Abstract
Zygomycosis, an infection that is associated with significant morbidity and mortality, is becoming common in immunocompromised patients. Posaconazole is a new extended-spectrum azole antifungal that has demonstrated in vitro and in vivo activity against zygomycetes. This report provides the results from the first 24 patients with active zygomycosis who were enrolled in two open-label, nonrandomized, multicentered compassionate trials that evaluated oral posaconazole as salvage therapy for invasive fungal infections. Posaconazole was usually given as an oral suspension of 200 mg four times a day or 400 mg twice a day. Eleven (46%) of the infections were rhinocerebral. Duration of posaconazole therapy ranged from 8 to 1,004 days (mean, 292 days; median, 182 days). Rates of successful treatment (complete cure and partial response) were 79% in 19 subjects with zygomycosis refractory to standard therapy and 80% in 5 subjects with intolerance to standard therapy. Overall, 19 of 24 subjects (79%) survived infection. Survival was also associated with surgical resection of affected tissue and stabilization or improvement of the subjects' underlying illnesses. Failures either had worsening of underlying illnesses or requested all therapy withdrawn; none of the failures received more than 31 days of posaconazole. Posaconazole oral solution was well tolerated and was discontinued in only one subject due to a drug rash. Posaconazole appears promising as an oral therapy for zygomycosis in patients who receive required surgery and control their underlying illness.
Zygomycosis (also known as mucormycosis) is an infection caused by saprophytic fungi of the class Zygomycetes, which are primarily opportunists that invade immunocompromised hosts and produce angioinvasive disease (49). Transmission is mainly through inhalation of small airborne spores, by traumatic skin implantation, or by ingestion and translocation of the organism through the gut. Patients at highest risk for zygomycosis are those with (i) immunosuppression related to neutropenia, corticosteroid use, hematologic malignancies, and solid-organ transplants, (ii) diabetes mellitus, especially those with ketoacidosis, (iii) conditions of iron overload with associated desferoxamine use, and (iv) skin disruption by trauma or other serious conditions, such as burns or heatstroke. In recipients of a hematopoietic stem cell transplant (HSCT), infection often occurs during periods of graft-versus-host disease due to escalation of immunosuppressant regimens (1, 4, 12, 21, 25, 27, 36, 41, 44). Common sites of infection include pulmonary, rhinocerebral, or disseminated disease (36). The outcome of zygomycosis is closely related to the overall health of the patients and the control of their underlying diseases.
Roden and associates analyzed 929 cases of zygomycosis reported since 1885 (38). Survival was reported in 65% of patients with no underlying condition, 56% with diabetes, and 34% with malignancy. Survival varied with infection site: localized skin, 90%; rhinocerebral, 38%; lung, 24%; gastrointestinal, 15%; and disseminated, 4%. Survival was only 3% without any therapy and 70% in those patients with both required surgery and antifungal therapy (38). Recognized factors important in determining outcome are rapidity of diagnosis, immunocompetence, surgical debridement of devitalized tissue, and expeditious initiation of effective antifungal therapy.
Results of several recent studies suggest that the incidence of zygomycosis is increasing, especially among recipients of HSCTs (22, 27, 28, 42). The prognosis in neutropenic patients is especially poor, with a fatality rate of 56% to 100% (16, 23, 24, 29, 31). To date, the antifungal of choice has been amphotericin B at the highest tolerable doses (3, 12, 36). Because morbidity and mortality remain high, especially in immunocompromised patients, new and better treatments are needed to reduce death and disfigurements.
Posaconazole is a new extended-spectrum azole antifungal that has demonstrated more in vitro and in vivo activity than itraconazole against zygomycetes (34, 45, 46). Like other azoles, posaconazole inhibits sterol 14α-demethylation, resulting in faulty cell membrane synthesis; however, its sterol inhibitory activity in zygomycetes is better than that of itraconazole (26). Posaconazole is highly lipophilic, orally absorbed, and extensively distributed in tissues. Two clinical trials have evaluated oral posaconazole treatment as salvage therapy for invasive fungal infection (IFI) in patients who either were intolerant of standard therapy or had disease refractory to standard antifungal therapies (35, 43). This paper reports the first 24 cases of zygomycosis entered into these trials that were considered to have active infection at the time of enrollment.
(This information was presented in part as an abstract [R. N. Greenberg, G. Anstead, R. Herbrecht, A. Langston, K. Marr, K. Mullane, I. Raad, G. Schiller, M. Schuster, J.-A. H. van Burik, J. R. Wyngard, R. Hare, and G. Corcoran, Posaconazole (POS) experience in the treatment of zygomycosis, abstr. M-1757, p. 476. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., 2003].)
MATERIALS AND METHODS
Study design.
This report presents data collected from subjects included in two open-label, nonrandomized, multicentered compassionate trials to evaluate posaconazole in individuals with IFIs who were intolerant of or refractory to currently available antifungal therapy. The first trial began enrollment in February 1999, and the second trial remains open in 2005. The 24 subjects presented in this report were enrolled between 11 February 1999 and 23 April 2001 and were the first individuals with active zygomycosis infection evaluated for the safety and efficacy of posaconazole as salvage therapy for zygomycosis. All 24 patients either had a positive culture, obtained by a sterile technique from a normally sterile site, that was clinically or radiologically consistent with zygomycosis infection or had histopathology from a biopsy showing presence of predominantly aseptate (pauciseptate) wide, ribbon-like hyphae and the presence of tissue necrosis and angioinvasion (9).
Patient selection.
