Abstract
Serine/threonine kinase Akt is a downstream effector protein of phosphatidylinositol-3-kinase (PI-3K). Many integrins can function as positive modulators of the PI-3K/Akt pathway. Integrin α2β1 is a collagen receptor that has been shown to induce specific signals distinct from those activated by other integrins. Here, we found that, in contrast what was found for cells adherent to fibronectin, α2β1-mediated cell adhesion to collagen leads to dephosphorylation of Akt and glycogen synthase kinase 3β (GSK3β) and concomitantly to the induction of protein serine/threonine phosphatase 2A (PP2A) activity. PP2A activation can be inhibited by mutation in the α2 cytoplasmic domain and by a function-blocking anti-α2 antibody. Akt can be coprecipitated with PP2A, and coexpression of Akt with PP2Ac (catalytic subunit) inhibits Akt kinase activity. Integrin α2β1-related activation of PP2A is dependent on Cdc42. These results indicate that cell adhesion to collagen modulates Akt activity via the α2β1-induced activation of PP2A.
The integrins are a large family of heterodimeric transmembrane receptors composed of α and β subunits (22). In addition to mediating cell-matrix interactions, integrins have been shown to activate intracellular signaling pathways which, in collaboration with growth factor-induced signals, regulate cellular functions (46). Some integrin signaling cascades are activated via the β subunit cytoplasmic domain, and they are therefore triggered by several integrin heterodimers. These signals include the activation of protein tyrosine kinases of the Src and focal adhesion kinase (FAK) families (9, 47). More-recent studies have revealed signaling events that are activated specifically by an α subunit (19). Integrins may associate with other membrane proteins, such as caveolin-1, and a subset of integrins can activate extracellular signal-related kinase, one of the mitogen-activated protein kinases, via Fyn and Shc (53, 54). Some integrins interact with other membrane proteins to regulate distinct signaling cascades. For example laminin receptor α3β1 associates with tetraspanin proteins and activates phosphatidylinositol-3-kinase (PI-3K) and PI-4K (4). We have shown that α2β1 integrin specifically activates the p38 pathway via a mechanism involving the α2 cytoplasmic tail and Cdc42 (25). The p38 signaling pathway seems to regulate the expression of type I collagen and collagenase-3 (25, 42), and it is required for cell migration on collagen (29).
The PI-3K/Akt pathway is activated by a wide range of extracellular stimuli, including the integrins (12), and it has been linked to cell survival (13). Recently it was demonstrated that the different variants of the cytoplasmic domain in the β1 subunit can equally activate Akt (14, 16) and that the binding of α5β1 to fibronectin activates Akt, unlike the binding of α2β1 to monomeric collagen (15). Thus the activation of Akt may be dependent on the integrin α subunit.
Reversible phosphorylation of proteins is a major mechanism for the control of cellular signaling pathways and maintenance of homeostasis (21). Although numerous kinases have been implicated in integrin signaling, the function and possible regulation of the corresponding phosphatases are largely unknown. Adhesion of cultured fibroblasts to extracellular matrix proteins has been shown to induce recruitment and activation of SHP-2, a nontransmembrane protein tyrosine phosphatase (39, 51). SHP-2 seems to play an active role in integrin-mediated signaling events, such as cell adhesion and migration (36, 62). Very little is known about the role of protein serine/threonine phosphatases in integrin signaling. Recent data have indicated a positive role for protein serine/threonine phosphatase 2A (PP2A) in integrin inside-out signaling. Inhibition of PP2A activity induces a selective loss of β1 integrins from focal adhesion sites (38) and inhibits cell adhesion (11); in addition PP2A has been shown to colocalize with β1 integrin at adhesion sites (38). However, the role of serine/threonine phosphatases in modulating integrin outside-in signals remains to be studied. Many studies have demonstrated the importance of PP2A in regulating a variety of cellular functions (52). Therefore it is likely that PP2A activity is tightly controlled in vivo.
