Abstract
The superantigenic staphylococcal enterotoxins are important virulence factors and contribute to various diseases, including food poisoning and toxic shock. Dexamethasone, an anti-inflammatory agent, attenuated staphylococcal enterotoxin B (SEB)-induced hypothermia and serum proinflammatory cytokines and improved survival from 0% to 86% in a lethal mouse model of SEB-mediated shock.
Staphylococcal enterotoxin B (SEB) and related superantigenic toxins are potent activators of the immune system and cause a myriad of maladies, ranging from food poisoning to potentially life-threatening toxic shock (13, 17, 21, 24). These toxins bind directly to the major histocompatibility complex (MHC) class II molecules on antigen-presenting cells (17, 20, 22) and stimulate T cells via specific Vβ regions of the T-cell receptors (TCR) (5, 17), resulting in activation of both monocytes/macrophages and T lymphocytes. The specific interaction of these microbial toxins with multiple cell types in the host leads to excessive production of proinflammatory cytokines, chemokines, and tissue factor, causing clinical symptoms that include fever, hypotension, and shock (13, 18, 24). Two key inflammatory cytokines, interleukin 1 (IL-1) and tumor necrosis factor alpha (TNF-α), are direct mediators of fever, hypotension, and shock (15). Additionally, gamma interferon (IFN-γ) from superantigen-activated T cells acts synergistically with IL-1 and TNF-α to enhance immune reactions and tissue injury.
At present, there is no available therapeutic for treating staphylococcal exotoxin-induced shock except for the use of intravenous immunoglobulins (6). Most therapeutic strategies for experimental animal models of SEB-induced shock have targeted proinflammatory cytokines, as there is a strong correlation between toxicity and increased serum levels of these inflammatory mediators (4, 11, 16, 19, 26). These therapeutics include cytokine inhibitors and signal transduction inhibitors that target SEB-induced cellular activation pathways (10, 16). Experimental approaches aimed at disrupting toxin interactions with receptors and costimulatory molecules on macrophages and lymphocytes have also been used by different laboratories (2, 9, 10, 28). Thus, bispecific chimeric inhibitors, composed of the DRα1 domain of MHC class II and the Vβ domain of the TCR connected by a flexible linker, were designed to bind SEB competitively and prevent binding to MHC class II of antigen-presenting cells and the TCR on T cells (9). Conserved peptides corresponding to residues 150 to 161 of SEB can act as an antagonist and prevent SEA-, SEB-, or toxic shock syndrome toxin 1-induced lethal shock in mice when given intravenously 30 min after an intraperitoneal (i.p.) toxin dose (2). This segment of SEB is not associated with the classically defined MHC class II or TCR binding domains, but it may block costimulatory signals necessary for T-cell activation. However, a subsequent study of these peptides indicates that they are ineffective inhibitors of SEB-induced effects both in vitro and in vivo (23).
We previously showed that dexamethasone potently inhibits staphylococcal exotoxin-induced T-cell proliferation, cytokine release, and activation markers in human peripheral blood mononuclear cells (14). The current study was undertaken to evaluate the therapeutic efficacy of dexamethasone, a well-known anti-inflammatory agent, for treating SEB-mediated toxic shock. Body temperatures and circulating levels of TNF-α, IFN-γ, IL-1, IL-2, and IL-6 were assessed in a lipopolysaccharide (LPS)-potentiated lethal mouse model after an i.p. injection of SEB. Purified SEB was obtained from Toxin Technology (Sarasota, Fla.) and had an endotoxin content of <1 ng/mg protein, as determined by the Limulus amoebocyte lysate gelation test (Biowhittaker, Walkersville, Md.). Escherichia coli O55:B5 LPS was purchased from Difco Laboratories (Detroit, Mich.) and reconstituted in sterile phosphate-buffered saline (PBS). Dexamethasone (Sigma, St. Louis, Mo.) was prepared in dimethyl sulfoxide at 50 mg/ml. All injections (0.2 ml/mouse) were given i.p., and all dilutions were made in saline.
