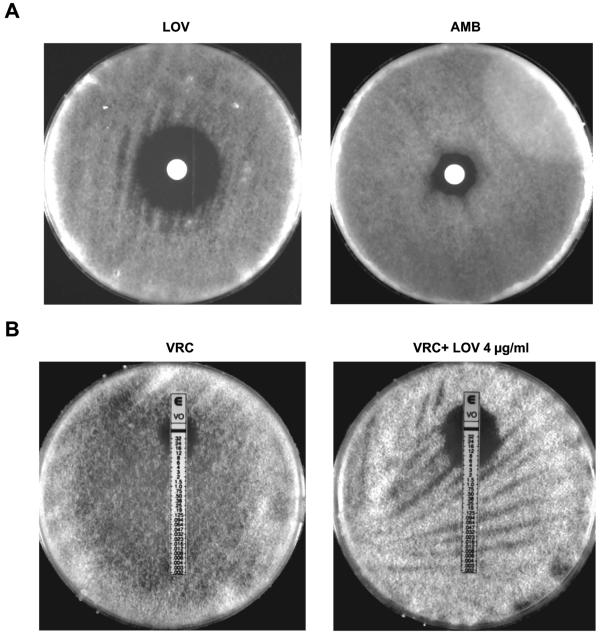
The activity of LOV against zygomycetes was also clearly observable with disk diffusion susceptibility testing (Fig. 1A). Notably, LOV produced a clear zone of inhibition of growth against all Zygomycetes isolates (mean radius ± standard deviation for LOV, 12.570 ± 3.861 mm).
FIG. 1.
(A) Effects of LOV compared with effects of AMB against a representative Zygomycetes isolate (M. circinelloides 424760) as seen with disk diffusion susceptibility testing. Each disk contained 125 μl of LOV, resulting in a final LOV concentration in each plate of 20 μg/ml from a stock solution of 4 mg/ml, and 50 μl of AMB, resulting in a final AMB concentration in each plate of 10 μg/ml from a stock solution of 5 mg/ml. (B) Change in the VRC MIC against a representative Zygomycetes isolate (M. circinelloides 424760) with simultaneous exposure to LOV as assessed by Etest susceptibility testing. Each Zygomycetes isolate was tested on regular RPMI agar plates and on RPMI agar plates containing a noninhibitory concentration of LOV (4 μg/ml). All of the experiments were performed in triplicate on different days.
LOV results in significant, concentration-dependent reduction of Zygomycetes biomass by the XTT assay.
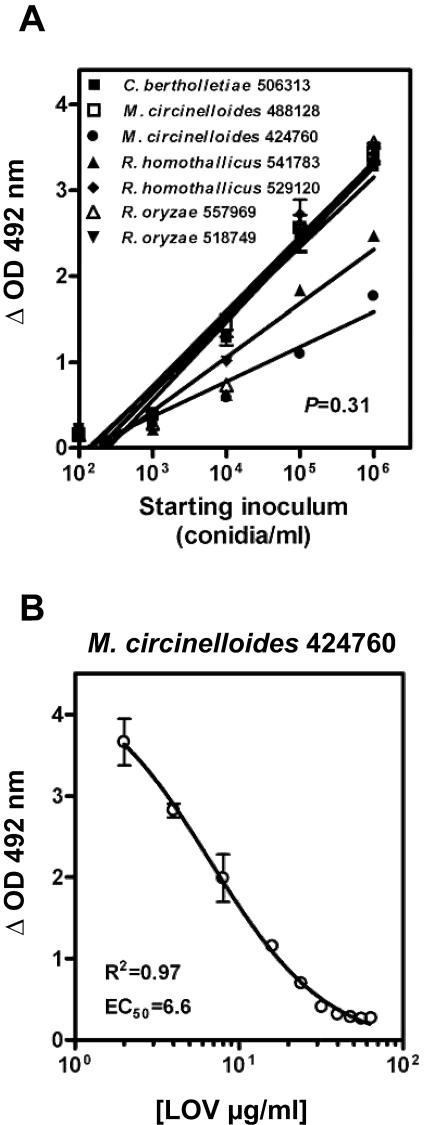
We found a linear relationship between XTT reduction to formazan (the colorimetrically assayed product) and starting inoculum of zygomycetes for all of the isolates tested over a range of inocula (Fig. 2A). Therefore, a reduction of the formazan absorbance from 3.0 to ≤0.5 optical density as a result of LOV activity against zygomycetes correlated with an approximately-2.5-log reduction in hyphal biomass.