Cell adhesion to three-dimensional (3D) fibrillar collagen, unlike adhesion to monomeric two-dimensional collagen, inhibits cell proliferation in different cell types (15, 20, 30) and induces specific integrin-mediated signals, which regulate gene expression (25, 42, 44). Here, a novel α2β1-mediated signaling mechanism is introduced. Using human primary fibroblasts and human osteosarcoma (Saos-2) cell clones expressing either the wild-type α2 subunit or a signaling-deficient α2/α1 chimera, we have analyzed the ability of α2β1 integrin to regulate signals that have been linked with cell proliferation and survival. We and others have shown that α2β1 integrin is not involved in the regulation of the extracellular signal-related kinase mitogen-activated protein kinase pathway in response to collagen (25, 42, 53). However, here we show that cell adhesion to 3D collagen attenuates Akt and glycogen synthase kinase 3β (GSK3β) phosphorylation by a mechanism involving α2β1-induced activation of PP2A.
MATERIALS AND METHODS
Plasmids, adenoviruses, and antibodies.
The α2 integrin and the chimerical α2/α1 integrin expression constructs have been described previously (25, 43). In α2/α1 the intracellular domain of α2 was replaced with one from α1 integrin. Cdc42Asn17, Cdc42QL, Rac1Asn17, and RhoAAsn19 were provided by J. C. Lacal (Consejo Superior de Investigaciones Cientificas, Madrid, Spain). pCMVHA-Akt and pCMVHA (hemagglutinin [HA] vector) were provided by K. Vuori (Burnham Institute), the HA-PP2Ac expression construct was provided by D. Brautigan (University of Virginia) (8), and pCMV was from Invitrogen. The adenovirus vector for the expression of membrane-targeted, active Akt (myrAkt) was obtained from K. Walsh (Tufts University School of Medicine) and was constructed as described previously (17, 18). The antibodies against Akt, the HA probe, and Cdc42 were purchased from Santa Cruz Biotechnology. Anti-PP2A was a gift from D. Brautigan. The phospho-specific antibodies were from New England Biolabs. The anti-α2 (MCA743) and anti-α1 (MCA1133) integrin antibodies were from Serotec.
Collagen gels.
Collagen gels were prepared from bovine dermal collagen, which contains 95% type I collagen and 5% type III collagen (Cellon). Eight volumes of Cellon were mixed with 1 volume of 10-fold-concentrated Dulbecco's modified Eagle medium (DMEM) and 1 volume of 0.05 M NaOH in HEPES buffer (0.2 M) and kept on ice. Cells were trypsinized, resuspended in 1/10 gel volume of DMEM supplemented with inhibitors or anti-integrin antibodies, mixed into neutralized Cellon solution, and transferred into cell culture plates. The collagen was allowed to polymerize for 1 h at 37°C, after which 1 gel volume of DMEM was added, the gels were detached from the sides of the cell culture plates, and incubation was continued for various times. Cells from various Saos-2 cell clones were cultured on plastic and harvested directly from the plate or kept in suspension (1 ml of DMEM) for 1 h. The release of cells from collagen gel was performed as described previously (25).
Immunoblot analysis.
Cells were released from collagen, washed once with ice-cold phosphate-buffered saline, and lysed in Laemmli sample buffer. The samples were sonicated, fractionated by sodium dodecyl sulfate-10 or 12% polyacrylamide gel electrophoresis, and transferred to a Hybond ECL membrane (Amersham Corp.). Western blotting was performed as described previously (25) with New England Biolabs antibodies at the dilution of 1:1,000 and other antibodies at the dilution of 1:250. Specific binding of antibodies was detected with peroxidase-conjugated secondary antibodies and visualized by an enhanced chemiluminescence detection system (Amersham).
Transfections and kinase assay.
