Abstract
Several antibiotics show significant pharmacokinetic interactions when they are given orally concomitantly with antacids. The objective of this study was to evaluate the effects of antacid (containing magnesium) on the pharmacokinetics of linezolid. A single dose of 600 mg linezolid was given orally alone and 10 min after administration of the antacid Maalox 70mVal, which contains 600 mg magnesium hydroxide and 900 mg aluminum hydroxide, to nine healthy males and nine healthy females in a crossover and randomized study. Linezolid plasma concentrations were determined by high-performance liquid chromatography, and pharmacokinetic parameters were calculated for both treatments. Coadministration with antacids did not change the pharmacokinetics of linezolid. The ratios (90% confidence intervals) of the individual values of the area under the concentration-time curve and the maximum concentration in plasma (Cmax) (linezolid plus antacid versus linezolid alone) were 1.01 (0.99 to 1.02) and 0.99 (0.96 to 1.02), respectively. Likewise, no significant difference in any of the other pharmacokinetic parameters was observed between the treatment groups (the time to Cmax, lag time, volume of distribution [V/F], and clearance [CL/F]). However, a significant sex difference was observed for AUC, Cmax, V/F, and CL/F; and these differences could be almost completely explained by the differences in body weight between males and females. No clinically relevant adverse effects were detected under either condition. The coadministration of antacids had no effect on the pharmacokinetics of linezolid. This demonstrates that the oral absorption of linezolid was not affected by the presence of antacids containing magnesium hydroxide and aluminum hydroxide. Antacids can be safely administered together with linezolid.
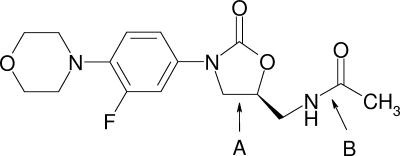
Linezolid is the first approved antibacterial drug from the oxazolidinone class (Fig. 1). It has a wide antimicrobial spectrum encompassing a variety of gram-positive bacteria. Studies on its mechanism of action suggest that it acts by selectively inhibiting bacterial protein synthesis, most likely via blockade of the 30S subunit-mediated initiation (11). The exact mechanism of action appears to be unique, and no cross-resistance with other translation-inhibiting antibiotics has been observed (7, 8, 10).
FIG. 1.
Chemical structure of linezolid. The molecule contains an oxazolidinone ring system (A) and a peptide bond (B). Both may serve as potential ligands for metal complexation.
The coadministration of antibiotics and antacids, proton pump inhibitors, or H2 antagonists may significantly reduce the oral absorption of antibiotics, resulting in a loss of effect. This was demonstrated for tetracyclines (9, 20). The proposed mechanism was the pH-dependent formation of chelates with metal ions, such as Fe2+, Al3+, Ca2+, and Mg2+, which leads to poorly soluble complexes that are not well absorbed from the gut lumen (6). Another mechanism may be an effect on gastrointestinal motility (for aluminum hydroxide-containing antacids). Changes in the intraluminal pH in the gastrointestinal tract alone do not affect the oral bioavailability of tetracyclines, as was seen after the coadministration of tetracycline and the H2 antagonists cimetidine and ranitidine (9, 13). Antacids even reduced the bioavailability of intravenously applied doxycycline by affecting the enterohepatic recycling (21). Multiple administrations of antacids may also alter urinary pH and increase the renal elimination of tetracyclines (17). The oral bioavailability of the fluoroquinolones amifloxacin, ciprofloxacin, norfloxacin, and ofloxacin was significantly reduced by coadministration with (aluminum-hydroxide containing) antacids (15, 18, 19, 28). This reduced bioavailability may be caused by the formation of insoluble chelates with the 3-carbonyl and 4-oxo groups of the antibiotics and aluminum and magnesium ions. Enoxacin showed a decreased solubility with a higher pH (14). However, the pharmacokinetics of gemifloxacin were only slightly affected and were not significantly affected clinically by the concomitant use of antacids and the proton pump inhibitor omeprazole (1, 2). Antacids also had no effect on the cephalosporins cefopril, cefixime, and cephalexin (16, 23), as well as amoxicillin or amoxicillin-clavulanic acid (9, 27). A significant reduction of bioavailability was, however, observed for cefpodoxime proxetil after coadministration with antacids or famotidine, an H2 antagonist (22, 26), which was thought to be due to the pH-dependent reduction of tablet disintegration and drug solubility in the gut lumen. The pharmacokinetics of azithromycin were only slightly impaired by antacids (reduced peak levels) (12); those of roxithromycin were not affected (4).