Pathogen-free BALB/c mice, 18 to 22 g, were obtained from Charles River (NCI-Frederick, Frederick, Md.). Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations. All experiments involving animals adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals (21a). Implantable programmable temperature transponders (IPTT-200) were purchased from Bio Medic Data Systems, Inc. (Seaford, Del.). Temperature transponder chips (one per mouse) were implanted subcutaneously at least 2 weeks before the experiment was initiated, and body temperature was monitored twice daily, starting 72 h before initiation of each experiment and continuing for at least 10 days postexposure.
The lethal shock model consisted of an i.p. dose of 1 μg of SEB/mouse, followed 4 h later by an i.p. injection of LPS (80 μg/mouse) as described previously (26, 27). The 50% lethal dose of LPS in mice is 300 μg/mouse without the use of sensitizing agents (8). Dexamethasone (50 μg/mouse, except where noted) was given i.p. three times at the designated time points, with the first dose administered at different time points (3.75 h, 4.25 h, 4.5 h, or 5 h) after SEB administration. A second dose of dexamethasone was given 20 h after SEB administration, and a third dose was given 24 h after the second dose. All drug-treated mice received three doses of dexamethasone regardless of the time when the first dose was given. Temperature data were calculated as the mean reading ± standard deviation (SD) for each group (7 to 10 mice per group). Animals were monitored for illness and death daily for a minimum of 10 days after toxin injection. Blood was collected and pooled from each group (five mice per group and time point) at 8 and 24 h after SEB administration, as previous studies showed that most serum cytokines peak 6 to 8 h after SEB administration in this mouse model (4, 26). Blood was allowed to clot and then centrifuged in microtiter serum separator tubes (Becton Dickinson, Bedford, Mass.). The sera were pooled and stored at −70°C until further analysis. Serum levels of cytokines TNF-α, IFN-γ, IL-1α, IL-2, and IL-6 in pooled samples from five mice per time point were determined by enzyme-linked immunosorbent assay according to the manufacturer's specifications (R&D Systems, Minneapolis, Minn.). Data were analyzed for significant differences by the Student t test with the Stata program (Stata Corp., College Station, Tex.). Temperature data were expressed as the mean reading ± SD. Cytokine data were expressed as the mean reading ± SD and then analyzed for significant differences between the control (SEB-plus-LPS-treated) and the dexamethasone-treated groups. Differences were considered significant if P was <0.05. The Fisher exact test was used for survival analysis of dexamethasone-treated mice. Differences between dexamethasone-treated and untreated control groups were considered significant if P was <0.05.
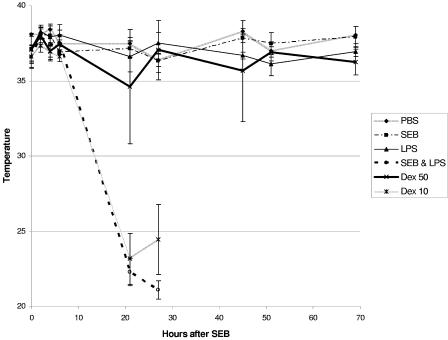
Based on the anti-inflammatory effects of dexamethasone and because it is used clinically for treating a variety of inflammatory diseases, we tested its effectiveness in a well-established model of SEB-induced shock in BALB/c mice. Body temperature was used, as it accurately predicts mortality due to SEB-induced shock (27). Preliminary experiments were conducted to establish the dose of dexamethasone needed to prevent lethal shock in mice. Figure 1 shows that SEB-plus-LPS-treated mice (n = 7) became hypothermic, with temperature drops starting at 8 to 10 h after SEB administration, whereas control mice treated with PBS, SEB, or LPS alone had normal temperatures. When animals were each given 50 μg of dexamethasone 3.75 h after SEB, 86% of the mice survived. A lower dose of dexamethasone (10 μg/mouse) given at 3.75 h after SEB resulted in a survival rate of only 28%. Importantly, mice treated with the higher dose of dexamethasone (50 μg/mouse) had a smaller drop in temperature at 21 h, and normal temperatures were maintained at 27 h and during the remaining observation period. This treatment with dexamethasone also alleviated objective signs and symptoms of distress (lethargy and ruffled fur) in the SEB-plus-LPS-exposed mice. Improvement was seen within 6 h after high-dose (50-μg/mouse) dexamethasone treatment. In contrast, the lower dose of dexamethasone (10 μg/mouse) did not improve the hypothermic response to SEB plus LPS at 21 h, but normal temperatures were seen in the two survivors at 45 h (data not shown). Mice treated with this lower dose of dexamethasone also showed signs of distress similar to those of the SEB-plus-LPS-treated mice. The high dose of dexamethasone (50 μg/mouse) was chosen for subsequent experiments to determine the therapeutic window for rescuing BALB/c mice from toxic shock.