For the Akt kinase assay, subconfluent cells plated on 10-cm-diameter dishes were transfected using 18 μl of Fugene 6 transfection reagent (Boehringer Mannheim) and 6 μg of either empty vector, HA vector, or PP2Ac expression vector together with 3 μg of pCMVHA-Akt. At 36 h after transfections, the cells were treated with collagen gel for 1.5 h, and a kinase assay was performed as described previously (18). Briefly, equal amounts of protein in cell lysis buffer (1% NP-40; 10% glycerol; 137 mM NaCl; 20 mM Tris-HCl [pH 7.4]; 20 mM NaF; 1 mM phenylmethylsulfonyl fluoride[PMSF]; leupeptin, antipain, and pepstatin [2 μg/ml each]) from each sample were preincubated with protein G-Sepharose for 30 min at 4°C. After centrifugation, an anti-HA antibody was added together with protein G-Sepharose and 2 mg of bovine serum albumin/ml. Immunoprecipitation was performed at 4°C for 2 h. The immunoprecipitates were washed twice with cell lysis buffer, twice with H2O, and twice with kinase buffer (20 mM HEPES [pH 7.2], 10 mM MgCl2, 10 mM MnCl2), and they were incubated in 50 μl of kinase buffer containing 2 μg of myelin basic protein (Boehringer Mannheim) and [γ-32P]ATP (5 μM, 10 μCi; Amersham) at room temperature for 30 min. Kinase reactions were terminated by adding sodium dodecyl sulfate sample solution. The reactions were run on 15% polyacrylamide gels and subjected to autoradiography. Okadaic acid (1 nM) was included at every stage to inhibit any in vitro phosphatase activity from influencing the kinase activity after the lysis of the cells. The in vitro kinase assays for PI-3K (3, 58) were performed in the presence of [γ-32P]ATP and PI (Avanti Polar Lipids). The formed [32P]PI was measured using thin-layer chromatography and autoradiography.
Phosphatase assay.
Subconfluent human osteosarcoma cells and human primary fibroblasts were treated with collagen for various times in the presence or absence of antibodies (function-blocking anti-α2, nonfunctional anti-α1 immunoglobulin G [IgG]; 5 μg/ml) or inhibitors (bisindolylmaleimide [BIM], 10 μM; SB203580, 20 μM; Calbiochem) or were lysed directly on the plate. Alternatively, cells plated on 10-cm-diameter dishes were transfected using 20 μl of Fugene 6 transfection reagent (Boehringer Mannheim) and 10 μg of expression vector pCEV either alone or with Cdc42Asn17, Cdc42QL, Rac1Asn17, or RhoAAsn19. Thirty-six hours after transfections, the cells were placed inside a collagen gel for 2 h. Cells were released from collagen as described previously (25) and lysed in phosphatase lysis buffer (20 mM HEPES [pH 7.4]; 10% glycerol; 0.1% NP-40; 1 mM EGTA; 30 mM β-mercaptoethanol; 1 mM PMSF; leupeptin, antipain, and pepstatin [2 μg/ml each]). The protein phosphatase assay system (Life Technologies) was used to determine phosphatase activity according to manufacturer's instructions. Briefly, 32P-labeled phosphorylase (a substrate for both PP1 and PP2) was allowed to react with 2 to 20 μg of protein from cell lysate for 10 min in 30°C, after which the proteins were precipitated with 20% trichloroacetic acid and the radioactivity released was measured by scintillation counting. Protein content was determined by the Bradford method, and phosphatase activity relative to protein content was calculated.
Immunoprecipitation.
For immunoprecipitation, the cells were transfected with Fugene 6 and 10 μg of the wt-PP2Ac-HA expression construct as described above (provided by D. Brautigan, University of Virginia) (8). Eight hours after transfection the cells were infected with myrAkt adenovirus at a multiplicity of infection of 50 and incubated for 16 h. Serum-free DMEM was added, and the incubation was continued for 24 h. The cells were treated with 3D collagen for 1 h under serum-free conditions, and the cells were lysed in solubilization buffer (10 mM Tris-HCl [pH 7.4], 50 mM NaCl, 1% Triton X-100, 30 mM sodium pyrophosphate, 100 μM Na3VO4, 1 mM PMSF) (1). Immunoprecipitation was performed as described for the Akt kinase assay, with either a specific antibody or control IgG from the same species, except that the washes were performed six times with solubilization buffer. Western blot detection was performed with antibodies against Akt and the HA probe and antiserum against the PP2A catalytic subunit.