The absorption of drugs composed of phenol rings and/or heterocycles containing atoms with free electron pairs, among which are the fluoroquinolones (15, 18, 19, 28) and tetracyclines (6), has been shown to be decreased by polyvalent cations. The linezolid molecule contains an oxazolidinone ring system and a peptide bond (Fig. 1). Both may serve as potential ligands for metal complexation. Therefore, it was the objective of the present study to investigate the effects of aluminum- and magnesium-containing antacids on the pharmacokinetics of 600 mg linezolid when it was given as a single dose to healthy volunteers (males and females).
MATERIALS AND METHODS
Study design.
This was a single-center, single-dose, open-label, randomized, two-way crossover drug interaction study. The subjects were randomized to receive one of the two treatments, 600 mg linezolid alone or 600 mg linezolid 10 min after administration of the antacids. Both linezolid administrations were separated by a washout period of at least 5 days and were given after an overnight fast of at least 12 h.
Subjects were healthy, as assessed by prestudy evaluation. They had normal results in their physical evaluation (including 12-lead electrocardiogram and vital signs) and laboratory evaluation (blood hematology and chemistry and urinalysis), and females had a negative pregnancy test result. All measurements were performed in the Clinical Laboratory of the University Hospital, Basel, Switzerland. No concomitant medication except for oral contraceptives and medication to treat adverse effects was allowed. Mineral supplements and vitamins were avoided 72 h before administration of the drugs. A special exclusion criterion was a history of duodenal or stomach ulcers. Prior to the start of the study, the study protocol and informed consent form were approved by the State Ethics Committee of Basel (Ethische Kommission beider Basel), and written informed consent was obtained from each subject before enrollment in the study.
Study design and drug administration.
Linezolid (Zyvoxid) 600 mg tablets were supplied by Pfizer AG, Zürich, Switzerland. The antacid, Maalox 70mVal suspension, was supplied by Cassela-med GmbH & Co. KG, Cologne, Germany. It was administered as a 10-ml suspension containing 900 mg aluminum hydroxide and 600 mg magnesium hydroxide.
During hospitalization, the subjects refrained from smoking and were not allowed to consume alcohol or xanthine-containing food or beverages, grapefruit, and orange juice.
Drug administration was performed in the morning after a fasting period of 12 h. Mineral water was allowed ad libitum. Adverse events were monitored throughout the study. Blood samples were drawn from the antecubital area of the arm before administration (baseline) and up to the 15.0-h measurement. A 7-ml blood sample was collected from each subject into EDTA-coated glass tubes 10 to 30 min before dosing (baseline) and at 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, 12.0, 15.0, 24.0, 30.0, and 36.0 h postdosing. Blood pressure and heart rate were measured while the volunteer was in the supine position before drug administration and at 2.0, 4.0, 6.0, 8.0, 12.0, 15.0, 24.0, 30.0, and 36.0 h after drug administration. A physical examination, vital signs (blood pressure, pulse rate), as well as clinical safety laboratory tests with blood and urine were performed after the washout phase and within 7 days after the last treatment period.
Sample analysis.
Linezolid plasma concentrations were determined by high-performance liquid chromatography (Laboratory of Bristol Centre for Antimicrobial Research and Evaluation, Department of Medical Microbiology, Southmead Hospital, Bristol, United Kingdom) (30). In brief, chromatography was performed on a Hypersil 5ODS column (HPLC Technology Ltd., Macclesfield, United Kingdom) by using a mobile phase of methanol-water-phosphoric acid (30:69:1) with the addition of 2 g/liter of heptane sulfonic acid (Sigma Chemical Co), and the pH was adjusted to 4.5. Detection was by determination of UV absorbance at 254 nm, with quantification by the external standard method. Serum samples were diluted with an equal volume of acetonitrile and were centrifuged at 5,000 × g, and 10 μl of the supernatant was injected into the chromatograph.