FIG. 2.
(A) Regression plots of the relationship between fungal inoculum and formazan production for all of the Zygomycetes isolates at 12 h. The slopes of the regression plots ranged from 0.62 to 0.95 and were not significantly different by analysis of variance (P = 0.31). The coefficients of determination (R2) were high for all of the tested isolates (range, 0.89 to 0.96). (B) XTT-based analysis of the in vitro activity of LOV in RPMI media against a representative Zygomycetes isolate (M. circinelloides 424760). Sigmoid concentration-inhibitory-effect curves were generated by fitting data to a four-parameter logistic regression model (Hill equation). The symbols represent the means ± standard deviations for experiments performed in triplicate in each case. OD, optical density.
LOV exhibited potent antifungal activity against all of the Zygomycetes isolates (median EC50, 29.4 μg/ml [range, 6.6 to 38.9 μg/ml]) in a steep concentration-inhibitory-effect curve (Hill slope range, −1.2 to −4.7). A substantial reduction (≥2.5 log) of the hyphal biomass was evident for all of the isolates as the LOV concentration approached the MFC (Fig. 2B). Again, LOV appeared to be more effective against Mucor spp. than against Rhizopus and Cunninghamella spp. The in vitro activity of LOV was consistent among all three media tested (Table 2).
LOV has fungicidal effects against Zygomycetes hyphae by DiBAC staining.
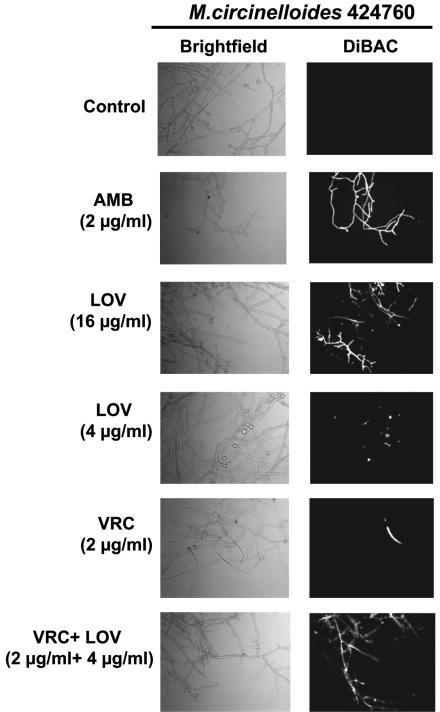
While control hyphae cells (without drug preexposure) demonstrated absence of fluorescence after DiBAC staining, there was clearly evident Zygomycetes hyphal damage in high concentrations of LOV (4× EC50; 16 μg/ml) of each isolate tested; at a subinhibitory LOV concentration (4 μg/ml), the fluorescence indicative of hyphal damage caused by LOV was minimal to absent (Fig. 3). AMB exhibited a pronounced hyphal damage at a concentration equal to the MFC (2 μg/ml), whereas VRC had no fungicidal activity at a clinically achievable concentration (2 μg/ml).
FIG. 3.