To be eligible for treatment with posaconazole, adult and pediatric subjects had to be diagnosed with proven, probable, or possible IFIs, as defined by an international consensus committee (2), that were refractory to standard antifungal therapy during appropriate antifungal therapy for at least 7 days, for which there were no effective antifungal agents, or that were intolerant to standard therapies because of toxicities. Exclusion criteria included significantly abnormal liver function tests or prolonged QTc intervals, concurrent or recent (within 1 week of enrollment) administration of drugs known to interact with azoles (including vinca alkaloids and astemizole), and a history of serious hypersensitivity or idiosyncratic reactions to azoles. All sites were required to have Institutional Review Board approval to conduct the trials, and all subjects were required to provide informed consent before their enrollment.
Treatment.
Posaconazole therapy was 800 mg/day in divided doses, either 400 mg twice daily or 200 mg four times per day, with food whenever possible; one exception was a pediatric patient who received a reduced dose of 200 mg three times per day (subject 18). The duration of treatment was based on response and on risk of zygomycosis relapsing due to continuing immunosuppression.
Pharmacokinetic sampling.
Serum trough levels of posaconazole were measured from seven patients enrolled in the two protocols (all with zygomycosis). Levels were drawn after at least 2 weeks of posaconazole treatment. Determination of serum posaconazole concentrations was performed as previously described (5). All available speciation data and sensitivity data are presented. MICs were determined by a broth microdilution method (45).
Data analysis.
Data for this report were gathered from study case report forms and from follow-up information provided by investigating physicians. A data review committee (R.N.G., K.M., and J.-A.H.V.) assessed all cases for inclusion and response to posaconazole. Complete response was defined as resolution of signs of infection with available microbiology data to support this observation and no relapse observed for at least 30 days after discontinuing posaconazole therapy. Partial response was defined as clinical and radiologic (if available) improvement during posaconazole treatment, with either no further evidence of active zygomycosis while on posaconazole or no follow-up information after discontinuing posaconazole. Failure was defined as the presence of zygomycosis at the time posaconazole treatment was discontinued or at the time of death. These definitions did not require any particular amount of improvement or specify a time limit for improvement. Adverse event information was acquired from case report forms and physicians' follow-up reports.
Statistical methods.
Survival was determined using the Kaplan-Meier method for three time-dependent events: survival from underlying disease, survival from zygomycosis, and actuarial survival (death from any cause). The association between a given time-dependent event and a given risk factor was determined by fitting a Cox proportional-hazards model to the data. Due to the small sample size (n = 24), each risk factor was considered separately for each survival endpoint. The risk factors considered were age, gender, reason for enrollment (intolerant to therapy versus refractory), surgery (no versus yes), the presence of diabetes mellitus, allogeneic HSCT, rhinocerebral infection, disseminated infection, and prophylaxis before zygomycosis infection. The association between each of these risk factors and outcome, recorded as success (complete cure or partial response) versus failure, was determined by Fisher's exact test and/or logistic regression analysis. Statistical significance was determined at the 0.05 level throughout.
RESULTS
Patients.
Nineteen patients were refractory to their therapy, and five were intolerant (all related to worsening renal function). Patient ages ranged from 7 years to 74 years, with a mean age of 46.8 years. Nineteen of 24 subjects were males (79%). The most common site of infection was rhinocerebral, documented in 11 cases (46%). There were four subjects with disseminated infections (rhinocerebral and lung; rhinocerebral, brain, lung, and skeletal muscle; brain and lung; and endocarditis with septic emboli.). Others had infection of the skin, bone, lung, trachea, or abdomen (Table 1).
TABLE 1.
Summary of 24 subjects treated with posaconazole (POS) for zygomycosis
| Subject no. | Age (yr)/sexa | Risk factor(s)b | Antifungal agents before POS (days) | Infection site | Organism | Surgery prior to POS | Reason for enrollment | Duration of POS therapy (days) | Outcomef (days after starting POS) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 57/M | CLL treated with alloBMT, ongoing GVHD | ITC (53), then AMB 1 mg/kg of body weight/day (3) | Rhinocerebral | Mucor sp. | Radical debridement of right face | Refractory: progressive sinusitis | 128 | CR (167) |
| 2 | 23/M | AML treated with alloBMT, ongoing GVHD | ITC (46), then LAM 12-15 mg/kg/day (13) | Skin (2.5-cm lesion) | —e | Radical skin excision that had normal margins | Refractory: continued fever | 86 | CR (121) |
| 3 | 58/M | Refractory anemia treated with alloBMT (×2) | FLC (95), then ABLC 5 mg/kg/day (12) with VRC (6), then CAS (4) | Rhinocerebral and peritonsillar abscess | Mucor sp. | Tonsillar debridement | Refractory: extension of sinus and tonsillar disease | 751 | Partial ethmoidectomy day 23; CR (791) |