The assay response was linear over the concentration range from 0.01 to 100 mg/liter, with a lower limit of detection of 0.03 mg/liter for linezolid. The intra- and interday accuracy and precision were assessed by the use of quality control standards, with limits for accuracy of 10% and coefficient of variability for precision of 10%.
Because antacids are locally acting drugs and are generally not expected to be absorbed in the systemic system, the Maalox 70mVal plasma concentration was not determined.
Pharmacokinetics.
The following pharmacokinetic parameters were determined. The maximum plasma concentration (Cmax) and the time of occurrence of Cmax (Tmax) were determined by inspection of raw data. The apparent terminal half-life (t1/2), clearance over the fraction absorbed (CL/F), the volume of distribution of the central compartment (V/F), and the area under the plasma concentration-time curve from time zero extrapolated to infinity (AUC0-∞) were estimated by noncompartmental analysis with WinNonlin software (version 4.01; Pharsight Corp., Cary, NC).
Data analysis and statistics.
The values of the pharmacokinetic parameters between the two treatment groups were compared by analysis of variance by using sex and body weight as covariates. In addition, AUC and Cmax were analyzed by the use of bioequivalence criteria: values were transformed logarithmically, and individual ratios (treatment with antacids versus treatment without antacids) were calculated. Bioequivalence was assumed when the geometric mean of the individual ratios as well as its 90% confidence interval was included in the interval 0.8 to 1.25. The level of significance was a P value of 0.05. All analyses were performed with SPSS software for Windows (version 12.0; SPSS Inc., Chicago, IL).
RESULTS
Eighteen healthy male (n = 9) and female (n = 9) subjects were enrolled in this study. The subjects had an average age of 26.8 years (age range, 21 to 47 years), an average body weight of 72.2 kg (body weight range, 53 to 87 kg), and an average height of 175.4 cm (height range, 164 to 184 cm). For details, see Table 1.
TABLE 1.
Demographics of subjectsa
| Subject | Age (yr) | Wt (kg) | Ht (cm) |
|---|---|---|---|
| Male (n = 9) | 29.6 ± 9.7 (21-47) | 78.4 ± 7.5 (69-87) | 180.7 ± 3.5 (175-184) |
| Female (n = 9) | 24 ± 1.0 (22-25) | 66 ± 6.2b (53-74) | 171.2 ± 4.5c (164-177) |
| All (n = 18) | 26.8 ± 7.2 (21-47) | 72.2 ± 9.2 (53-87) | 175.4 ± 6.3 (164-184) |
Data represent means±standard deviations (ranges).
Significantly different from male subjects (P < 0.002).
Significantly different from male subjects (P < 0.001).
The linezolid and antacid treatments were well tolerated by the subjects: mild adverse events were observed in 11 of the 18 subjects. Seven subjects experienced headache, which in one subject was treated with 500 mg acetaminophen. One subject complained about abdominal pain, and three subjects complained about increased stool frequency or diarrhea. One subject reported increased miction. There were no clinically relevant changes in vital signs.