Detection of hyphal damage to the representative isolate M. circinelloides 424760 by fluorescent microscopy with the cellular morbidity dye DiBAC following exposure to antifungals. Zygomycetes hyphae were prepared after 18 h of incubation in RPMI media, washed, and resuspended in RPMI media containing AMB (2 μg/ml), LOV (16 and 4 μg/ml), VRC (2 μg/ml), or VRC in combination with LOV (2 and 4 μg/ml, respectively). Untreated hyphae were used as controls. After 6 h of incubation, cells were washed and stained with DiBAC. Hyphae were then examined with the use of bright-field (light boxes) and epifluorescence (black boxes) microscopy at ×400 with Normanski optics and a fluorescein isothiocyanate filter. The fluorescence in the dark boxes is indicative of early hyphal damage by the corresponding antifungal agent.
Protection of Tl flies infected with Zygomycetes by LOV.
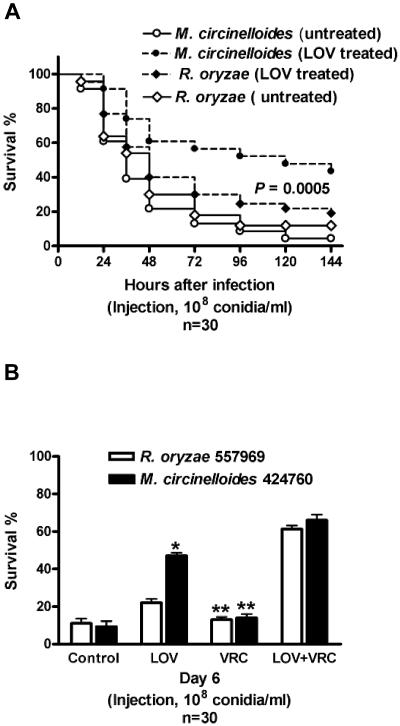
Injection of a concentrated solution of conidia (108 conidia/ml) of either R. oryzae or M. circinelloides resulted in a hyperacute infection with equally high mortality rates in Tl flies (>80%) within 3 days of infection (Fig. 4A). LOV-fed flies had a significantly better survival rate 6 days after infection with M. circinelloides (50%) than did control flies (<15%; P = 0.0005) (Fig. 4A). However, LOV had no activity in Tl flies infected with R. oryzae (Fig. 4A).
FIG. 4.
(A) LOV protection of Tl flies infected by injection of each representative Zygomycetes isolate (M. circinelloides 424760 or R. oryzae 557969; 108 conidia/ml). Survival curves of LOV-treated and untreated (control) flies are shown. The data represent the means of four independent experiments (30 flies per group). The P value was <0.0005 for LOV-treated flies infected with M. circinelloides versus control (untreated flies), and the P value was not significant for LOV-treated flies infected with R. oryzae versus control (untreated flies). (B) Survival of Tl flies treated with VRC alone, LOV alone, or VRC plus LOV 6 days after infection by injection of each representative Zygomycetes isolate (M. circinelloides 424760 or R. oryzae 557969; 108 conidia/ml). The data represent the means of four independent experiments (30 flies per group), and the bars represent the standard deviations. *, P of <0.01 for VRC plus LOV versus LOV alone; **, P of <0.001 for VRC plus LOV versus VRC alone.
LOV exhibits synergistic interaction with VRC in vitro when tested in combination against Zygomycetes isolates.
By XTT-based checkerboard, the combination of both LOV and VRC was synergistic against all of the Zygomycetes isolates tested (FIC index of ≤0.5), with FIC indices ranging from 0.104 to 0.290 (Table 3). Importantly, the interaction of LOV with VRC resulted in MICs (VRC MIC50, 1 μg/ml; LOV MIC50, 2 μg/ml) that were in the range of clinically achievable concentrations of both drugs (Table 3).
TABLE 3.
