Abstract
Shigella spp. infect approximately 450,000 persons annually in the United States, resulting in over 6,000 hospitalizations. Since 1999, the National Antimicrobial Resistance Monitoring System (NARMS) for Enteric Bacteria has tested every 10th Shigella isolate from 16 state or local public health laboratories for susceptibility to 15 antimicrobial agents. From 1999 to 2002, NARMS tested 1,604 isolates. Among 1,598 isolates identified to species level, 1,278 (80%) were Shigella sonnei, 295 (18%) were Shigella flexneri, 18 (1%) were Shigella boydii, and 7 (0.4%) were Shigella dysenteriae. Overall, 1,251 (78%) were resistant to ampicillin and 744 (46%) were resistant to trimethoprim-sulfamethoxazole (TMP-SMX). Prevalence of TMP-SMX- or ampicillin- and TMP-SMX-resistant Shigella sonnei isolates varied by geographic region, with lower rates in the South and Midwest regions (TMP-SMX resistance, 27% and 30%, respectively; ampicillin and TMP-SMX resistance, 25% and 22%, respectively) and higher rates in the East and West regions (TMP-SMX resistance, 66% and 80%, respectively; ampicillin and TMP-SMX resistance, 54% and 65%, respectively). Nineteen isolates (1%) were resistant to nalidixic acid (1% of S. sonnei and 2% of S. flexneri isolates); 12 (63%) of these isolates had decreased susceptibility to ciprofloxacin. One S. flexneri isolate was resistant to ciprofloxacin. All isolates were susceptible to ceftriaxone. Since 1986, resistance to ampicillin and TMP-SMX has dramatically increased. Shigella isolates in the United States remain susceptible to ciprofloxacin and ceftriaxone.
Shigellosis is an important cause of gastroenteritis, resulting in an estimated 450,000 cases in the United States each year (27). Shigella infections can lead to illness ranging from mild, self-limited diarrhea to severe dysentery with frequent passage of blood and mucus, high fever, cramps, tenesmus, and in rare cases, bacteremia. Complications of shigellosis are seen most frequently in children, the elderly, and the immunocompromised. Prompt treatment with effective antimicrobial agents may shorten the duration of clinical symptoms and carriage and reduce the spread of infection (36).
Antimicrobial resistance has complicated the selection of empirical agents for treatment of shigellosis, particularly in children. When the prevalence of resistance to ampicillin among Shigella isolates increased in the 1970s, trimethoprim-sulfamethoxazole (TMP-SMX) became the alternative (31). In 1986, national laboratory-based surveillance for antimicrobial susceptibility of Shigella isolates revealed that 32% were resistant to ampicillin and 7% were resistant to TMP-SMX (46). In 1995, laboratory-based surveillance demonstrated resistance to ampicillin in 67% and resistance to TMP-SMX in 35% of Shigella isolates (15). More recent data from Oregon revealed high rates of TMP-SMX (63%), ampicillin (59%), and multidrug (13%) resistance among Shigella isolates from this state (35).
National surveillance for antimicrobial resistance among Shigella isolates began in 1999 as part of the National Antimicrobial Resistance Monitoring System (NARMS) for Enteric Bacteria (10). To describe trends in antimicrobial resistance among Shigella isolates from 1999 through 2002, we analyzed NARMS data for the first 4 years of surveillance. Our findings indicate an increase in the rates of resistance of Shigella isolates to ampicillin and TMP-SMX and evidence of emerging resistance to nalidixic acid.
MATERIALS AND METHODS
Surveillance and antimicrobial susceptibility testing.
NARMS was established in 1996 to monitor antimicrobial resistance of human nontyphoid Salmonella and Escherichia coli O157 isolates (10). Shigella was added to NARMS surveillance in 1999, and annual reports are available electronically (10). Public health laboratories in 15 states (Colorado, Connecticut, Florida, Georgia, Kansas, Maryland, Massachusetts, Minnesota, New Jersey, New York, Oregon, Tennessee, Washington, and West Virginia) and two local health departments (Los Angeles County and New York City) participated in Shigella surveillance in NARMS. In all sites, participating public health laboratories were requested to send every 10th Shigella isolate received from clinical laboratories to the Centers for Disease Control and Prevention (CDC) for antimicrobial susceptibility testing. Duplicate isolates from the same patient are not routinely excluded by laboratories; however, duplicate submission of Shigella isolates is rarely identified at the CDC. Participating public health laboratories reported the patient's age, date of birth, sex, and county of residence, the specimen source (stool, blood, or other), the date of isolate collection, and the date of isolate receipt at the state public health laboratories. For our analysis, we combined isolates from New York City and New York State as New York, for a total of 16 sites. Postcensus estimates for the population in these 16 sites in 2000 was 105 million persons, or 37% of the U.S. population in 2000 (48).