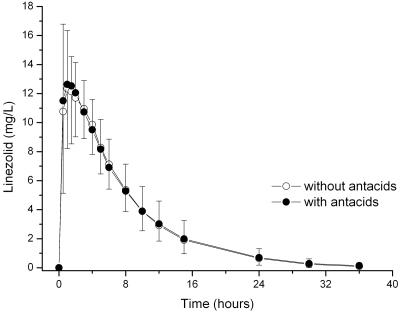
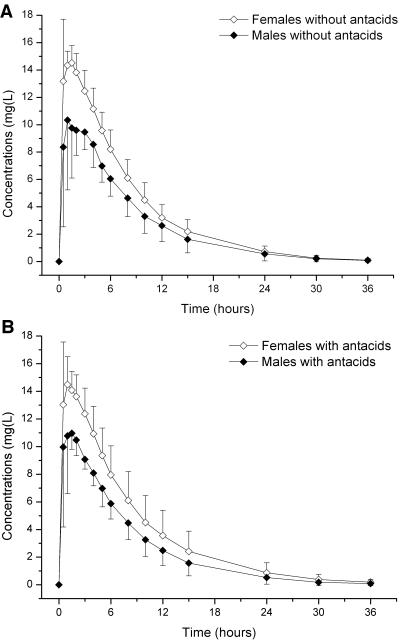
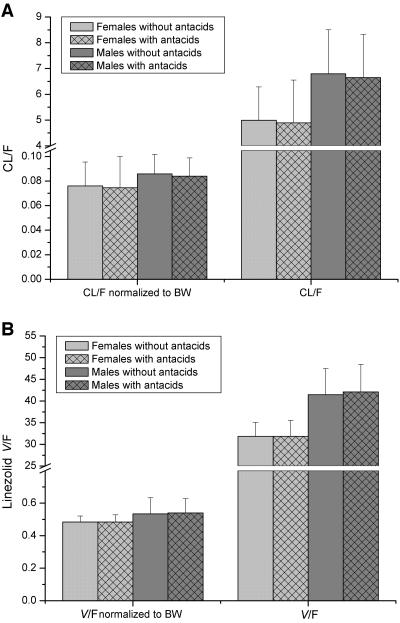
The pharmacokinetics of linezolid were not changed by coadministration of antacids: AUC and Cmax fulfilled the bioequivalence criteria and were not statistically significantly different between the two treatments. No significant differences in any of the other pharmacokinetic parameters were seen (Table 2; Fig. 2). However, with regard to sex and body weight, some of the pharmacokinetic parameters differed significantly (Table 2; Fig. 3 and 4). Female subjects showed significantly higher Cmax values and larger AUC and CL/F values than male subjects. On the other hand, in the female subjects V/F was significantly decreased. Analysis of variance by using sex and body weight as covariates revealed that there was a significant effect of weight on AUC (P = 0.012) and CL/F (P = 0.02) and a significant effect of sex on V/F (P = 0.002). When the results were normalized for body weight, the differences in AUC (P < 0.001) and Cmax (P = 0.001) by gender were still present; however, the extent of this effect of gender on CL/F and V/F was almost negligible after normalization to body weight (Fig. 4A and B). The half-life of linezolid was identical in the male and the female subjects.
TABLE 2.
Pharmacokinetics parameters of linezolida
| Subject and pharmacokinetic parameter | Linezolid (mean ± SD) | Linezolid + Maalox (mean ± SD) | Geometric mean ratio | 90% CI (P value) |
|---|---|---|---|---|
| Females (n = 9) | ||||
| Cmax (mg/liter) | 16.2 ± 2.4 | 15.5 ± 2.2 | 0.98 | 0.96-1.01 |
| NS | ||||
| AUC0-∞ (mg · h/liter) | 126.7 ± 28.8 | 133.0 ± 36.3 | 1.01 | 0.98-1.03 |
| NS | ||||
| Tmax (h) | 1.22 ± 0.57 | 1.02 ± 0.42 | NS | |
| t1/2 (h) | 4.65 ± 1.16 | 4.95 ± 1.64 | NS | |
| V/F (liters) | 31.8 ± 3.26 | 31.9 ± 3.67 | NS | |
| CL/F (liters/h) | 4.99 ± 1.30 | 4.89 ± 1.66 | NS | |
| Males (n = 9) | ||||
| Cmax (mg/liter) | 13.4 ± 1.9 | 13.3 ± 2.7 | 0.99 | 0.93-1.06 |
| NS | ||||
| AUC0-∞ (mg · h/liter) | 93.3 ± 22.9 | 95.1 ± 23.5 | 1.00 | 0.98-1.03 |
| NS | ||||
| Tmax (h) | 1.00 ± 0.50 | 1.2 ± 0.87 | NS | |
| t1/2 (h) | 4.52 ± 1.54 | 4.66 ± 1.44 | NS | |
| V/F (liters) | 41.5 ± 6.0 | 42.1 ± 6.3 | NS | |
| CL/F (liters/h) | 6.79 ± 1.71 | 6.65 ± 1.67 | NS | |
| All subjects (n = 18) | ||||
| Cmax (mg/liter) | 14.8 (2.5) | 14.4 ± 2.6 | 0.99 | 0.96-1.2 |
| NS | ||||
| AUC0-∞ (mg · h/liter) | 110.0 (30.5) | 114.1 ± 35.5 | 1.01 | 0.99-1.02 |
| NS | ||||
| Tmax (h) | 1.11 ± 0.53 | 1.09 ± 0.67 | NS | |
| t1/2 (h) | 4.58 ± 1.32 | 4.80 ± 1.50 | NS | |
| V/F (liters) | 36.7 ± 6.9 | 37.0 ± 7.3 | NS | |
| CL/F (liters/h) | 5.89 ± 1.74 | 5.77 ± 1.85 | NS |
Significant differences due to sex for V/F (P = 0.002); significant differences due to body weight for AUC (P = 0.012) and CL/F (P = 0.02); when normalized by body weight, a significant effect of sex on AUC (P < 0.001) and Cmax (P = 0.001) was observed. Abbreviations: NS, not statistically significant different; CI, confidence interval; CV, coefficient of variation.