In vitro interaction between VRC and LOV against Zygomycetes isolatesa
| Isolate | Result for:
|
|||||
|---|---|---|---|---|---|---|
| XTT-based checkerboard
|
Etest/agar dilutiond
|
|||||
| MIC (μg/ml)
|
Lowest ΣFIC for VRC/LOV (interpretation)c | MIC (μg/ml)
|
||||
| VRC | LOVb | VRC/LOV | VRC | VRC/LOV | ||
| C. bertholletiae 506313 | 8 | 48 | 1/2 | 0.175 (S) | 16 | 3 |
| R. homothallicus 541783 | 8 | 56 | 1/2 | 0.155 (S) | 32 | 12 |
| R. oryzae 518749 | 16 | 48 | 1/2 | 0.104 (S) | 32 | 8 |
| R. homothallicus 529120 | 8 | 48 | 0.5/2 | 0.104 (S) | 24 | 6 |
| R. oryzae 557969 | 8 | 56 | 2/2 | 0.290 (S) | 32 | 8 |
| M. circinelloides 424760 | 16 | 32 | 1/2 | 0.125 (S) | 12 | 4 |
| M. circinelloides 488128 | 8 | 56 | 1/4 | 0.132 (S) | 32 | 4 |
Assessed by using XTT-based checkerboard and Etest/agar dilution susceptibility methods.
The following LOV concentrations were used in checkerboard dilution studies: 2, 4, 8, 16, 32, 48, 56, and 64 μg/ml.
S, synergy.
P = 0.0006. MICs of VRC against Zygomycetes spp. by Etest/agar dilution method are given as results for VRC alone versus results for VRC in combination with LOV (4 μg/ml).
LOV and VRC have significant synergistic effects against Zygomycetes by Etest/plate dilution method.
The effects of LOV on VRC MICs against all of the Zygomycetes isolates by Etest/plate dilution method are shown in Table 3 and Fig. 1B. There was a significant, pronounced reduction in VRC MICs (threefold to sixfold) following concomitant exposure of all of the Zygomycetes isolates to both LOV and VRC (P < 0001).
LOV combination with VRC is fungicidal against Zygomycetes by DiBAC staining.
The combination of VRC and LOV at subinhibitory concentrations for both drugs (2 and 4 μg/ml, respectively) potentiated fungicidal activity against Zygomyceteshyphae, as evidenced by DiBAC staining (Fig. 3). In pilot experiments, VRC alone was shown to cause minimal fluorescence indicative of cellular wall and membrane damage in Zygomycetes hyphae at concentrations up to 8 μg/ml. The effects of VRC combined with LOV on hyphal damage were comparable with those of AMB at concentrations equal to the MFC (2 μg/ml).
Protection of Tl flies infected with M. circinelloides by the combination of LOV and VRC.
Previous studies showed that concentrations of VRC up to 2 mg/ml were not toxic in adult flies (21). We found that Tl flies fed with LOV plus VRC and infected with either R. oryzae or M. circinelloides had a significantly better survival rate than did infected flies fed with LOV alone (P < 0.01). VRC alone had no activity in infected Tl flies (Fig. 4B).
DISCUSSION
The development of oral antifungal therapeutic strategies is a major unmet medical need for zygomycosis, an opportunistic mycosis for which oral antifungal options are extremely limited (11). In the present study, we found that the in vitro susceptibilities of the Zygomycetes isolates to the antifungal drugs tested were in agreement with those from previous studies (6, 11, 30). Indeed, of the antifungal agents tested, only AMB exhibited significant fungicidal activity. Although it has been reported that Zygomycetes spp. might be susceptible to ITC in vitro (6), ITC exhibited marginal fungistatic activities against the isolates tested in our study. However, the Rhizopus, Mucor, and Cunninghamella isolates that we tested were more resistant to ITC than were Absidia and Rhizomucor isolates tested elsewhere (6, 30). Our study also confirmed the well-described in vitro resistance of Zygomycetes spp. to VRC and CAS (6, 11, 30).
We also found that LOV had considerable in vitro fungicidal activity against all seven Zygomycetes isolates tested. Although we did not observe dramatic interspecies differences in susceptibility to LOV, the Mucor isolates appeared to be more susceptible to LOV than the other isolates were. In comparison, a recent study that used nonstandardized methods found that Rhizomucor pusillus strains were remarkably more sensitive to LOV than Rhizomucor miehei strains were according to agar diffusion susceptibility testing (23).