Shigella isolates were tested at the CDC with a semiautomated system (Sensititre; Trek Diagnostics, Westlake, OH) to determine the MIC ranges of 15 antimicrobial agents: amikacin, ampicillin, amoxicillin-clavulanic acid, ceftiofur, ceftriaxone, cephalothin, chloramphenicol, ciprofloxacin, gentamicin, kanamycin, nalidixic acid, streptomycin, sulfamethoxazole, tetracycline, and trimethoprim-sulfamethoxazole. Ceftiofur is the only expanded-spectrum cephalosporin approved for systemic use in food animals in the United States and is included in NARMS because the use of this antimicrobial in food animals has been implicated as a factor responsible for the emergence of ceftriaxone-resistant enteric pathogens, such as Salmonella (16). This has not been documented for Shigella. MIC results were dichotomized (isolates with intermediate susceptibility were categorized as sensitive), and CLSI (formerly NCCLS) criteria were used when applicable (14). Ceftiofur resistance was defined as a MIC of ≥8 μg/ml based on population distributions of MICs for gram-negative isolates. Decreased susceptibility to ciprofloxacin was defined as a MIC of ≥0.125 μg/ml.
Statistical analysis.
Statistical analysis focused on the relationships between antimicrobial resistance patterns and Shigella serotype, geographic location, and age and sex of the patient. We defined a pansensitive isolate as one that was sensitive to all antimicrobial agents included in the analysis. We defined multidrug resistance as resistance to two or more classes of antimicrobial agents (tested separately) among all classes included in the analysis. To calculate incidence rates by geographic region, we grouped participating sites into four regions: West (Los Angeles County, Oregon, and Washington), Midwest (Colorado, Kansas, and Minnesota), South (Florida, Georgia, Maryland, Tennessee, and West Virginia), and Northeast (Connecticut, Massachusetts, New York, and New Jersey). Region-specific rates of isolation per 100,000 population were determined for the study period with 2000 census data from the U.S. Bureau of the Census (48).
We compared the number of Shigella isolates submitted to NARMS from 1999 to 2002 to the number of laboratory-confirmed Shigella infections reported to the CDC via the Public Health Laboratory Information System (PHLIS) (11). Unlike the NARMS surveillance system, PHLIS is used by public health laboratory directors and state and territorial epidemiologists from every state in the United States to report all laboratory-confirmed Shigella isolates. Therefore, the comparison with PHLIS provides information about the generalizability of the data gathered through NARMS. In addition, we compared the proportion of Shigella isolates resistant to TMP-SMX and ampicillin found in our study to two previous national laboratory-based surveillance studies to examine trends in resistance to these agents (15, 46).
Statistical analyses were conducted using SAS version 8 (SAS Institute Inc., Cary, NC) statistical software. We used χ2 tests to compare proportions and Fisher's exact test when appropriate. Continuous variables were compared by the Wilcoxon rank-sum test. P values are based on two-tailed test results, and P values of <0.05 were considered statistically significant.
RESULTS
During the 4-year period 1999 to 2002, the CDC received and tested 1,604 Shigella isolates from participating NARMS sites included in this analysis. This represents approximately 9% of 17,432 culture-confirmed cases reported to PHLIS by these sites. Among the 1,604 isolates, 275 (17%) were from New York (of which 192 [70%] were from New York City), 197 (12%) were from New Jersey, 177 (11%) were from Georgia, 166 (10%) were from Minnesota, 147 (9%) were from Massachusetts, and 642 (40%) were from other sites. Among 1,598 isolates identified to species level, 1,278 (80%) were Shigella sonnei (compared with approximately 82% reported through PHLIS for this time period and from these sites), 295 (18%) were Shigella flexneri (versus 16%), 18 (1%) were Shigella boydii (versus 0.7%), and 7 (0.4%) were Shigella dysenteriae (versus 0.4%). Most Shigella isolates were isolated from specimens collected from stool (97%).
Geographic distribution.
During the 4-year study period, the number of Shigella isolates received per 100,000 population was 1.75 in the Northeast, 1.51 in the South, 2.30 in the Midwest, and 1.10 in the West. S. sonnei accounted for 76% of all Shigella isolates from the Northeast, 89% from the South, 87% from the Midwest, and 62% from the West. S. flexneri accounted for 22% of all Shigella isolates from the Northeast, 10% from the South, 12% from the Midwest, and 35% from the West. S. boydii accounted for 1% of isolates from the Northeast, 1% from the South, 0.7% from the Midwest, and 2% from the West. S. dysenteriae accounted for 1% of isolates from the Northeast and 0% from the South, Midwest, and West.
Age and gender.
Information on patient age was available for 1,442 (90%) of 1,598 isolates identified to species level that were received during the study period. The median age was 8 years (range, <1 year to 99 years), and 51% were male. Infants and children from 1 to 4 years of age accounted for the highest proportion of S. sonnei (32%) and S. flexneri (29%) isolates submitted to NARMS. In the PHLIS data reported in 2002, infants and children aged 1 to 4 years accounted for a similarly high proportion of infections with S. sonnei (33%) and S. flexneri (26%) (11). Consistent with PHLIS data, persons from whom S. sonnei isolates were submitted to NARMS were younger than those with S. flexneri (median age, 8 years versus 12 years; P = 0.03). Overall, more S. flexneri isolates in NARMS were from males than females (20% versus 17%; P = 0.09), particularly among persons 20 to 49 years of age (31% versus 16%; P < 0.01).
Seasonality.