| 4 | 46/F | CLL treated with alloBMT ×2, ongoing GVHD | ITC (48) with CAS (48) and with LAM 7.5 mg/kg/day (27) | Rhinocerebral and jaw | Mucor sp. | Sinus and ethmoid debridement ×2 | Refractory: progression of maxillary sinus disease | 256 | CR (286) |
| 5 | 54/F | CML treated with alloBMT, ongoing GVHD | ABLC 5 mg/kg/day (8), then LAM 7.5 mg/kg/day (18) | Disseminated (brain abscess, rhinocerebral, lung, and gastrocnemius muscle) | Cunninghamella sp. | None | Refractory: progression of brain lesion | 148 | PR; died day 148 due to failure to thrive |
| 6 | 17/M | AML treated with T-cell-depleted alloPBSCT, ongoing GVHD | ITC (279), then LAM 5 mg/kg/day (27) | Lung | Rhizopus stolonifer | Partial lobectomy | Refractory: progression of lung lesions | 1004 | PR |
| 7 | 29/M | CML treated with alloBMT, subsequent AML treated with alloBMT with donor lymphocyte infusion ×2, maintenance chemotherapy for relapsing AML | LAM 5 mg/kg/day, (10) | Rhinocerebral pharyngeal | — | None | Refractory: progression of sinus pharyngeal disease | 37 | PR; died day 37 from UD and bacterial pneumonia |
| 8 | 55/M | MDS treated with alloPBSCT, subsequent AML treated with alloPBSCT, ongoing GVHD | ITC (1,483), then VRC (36) | Rhinocerebral and jaw | Rhizopus sp. | Partial debridement of jaw and palate | Refractory: progression of palate, sinus, and brain lesions | 631 | ABLC 5 mg/kg/day and hyperbaric oxygen treatments days 92 to 268; CR (814) |
| 9 | 22/M | CML, subsequent ALL treated with alloBMT, ongoing GVHD | AMB 0.5 to 1.3 mg/kg/day (20) | Disseminated (lung, skin, and brain) | Cunninghamella sp. | None | Refractory: progression of lung lesions | 13 | Failure; died day 14 from clinical progression of skin and brain infection |
| 10 | 55/M | Metastatic renal cell carcinoma after nephrectomy treated with alloBMT, ongoing GVHD | VRC (136), then ITC (25) | GId (duodenal biopsy) | Rhizopus microsporus | None | Intolerant: elevated serum creatinine | 12 | Failure; POS discontinued day 12 after duodenal biopsy showed zygomycosis (no additional information) |
| 11 | 62/M | AML treated with alloBMT | FLC (18), then LAM 5 mg/kg/day, (6) with topical AMB 0.65 mg/kg/day (5) | Rhinocerebral | Mucor sp. | Enucleation, sphenoid and orbital debridement ×2 | Refractory: progression of sinus and orbital disease | 30 | Failure; died day 31 from zygomycosis |
| 12 | 57/M | SOT (kidney, heart) | AMB 1.3 mg/kg/day (34) | Chest, pericardium, and peritoneum | Rhizopus sp. | Chest, pericardial, and peritoneal debridement ×2 | Refractory: progression of chest and peritoneal lesions | 166 | CR (197) |
| 13 | 56/M | SOT (liver) | LAMc(111) | Rhinocerebral | — | Rhinotomy, maxillary debridement ×2 | Refractory: continued bony invasion | 66 | CR (100) |
| 14 | 42/M | SOT (kidney, pancreas) | ABLC 5 to 10 mg/kg/day (33) | Intestines and liver | Rhizopus sp. | Excision of necrotic jejunum | Refractory: enlarging liver abscess | 672 | CR (820) |
| 15 | 54/M | SOT (kidney) | FLC (2), then VRC (7), then ABLC 5 mg/kg/day (14) | Skin and bone | Mucor sp. | Debridement of skin and bone ×2 | Refractory: progression of bony invasion | 279 | Debridement of skin and bone days 73 and 85; PR |
| 16 | 61/M | Type 1 DM | LAMc(16) | Rhinocerebral | — | Caldwell Luc procedure | Intolerant: elevated serum creatinine | 294 | CR (1,212) |
| 17 | 67/M | Type 2 DM | AMBc(60) | Rhinocerebral | — | Debridement of antrum, sinuses, and palate ×2 | Refractory: progression of orbital, sinus, and palatal disease | 157 | POS stopped on day 157 due to a purpuric rash over face and upper shoulders; CR (331) |
| 18 | 7/F | Type 1 DM, chronic use of inhaled steroids for asthma | LAM 10 to 15 mg/kg/day (10) and intraorbital AMB | Rhinocerebral | Mucor sp. | Debridement of paranasal sinuses and orbit ×5 | Refractory: progression of orbital and sinus disease | 547 | POS, 200 mg TID, and LAM continued; hyperbaric oxygen and intraorbital AMB (day 1 to 14); PR |
| 19 | 74/F | Type 2 DM with renal disease | LAM 7 mg/kg/day, (5) | Trachea and neck | — | None | Intolerant: elevated serum creatinine | 352 | PR; died day 352 from noninfectious causes |
| 20 | 18/M | Type 1 DM, ALL, undergoing chemotherapy | AMB 1 to 1.5 mg/kg/day (21) | Skin | Rhizomucor sp. | Debridement | Intolerant: elevated serum creatinine | 901 | CR (1,084) |
| 21 | 50/M | AML | AMB (6), then LAM 7 mg/kg/day (69) | Rhinocerebral | Rhizopus sp. | Debridement of sinuses ×2 and partial extirpation of right eye | Refractory: progression of sinus and orbital disease | 197 | PR; died day 198 from UD |
| 22 | 65/M | Stage 4 NK-cell leukemia | LAM 6 mg/kg/day (59) | Lung | — | Wedge resection | Intolerant: renal disease | 245 | PR; died day 245 from UD |
| 23 | 62/F | Non-Hodgkin lymphoma | CAS (17) with ABLC 6 mg/kg/day (17) | Disseminated (rhinocerebral and lung) | Rhizomucor pusillus | None | Refractory: orbital extension of infection | 28 | Failure; died from zygomycosis day 29 |
| 24 | 32/M | RBC aplasia treated with antithymocyte globulin, cyclosporine, prednisone | FLC (>30), then CAS (2) with LAM 3 to 10 mg/kg/day (14 days prior to POS and continued for 8 days with POS) | Disseminated (endocarditis with septic emboli) | Cunninghamella sp. | Aortic valve replacement | Refractory: progression of brain and heart lesions | 8 | Failure; died day 8 from zygomycosis |
M, male; F, female.