FIG. 2.
Mean ± standard deviation plasma concentration-versus-time profiles for linezolid given alone and 10 min after administration of antacids to 18 healthy volunteers.
FIG. 3.
Differences in the pharmacokinetics of linezolid by sex. Mean ± standard deviation plasma concentrations of linezolid given alone or 10 min after administration of antacids to nine healthy female subjects and nine male subjects. (A) Linezolid administration alone; (B) after administration of antacids.
FIG. 4.
Oral clearance and volume of distribution of linezolid normalized by subject body weight (BW) and gender difference with and without coadministration of antacids. (A) CL/F; (B) V/F.
DISCUSSION
The aim of this study was to investigate the potential pharmacokinetic interaction between the antibiotic linezolid and the antacid Maalox 70mVal. These interactions could be very important clinically since they could affect the efficacy of linezolid. Modifications in the gastrointestinal tract, such as a change in pH or changes in gastrointestinal motility caused by the antacid, could be the reason for the accelerated, increased, or reduced bioavailabilities of different drugs. Since linezolid is mostly used in case of severe infections or resistance to other antibiotics, it is mandatory to detect any interaction which could alter its efficacy.
In the present study, we could show that the pharmacokinetics of linezolid were not affected by coadministration of Maalox 70mVal. In the presence and in the absence of the antacid, the Cmax values of linezolid were 15.5 ± 2.2 mg/liter and 16.2 ± 2.4 mg/liter, respectively; the AUC0-∞ values of linezolid were 133.0 ± 36.3 mg · h/liter and 126.7 ± 28.8 mg · h/liter, respectively; and the apparent terminal half-lives of linezolid were 4.95 ± 1.64 h and 4.65 ± 1.16 h, respectively. This demonstrates that there is no pharmacokinetic interaction between linezolid and Maalox 70mVal.
The subjects received both treatments under fasting conditions. The values of the pharmacokinetic parameters obtained in this study were comparable to those observed by Burkhardt et al. (5) and Sisson et al. (24). As in those studies, a significant sex effect was observed in the present study (Fig. 3; Table 2); however, the extent of this difference was smaller in our study than in those studies. Since the half-lives were not different between the male and the female subjects, the significantly lower Cmax and AUC in the male subjects may be explained at least in part by the significantly increased volume of distribution and body weight of the male subjects compared with those of the female subjects. Although this effect by gender was statistically significant, it was small and is most likely not clinically relevant (24). Therefore, no dose adjustment seems to be required.
Finally, no clinically significant adverse events were recorded. Furthermore, no drug-related changes in vital signs or in clinical chemistry, urinalysis, or hematology values were observed. This confirms the fact that linezolid is well tolerated (5), even in cancer patients (25), and only a few adverse effects were reported (3, 29).
In summary, these results demonstrate that the coadministration of linezolid and Maalox 70mVal has no effect on the pharmacokinetics of linezolid under fasting conditions. Although there are differences in the pharmacokinetics between male and female subjects, for both sexes there were no pharmacokinetic differences between the two treatment groups (Table 2; Fig. 3). This demonstrates that the oral absorption of linezolid is not affected by the presence of antacids containing magnesium and aluminum hydroxide. Therefore, both drugs can safely be administered together.