Furthermore, we observed that the activity of LOV against Zygomycetes isolates was consistent across several different culture media. Of note, there was a trend toward lower LOV MICs and EC50s in YNB media. Our group and others have postulated that because enriched YNB medium facilitates fungal growth compared with RPMI and RPMI-2, it might result in increased metabolic activity of fungal cells and better drug penetration into the intracellular sites of drug action (20, 24).
To better characterize the effects of LOV against Zygomycetes spp., we employed a strategy combining different viability dye staining methods (18). We found that the XTT colorimetric assay was able to accurately quantify the Zygomycetes fungal biomass. However, the incubation period was much shorter than with other filamentous fungi (18). In agreement with studies of other filamentous fungi (18, 25), we found that the LOV EC50s correlated better with the MICs for Zygomycetes spp.
We then microscopically confirmed the fungicidal activity of LOV against Zygomycetes spp. by staining LOV-treated hyphae with the fluorescence morbidity dye DiBAC. In correlation with XTT-based analysis, DiBAC staining showed that LOV had minimal fungicidal activity at a low concentration (2 μg/ml), whereas it caused prominent hyphal damage at fungicidal concentrations (4× EC50; 16 μg/ml). The fungicidal effects of LOV were comparable with those of AMB at a fungicidal concentration (2 μg/ml).
To examine whether LOV has the same activity against Zygomycetes spp. in vivo, we adapted an established mini-host model of zygomycosis in Drosophila melanogaster. Importantly, this model organism has been successfully used to assess the in vivo effects of LOV in the field of neurological research (2). We found that LOV had a significant protective effect in flies infected with a M. circinelloides strain. However, LOV had no activity in Tl flies infected by a less susceptible in vitro R. oryzae strain. Although Drosophila offers several advantages over conventional, logistically more difficult animal models in the screening of candidate compounds for antifungal activity because of its simplicity and rapidity, it does not allow for quantification of orally absorbed drugs. As a result, pharmacodynamic and pharmacokinetic studies with Drosophila are challenging, whereas little is known of the metabolism of LOV in this model. Thus, our promising findings of LOV activity in Tl flies will need further validation with mammalian models of zygomycosis.
As statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme A reductase, a rate-limiting enzyme in ergosterol biosynthesis (26), azoles block a sequential target in the ergosterol biosynthetic pathway (C-14 demethylation) (22). We analyzed the interaction of LOV with VRC, a triazole with no meaningful activity against Zygomycetes spp., because the potential of statins to overcome azole resistance has been shown previously for Candida (5) and might have even greater clinical significance. By employing checkerboard dilution and Etest-based methods, we found that at subinhibitory concentrations, LOV significantly potentiated the activity of VRC against all of the Zygomycetes isolates tested in vitro. We further verified this observation by using DiBAC staining. Importantly, the concentrations of LOV used in synergy studies with VRC were within the range of serum concentrations of LOV reported for mammalian models (2 to 20 μM or 0.5 to 5 μg/ml) and for humans (3.9 μM or 1 μg/ml) (4). Finally, we found that LOV and VRC exhibited synergistic effects against two representative Zygomycetes isolates in vivo in the Drosophila model of zygomycosis. These results are in agreement with studies that reported in vitro synergy of statins with different azoles against Candida and Cryptococcus species (5). Importantly, there is also evidence of in vivo synergy of LOV and azoles against Trypanosoma cruzi, a protozoan parasite in which, similar to fungi, ergosterol is an essential cell membrane component (31).
