Abstract
Naphthyridones that were recently described as a class of translation inhibitors in gram-positive bacteria mediate their mode of action via GyrA in Haemophilus influenzae and Escherichia coli. These are the first examples of compounds in which modes of action in different bacterial pathogens are mediated through widely different targets.
Naphthyridones A-72310 and A-692345 were studied previously as racemic mixtures and were described as a novel class of translation inhibitors (3, 12). Here, both sets of two enantiomers were separated, resulting in four compounds (Fig. 1). All compounds were tested in a transcription-translation assay using S30 fractions isolated from Escherichia coli and were confirmed to inhibit translation, with 50% inhibitory concentrations ranging from 7.5 to 26 μM (Table 1). Antimicrobial activities of the compounds tended to be more potent against gram-negative species than gram-positive bacteria (Table 1). Strains of Haemophilus influenzae and E. coli lacking AcrB and TolC, respectively, were used to assess efflux via systems containing these subunits (4, 11). Absence of the pump increased activity only modestly in H. influenzae (1- to 4-fold) but more dramatically in E. coli (4- to 32-fold), indicating the occurrence of net efflux of naphthyridones.
FIG. 1.
Chemical structures of compounds 1 to 4.
TABLE 1.
Biochemical and microbiological profiles of naphthyridonesc
| Agent | IC50 for E. colia
|
MIC againstb:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TT | SC | S. pneumoniae | S. aureus | H. influenzae | H. influenzae acrB::cap | Moraxella catarrhalis | E. coli | E. coli tolC::Tn10 | |
| Compound 1 | 26 | 4.8 | 32 | >64 | 2 | 2 | 4 | 16 | 4 |
| Compound 2 | 17 | 18 | 16 | >64 | 8 | 4 | 8 | 32 | 8 |
| Compound 3 | 17 | 3.7 | 8 | 32 | 1 | 0.25 | 0.5 | 4 | 0.13 |
| Compound 4 | 7.5 | ND | 8 | 16 | 2 | 1 | 1 | 4 | 0.5 |
| Ciprofloxacin | >400 | 0.12 | 1 | 0.5 | ND | 0.002 | 0.015 | 0.015 | 0.001 |
| Ofloxacin | >400 | 0.18 | 2 | 0.25 | 0.03 | 0.0075 | 0.06 | 0.06 | 0.0038 |
| Linezolid | 4.3 | ND | 1 | 2 | 16 | 4 | 4 | >64 | 16 |
Numbers indicate 50% inhibitory concentrations (μM) obtained in an in vitro transcription-translation assay (TT) and an in vitro supercoiling assay (SC).
Microbiological data are MICs (in μg · ml−1) obtained against a number of species.
cap, chloramphenicol resistance; ND, not determined.
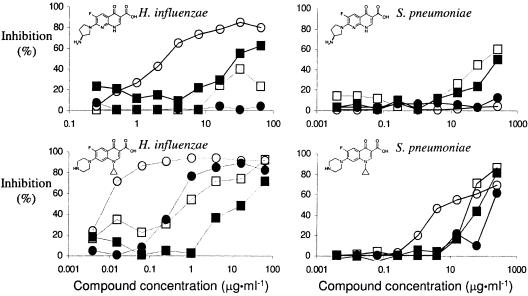
All compounds were tested in a set of radiolabeled precursor assays (6) using Streptococcus pneumoniae. Leucine and valine, thymidine, uridine, N-acetylglucosamine, and acetic acid were used as precursors to measure inhibition of the synthesis of protein, DNA, RNA, cell walls, and fatty acids, respectively. All naphthyridones inhibited incorporation of leucine and valine at lower compound concentrations than they inhibited any other processes. These results confirm a previous study (3) which showed that the compounds mediated their modes of action (MOA) via inhibition of protein synthesis. As expected, the structurally similar quinolone ciprofloxacin preferentially inhibited incorporation of thymidine (Fig. 2). A similar outcome was obtained with Staphylococcus aureus (data not shown).
FIG. 2.