ALL, acute lymphocytic leukemia; alloBMT, allogenic bone marrow transplant; alloPBSCT, allogenic peripheral-blood stem cell transplant; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; DM, diabetes mellitus; GVHD, graft-versus-host disease; MDS, myelodysplastic syndrome; NK, natural killer; RBC, red blood cell; SOT, solid-organ transplant.
Doses for LAM or AMB were not available for patients 13, 16, and 17.
GI, gastrointestinal tract.
—, patient had a negative culture but had histopathology for zygomycosis.
CR, complete response; PR, partial response; TID, three times a day; UD, underlying disease.
Subjects had a wide range of underlying risk factors for zygomycosis infection (Table 1). Eleven subjects had undergone HSCTs, which included bone marrow transplants (BMTs), and four others had diabetes mellitus without ketoacidosis. Six subjects with allogeneic HSCT had rhinocerebral infection, two had disseminated infection, and one subject each had lung, skin, or gastrointestinal infection. Three of the four subjects with diabetes mellitus as their main risk factor had rhinocerebral infections, while one patient had infection of the neck and trachea. Four others had solid-organ transplants, and their sites of infection were gastrointestinal, skin and underlying bone, rhinocerebral, and pericardial infections. One subject with red blood cell aplasia had infection of the heart. Other risk factors and sites of infection included acute myelogenous leukemia (AML) (rhinocerebral), acute lymphocytic leukemia (skin), NK-cell leukemia (lung), and non-Hodgkin lymphoma (disseminated).
Prior antifungal drug exposure.
Nine of 24 (38%) subjects received antifungal prophylaxis before the diagnosis of zygomycosis, including fluconazole (FLC), voriconazole (VRC), and itraconazole (ITC). Antifungal treatment that was administered before posaconazole was started included amphotericin B (AMB) in standard or lipid formulations (liposomal AMB [LAM] or AMB lipid complex [ABLC]), VRC, ITC, FLC, and caspofungin (CAS). At time of enrollment, patients were receiving LAM (12 patients); AMB (5 patients); ABLC (2 patients); ABLC and CAS (2 patients); LAM, CAS, and ITC (1 patient); ITC (1 patient); and VRC (1 patient).
Duration of posaconazole treatment.
Posaconazole was administered for an average of 292 days (range of 8 to 1,004 days) in this series of 24 subjects. The median duration of treatment was 182 days. Sixteen subjects (66%) continued posaconazole treatment for over 100 days, and one patient took the drug for 1,004 days. Random serum troughs from seven subjects enrolled in the protocols were assayed for their concentrations of posaconazole after more than 14 days of 800 mg/day posaconazole, and levels ranged from 0.309 μg/ml to 2.990 μg/ml. The mean ± standard error of the mean for the trough levels was 1.139 ± 0.341 μg/ml.
Outcome.
Table 1 lists the results of posaconazole treatment for individual subjects. The table includes information on timing of surgery in relation to starting posaconazole. Subjects were included in these salvage protocols because they had zygomycosis refractory to therapy (n = 19) or were intolerant of standard therapy (n = 5). Overall, 19 (79%) of 24 patients had a complete (11 patients) or partial (8 patients) response. Complete or partial response was seen in 79% of those subjects enrolled due to refractory zygomycosis and in 80% of those enrolled due to intolerance to standard antifungal therapy. Subjects with underlying risk factors that included long-term immunosuppression, such as hematological malignancies with allogeneic HSCT, and subjects with solid-organ transplants also had high rates of complete or partial response (73% and 100%, respectively). Four of 5 subjects who switched from amphotericin B to posaconazole and 14 of 17 who switched from a lipid formulation of amphotericin B to posaconazole (11 of 13 from liposomal amphotericin B and 3 of 4 from amphotericin B lipid complex) experienced either a complete or partial response.
For the 19 of 24 patients (79%) who did not fail, the underlying illness did not worsen and in most instances improved or resolved. Most investigators reported that improvement was evident within 2 weeks of initiating posaconazole. Failure was associated with progression of underlying illness and no more than 31 days of posaconazole. Four of the five failures are known to have died from zygomycosis.
Nine of 24 subjects died during the study. Two died from persistent zygomycosis when treatment was withdrawn at the request of the individual or legally appointed guardian (after 8 and 14 days of posaconazole therapy), and two died of persistent zygomycosis after 29 and 31 days of posaconazole therapy. Four subjects died from their underlying illnesses after initially improving during posaconazole treatment; they had received posaconazole for 37, 198, 245, and 352 days. One subject died on day 148 from aspiration pneumonia; this individual had been diagnosed with fungal lesions in the brain as part of disseminated zygomycosis. The brain lesions did not progress while taking posaconazole. This subject was considered a partial response.
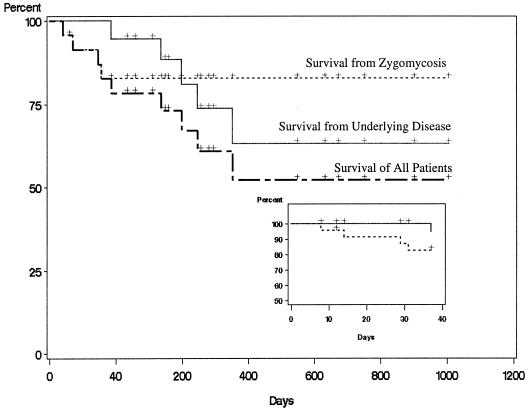
Kaplan-Meier plots of survival in the 24 subjects analyzed are provided in Fig. 1 for each of the three time-dependent events of interest. Most of the events occurred within 90 days after beginning therapy. The 90-day survival rates were 94.7% (± 5.1%) for survival from underlying disease, 82.8% (± 7.8%) for survival from zygomycosis, and 78.4% (± 8.6%) for survival from all causes of death. After 90 days, no further deaths were due to zygomycosis, but deaths due to underlying diseases continued to occur.