Among the 1,278 S. sonnei isolates, 233 (18%) were isolated in the winter quarter (December, January, and February), 282 (22%) in the spring (March, April, and May), 412 (32%) in the summer (June, July, and August), and 351 (27%) in the fall (September, October, and November). Among S. flexneri isolates, similar proportions of isolates (24% to 27%) were isolated in each quarter.
Antimicrobial resistance.
Among the 1,604 Shigella isolates tested, 115 (7%) were pansusceptible (97 [8%] of 1,278 S. sonnei, 14 [5%] of 295 S. flexneri, 3 [17%] of 18 S. boydii, and 1 [14%] of 7 S. dysenteriae isolates). Large proportions of isolates were resistant to ampicillin (1,251 [78%]), streptomycin (891 [56%]), sulfamethoxazole (757 [47%]), and TMP-SMX (744 [46%]). Resistance to both ampicillin and TMP-SMX was seen in 613 (38%) of isolates (Table 1).
TABLE 1.
Antimicrobial resistance of Shigella isolates by species, United States, 1999 to 2002
| Antimicrobial agent or characteristic | Breakpoint(s) (R) | % Resistant
|
|||||
|---|---|---|---|---|---|---|---|
| All isolates (n = 1,604) | S. sonnei (n = 1,278) | S. flexneri (n = 295) | S. boydii (n = 18) | S. dysenteriae (n = 7) | Unknown (n = 6) | ||
| Amikacin | ≥64 | 0.06 | 0.08 | 0 | 0 | 0 | 0 |
| Amoxicillin-clavulanic acid | ≥32/16 | 2 | 2 | 5 | 0 | 0 | 0 |
| Ampicillin | ≥32 | 78 | 79 | 76 | 0 | 71 | 100 |
| Ceftiofura | ≥8 | 0.06 | 0 | 0.3 | 0 | 0 | 0 |
| Ceftriaxone | ≥64 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cephalothin | ≥32 | 6 | 7 | 3 | 0 | 0 | 0 |
| Chloramphenicol | ≥32 | 14 | 1 | 70 | 0 | 43 | 67 |
| Ciprofloxacin | ≥4 | 0.06 | 0 | 0.3 | 0 | 0 | 0 |
| Gentamicin | ≥16 | 0.2 | 0.2 | 0.3 | 0 | 0 | 0 |
| Kanamycin | ≥64 | 0.9 | 0.8 | 1 | 0 | 0 | 0 |
| Nalidixic acid | ≥32 | 1 | 1 | 2 | 6 | 0 | 0 |
| Streptomycina | ≥64 | 56 | 55 | 55 | 72 | 71 | 83 |
| Sulfamethoxazole | ≥512 | 47 | 45 | 54 | 78 | 71 | 50 |
| Tetracycline | ≥16 | 45 | 33 | 92 | 50 | 71 | 83 |
| TMP-SMX | ≥4/76 | 46 | 48 | 40 | 39 | 57 | 50 |
| Ampicillin and TMP-SMX | ≥32 and ≥4/76 | 38 | 39 | 35 | 0 | 43 | 50 |
| % Pansusceptible | 7 | 8 | 5 | 17 | 14 | 0 | |
| % Multiresistantb | 64 | 59 | 85 | 83 | 71 | 100 | |
No CLSI interpretive standards for this antimicrobial agent. The MIC for ceftiofur was based on population distributions of MICs for gram-negative isolates. The MIC of streptomycin is based on the Canadian Integrated Program for Antimicrobial Resistance (19).
Defined as resistant to two or more antimicrobial agents.
Similarly high proportions of S. sonnei and S. flexneri isolates were resistant to ampicillin (79% and 76%; P = 0.18), but resistance to TMP-SMX was more common among S. sonnei than among S. flexneri isolates (48% versus 40%; P = 0.02) (Table 1). Resistance to both ampicillin and TMP-SMX was similar among S. sonnei and S. flexneri isolates (39% and 35%; P = 0.19). However, resistance to chloramphenicol was common among S. flexneri isolates and extremely rare among S. sonnei isolates (70% versus 1%; P < 0.01).
None of the Shigella isolates tested were resistant or had decreased susceptibility to ceftriaxone (MIC ≥ 4 μg/ml). Only one isolate (S. flexneri) was resistant to ciprofloxacin; it was obtained from a child who recently traveled to China (7). In addition, 19 (1%) isolates (13 S. sonnei, 5 S. flexneri, and 1 S. boydii isolate) were resistant to nalidixic acid; 12 (63%) of these isolates also had decreased susceptibility (MIC > 0.125 μg/ml) to ciprofloxacin.
Among the 1,604 isolates tested, 1,031 (64%) were resistant to two or more agents (multidrug resistant). Common resistance patterns included the combination of ampicillin, streptomycin, sulfamethoxazole, and tetracycline resistance (322 [31%]), ampicillin, streptomycin, and sulfamethoxazole resistance (149 [14%]), and ampicillin and streptomycin resistance (108 [10%]). Resistance to the combination of ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline was seen in 85 (8%) isolates.