RESULTS
Adhesion of α2β1 integrin to collagen promotes dephosphorylation of Akt.
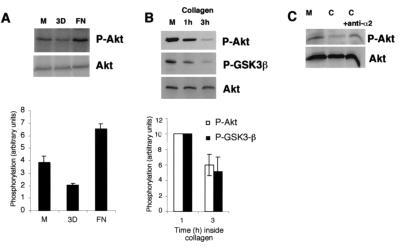
A previous study suggested that integrin α5β1-mediated cell adhesion to fibronectin induces Akt phosphorylation, while adhesion to monomeric collagen has no effect (32). Since monomeric two-dimensional and fibrillar three-dimensional (3D) collagen may have distinct properties in triggering integrin signaling (25, 42), immunoblotting was performed to determine whether cell adhesion to 3D collagen influences the Akt kinase. We examined the phosphorylation of Ser473 on Akt with a phospho-specific antibody, since this residue is considered to indicate active kinase (6). Serum-starved human primary fibroblasts were harvested in the monolayer or trypsinized and replated on fibronectin-coated plates or seeded inside collagen (for 2 h), and Akt phosphorylation was studied by immunoblotting. Cells adhering on fibronectin showed increased Akt phosphorylation (67% ± 11% [mean ± standard error of the mean {SEM}]; four separate experiments) compared to cells cultured on plastic, while phosphorylation of Ser473 was reduced by 48% ± 3% (mean ± SEM; four separate experiments) in cells cultured inside 3D fibrillar collagen (Fig. 1A). We also analyzed the effect of collagen-mediated reduction of Akt phosphorylation on GSK3β, a well-characterized Akt substrate that is inactivated by the active Akt kinase via phosphorylation at Ser9 (12). In only 1 h, 3D collagen induced a rapid decrease of phosphorylation of Akt, and after 3 h nearly all Akt was in a nonphosphorylated state (Fig. 1B). Inactivation of Akt resulted in decreased phosphorylation of GSK3β (Fig. 1B). In seven separate experiments the phosphorylation of Akt and GSK3β in cells cultured inside collagen was reduced by 40% ± 16% and 47% ± 17% (means ± SEMs), respectively. To evaluate the role of α2β1 in collagen-induced Akt inactivation, we treated human fibroblasts with an anti-α2 integrin function-blocking monoclonal antibody (MAb). Importantly, this antibody has previously been shown to block α2β1-initiated signals inside collagen (42). The antibody blocked collagen-induced Akt dephosphorylation (Fig. 1C). The results indicate that α2β1 integrin function is involved in the observed reduction of Akt phosphorylation.
FIG. 1.
Differential regulation of Akt phosphorylation by extracellular matrix molecules. (A) Fibroblasts were deprived of serum for 16 h and then harvested from the cell culture dish (M, monolayer), seeded inside 3D collagen gel, or plated on fibronectin (FN) for 2 h. P-Akt, phosphorylated Akt. (B) Primary fibroblasts were deprived of serum for 16 h and then harvested from the cell culture dish (M) or seeded inside 3D collagen gel for 1 and 3 h. P-GSK3β, phosphorylated GSK3β. (C) Primary fibroblasts were deprived of serum for 16 h and then harvested from the cell culture dish (M) or seeded inside collagen gel (C) or treated with an anti-α2 function-blocking MAb (C + anti-α2) and placed inside collagen for 1.5 h. Western blot analyses with an antibody that recognizes Akt when it is phosphorylated at Ser473 or with an antibody that recognizes GSK3β when it is phosphorylated are shown. Immunoblotting with an antibody that recognizes all forms of Akt was performed for reference. Densitometric quantitation of phosphorylated Akt and GSK3β levels relative to loading is also shown (A and B).