Although sequential inhibition of sterol biosynthesis may be plausible (22), the mechanisms of the antifungal activity of LOV remain largely unexplored because of the pleiotropic effects of LOV in cellular metabolism. For example, LOV is a well-known inhibitor of protein isoprenylation, a highly conserved essential process for cell proliferation, differentiation, and apoptosis in eukaryotes from fungi to humans (4, 10, 26) as well as in prokaryotes (34). Importantly, LOV was recently shown to induce apoptosis of several human cancer cell lines by mechanisms involving inhibition of Ras and several other isoprenoids (1, 4, 13, 14, 26). Similarly, LOV was recently shown to suppress the expression of three Ras genes in M. racemosus and to induce apoptosis-like cell death (29). However, the exact mechanisms that mediate apoptosis in fungi seem to be complex and have not been elucidated. Exploring the underlying mechanisms of LOV activity against Zygomycetesmight pave the way for the development of compounds with more selective action. This is particularly important in view of the fact that significant toxicity has been reported as a result of statin interactions with azoles or other drugs that are metabolized in the liver by cytochrome P450 (4). Finally, expanding these observations to other members of the statin family with or without other azoles that exhibit activity against Zygomycetes (e.g., posaconazole) or other classes of antifungal agents would be of interest.
In conclusion, the significant antifungal activity of LOV against Zygomycetes as well as its synergy with triazoles may result in significant clinical applications in the near future.
Acknowledgments
We are grateful to Nathaniel D. Albert for technical assistance and Don Norwood for editorial assistance.
This work was supported by The University of Texas M. D. AndersonCancer Center Faculty E. N. Cobb Scholar Award Research Endowment (to D.P.K.).
REFERENCES
- 1.Agarwal, B., S. Bhendwal, B. Halmos, S. F. Moss, W. G. Ramey, and P. R. Holt. 1999. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin. Cancer Res. 5:2223-2229. [PubMed] [Google Scholar]
- 2.Belgacem, Y. H., and J. R. Martin. 2002. Neuroendocrine control of a sexually dimorphic behavior by a few neurons of the pars intercerebralis in Drosophila. Proc. Natl. Acad. Sci. USA 99:15154-15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, K. K. W., A. M. Oza, and L. L. Siu. 2003. The statins as anticancer agents. Clin. Cancer Res. 9:10-19. [PubMed] [Google Scholar]
- 5.Chin, N. X., I. Weitzman, and P. Della-Latta. 1997. In vitro activity of fluvastatin, a cholesterol-lowering agent, and synergy with fluconazole and itraconazole against Candida species and Cryptococcus neoformans. Antimicrob. Agents Chemother. 41:850-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dannaoui, E., J. Meletiadis, J. W. Mouton, J. F. Meis, P. E. Verweij, et al. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 51:45-52. [DOI] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A., and A. Rezusta. 2002. E-test method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: comparison with NCCLS broth microdilution method. J. Clin. Microbiol. 40:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton, R. G., H. F. Kung, D. L. Longo, and M. R. Smith. 1992. Regulation of intracellular actin polymerization by prenylated cellular proteins. J. Cell Biol. 117:347-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gower, T. L., and B. S. Graham. 2001. Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro. Antimicrob. Agents Chemother. 45:1231-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg, R. N., L. J. Scott, H. H. Vaughn, and J. A. Ribes. 2004. Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Curr. Opin. Infect. Dis. 17:517-525. [DOI] [PubMed] [Google Scholar]
- 12.Grellier, P., A. Valentin, V. Millerioux, J. Schrevel, and D. Rigomier. 1994. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors lovastatin and simvastatin inhibit in vitro development of Plasmodium falciparum and Babesia divergens in human erythrocytes. Antimicrob. Agents Chemother. 38:1144-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katano, H., L. Pesnicak, and J. I. Cohen. 2004. Simvastatin induces apoptosisofEpstein-Barr virus (EBV)-transformed lymphoblastoid cell lines and delays development of EBV lymphomas. Proc. Natl. Acad. Sci. USA 101:4960-4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, S. W., M. M. Kim, H. J. Choi, S. S. Yoon, M. H. Lee, K. Park, C. H. Park, and W. K. Kang. 2001. Phase II study of high dose lovastatin in patients with advanced gastric adenocarcinoma. Investig. New Drugs 19:81-83. [DOI] [PubMed] [Google Scholar]