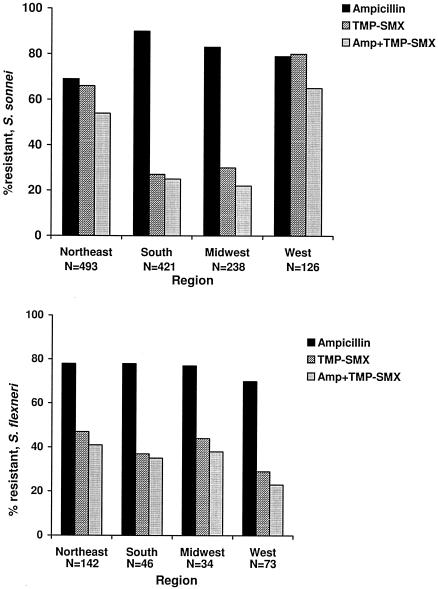
The prevalence of TMP-SMX- or ampicillin- and TMP-SMX-resistant Shigella sonnei isolates varied by geographic region (Fig. 1). Among S. sonnei isolates, 66% of 493 in the Northeast, 27% of 421 in the South, 30% of 238 in the Midwest, and 80% of 126 in the West were resistant to TMP-SMX. Among S. sonnei isolates, 54% of 493 in the Northeast, 25% of 421 in the South, 22% of 238 in the Midwest, and 65% of 126 in the West were resistant to both ampicillin and TMP-SMX. No geographic variability was found among S. flexneri isolates; 47% of 142 in the Northeast, 37% of 46 in the South, 44% of 34 in the Midwest, and 29% of 73 in the West were resistant to TMP-SMX. Among S. flexneri isolates, 41% of 142 in the Northeast, 35% of 46 in the South, 38% of 34 in the Midwest, and 23% of 73 in the West were resistant to both ampicillin and TMP-SMX.
FIG. 1.
Percentage of S. sonnei and S. flexneri isolates resistant to TMP-SMX, ampicillin (Amp), or both by region from 1999 to 2002.
Resistance rates among S. sonnei isolates differed between sexes. Specifically, S. sonnei isolates from males were more likely to be resistant to TMP-SMX or to both ampicillin and TMP-SMX than S. sonnei isolates from females (53% versus 45% resistance to TMP-SMX, P = 0.01, and 45% versus 36% resistance to ampicillin and TMP-SMX, P < 0.01, respectively). There were no significant sex differences in infection with TMP-SMX-resistant S. flexneri in males and females (42% and 39%; P = 0.58) or with ampicillin- and TMP-SMX-resistant S. flexneri in males and females (38% and 33%; P = 0.43). Persons infected with TMP-SMX-resistant S. sonnei were significantly older than persons infected with TMP-SMX-susceptible strains (9 years versus 7 years; P < 0.01). Otherwise, there were no statistically significant differences in median age between resistant and susceptible strains of S. sonnei or S. flexneri. The relatively greater prevalence of antimicrobial-resistant S. sonnei in males was observed in each of the four regions.
Overall, the proportion of Shigella isolates resistant to ampicillin, TMP-SMX, and both agents increased since national surveys were conducted in 1986 and 1995. Over this 16-year period, the proportion resistant to ampicillin increased from 32% to 67% to 78%, the proportion resistant to TMP-SMX increased from 7% to 35% to 46%, and the proportion resistant to both agents increased from 6% to 19% to 38% (15, 46).
DISCUSSION
Antimicrobial resistance among Shigella spp. is common in the United States, and between 1999 and 2002, only 7% of isolates reported through NARMS were pansensitive. Resistance to TMP-SMX or to both ampicillin and TMP-SMX was more common among S. sonnei than S. flexneri isolates, while resistance to chloramphenicol was more common among S. flexneri isolates, findings consistent with previous surveillance studies (15, 46). The proportion of isolates resistant to ampicillin, TMP-SMX, or both has increased substantially in the last decade, and these agents are no longer appropriate for empirical treatment in most parts of the country. The comparison of NARMS data with national laboratory-based surveillance data collected through PHLIS for the same time period suggests that NARMS captures a demographically representative sample of the total population of individuals infected with Shigella in the United States.
Few Shigella isolates were resistant to nalidixic acid (n = 19) or ciprofloxacin (n = 1), and none was resistant to ceftriaxone. While these agents may offer reliable results when used for empirical treatment of shigellosis, clinicians and public health officials should anticipate increasing resistance to them as their use increases. Clinical experience also supports the use of macrolides, such as azithromycin, for therapy of shigellosis; however, NARMS does not currently include data on susceptibility to any representative of this class of antimicrobial agents (24).
A majority of isolates that were resistant to nalidixic acid also had decreased susceptibility to ciprofloxacin. Although clinical failures to treat nalidixic acid-resistant Shigella infection with fluoroquinolones have not been reported, clinicians should be aware that failures have been reported for Salmonella infections caused by strains that were resistant to nalidixic acid and had decreased susceptibility to ciprofloxacin (13, 40).