FIG. 1.
Dexathemasone attenuated the hypothermic response of BALB/c mice treated with SEB plus LPS. Body temperatures of mice exposed to PBS, SEB, LPS, SEB plus LPS, SEB plus LPS plus 50 μg of dexamethasone, and SEB plus LPS plus 10 μg of dexamethasone are shown. Points represent the means ± SDs for each group (seven mice/group except at time points beyond 51 h, when one mouse died in the group treated with 50 μg of dexamethasone). Temperature readings of two survivors of the group treated with 10 μg of dexathemasone were not included.
We next determined the time window for therapeutic efficacy of dexamethasone treatment against SEB-induced shock. When dexamethasone (50 μg/mouse) was given 3.75 or 4.25 h after SEB, 80% of mice (n = 10) survived (P < 0.001), whereas treatment at 4.5 h and 5 h resulted in 10% and 20% survival, respectively. Dexamethasone given at these time points also delayed the time of death of nonsurvivors compared to that of SEB-plus-LPS controls (Fig. 2). The body temperatures of mice treated with dexamethasone at 4.5 or 5 h after SEB administration remained low at 22 to 27 h, but temperatures rose to normal by 44 h as nonsurvivors were eliminated from the temperature measurements (data not shown). At this time point, the numbers of survivors differed in the treatment groups: 8 of 10 mice survived in the group treated with dexamethasone at 4.25 h post-SEB administration, whereas only 4 of 10 and 3 of 10 mice remained in the groups that were given dexamethasone at 4.5 h and 5 h, respectively. At 76 h after SEB administration, only three and two survivors were left in the 4.5-h and 5-h dexamethasone treatment groups, respectively.
FIG. 2.
Survival analysis of BALB/c mice (n = 10) treated with dexamethasone (50 μg/mouse) at 3.75 h, 4.25 h, 4.5 h, and 5 h after SEB administration in the LPS-potentiated lethal shock model. S+L, SEB plus LPS.
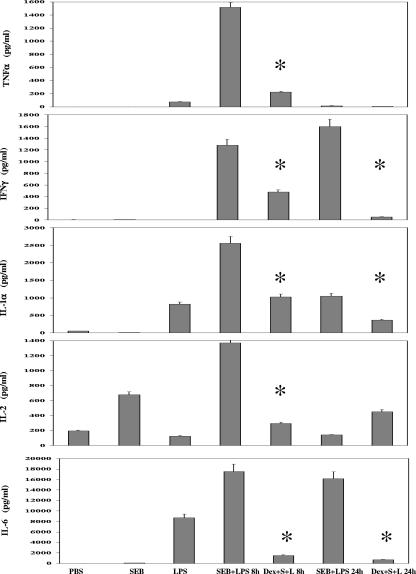
Previous reports show that superantigens trigger a cascade of proinflammatory cytokines resulting in toxic shock (4, 11, 18, 19, 26). We therefore analyzed the effects of dexamethasone on cytokine levels in mice. Dexamethasone significantly (P < 0.05) reduced serum concentrations of TNF-α, IFN-γ, IL-1α, IL-2, and IL-6 levels by 86%, 63%, 52%, 83%, and 90%, respectively, at 8 h after SEB administration, with further reduction of most cytokines 24 h after SEB treatment (Fig. 3).
FIG. 3.
Inhibition of serum TNF-α, IFN-γ, IL-1, IL-2, and IL-6 by dexamethasone (50 μg/mouse administered 3.75 h after SEB) in SEB-plus-LPS-treated mice. Circulating cytokines at 8 and 24 h after SEB administration were measured by enzyme-linked immunosorbent assay. Values represent the means ± SDs for duplicate samples. The asterisk indicates a P of <0.05 compared with results for mice treated with SEB plus LPS. Results represent three experiments.