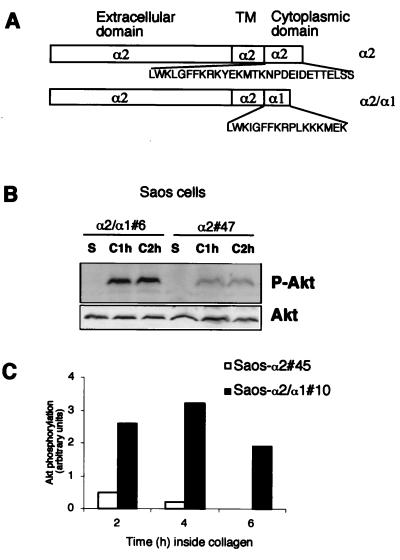
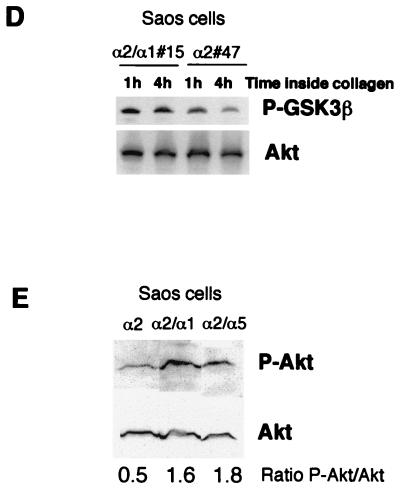
To confirm that the effect of collagen on Akt activation is mediated by α2β1 integrin, we used osteosarcoma cells (Saos) expressing α1β1 and lacking endogenous α2β1 integrin. The cells were transfected to have stable expression of wild-type α2 (Saos-α2) or α2 with the α1 cytoplasmic domain (Saos-α2/α1) (Fig. 2A) (25). The mutant α2/α1β1 integrin mediates cell adhesion to collagen as efficiently as the wild-type receptor but lacks α2 signaling functions (25). In agreement with a previous study (27), detachment and maintenance of the cells in suspension resulted in complete Akt inactivation in 1 h (Fig. 2B, lane S). After 2 h inside collagen, phosphorylation of Ser473 on Akt was clearly reduced in Saos-α2 cells compared to that in Saos-α2/α1 cells, indicating the importance of the α2 cytoplasmic domain in Akt inactivation (Fig. 2B). This was further confirmed using different stable cell clones. Akt phosphorylation was monitored over a course of some hours, and, according to the densitometric analysis, after 6 h no phosphorylated Akt was detected in Saos-α2 cells, while Akt remained phosphorylated at Ser473 in Saos-α2/α1 cells (Fig. 2C). In correlation with that of Akt, the phosphorylation of GSK3β decreased inside collagen in Saos-α2 cells while phosphorylation at Ser9 was maintained in cells expressing the mutant α2/α1 integrin chimera (Fig. 2D). Densitometric analysis performed in six separate experiments showed that the amount of phosphorylated GSK3β in α2-expressing cells was 40% ± 22% (mean ± SEM) lower after 1 h, and 36% ± 8% (mean ± SEM) lower after 4 h, inside collagen than the amount in Saos-α2/α1 cells. These results indicate that functional α2β1 integrin is essential for the collagen-induced rapid reduction of Akt and GSK3β phosphorylation, while integrin α1β1 does not seem to contribute to the negative regulation of Akt in the cells studied. The active role of the cytoplasmic domain of α2 in the regulation of Akt was further confirmed using Saos cells expressing an α2/α5 integrin chimera (5). When these cells were exposed to 3D collagen, the levels of phosphorylated Akt were the same as those in α2/α1 cells and higher than those in Saos-α2 cells (Fig. 2E).
FIG. 2.