There were regional differences in the prevalence of TMP-SMX resistance among Shigella sonnei isolates. TMP-SMX resistance was found in 30% of S. sonnei isolates in the Midwest NARMS sites compared to 66% and 80% of S. sonnei isolates from the Northeast and West, respectively. A similar trend was seen for isolates of S. sonnei resistant to both ampicillin and TMP-SMX. The geographic differences in resistance patterns may be a result of clonal spread of isolates. One study conducted during an outbreak of shigellosis in a day-care center revealed that pulsed-field gel electrophoresis and antimicrobial susceptibility patterns were identical in all outbreak strains, while nonoutbreak strains isolated during the outbreak period had indistinguishable pulsed-field gel electrophoresis patterns but different antimicrobial susceptibility patterns. Therefore, the occurrence of outbreaks of shigellosis in areas captured by NARMS sites in the Northeast or West regions could account for the geographic differences in resistance patterns observed. Because molecular typing of organisms is not routinely performed by NARMS, we cannot exclude this possibility.
Our study confirms that infection with S. sonnei is particularly common among young children and among women aged 20 to 40 years, while S. flexneri infections occur more frequently in adult men aged 30 to 49 years. The higher median age among persons infected with S. flexneri and the relatively greater proportion of S. flexneri isolates in adult men may reflect the occurrence of sexually transmitted shigellosis in the population of men who have sex with men (MSM), although outbreaks of S. sonnei infections have also recently been reported in this community (12, 45). Our data indicate that persons with TMP-SMX-resistant S. sonnei infection were more likely to be men and were older than persons with TMP-SMX-susceptible infections. The recently reported outbreak of S. sonnei among MSM in California was caused by a TMP-SMX-resistant strain. Furthermore, a high rate of human immunodeficiency virus infection has been reported among MSM with S. sonnei infection in the Bay Area (6). The widespread use of TMP-SMX for prophylaxis of pneumocystis pneumonia among MSM may predispose this population of adult men to TMP-SMX-resistant S. sonnei infections. An extensive outbreak of TMP-SMX-sensitive S. sonnei infection among day-care center attendees in the mid-Atlantic regions from 2001 to 2003 may also have contributed to the relatively lower percentage of TMP-SMX resistance among S. sonnei isolates from the South and Midwest and to the lower median age of persons infected with TMP-SMX-susceptible strains (9).
Transmission of shigellosis is primarily fecal-oral, and no Shigella vaccines are available in the United States. Because it takes only few organisms to transmit the infection, hand washing has been promoted as the single most important control measure to reduce the spread of shigellosis (23) and is especially critical in limiting the spread of shigellosis among young children in day-care centers (28). Many state health departments require exclusion of food handlers, health-care workers, child-care providers, and children who attend day care centers while they are symptomatic and until one or more negative stool cultures have been obtained (41). In these situations, appropriate antimicrobial use can greatly reduce the inconvenience and cost incurred during outbreaks, since most persons will cease to excrete Shigella within 72 h of starting appropriate antimicrobial therapy compared with carriage of up to several weeks that can occur without therapy (26).
The high prevalence of antimicrobial resistance among Shigella isolates noted in this study limits safe and efficacious treatment options for shigellosis, particularly for children. Where resistance to ampicillin and TMP-SMX is common, appropriate antimicrobial agents for the treatment of shigellosis are limited to nalidixic acid or fluoroquinolones, ceftriaxone, or azithromycin (18, 24, 42). Nalidixic acid (55 mg/kg/24 h in four divided doses for 5 days), a narrow-spectrum quinolone, is effective and approved in the United States for treatment of children older than 3 months (18). The broader-spectrum fluoroquinolones, while effective in treating shigellosis in children, are not approved by the U.S. Food and Drug Administration for use in children aged 18 years or younger because some fluoroquinolones have been shown to cause cartilage damage in juvenile animals (8). However, ciprofloxacin, the fluoroquinolone most extensively studied in children, has been successfully used to treat acute invasive diarrhea in children without the development of joint abnormalities (25, 37). Fluoroquinolones may be justified in children, after risks and benefits of treatment are discussed with parents, when no other oral agent is available and in cases of severe shigellosis caused by a multidrug-resistant strain (4).
Mobile genetic units, including plasmids, gene cassettes in integrons, and transposons, are important in the spread of resistant determinants among Shigella isolates (32, 47). Trimethoprim and sulfamethoxazole resistance is most commonly acquired through a plasmid-encoded variant of the dihydrofolate reductase enzyme (20). Ampicillin resistance arises as a result of beta-lactamases similar to TEM-1 or OXA-1, whose genes may be located on chromosomes, plasmids, or transposons (30). Although resistance to quinolones is commonly mediated through chromosomal mutations rather than mobile genetic units, certain plasmids have been shown to contribute to quinolone resistance by increasing the rate of spontaneous mutation (3, 5).
Reports of antimicrobial resistance trends in Shigella isolates from other countries raise the specter of wider resistance to nalidixic acid, fluoroquinolones, and ceftriaxone in the future.