FIG. 1.
Kaplan-Meier survival curves for underlying disease, zygomycosis infection, and actuarial disease (all 24 subjects). Crosses indicate deaths or end of treatment with posaconazole. The solid line represents percent survival from underlying disease; it does not consider any specific antifungal therapy or surgery. Those deaths were due to complications from patients underlying condition and not due to zygomycosis. The dotted line represents percent survival from zygomycosis. Those deaths were due only to zygomycosis. The hatched line represents survival considering all causes. The inset expands the view of percent survival during the first 40 days of posaconazole treatment. All deaths from zygomycosis occurred early in treatment, suggesting posaconazole treatment for more than 30 days is associated with long-term survival. Deaths not due to zygomycosis but from underlying disease were more likely after 40 days of posaconazole treatment.
Of the 18 individuals who underwent surgical debridement, 15 had residual disease at the start of posaconazole therapy. Only 2 (13%) of these 15 patients had a failed outcome, compared to 3 of 6 (50%) having no surgery (P = 0.11 by Fisher's exact test). Surgery (n = 18) was marginally significantly associated with a reduced risk of experiencing a failed outcome (odds ratio, 0.12; 95% confidence interval, 0.01 to 1.10). Surgery was marginally significantly associated with a protective effect for the hazard of dying from underlying disease (P = 0.058; hazard rate, 0.17; 95% confidence interval, 0.03 to 1.07). That is, a subject was 5.7 times more likely to survive the underlying disease if he or she had surgery. Surgery was significantly associated with better overall survival (P = 0.02; hazard rate, 0.21; 95% confidence interval, 0.06 to 0.78).
Three of the 4 (75%) subjects with a disseminated infection had a failed outcome, compared to 2 of 20 (10%) subjects without a disseminated infection (P = 0.0018 by Fisher's exact test). Hence, a subject with a disseminated infection was 27 times more likely to have a failed outcome (95% confidence interval for the odds ratio, 1.8 to 399.2). Having disseminated disease was significantly associated with a reduced chance of overall survival (P = 0.0018; hazard rate, 28.56; 95% confidence interval, 3.49 to 233.88). No other risk factors were significantly associated with overall survival or outcome.
Microbiology.
Zygomycetes were isolated in culture from 17 of 24 (71%) subjects and were Rhizopus spp. (n = 6), Mucor spp. (n = 6), Cunninghamella spp. (n = 3), and Rhizomucor spp. (n = 2). Five of six Rhizopus sp. isolates, five of six Mucor sp. isolates, one of three Cunninghamella sp. isolates, and one of two Rhizomucor sp. isolates were associated with either complete or partial responses. The MIC for one Cunninghamella sp. isolate (patient 9, failure) was 1 μg/ml, and the MIC for one Rhizopus sp. isolate (patient 12, complete response) was 4 μg/ml. Data from the other isolates are not available. Also, to date, there are no interpretive guidelines for these data.
Adverse events.
One subject (patient 5) with a brain lesion at enrollment gradually lost visual acuity after 1 month of treatment. No definitive cause was identified, and the investigator rated the event as probably not related to the posaconazole. The individual continued to improve otherwise and remained on therapy with posaconazole for a total of 148 days. One subject, who was treated with posaconazole for 157 days, developed a non-life-threatening purpuric rash over the face and shoulders. The rash was first noticed towards the end of therapy and resolved shortly after posaconazole was discontinued (subject 17). In general, the oral suspension was well tolerated and no subject discontinued therapy due to the taste of the medication.
DISCUSSION
Twenty-four subjects with zygomycosis received salvage treatment with posaconazole. Nineteen of the 24 subjects (79%) who were treated with posaconazole, 17 of 19 (89%) as monotherapy, survived infection. Our data compare favorably with those reported for salvage treatments of zygomycosis with lipid formulations of amphotericin B. Five phase I and phase II studies demonstrated successful outcomes (cure or improvement) in 12 of 20 (60%) subjects treated with amphotericin B colloidal dispersion for invasive zygomycosis (14). In an open-label study of 556 subjects with IFIs who had refractory infections or were intolerant of conventional therapies, ABLC treatment resulted in complete or partial responses in 17 of 24 (71%) subjects with zygomycosis (50).
Posaconazole is highly lipophilic, orally absorbed, and extensively distributed in tissues. In an open-label, multicenter study evaluating the efficacy and safety of posaconazole as salvage therapy for patients with IFIs, interim analysis of the first 51 subjects demonstrated that the clinical response was ≥50% after 4 and 8 weeks of treatment (35). In phase II and phase III trials involving immunocompromised individuals, posaconazole had a safety profile similar to that of fluconazole (13, 32, 43, 47). In our 24 subjects, the only serious adverse event was a drug rash that was reported as probably associated with posaconazole (subject 17).