Inhibition of precursor incorporation into macromolecules by naphthyridones differs in H. influenzae and S. pneumoniae. Inhibition of synthesis was measured for protein (leucine [□]), valine (▪), RNA (uridine [•]), and DNA (methylthymidine [○]) in H. influenzae (left) and S. pneumoniae (right); for clarity, precursors for incorporation into fatty acid (acetic acid) and peptidoglycan/cell wall (N-acetylglucosamine), which were inhibited to much lower extents, have been omitted. Results obtained with compound 1 (top) are typical for all four naphthyridone compounds; ciprofloxacin (bottom) served as a control for inhibition of DNA synthesis in both S. pneumoniae and H. influenzae. Results with additional control compounds (erythromycin, triclosan, carbonyl cyanide m-chlorophenylhydrazone, rifampin, and penicillin G) are not shown.
The hypothesis that protein synthesis was the target of the naphthyridones in S. pneumoniae was confirmed by the isolation and analysis of mutants of S. pneumoniae. Mutants resistant to the most potent compound, compound 3, were isolated after incubation for 24 h at a frequency of 10−7 from blood agar plates containing compound 3 at 32 μg · ml−1. Although all mutants were cross-resistant against other naphthyridones in the microdilution assay (Table 2), about half of them showed a spotty growth phenotype at higher naphthyridone concentrations. Based on previous data (3) all 16S rRNA genes and the gene encoding the S3 ribosomal protein were sequenced in one representative strain of each phenotype. The strain showing spotty growth had a G1049→A mutation, corresponding to G1053 in E. coli, in all four copies of the 16S rRNA, whereas no mutation was found in the S3 gene. Conversely, the seemingly more stable mutant did not have any mutation in the 16S rRNA genes but contained an in-frame deletion in the gene encoding the S3 ribosomal protein, removing amino acids GYS159. Although G1053 is immediately next to the main tetracycline binding site (9), the susceptibility to this antibiotic was not changed (Table 2). Interestingly, although the deletion in S3 does not seem close enough to G1053 (2) to comprise part of a putative naphthyridone binding site, its proximity to G1053 may be sufficient to influence that region of the ribosome, due to more indirect alterations of the structure. No cross-resistance to fluoroquinolones was observed (Table 2).
TABLE 2.
Cross-resistance of naphthyridone-resistance mutants of S. pneumoniae, E. coli tolC, and H. influenzae acrB
| Characteristic or agent | Description or MICa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
S. pneumoniae
|
E. coli tolC::Tn10
|
H. influenzae acrB::cap
|
|||||||
| Parent | 3A | 3B | Parent | 1A | 3A | Parent | 1A | 3A | |
| RNA/protein | 16S rRNA | S3 | GyrA | GyrA | GyrA | GyrA | |||
| Mutation | G1049→A | ΔGYS159 | S83→A | S83→L | D88→Y | E153→G | |||
| Compound 1 | 32 | >64 | >64 | 4 | >64 | >64 | 2 | 8 | 4 |
| Compound 2 | 16 | >64 | 32 | 8 | >64 | >64 | 4 | 16 | 16 |
| Compound 3 | 8 | 64 | 32 | 0.125 | 8 | 8 | 0.25 | 4 | 1 |
| Compound 4 | 8 | 16 | 64 | 0.5 | 32 | 16 | 1 | 4 | 4 |
| Moxifloxacin | 0.125 | 0.125 | 0.125 | 0.0038 | 0.0075 | 0.06 | 0.0038 | 0.03 | 0.015 |
| Ciprofloxacin | 1 | 1 | 1 | 0.001 | 0.0075 | 0.06 | 0.002 | 0.03 | 0.015 |
| Gemifloxacin | 0.031 | 0.031 | 0.031 | 0.0002 | 0.001 | 0.015 | 0.0005 | 0.0037 | 0.0019 |
| Ofloxacin | 2 | 2 | 2 | 0.0038 | 0.015 | 0.06 | 0.0075 | 0.03 | 0.03 |
| Linezolid | 1 | 1 | 1 | 16 | 16 | 16 | 4 | 4 | 4 |
| Thiamphenicol | 2 | 2 | 2 | 16 | 32 | 16 | NA | NA | NA |
| Tetracycline | 0.125 | 0.125 | 0.06 | NA | NA | NA | 0.25 | 0.25 | 0.25 |
| Kanamycin | 64 | 64 | 32 | 0.25 | 0.5 | 0.5 | 4 | 4 | 4 |
Designations 1A, 3A, and 3B indicate the number of the compound that was used for selection and isolation plus a letter distinguishing different isolates. MICs are in μg · ml−1. NA, not applicable (due to antibiotic resistance marker); cap, chloramphenicol resistance.