A recent report from the United Kingdom revealed a 13% nalidixic acid resistance among Shigella sonnei isolates, with all isolates also exhibiting decreased susceptibility to ciprofloxacin (MIC, 0.25 to 1.0 mg/liter) (13). A study from Japan reports a 26% prevalence of nalidixic acid resistance among Shigella sonnei isolates (21). Fluoroquinolone resistance in S. dysenteriae isolates has been reported recently from Bangladesh, India, and Nepal (29, 38, 43, 44). Finally, with the gradual increase in extended-spectrum beta-lactamases detected in Klebsiella and other Enterobacteriaceae, an increase in extended-spectrum beta-lactamase-producing Shigella strains has been reported since 1999 (2). Ceftriaxone-resistant strains of S. sonnei and S. flexneri have been reported from Korea, Argentina, France, Turkey, and Taiwan (1, 17, 22, 33, 34). The beta-lactamases described in these reports included CTX-M-14, CTX-M-2, SHV-2, CTX-M-3, and CMY-2 type AmpC.
Our study has several limitations. Laboratory-based surveillance systems, such as NARMS, may overestimate the prevalence of resistance among Shigella isolates because persons infected with resistant strains who fail empirical treatment are more likely to get a stool culture for continued diarrhea than are persons infected with susceptible strains who respond to empirical therapy. In addition, NARMS sites include a nonweighted 37% sample of the U.S. population, limiting generalizability of the data. Nonetheless, there was good agreement between demographic data for patients captured by NARMS and the national Shigella laboratory-based surveillance system reported to PHLIS. This high degree of correlation suggests that NARMS surveillance captures a representative sample of all Shigella infections in the United States and that generalizations from NARMS data on antimicrobial resistance among Shigella isolates may be valid despite the fact that NARMS captures only every 10th isolate of Shigella received by participating public health laboratories. In 2003, NARMS surveillance was extended to all 50 state health department laboratories. Finally, interpretation of antimicrobial susceptibility breakpoints without also considering pharmacokinetics and pharmacodynamics of the drug is not straightforward. For example, in vitro resistance testing using axenic medium may reveal that extracellular Shigella isolates are susceptible to aminoglycosides. However, in vivo, the same Shigella isolate, which is a facultative intracellular pathogen, may be resistant to aminoglycosides due to their inability to permeate mammalian cells (39, 49). The CLSI recommends that susceptibility of Shigella to aminoglycosides not be reported to clinicians as “sensitive” because of this discrepancy (14).
Treatment for shigellosis is critical in persons who have severe disease, especially in children and the immunosuppressed. The small but evident increase in the proportion of Shigella isolates that are resistant to nalidixic acid may portend the loss of an important class of antimicrobial agents against Shigella. The continued monitoring of emerging resistance in Shigella isolates through NARMS will be essential to timely and appropriate recommendations for antimicrobial therapy.
Acknowledgments
Funding is from the Centers for Disease Control and Prevention.
We are indebted to the participating local and state health departments and public health laboratories and Timothy Barrett from the Food-borne and Diarrheal Diseases Branch, Division of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, Centers for Disease Control and Prevention, for their assistance and comments on the manuscript.
REFERENCES
- 1.Acikgoz, Z. C., Z. Gulay, M. Bicmen, S. Gocer, and S. Gamberzade. 2003. CTX-M-3 extended-spectrum beta-lactamase in a Shigella sonnei clinical isolate: first report from Turkey. Scand. J. Infect. Dis. 35:503-505. [DOI] [PubMed] [Google Scholar]
- 2.Ahamed, J., and M. Kundu. 1999. Molecular characterization of the SHV-11 β-lactamase of Shigella dysenteriae. Antimicrob. Agents Chemother. 43:2081-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambler, J. E., Y. J. Drabu, P. H. Blakemore, and R. J. Pinney. 1993. Mutator plasmid in a nalidixic acid-resistant strain of Shigella dysenteriae type 1. J. Antimicrob. Chemother. 31:831-839. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics. 2000. Shigella infections, p. 510-512, 645. In L. Pickering (ed.), 2000 Red book: report of the Committee on Infectious Diseases, 25th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
- 5.Ashraf, M. M., Z. U. Ahmed, and D. A. Sack. 1991. Unusual association of a plasmid with nalidixic acid resistance in an epidemic strain of Shigella dysenteriae type 1 from Asia. Can. J. Microbiol. 37:59-63. [DOI] [PubMed] [Google Scholar]
- 6.Baer, J. T., D. J. Vugia, A. L. Reingold, T. Aragon, F. J. Angulo, and W. Z. Bradford. 1999. HIV infection as a risk factor for shigellosis. Emerg. Infect. Dis. 5:820-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker, N., J. M. Nelson, K. Joyce, K. Gay, F. J. Angulo, and the NARMS Working Group. 2003. Quinolone resistance among Shigella NARMS 1999-2001, abstr. 8. Abstr. Natl. Found. Infect. Dis. Conf. Antimicrob. Resist.
- 8.Burkhardt, J., J. N. Walterspiel, and U. B. Schaad. 1997. Quinolone arthropathy in animals versus children. Clin. Infect. Dis. 25:1196-1204. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2004. Day care-related outbreaks of thamnose-negative Shigella sonnei-six states, June 2001-March 2003. Morb. Mortal. Wkly. Rep. 53:60-63. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2001. National Antimicrobial Resistance Monitoring System annual report 2001. Centers for Disease Control and Prevention, Atlanta, Ga. [Online.] www.cdc.gov/narms/. Accessed 21 June 2005.