Acknowledgments
We thank Luisa Baselgia and Claudia Bläsi for expert technical assistance.
This work was financially supported by an unconditional research grant of Pfizer Ltd., Switzerland.
REFERENCES
- 1.Allen, A., M. Vousden, and A. Lewis. 1999. Effect of omeprazole on the pharmacokinetics of oral gemifloxacin in healthy volunteers. Chemotherapy 45:496-503. [DOI] [PubMed] [Google Scholar]
- 2.Allen, A., M. Vousden, A. Porter, and A. Lewis. 1999. Effect of Maalox on the bioavailability of oral gemifloxacin in healthy volunteers. Chemotherapy 45:504-511. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein, W. B., R. F. Trotta, J. T. Rector, J. A. Tjaden, and A. J. Barile. 2003. Mechanisms for linezolid-induced anemia and thrombocytopenia. Ann. Pharmacother. 37:517-520. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh, M., H. Lode, G. Hoffken, S. Daeschlein, and P. Koeppe. 1992. Pharmacokinetics of roxithromycin and influence of H2-blockers and antacids on gastrointestinal absorption. Eur. J. Clin. Microbiol. Infect. Dis. 11:465-468. [DOI] [PubMed] [Google Scholar]
- 5.Burkhardt, O., K. Borner, N. von der Hoh, P. Koppe, M. W. Pletz, C. E. Nord, and H. Lode. 2002. Single- and multiple-dose pharmacokinetics of linezolid and co-amoxiclav in healthy human volunteers. J. Antimicrob. Chemother. 50:707-712. [DOI] [PubMed] [Google Scholar]
- 6.Chin, T. F., and J. L. Lach. 1975. Drug diffusion and bioavailability: tetracycline metallic chelation. Am. J. Hosp. Pharm. 32:625-629. [PubMed] [Google Scholar]
- 7.Daly, J. S., G. M. Eliopoulos, E. Reiszner, and R. C. Moellering, Jr. 1988. Activity and mechanism of action of DuP 105 and DuP 721, new oxazolidinone compounds. J. Antimicrob. Chemother. 21:721-730. [DOI] [PubMed] [Google Scholar]
- 8.Daly, J. S., G. M. Eliopoulos, S. Willey, and R. C. Moellering, Jr. 1988. Mechanism of action and in vitro and in vivo activities of S-6123, a new oxazolidinone compound. Antimicrob. Agents Chemother. 32:1341-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deppermann, K. M., H. Lode, G. Hoffken, G. Tschink, C. Kalz, and P. Koeppe. 1989. Influence of ranitidine, pirenzepine, and aluminum magnesium hydroxide on the bioavailability of various antibiotics, including amoxicillin, cephalexin, doxycycline, and amoxicillin-clavulanic acid. Antimicrob. Agents Chemother. 33:1901-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eustice, D. C, P. A. Feldman, I. Zajac, and A. M. Slee. 1988. Mechanism of action of DuP 721: inhibition of an early event during initiation of protein synthesis. Antimicrob. Agents Chemother. 32:1218-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eustice, D. C., P. A. Feldman, and A. M. Slee. 1988. The mechanism of action of DuP 721, a new antibacterial agent: effects on macromolecular synthesis. Biochem. Biophys. Res. Commun. 150:965-971. [DOI] [PubMed] [Google Scholar]
- 12.Flockhart, D. A., Z. Desta, and S. K. Mahal. 2000. Selection of drugs to treat gastro-oesophageal reflux disease: the role of drug interactions. Clin. Pharmacokinet. 39:295-309. [DOI] [PubMed] [Google Scholar]
- 13.Garty, M., and A. Hurwitz. 1980. Effect of cimetidine and antacids on gastrointestinal absorption of tetracycline. Clin. Pharmacol. Ther. 28:203-207. [DOI] [PubMed] [Google Scholar]
- 14.Grasela, T. H., Jr., J. J. Schentag, A. J. Sedman, J. H. Wilton, D. J. Thomas, R. W. Schultz, M. E. Lebsack, and A. W. Kinkel. 1989. Inhibition of enoxacin absorption by antacids or ranitidine. Antimicrob. Agents Chemother. 33:615-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gugler, R., and H. Allgayer. 1990. Effects of antacids on the clinical pharmacokinetics of drugs. An update. Clin. Pharmacokinet. 18:210-219. [DOI] [PubMed] [Google Scholar]