The MIC50 and MIC90 of posaconazole for zygomycetes are 0.25 and 1 to 4 μg/ml, respectively. Posaconazole appears slightly more active in vitro than itraconazole (1.6-fold lower MICs) and significantly more active than voriconazole and fluconazole against this group of organisms (45, 46). Rhizopus spp. are significantly less susceptible to posaconazole than other zygomycete species (6, 7). Posaconazole treatment of mice experimentally infected with zygomycetes has resulted in prolonged survival and reduced tissue burden for disseminated Mucor spp., no effects against Rhizopus oryzae (MIC of 0.25 μg/ml), partial benefit against Absidia corymbifera (MIC of 0.12 μg/ml), and a dose-response effect against Rhizopus microsporus (0.25 μg/ml) (45, 46).
The mean serum trough levels of posaconazole after at least 2 weeks of treatment were collected from seven patients entered into these trials, and the overall mean level was 1.139 μg/ml, with a range from 0.309 μg/ml to 2.990 μg/ml. All of these seven patients had zygomycosis, and all were complete cures. Clinical success rates of 83% (five of six patients) for Rhizopus spp., 83% (five of six patients) for Mucor spp., 50% (one of two patients) for Rhizomucor spp., and 33% (one of three patients) for Cunninghamella spp. were observed. Due to the small sample size and lack of peak serum levels, correlation of serum level with outcome based on species was not attempted.
Although itraconazole has an in vitro antifungal activity similar to that of posaconazole, it has different pharmacokinetics, and documentation of the clinical effectiveness of itraconazole against zygomycosis is limited to case reports (7, 8, 15, 39, 45). Voriconazole and fluconazole do not have significant in vitro activity against zygomycosis (7, 19, 33, 45). Reports from uncontrolled studies of combination therapy with azoles, echinocandins, or both plus amphotericin B show various outcomes (15, 18, 39, 48). Breakthrough zygomycosis has been described for patients receiving voriconazole, fluconazole, itraconazole, and caspofungin (17, 28, 30, 37, 40). Adjuvant therapy with granulocyte-macrophage colony-stimulating factor, interferon, granulocyte colony-stimulating factor, and/or hyperbaric oxygen therapy has been used successfully with some patients (10, 11, 20); however, there are no controlled studies to help define the use of adjuvant cytokine therapy. Thus, prior to the development of posaconazole, parenteral amphotericin B or a lipid formulation of amphotericin B has been the primary treatment option for patients with zygomycosis infections.
Mortality and morbidity associated with zygomycosis remain unacceptably high despite treatment with maximally tolerated doses of amphotericin B formulations and surgery. In our series, 4 of 5 subjects switching from amphotericin B to posaconazole and 14 of 17 subjects switching from a lipid formulation of amphotericin B to posaconazole experienced either a complete or a partial response during posaconazole therapy. These responses were associated with required surgical debridement and no worsening or improvement of underlying conditions. It is unknown whether the long half-life of polyenes in tissue might have contributed in a synergistic manner to the success seen with posaconazole.
Limitations to uncontrolled compassionate-use protocols such as these include lack of a complete set of pharmacologic data for these patients (collection of these data was not required by the protocols), lack of species identification and sensitivity data, and lack of some information on dosing of initial antifungal therapy. The small numbers of study patients limit the power of the statistical analyses. However, our case series suggests that oral posaconazole, given at 800 mg/day in divided doses, is an option for individuals who have failed amphotericin B therapy or who have dose-limiting toxicity. Further investigations are warranted to evaluate the role of posaconazole therapy for zygomycosis.
Acknowledgments
Schering-Plough Research Institute, Kenilworth, N.J., administered the clinical development program for posaconazole and funded these studies.
The contribution of the following individuals who participated in these studies as coinvestigators is gratefully acknowledged: Glenn Neel, William Lau, Stefano Tarantolo, Andrew Gilman, John Gullett, Joseph Solomkin, Carlos Bachier, and Michael Wong. We also express our gratitude to Cathy Bruno, Elizabeth Downs, and Lauren Scott for their assistance in manuscript preparation. Hua Zhu from the Department of Statistics, University of Kentucky, assisted R. J. Kryscio with the statistical analyses.
We received a clinical grant from the study sponsor for treatment of patients (M.J.A., G.A., R.N.G., R. Herbrecht, A.L., K.A.M., I.R., G.S., M.S., and J.-A.H.V.), a consultantship from the study sponsor (R. Herbrecht, K.A.M., S.G.R., M.S., and J.-A.H.V.), an outside research grant from the study sponsor (J.R.W.), stock in the study sponsor (G.C.), and we participated in company functions (e.g., advisory boards) for the study sponsor (C.E.G., R. Herbrecht, A.L., K.A.M., R.N.G., K.M., and J.-A.H.V.). R. Hare is an employee of the study sponsor, and G.C. was employed by the study sponsor at the time of the studies. R.J.K. has no conflict of interest.