- 11.Centers for Disease Control and Prevention. 2003. Public Health Information System (PHLIS). Centers for Disease Control and Prevention, Atlanta, Ga. [Online.] http://www.cdc.gov/ncidod/dbmd/phlisdata/. Accessed 21 June 2005.
- 12.Centers for Disease Control and Prevention. 2001. Shigella sonnei outbreak among men who have sex with men-San Francisco, California, 2000-2001. Morb. Mortal. Wkly. Rep. 50:922-926. [PubMed] [Google Scholar]
- 13.Cheasty, T., M. Day, and E. J. Threlfall. 2004. Increasing incidence of resistance to nalidixic acid in shigellas from humans in England and Wales: implications for therapy. Clin. Microbiol. Infect. 10:1033-1035. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing: fifteenth informational supplement. CLSI/NCCLS document M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 15.Cook, K., T. Boyce, N. Puhr, R. Tauxe, and E. Mintz. 1996. Increasing antimicrobial-resistant Shigella infections in the United States, abstr. E20. Program Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 16.Dunne, E. F., P. D. Fey, P. Kludt, R. Reporter, F. Mostashari, P. Shillam, J. Wicklund, C. Miller, B. Holland, K. Stamey, T. J. Barrett, J. K. Rasheed, F. C. Tenover, E. M. Ribot, and F. J. Angulo. 2000. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC beta-lactamase. JAMA 284:3151-3156. [DOI] [PubMed] [Google Scholar]
- 17.Fortineau, N., T. Naas, O. Gaillot, and P. Nordmann. 2001. SHV-type extended-spectrum beta-lactamase in a Shigella flexneri clinical isolate. J. Antimicrob. Chemother. 47:685-688. [DOI] [PubMed] [Google Scholar]
- 18.Guerrant, R., T. Van Gilder, T. S. Steiner, N. M. Thielman, L. Slutsker, R. V. Tauxe, T. Hennessy, P. M. Griffin, H. DuPont, R. B. Sack, P. Tarr, M. Neill, I. Nachamkin, L. B. Reller, M. T. Osterholm, M. L. Bennish, and L. K. Pickering. 2001. Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 32:331-351. [DOI] [PubMed] [Google Scholar]
- 19.Health Canada. 2002. Canadian Integrated Program for Antimicrobial Resistance Surveillance annual report. Health Canada, Ottawa, Ontario, Canada. [Online.] http://www.phac-aspc.gc.ca/cipars-picra/2002_e.html.
- 20.Heikkila, E., A. Siitonen, M. Jahkola, M. Fling, L. Sundstrom, and P. Huovinen. 1990. Increase of trimethoprim resistance among Shigella species, 1975-1988: analysis of resistance mechanisms. J. Infect. Dis. 161:1242-1248. [DOI] [PubMed] [Google Scholar]
- 21.Hirose, K., J. Terajima, H. Izumiya, K. Tamura, E. Arakawa, N. Takai, and H. Watanabe. 2005. Antimicrobial susceptibility of Shigella sonnei isolates in Japan and molecular analysis of S. sonnei isolates with reduced susceptibility to fluoroquinolones. Antimicrob. Agents Chemother. 49:1203-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, I.-F., C.-H. Chiu, M.-H. Wang, C.-Y. Wu, K.-S. Hsieh, and C. C. Chiou. 2005. Outbreak of dysentery associated with ceftriaxone-resistant Shigella sonnei: first report of plasmid-mediated CMY-2-type AmpC β-lactamase resistance in S. sonnei. J. Clin. Microbiol. 43:2608-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan, M. 1982. Interruption of shigellosis by handwashing. Trans. R. Soc. Trop. Med. Hyg. 76:164-168. [DOI] [PubMed] [Google Scholar]
- 24.Khan, W. A., C. Seas, U. Dhar, M. A. Salam, and M. L. Bennish. 1997. Treatment of shigellosis. V. Comparison of azithromycin and ciprofloxacin. A double-blind, randomized, controlled trial. Ann. Intern. Med. 126:697-703. [DOI] [PubMed] [Google Scholar]
- 25.Leibovitz, E., J. Janco, L. Piglansky, J. Press, P. Yagupsky, H. Reinhart, I. Yaniv, and R. Dagan. 2000. Oral ciprofloxacin vs. intramuscular ceftriaxone as empiric treatment of acute invasive diarrhea in children. Pediatr. Infect. Dis. J. 19:1060-1067. [DOI] [PubMed] [Google Scholar]
- 26.Lolekha, S., S. Vibulbandhitkit, and P. Poonyarit. 1991. Response to antimicrobial therapy for shigellosis in Thailand. Rev. Infect. Dis. 13(Suppl. 4):S342-S346. [DOI] [PubMed] [Google Scholar]
- 27.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohle-Boetani, J., M. Stapleton, R. Finger, et al. 1995. Communitywide shigellosis: control of an outbreak and risk factors in child day-care centers. Am. J. Public Health 85:812-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naheed, A., P. Kalluri, K. A. Talukder, A. S. Faruque, F. Khatun, G. B. Nair, E. D. Mintz, and R. F. Breiman. 2004. Fluoroquinolone-resistant Shigella dysenteriae type 1 in northeastern Bangladesh. Lancet Infect. Dis. 4:607-608. [DOI] [PubMed] [Google Scholar]