Similar studies using H. influenzae and E. coli (data not shown) yielded different results. Inhibition of incorporation of radiolabeled precursors showed that, in these species, thymidine incorporation, and thus, DNA synthesis, was inhibited similarly to the way it was inhibited by quinolones (Fig. 2). The hypothesis that inhibition of DNA synthesis rather than protein synthesis was the target of the naphthyridones in these two gram-negative species was confirmed by isolation of mutants of AcrB- and TolC-negative H. influenzae and E. coli, respectively. Mutants to either compound 1 (at 16 and 32 μg · ml−1 for H. influenzae acrB::cap [chloramphenicol resistant] and E. coli tolC::Tn10, respectively) or compound 3 (at 2 and 0.5 μg · ml−1 for H. influenzae acrB::cap and E. coli tolC::Tn10, respectively) were isolated at frequencies of 10−7 to 10−8. In contrast to the naphthyridone-resistant mutant isolates obtained from S. pneumoniae, all mutants were found to be cross-resistant to fluoroquinolones, suggesting involvement of GyrA or ParC (7). One mutant of each compound-species combination was analyzed further (Table 2) and all mutants were found to contain mutations in gyrA. Three out of four had previously described mutations in the quinolone-resistance-determining region (8, 15, 16) and one novel mutation, E153→G, was found. Compounds 1 to 3 were tested in an in vitro supercoiling assay (10) and were found to inhibit E. coli GyrA-GyrB (Table 1).
Transcriptional profiles were determined for cultures of E. coli tolC::Tn10 which were treated for 30 min at compound concentrations that equaled their MICs (1). Multivariate analysis of all transcript levels (14) showed that treatment with naphthyridones altered the profile similarly to treatment with inhibitors of DNA synthesis ciprofloxacin, ofloxacin, or nalidixic acid, and the result was quite distinct from those of protein synthesis inhibitors chloramphenicol and tetracycline (data not shown). A similar result was obtained when the analysis was more focused, using the restricted gene set defined by Dandliker et al. (3). Whereas in B. subtilis elevated transcript levels of many ribosomal proteins were found upon treatment with naphthyridones (3), in E. coli a >2-fold increase of many of these genes was found with tetracycline and with chloramphenicol (32 and 27 out of 34 genes, respectively), but no increase was detected after treatment with naphthyridones (data not shown), suggesting that in E. coli, these naphthyridones do not inhibit growth via inhibition of protein synthesis.
Here, we confirm an earlier report that naphthyridones are in vitro inhibitors of translation in S. pneumoniae and E. coli, and that in gram-positive species, the antibacterial action is mediated via inhibition of this target (3). However, naphthyridones also inhibit DNA gyrase of E. coli in an in vitro supercoiling assay (Table 1). Since DNA gyrase is a well-established target of both coumarins (13) and quinolones (5), this implies that there are (at least) two possible targets via which naphthyridones can act. The data presented here show that, whereas the in situ inhibition of translation may occur in E. coli and H. influenzae, the antibacterial action is mediated via inhibition of DNA gyrase. Combining our results with those of Dandliker et al. (3) suggests that naphthyridones mediate their antibacterial MOA via inhibition of translation in gram-positive species and via inhibition of DNA supercoiling in gram-negative species. To our knowledge, this is the first example of compounds with widely different MOA in different bacterial pathogens. One implication is that in order to elucidate relationships between chemical structure, biochemical activity, and antimicrobial activity in different pathogens, the MOA in each species needs to be determined.