REFERENCES
- 1.Anstead, G. M., D. A. Sutton, E. H. Thompson, I. Weitzman, R. A. Otto, and S. K. Ahuja. 1999. Disseminated zygomycosis due to Rhizopus schipperae after heatstroke. J. Clin. Microbiol. 37:2656-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, J. P., K. A. Vardulaki, C. Conlon, J. Cooke, P. Daza-Ramirez, E. G. Evans, P. M. Hawkey, R. Herbrecht, D. I. Marks, J. M. Moraleda, G. R. Park, S. J. Senn, and C. Viscoli. 2003. A systematic review of the antifungal effectiveness and tolerability of amphotericin B formulations. Clin. Ther. 25:1295-1320. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti, A., A. Das, A. Sharma, N. Panda, S. Das, K. L. Gupta, and V. Sakhuja. 2001. Ten years' experience in zygomycosis at a tertiary care centre in India. J. Infect. 42:261-266. [DOI] [PubMed] [Google Scholar]
- 5.Courtney, R., E. Radwanski, J. Lim, and M. Laughlin. 2004. Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men. Antimicrob. Agents Chemother. 48:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dannaoui, E., J. F. Meis, D. Loebenberg, and P. E. Verweij. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannaoui, E., J. Meletiadis, J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 51:45-52. [DOI] [PubMed] [Google Scholar]
- 8.Eisen, D. P., and J. Robson. 2004. Complete resolution of pulmonary Rhizopus oryzae infection with itraconazole treatment: more evidence of the utility of azoles for zygomycosis. Mycoses 47:159-162. [DOI] [PubMed] [Google Scholar]
- 9.Frater, J. L., G. S. Hall, and G. W. Procop. 2001. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch. Pathol. Lab. Med. 125:375-378. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Diaz, J. B., L. Palau, and G. A. Pankey. 2001. Resolution of rhinocerebral zygomycosis associated with adjuvant administration of granulocyte-macrophage colony-stimulating factor. Clin. Infect. Dis. 32:e145-e150. [DOI] [PubMed] [Google Scholar]
- 11.Gaviria, J. M., L. A. Grohskopf, R. Barnes, and R. K. Root. 1999. Successful treatment of rhinocerebral zygomycosis: a combined-strategy approach. Clin. Infect. Dis. 28:160-161. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez, C. E., M. G. Rinaldi, and A. M. Sugar. 2002. Zygomycosis. Infect. Dis. Clin. N. Am. 16:895-914. [DOI] [PubMed] [Google Scholar]
- 13.Graybill, J. R., I. Raad, R. Negroni, G. Corcoran, and L. Pedicone. 2004. Posaconazole (POS) long-term safety in patients with invasive fungal infections (IFIs), abstr. M-1025, p. 415. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 14.Herbrecht, R., V. Letscher-Bru, R. A. Bowden, S. Kusne, E. J. Anaissie, J. R. Graybill, G. A. Noskin, B. A. Oppenheim, E. Andrès, and L. A. Pietrelli. 2001. Treatment of 21 cases of invasive mucormycosis with amphotericin B colloidal dispersion. Eur. J. Clin. Microbiol. Infect. Dis. 20:460-466. [DOI] [PubMed] [Google Scholar]
- 15.Hunter, A. J., and R. E. Bryant. 2002. Abdominal wall mucormycosis successfully treated with amphotericin and itraconazole. J. Infect. 44:203-204. [DOI] [PubMed] [Google Scholar]
- 16.Husain, S., B. D. Alexander, P. Munoz, R. K. Avery, S. Houston, T. Pruett, R. Jacobs, E. A. Dominguez, J. G. Tollemar, K. Baumgarten, C. M. Yu, M. M. Wagener, P. Linden, S. Kusne, and N. Singh. 2003. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin. Infect. Dis. 37:221-229. [DOI] [PubMed] [Google Scholar]
- 17.Imhof, A., S. A. Balajee, D. N. Fredricks, J. A. Englund, and K. A. Marr. 2004. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin. Infect. Dis. 39:743-746. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs, P., L. Wood, A. Du Toit, and K. Esterhuizen. 2003. Eradication of invasive mucormycosis—effectiveness of the echinocandin FK463. Hematology 8:119-123. [DOI] [PubMed] [Google Scholar]
- 19.Jeu, L., F. J. Piacenti, A. G. Lyakhovetskiy, and H. B. Fung. 2003. Voriconazole. Clin. Ther. 25:1321-1381. [DOI] [PubMed] [Google Scholar]
- 20.John, B. V., G. Chamilos, and D. P. Kontoyiannis. 2005. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin. Microbiol. Infect. 11:515-517. [DOI] [PubMed] [Google Scholar]
- 21.Joshi, N., G. M. Caputo, M. R. Weitekamp, and A. W. Karchmer. 1999. Infections in patients with diabetes mellitus. N. Engl. J. Med. 341:1906-1912. [DOI] [PubMed] [Google Scholar]
- 22.Kontoyiannis, D. P., M. S. Lionakis, R. E. Lewis, G. Chamilos, M. Healy, C. Perego, A. Safdar, H. Kantarjian, R. Champlin, T. J. Walsh, and I. I. Raad. 2005. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J. Infect. Dis. 191:1350-1360. [DOI] [PubMed] [Google Scholar]
- 23.Kontoyiannis, D. P., V. C. Wessel, G. P. Bodey, and K. V. Rolston. 2000. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin. Infect. Dis. 30:851-856. [DOI] [PubMed] [Google Scholar]
- 24.Lee, F. Y., S. B. Mossad, and K. A. Adal. 1999. Pulmonary mucormycosis: the last 30 years. Arch. Intern. Med. 159:1301-1309. [DOI] [PubMed] [Google Scholar]
- 25.Maertens, J., H. Demuynck, E. K. Verbeken, P. Zachee, G. E. Verhoef, P. Vandenberghe, and M. A. Boogaerts. 1999. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 24:307-312. [DOI] [PubMed] [Google Scholar]
- 26.Mann, P. A., R. Patel, G. Chen, and P. M. McNicholas. 2004. Posaconazole is a potent inhibitor of sterol 14α-demethylation in zygomycetes, abstr. M-1978, p. 442. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 27.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 28.Marty, F. M., L. A. Cosimi, and L. R. Baden. 2004. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N. Engl. J. Med. 350:950-952. [DOI] [PubMed] [Google Scholar]
- 29.Nosari, A., P. Oreste, M. Montillo, G. Carrafiello, M. Draisci, G. Muti, A. Molteni, and E. Morra. 2000. Mucormycosis in hematologic malignancies: an emerging fungal infection. Haematologica 85:1068-1071. [PubMed] [Google Scholar]
- 30.Oliveira, J. S., F. R. Kerbauy, A. L. Colombo, D. M. Bahia, G. S. Pinheiro, M. R. Silva, M. S. Ribeiro, G. Raineri, and J. Kerbauy. 2002. Fungal infections in marrow transplant recipients under antifungal prophylaxis with fluconazole. Braz. J. Med. Biol. Res. 35:789-798. [DOI] [PubMed] [Google Scholar]
- 31.Pagano, L., P. Ricci, A. Tonso, A. Nosari, L. Cudillo, M. Montillo, A. Cenacchi, L. Pacilli, F. Fabbiano, A. Del Favero, et al. 1997. Mucormycosis in patients with haematological malignancies: a retrospective clinical study of 37 cases. Br. J. Haematol. 99:331-336. [DOI] [PubMed] [Google Scholar]
- 32.Perfect, J. R., D. R. Graham, G. Corcoran, L. Pedicone, and I. Raad. 2004. Posaconazole (POS) safety and efficacy in elderly (≥65 years of age) patients with invasive fungal infections (IFIs), abstr. 630, p. 164. Abstr. 42nd Annu. Meet. Infect. Dis. Soc. Am. Infectious Diseases Society of America, Alexandria, Va.