- 15.Kontoyiannis, D. P., R. E. Lewis, N. Sagar, G. S. May, R. A. Prince, and K. V. I. Rolston. 2000. Itraconazole-amphotericin B antagonism in Aspergillus fumigatus: an E-test-based strategy. Antimicrob. Agents Chemother. 44:2915-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontoyiannis, D. P., M. S. Lionakis, R. E. Lewis, G. Chamilos, M. Healy, C. Perego, A. Safdar, H. Kantarjian, R. Champlin, T. J. Walsh, and I. I. Raad. 2005. Zygomycosis in the era of Aspergillus-active antifungal therapy in a tertiary care cancer center: a case-control observational study of 27 recent cases. J. Infect. Dis. 191:1350-1360. [DOI] [PubMed] [Google Scholar]
- 17.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 18.Lewis, R. E., N. P. Wiederhold, and M. E. Klepser. 2005. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium spp. Antimicrob. Agents Chemother. 49:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis, R. E., D. J. Diekema, S. A. Messer, M. A. Pfaller, and M. E. Klepser. 2002. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 49:345-351. [DOI] [PubMed] [Google Scholar]
- 20.Lionakis, M. S., R. E. Lewis, G. Samonis, and D. P. Kontoyiannis. 2003. Pentamidine is active in vitro against Fusarium species. Antimicrob. Agents Chemother. 47:3252-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lionakis, M. S., R. E. Lewis, G. S. May, N. P. Wiederhold, N. D. Albert, G. Halder, and D. P. Kontoyiannis. 2005. Toll-deficient Drosophila flies as a fast, high-throughput model for the study of antifungal drug efficacy against invasive aspergillosis and Aspergillus virulence. J. Infect. Dis. 191:1188-1195. [DOI] [PubMed] [Google Scholar]
- 22.Lorenz, R. T., and L. W. Parks. 1990. Effects of lovastatin (mevinolin) on sterol levels and on activity of azoles in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 34:1660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukacs, G., T. Papp, I. Nyilasi, E. Nagy, and C. Vagvolgyi. 2004. Differentiation of Rhizomucor species on the basis of their different sensitivities to lovastatin. J. Clin. Microbiol. 42:5400-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meletiadis, J., J. F. Meis, J. W. Mouton, and P. E. Verweij. 2001. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 39:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, B. A. Bouman, J. P. Donnelly, P. E. Verweij, and Eurofung Network. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miida, T., S. Hirayama, and Y. Nakamura. 2004. Cholesterol-independent effects of statins and new therapeutic strategies: ischemic stroke and dementia. J. Atheroscler. Thromb. 5:253-264. [DOI] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 28.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose, L. V., and J. E. Linz. 1998. Lovastatin triggers apoptosis-like cell death process in the fungus Mucor racemosus. Fungal Genet. Biol. 25:119-133. [DOI] [PubMed] [Google Scholar]
- 30.Sun, Q. N., A. W. Fothergill, D. I. McCarthy, M. G. Rinaldi, and J. R. Graybill. 2002. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob. Agents Chemother. 46:1581-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbina, J. A., K. Lazardi, E. Marchan, G. Visbal, T. Aguirre, M. M. Piras, R. Piras, R. A. Maldonado, G. Payares, and W. de Souza. 1993. Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 37:580-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh, T. J., J. W. Hiemenez, N. L. Seibel, J. R. Perfect, G. Horwith, L. Lee, J. L. Silber, M. J. DiNubile, A. Reboli, E. Bow, J. Lister, and E. J. Anaissie. 1998. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 26:1383-1396. [DOI] [PubMed] [Google Scholar]
- 33.White, T. J., T. Bruns, S. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, Calif.
- 34.Wilding, E. J., J. R. Brown, A. P. Bryant, A. F. Chalker, D. J. Holmes, K. A. Ingraham, S. Iordanescu, C. Y. So, M. Rosenberg, and M. N. Gwynn. 2000. Identification, evolution, and essentiality of the mevalonate pathway for isopentenyl diphosphate biosynthesis in gram-positive cocci. J. Bacteriol. 182:4319-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]