- 16.Healy, D. P., J. V. Sahai, L. P. Sterling, and E. M. Racht. 1989. Influence of an antacid containing aluminum and magnesium on the pharmacokinetics of cefixime. Antimicrob. Agents Chemother. 33:1994-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffe, J. M., R. I. Poust, S. L. Feld, and J. L. Colaizzi. 1974. Influence of repetitive dosing and altered urinary pH on doxycycline excretion in humans. J. Pharm. Sci. 63:1256-1260. [DOI] [PubMed] [Google Scholar]
- 18.Lode, H. 1988. Drug interactions with quinolones. Rev. Infect. Dis. 10(Suppl. 1):S132-S136. [DOI] [PubMed] [Google Scholar]
- 19.Mizuki, Y., I. Fujiwara, and T. Yamaguchi. 1996. Pharmacokinetic interactions related to the chemical structures of fluoroquinolones. J. Antimicrob. Chemother. 37(Suppl. A):41-55. [DOI] [PubMed] [Google Scholar]
- 20.Neuvonen, P. J. 1976. Interactions with the absorption of tetracyclines. Drugs 11:45-54. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen, V. X., D. E. Nix, S. Gillikin, and J. J. Schentag. 1989. Effect of oral antacid administration on the pharmacokinetics of intravenous doxycycline. Antimicrob. Agents Chemother. 33:434-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saathoff, N., H. Lode, K. Neider, K. M. Depperman, K. Borner, and P. Koeppe. 1992. Pharmacokinetics of cefpodoxime proxetil and interactions with an antacid and an H2 receptor antagonist. Antimicrob. Agents Chemother. 36:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shyu, W. C., R. B. Wilber, K. A. Pittman, and R. H. Barbhaiya. 1992. Effect of antacid on the bioavailability of cefprozil. Antimicrob. Agents Chemother. 36:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sisson, T. L., G. L. Jungbluth, and N. K. Hopkins. 2002. Age and sex effects on the pharmacokinetics of linezolid. Eur. J. Clin. Pharmacol. 57:793-797. [DOI] [PubMed] [Google Scholar]
- 25.Smith, P. F., M. C. Birmingham, G. A. Noskin, A. K. Meagher, A. Forrest, C. R. Rayner, and J. J. Schentag. 2003. Safety, efficacy and pharmacokinetics of linezolid for treatment of resistant gram-positive infections in cancer patients with neutropenia. Ann. Oncol. 14:795-801. [DOI] [PubMed] [Google Scholar]
- 26.Sommers, D. K., M. Van Wyk, P. E. Williams, and S. M. Harding. 1984. Pharmacokinetics and tolerance of cefuroxime axetil in volunteers during repeated dosing. Antimicrob. Agents Chemother. 25:344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staniforth, D. H., H. L. Clarke, R. Horton, D. Jackson, and D. Lau. 1985. Augmentin bioavailability following cimetidine, aluminum hydroxide and milk. Int. J. Clin. Pharmacol. Ther. Toxicol. 23:154-157. [PubMed] [Google Scholar]
- 28.Stroshane, R. M., R. R. Brown, J. A. Cook, and P. S. Wissel. 1989. Effect of food, milk and antacid on the absorption of orally administered amifloxacin. Rev. Infect. Dis. 11(Suppl. 5):S1018-S1019. [Google Scholar]
- 29.Tartarone, A., G. Gallucci, G. Iodice, G. Romano, M. Coccaro, M. L. Vigliotti, G. Mele, R. Matera, and N. Di Renzo. 2004. Linezolid-induced bradycardia: a case report. Int. J. Antimicrob. Agents 23:412-413. [DOI] [PubMed] [Google Scholar]
- 30.Tobin, C. M., J. Sunderland, L. O. White, and A. P. MacGowan. 2001. A simple, isocratic high-performance liquid chromatography assay for linezolid in human serum. J. Antimicrob. Chemother. 48:605-608. [DOI] [PubMed] [Google Scholar]