Bacterial superantigens cause toxic shock and contribute to septic complications during infection. SEB is the most widely studied toxin among the staphylococcal exotoxins, and various in vivo animal models have been developed to identify therapeutic agents to prevent superantigen-induced shock (2, 4, 23, 26, 28). Proinflammatory cytokines are prime targets, as in vivo studies have shown a good correlation between the increased serum levels of proinflammatory cytokines TNF-α, IFN-γ, IL-1, and IL-6 and SEB-induced lethality (2, 4, 19, 26). Additionally, neutralizing antibodies against TNF-α also prevent SEB-induced lethality (19).
In this study, we used body temperature, which has been used as an indicator of infection since ancient times, as a marker for SEB-induced toxic shock in an LPS-potentiated mouse model. We found that dexamethasone prevented the hypothermic response to SEB, reduced serum proinflammatory cytokine levels, and ultimately improved survival. However, a major problem of in vivo testing of therapeutics against SEB-induced toxic shock is how well animal models mimic human disease. The SEB-induced toxic shock mouse models rely on the synergistic action of SEB and LPS in eliciting a massive cascade of proinflammatory cytokines with lethality as an endpoint (4, 19, 23, 26, 27, 28). Due to the insensitivity of mice to SEB and LPS, the doses of SEB and LPS used in these mouse models are higher than the nanogram quantities of these agents encountered in human cases of toxic shock syndrome and endotoxemia (24). In addition, toxic shock syndrome represents a spectrum and progression of clinical symptoms, including multiorgan failure, in humans exposed to bacterial toxins and/or concurrent bacterial infection, pathogenic features which are absent in most mouse models. Nevertheless, the doses of dexamethasone used in the present study are similar to the high doses of corticosteriods used clinically in the management of systemic inflammation (3). Additional work is necessary to establish the relevance of mouse models using high doses of bacterial toxins to identify therapeutic agents for the treatment of superantigen-induced disease in humans.
Although the anti-inflammatory effects of steroids such as dexamethasone on leukocytes are well known (7, 12, 25), the in vivo use of steroids in treating shock is controversial, with studies showing conflicting results as to their effectiveness (reviewed in reference 1). Our results using the LPS-potentiated model of SEB-induced toxic shock suggest a very narrow therapeutic window of treatment, which might shed some light on the timing of dexamethasone treatment for patients entering shock in a clinical setting. Our findings indicate that dexamethasone given at an early time point(s) after the entry of the inciting agent(s) can prevent cytokine release, hypothermia, and shock.
Acknowledgments
The views expressed in this publication are those of the authors and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. Government.
REFERENCES
- 1.Annane, D., E. Bellissant, P. E. Bollaert, J. B. Briegel, D. Keh, and Y. Kupfer. 2004. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ 329:480-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arad, G., R. Levy, D. Hillman, and R. Kaempfer. 2000. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat. Med. 6:414-421. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, I. L., M. Finland, M. Hamburger, E. H. Kass, M. Lepper, and B. A. Waisbren. 1962. A double-blind study of the effectiveness of cortisol in the management of severe infections. Trans. Assoc. Am. Physicians 75:198-207. [PubMed] [Google Scholar]
- 4.Blank, C., A. Luz, S. Bendigs, A. Erdmann, H. Wagner, and K. Heeg. 1997. Superantigen and endotoxin synergize in the induction of lethal shock. Eur. J. Immunol. 27:825-833. [DOI] [PubMed] [Google Scholar]
- 5.Choi, Y., B. Kotzin, L. Hernon, J. Callahan, P. Marrack, and J. Kappler. 1989. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc. Natl. Acad. Sci. USA 86:8941-8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darenberg, J., B. Soderquist, B. H. Normark, and A. Norrby-Teglund. 2004. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: implications for therapy of toxic shock syndrome. Clin. Infect. Dis. 38:836-842. [DOI] [PubMed] [Google Scholar]