Acknowledgments
We thank Richard Alm, Paul Fleming, Tom Keating, Kevin Keenan, Valerie Laganas, Bob McLaughlin, Scott Mills, Linda Otterson, Adam Shapiro, Wei Yang, and Elaina Zverina for their contributions to this work.
REFERENCES
- 1.Affymetrix. 2001. Genechip Escherichia coli antisense genome array. Expression analysis protocol 701119. Affymetrix, Santa Clara, Calif.
- 2.Brodersen, D. E., W. M. Clemons, Jr., A. P. Carter, B. T. Wimberley, and V. Ramakrishnan. 2002. Crystal structure of the 30S ribosomal subunit from Thermus thermophilus: structure of the proteins and their interaction with 16S RNA. J. Mol. Biol. 316:725-768. [DOI] [PubMed] [Google Scholar]
- 3.Dandliker, P. J., S. D. Pratt, A. M. Nilius, C. Black-Schaefer, X. Ruan, D. L. Towne, R. F. Clark, E. E. Englund, R. Wagner, M. Weitzberg, L. E. Chovan, R. K. Hickman, M. M. Daly, S. Kakavas, P. Zhong, Z. Cao, C. A. David, X. Xuei, C. G. Lerner, N. B. Soni, M. Bui, L. L. Shen, Y. Cai, P. J. Merta, A. Y. Saiki, and B. A. Beutel. 2003. Novel antibacterial class. Antimicrob. Agents Chemother. 47:3831-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fralick, J. A. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 178:5803-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gellert, M., K. Mizuuchi, M. H. O'Dea, T. Ito, and J. I. Tomizawa. 1977. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc. Natl. Acad. Sci. USA 74:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilliard, J. J., R. M. Goldschmidt, L. Licata, E. Z. Baum, and K. Bush. 1999. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob. Agents Chemother. 43:1693-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khodursky, A. B., E. L. Zechiedrich, and N. R. Cozzarelli. 1995. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:11801-11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, X., N. Mariano, J. J. Rahal, C. M. Urban, and K. Drlica. 2004. Quinolone-resistant Haemophilus influenzae in a long-term-care facility: nucleotide sequence characterization of alterations in the genes encoding DNA gyrase and DNA topoisomerase IV. Antimicrob. Agents Chemother. 48:3570-3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pioletti, M., F. Schlünzen, J. Harms, R. Zarivach, M. Glühmann, H. Avila, A. Bashan, H. Bartels, T. Auerbach, C. Jacobi, T. Hartsch, A. Yonah, and F. Franceschi. 2001. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 20:1829-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reece, R. J., and A. Maxwell. 1989. Tryptic fragments of the Escherichia coli DNA gyrase A protein. J. Biol. Chem. 264:19648-19653. [PubMed] [Google Scholar]
- 11.Sanchez, L., W. Pan, M. Vinas, and H. Nikaido. 1997. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J. Bacteriol. 179:6855-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen, L. L., C. Black-Schaefer, Y. Cai, P. J. Dandliker, and B. A. Beutel. 2005. Mechanism of action of novel series of naphtyridine-type ribosome inhibitors: enhancement of tRNA footprinting at the decoding site of 16S rRNA. Antimicrob. Agents Chemother. 49:1890-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugino, A., N. P. Higgins, P. O. Brown, C. L. Peebles, and N. R. Cozzarelli. 1978. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc. Natl. Acad. Sci. USA 75:4838-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umetrics AB. 2002. SIMCA-P version 10.0. Umetrics AB, Umea, Sweden.
- 15.Yoshida, H., M. Bogaki, M. Nakamura, and S. Nakamura. 1990. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob. Agents Chemother. 34:1271-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu, X., M. Susa, C. Knabbe, R. D. Schmid, and T. T. Bachmann. 2004. Development and validation of a diagnostic DNA microarray to detect quinolone-resistant Escherichia coli among clinical isolates. J. Clin. Microbiol. 42:4083-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]