Collagen-induced dephosphorylation of Akt is regulated by α2β1 integrin in human osteosarcoma Saos cells. (A) cDNA constructs used for expression of integrin α2 and α2/α1 (chimera) subunits in Saos cells, which lack endogenous α2β1 integrin. TM, transmembrane domain. (B to E) Saos-α2, Saos-α2/α1, and Saos-α2/α5 cell clones were serum starved, detached, and cultured inside 3D collagen gel (C, collagen) for the indicated times or left in suspension (S) for 1 h. For panel E cells were cultured inside collagen for 2 h. Western blot analysis with antibodies that recognize Akt (B and E) and GSK3β (D) when they are phosphorylated (P-Akt and P-GSK3β, respectively) is shown. (B, D, and E) Immunoblotting with an antibody that recognizes all forms of Akt was performed for reference. (C) A separate experiment with longer incubation times inside collagen was performed with different Saos cell clones. Densitometric quantitation of phosphorylated Akt levels at different time points relative to loading is shown. If the experiment was performed with several cell clones, the clone numbers are indicated (e.g., #6).
Integrin α2β1 regulates Akt via PP2A.
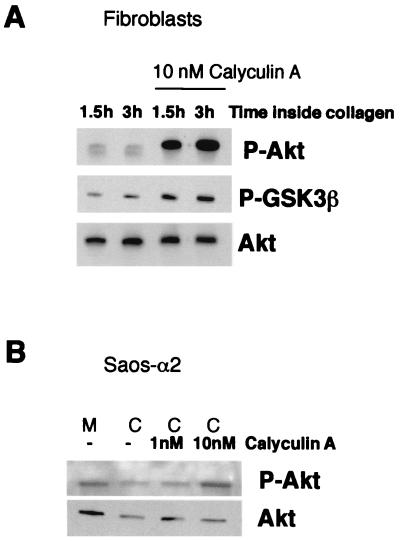
Akt activity can be decreased by either inhibition of upstream activators or dephosphorylation of Thr308 and Ser473 (37). To determine which mechanism is involved, activation of PI-3K was tested by a direct kinase assay performed with Saos-α2 and Saos-α2/α1 cells cultured inside collagen. No constant differences between Saos-α2 and Saos-α2/α1 cell clones were detected (mean of two Saos-α2 and two Saos-α2/α1 clones: 80 and 73 arbitrary units, respectively). Furthermore, inhibition of PI-3K with LY-294002 had no further effect on the low levels of phosphorylated Akt measured in Saos-α2 cells after 1.5 h inside collagen (not shown). We therefore concluded that α2β1 integrin regulates Akt phosphorylation downstream of PI-3K. Integrin α2β1-mediated reduction of Akt and GSK3β phosphorylation could, indeed, be prevented by protein serine/threonine phosphatase inhibitor calyculin A. This was observed in both the human primary fibroblasts and Saos-α2 cells cultured inside collagen (Fig. 3). The above experiments strongly indicated that α2β1 regulates Akt dephosphorylation rather than its activation via PI-3K and phosphoinositide-dependent kinase 1 but did not resolve the exact mechanism of Akt deactivation.
FIG. 3.
Regulation of Akt phosphorylation involves serine/threonine phosphatase activity. (A) Western blot analysis with an antibody that recognizes Akt or GSK3β when they are phosphorylated (P-Akt and P-GSK3β, respectively). Fibroblasts were deprived of serum for 16 h and seeded inside 3D collagen gel in the presence or absence of serine/threonine phosphatase inhibitor calyculin A (10 nM). (B) Western blot analysis with an antibody that recognizes phosphorylated Akt. Saos-α2 cells were deprived of serum for 16 h and then harvested from the cell culture dish (M, monolayer) and seeded inside 3D collagen (C) gel for 2 h. Cells were cultured in the presence or absence of serine/threonine phosphatase inhibitor calyculin A (1 and 10 nM). Immunoblotting with an antibody that recognizes all forms of Akt was performed for reference (A and B).