- 33.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 34.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and the Sentry Participants Group. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raad, I., S. Chapman, R. Bradsher, V. Morrison, M. Goldman, J. Graybill, J. R. Perfect, T. Patterson, T. Walsh, G. Corcoran, and P. Pappas. 2004. Posaconazole (POS) salvage therapy for invasive fungal infections (IFI), abstr. M-669, p. 412. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 36.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rickerts, V., A. Bohme, A. Viertel, G. Behrendt, V. Jacobi, K. Tintelnot, and G. Just-Nubling. 2000. Cluster of pulmonary infections caused by Cunninghamella bertholletiae in immunocompromised patients. Clin. Infect. Dis. 31:910-913. [DOI] [PubMed] [Google Scholar]
- 38.Roden, M. M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaufele, M. Sein, T. Sein, C. C. Chiou, J. H. Chu, D. P. Kontoyiannis, and T. J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 39.Roy, V., L. I. Ali, T. H. Carter, and G. B. Selby. 2000. Successful non-surgical treatment of disseminated polymicrobial fungal infection in a patient with pancytopenia and graft-versus-host disease. J. Infect. 41:273-275. [DOI] [PubMed] [Google Scholar]
- 40.Safdar, A., S. O'Brien, and I. F. Kouri. 2004. Efficacy and feasibility of aerosolized amphotericin B lipid complex therapy in caspofungin breakthrough pulmonary zygomycosis. Bone Marrow Transplant. 34:467-468. [DOI] [PubMed] [Google Scholar]
- 41.Sharma, R. R., S. J. Pawar, A. Delmendo, S. D. Lad, and S. D. Athale. 2001. Fatal rhino-orbito-cerebral mucormycosis in an apparently normal host: case report and literature review. J. Clin. Neurosci. 8:583-586. [DOI] [PubMed] [Google Scholar]
- 42.Siwek, G. T., K. J. Dodgson, M. de Magalhaes-Silverman, L. A. Bartelt, S. B. Kilborn, P. L. Hoth, D. J. Diekema, and M. A. Pfaller. 2004. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin. Infect. Dis. 39:584-587. [DOI] [PubMed] [Google Scholar]
- 43.Skiest, D., D. Ward, A. Northland, J. Reynes, and W. Greaves. 1999. Treatment of azole-refractory candidiasis in HIV disease, abstr. 1162, p. 491. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 44.Stern, L. E., and R. J. Kagan. 1999. Rhinocerebral mucormycosis in patients with burns: case report and review of the literature. J. Burn Care Rehabil. 20:303-306. [DOI] [PubMed] [Google Scholar]
- 45.Sun, Q. N., A. W. Fothergill, D. I. McCarthy, M. G. Rinaldi, and J. R. Graybill. 2002. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob. Agents Chemother. 46:1581-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun, Q. N., L. K. Najvar, R. Bocanegra, D. Loebenberg, and J. R. Graybill. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob. Agents Chemother. 46:2310-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vazquez, J. A., R. Northland, S. Miller, G. Dickinson, and G. Wright. 2000. Posaconazole compared to fluconazole for oral candidiasis in HIV-positive patients, abstr. 1107, p. 372. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 48.Voitl, P., C. Scheibenpflug, T. Weber, O. Janata, and A. M. Rokitansky. 2002. Combined antifungal treatment of visceral mucormycosis with caspofungin and liposomal amphotericin B. Eur. J. Clin. Microbiol. Infect. Dis. 21:632-634. [DOI] [PubMed] [Google Scholar]
- 49.Walsh, T. J., A. Groll, J. Hiemenz, R. Fleming, E. Roilides, and E. Anaissie. 2004. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 10(Suppl. 1):48-66. [DOI] [PubMed] [Google Scholar]
- 50.Walsh, T. J., J. W. Hiemenz, N. L. Seibel, J. R. Perfect, G. Horwith, L. Lee, J. L. Silber, M. J. DiNubile, A. Reboli, E. Bow, J. Lister, and E. J. Anaissie. 1998. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 26:1383-1396. [DOI] [PubMed] [Google Scholar]