- 7.Filep, J. G., A. Delalandre, Y. Payette, and E. Foldes-Filep. 1997. Glucocorticoid receptor regulates expression of L-selectin and CD11/CD18 on human neutrophils. Circulation 96:295-301. [DOI] [PubMed] [Google Scholar]
- 8.Freudenberg, M. A., D. Keppler, and C. Galanos. 1986. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect. Immun. 51:891-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong-Geller, E., M. Möllhoff, P. R. Shiflett, and G. Gupta. 2004. Design of chimeric receptor mimics with different TcRVβ isoforms: type-specific inhibition of superantigen pathogenesis. J. Biol. Chem. 279:5676-5684. [DOI] [PubMed] [Google Scholar]
- 10.Hong-Geller, E., and G. Gupta. 2003. Therapeutic approaches to superantigen-based diseases: a review. J. Mol. Recognit. 16:91-101. [DOI] [PubMed] [Google Scholar]
- 11.Huang, W. T., M. T. Lin, and S. J. Won. 1997. Staphylococcal enterotoxin A-induced fever is associated with increased circulating levels of cytokines in rabbits. Infect. Immun. 65:2656-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyce, D. A., G. Gimblett, and J. H. Steer. 2001. Targets of glucocorticoid action on TNF-alpha release by macrophages. Inflamm. Res. 50:337-340. [DOI] [PubMed] [Google Scholar]
- 13.Kotzin, B. L., D. Y. M. Leung, J. Kappler, and P. Marrack. 1993. Superantigens and their potential role in human disease. Adv. Immunol. 54:99-166. [DOI] [PubMed] [Google Scholar]
- 14.Krakauer, T. 1995. Differential inhibitory effects of interleukin-10, interleukin-4, and dexamethasone on staphylococcal enterotoxin-induced cytokine production and T cell activation. J. Leukoc. Biol. 57:450-454. [DOI] [PubMed] [Google Scholar]
- 15.Krakauer, T., J. Vilcek, and J. J. Oppenheim. 1998. Proinflammatory cytokines: TNF and IL-1 families, chemokines, TGFβ and others, p. 775-811. In W. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Press, Philadelphia, Pa.
- 16.Krakauer, T. 2005. Chemotherapeutics targeting immune activation by staphylococcal superantigens. Med. Sci. Monit. 11:290-295. [PubMed] [Google Scholar]
- 17.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-709. [DOI] [PubMed] [Google Scholar]
- 18.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 19.Miethke, T., C. Wahl, K. Heeg, B. Echtenacher, P. H. Krammer, and H. Wagner. 1992. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J. Exp. Med. 175:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollick, J. A., M. Chintagumpala, R. G. Cook, and R. R. Rich. 1991. Staphylococcal exotoxin activation of T cells. Role of exotoxin-MHC class II binding affinity and class II isotype. J. Immunol. 146:463-468. [PubMed] [Google Scholar]
- 21.Monday, S. R., and G. A. Bohach. 1999. Properties of Staphylococcus aureus enterotoxins and toxic shock syndrome toxin-1, p. 589-610. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins. Academic Press, London, United Kingdom..
- 21a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 22.Proft, T., and J. D. Fraser. 2003. Bacterial superantigens. Clin. Exp. Immunol. 133:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajagopalan, G., M. M. Sen, and C. S. David. 2004. In vitro and in vivo evaluation of staphylococcal superantigen peptide antagonists. Infect. Immun. 72:6733-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlievert, P. M. 1993. Role of superantigens in human disease. J. Infect. Dis. 167:997-1002. [DOI] [PubMed] [Google Scholar]
- 25.Schramm, R., and H. Thorlacius. 2003. Staphylococcal enterotoxin B-induced acute inflammation is inhibited by dexamethasone: important role of CXC chemokines KC and macrophage inflammatory protein 2. Infect. Immun. 71:2542-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiles, B. G., S. Bavari, T. Krakauer, and R. G. Ulrich. 1993. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect. Immun. 61:5333-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiles, B. G., Y. G. Campbell, R. M. Castle, and S. A. Grove. 1999. Correlation of temperature and toxicity in murine studies of staphylococcal enterotoxins and toxic shock syndrome toxin 1. Infect. Immun. 67:1521-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visvanathan, K., A. Charles, J. Bannan, P. Pugach, K. Kashfi, and J. B. Zabriskie. 2001. Inhibition of bacterial superantigens by peptides and antibodies. Infect. Immun. 69:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]