- 30.Navia, M. M., L. Capitano, J. Ruiz, M. Vargas, H. Urassa, D. Schellemberg, J. Gascon, and J. Vila. 1999. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. J. Clin. Microbiol. 37:3113-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson, J. D., H. Kusmiesz, L. H. Jackson, and E. Woodman. 1976. Trimethoprim-sulfamethoxazole therapy for shigellosis. JAMA 235:1239-1243. [PubMed] [Google Scholar]
- 32.Olarte, J., L. Filloy, and E. Galindo. 1976. Resistance of Shigella dysenteriae type 1 to ampicillin and other antimicrobial agents: strains isolated during a dysentery outbreak in a hospital in Mexico City. J. Infect. Dis. 133:572-575. [DOI] [PubMed] [Google Scholar]
- 33.Pai, H., E.-H. Choi, H.-J. Lee, J. Y. Hong, and G. A. Jacoby. 2001. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J. Clin. Microbiol. 39:3747-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radice, M., C. Gonzealez, P. Power, M. C. Vidal, and G. Gutkind. 2001. Third-generation cephalosporin resistance in Shigella sonnei, Argentina. Emerg. Infect. Dis. 7:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Replogle, M. L., D. W. Fleming, and P. R. Cieslak. 2000. Emergence of antimicrobial-resistant shigellosis in Oregon. Clin. Infect. Dis. 30:515-519. [DOI] [PubMed] [Google Scholar]
- 36.Salam, M. A., and M. L. Bennish. 1991. Antimicrobial therapy for shigellosis. Rev. Infect. Dis. 13(Suppl. 4):S332-S341. [DOI] [PubMed] [Google Scholar]
- 37.Salam, M. A., U. Dhar, W. A. Khan, and M. L. Bennish. 1998. Randomised comparison of ciprofloxacin suspension and pivmecillinam for childhood shigellosis. Lancet 352:522-527. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar, K., S. Ghosh, S. K. Niyogi, and S. K. Bhattacharya. 2003. Shigella dysenteriae type 1 with reduced susceptibility to fluoroquinolones. Lancet 361:785. [DOI] [PubMed] [Google Scholar]
- 39.Schwab, J. C., and G. L. Mandell. 1989. The importance of penetration of antimicrobial agents into cells. Infect. Dis. Clin. N. Am. 3:461-467. [PubMed] [Google Scholar]
- 40.Shakespeare, W. A., D. Davie, C. Tonnerre, M. A. Rubin, M. Strong, and C. A. Petti. 2005. Nalidixic acid-resistant Salmonella enterica serotype Typhi presenting as a primary psoas abscess: case report and review of the literature. J. Clin. Microbiol. 43:996-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shane, A. L., N. A. Tucker, J. A. Crump, E. D. Mintz, and J. A. Painter. 2003. Sharing Shigella: risk factors for a multicommunity outbreak of shigellosis. Arch. Pediatr. Adolesc. Med. 157:601-603. [DOI] [PubMed] [Google Scholar]
- 42.Shanks, G. D., O. B. Ragama, G. M. Aleman, S. L. Andersen, and D. M. Gordon. 1996. Azithromycin prophylaxis prevents epidemic dysentery. Trans. R. Soc. Trop. Med. Hyg. 90:316. [DOI] [PubMed] [Google Scholar]
- 43.Sur, D., S. K. Niyogi, S. Sur, K. K. Datta, Y. Takeda, G. B. Nair, and S. K. Bhattacharya. 2003. Multidrug-resistant Shigella dysenteriae type 1: forerunners of a new epidemic strain in eastern India? Emerg. Infect. Dis. 9:404-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talukder, K. A., B. K. Khajanchi, M. A. Islam, D. K. Dutta, Z. Islam, A. Safa, G. Y. Khan, K. Alam, M. A. Hossain, S. Malla, S. K. Niyogi, M. Rahman, H. Watanabe, G. B. Nair, and D. A. Sack. 2004. Genetic relatedness of ciprofloxacin-resistant Shigella dysenteriae type 1 strains isolated in south Asia. J. Antimicrob. Chemother. 54:730-734. [DOI] [PubMed] [Google Scholar]
- 45.Tauxe, R., R. C. McDonald, N. Hargrett-Bean, and P. A. Blake. 1988. The persistence of Shigella flexneri in the United States: increasing role of adult males. Am. J. Public Health 78:1432-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tauxe, R. V., N. D. Puhr, J. G. Wells, N. Hargrett-Bean, and P. A. Blake. 1990. Antimicrobial resistance of Shigella isolates in the USA: the importance of international travelers. J. Infect. Dis. 162:1107-1111. [DOI] [PubMed] [Google Scholar]
- 47.Toro, C. S., M. Farfan, I. Contreras, O. Flores, N. Navarro, G. C. Mora, and V. Prado. 2005. Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol. Infect. 133:81-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.United States Census Bureau. 2004. State and county QuickFacts. U.S. Census Bureau, Washington, D.C. [Online.] http://quickfacts.census.gov/qfd/.
- 49.Vaudaux, P., and F. A. Waldvogel. 1979. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 16:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]