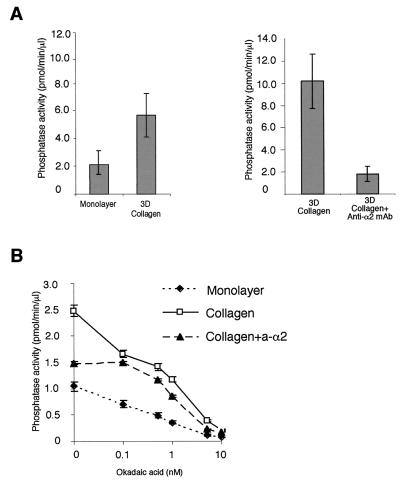
Using a specific assay for serine/threonine phosphatases, we asked whether cell adhesion to 3D collagen could induce serine/threonine phosphatase activity and, if so, whether the differences would be large enough to be detected against the background of all cellular phosphatase activity. We observed that human primary fibroblasts seeded inside collagen have two- to fivefold (range in three separate experiments, each with three parallel measurements)-higher phosphatase activity than cells cultured in a monolayer (Fig. 4A). Interestingly, the collagen-induced phosphatase activity could be almost completely blocked with a function-blocking antibody against anti-α2 integrin (Fig. 4A), while a control antibody (nonfunctional anti-α1 antibody) had no effect (not shown). The phosphatase assay measures PP2A activity but is not specific for it. However, PP2A can be distinguished from PP1 by its over-100-fold-higher susceptibility to okadaic acid (10). The integrin-induced phosphatase activity was inhibited by 52% with 0.1 nM okadaic acid (a very low inhibitor concentration, which does not influence PP1 [50% inhibitory concentration, 15 nM]), reducing the phosphatase activity to that measured in cells treated with collagen in the presence of an anti-integrin antibody (Fig. 4B). The data indicate that α2β1 integrin induces PP2A-type phosphatase activity in response to collagen.
FIG. 4.
Serine/threonine phosphatase PP2A is activated inside 3D collagen gel. Fibroblasts were serum starved and then harvested from the cell culture dish (monolayer) or seeded inside collagen gel for 1.5 h. Serum-starved fibroblasts were detached and placed inside collagen in the presence or absence of either an anti-α2 function-blocking MAb (collagen + anti-α2; 2.5 μg/ml) or a nonfunctional anti-α1 MAb (2.5 μg/ml; not shown). Thereafter, cells were lysed and equal amounts of protein were assayed for PP1-PP2A activity using 32P-labeled glycogen phosphorylase as a substrate. Phosphatase activity of cells seeded inside collagen versus that of cells cultured in a monolayer and the effect of anti-α2 MAb are shown (A). To distinguish between PP1 and PP2A type activity, the effect of okadaic acid (causes half-maximal inhibition of PP2A at 0.1 nM and has no effect on PP1 below 5 nM) was tested by an in vitro phosphatase reaction (B). Phosphatase activity relative to cell lysate protein content (mean ± SEM; n = 3) is shown.
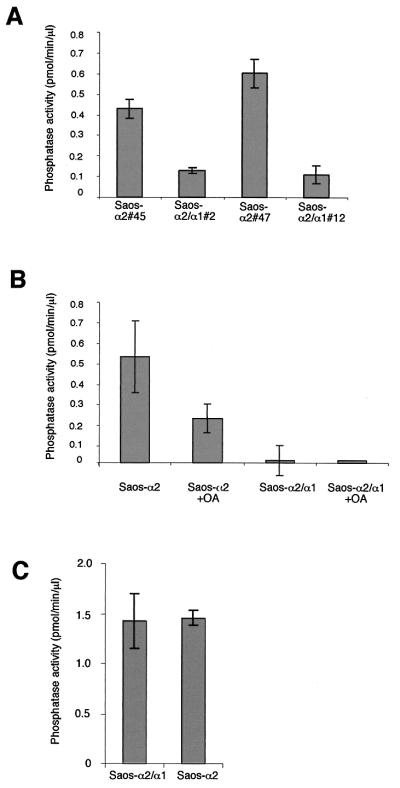
This was further supported by the fact that serine/threonine phosphatase activity was significantly higher (3.3- to 4.5-fold) in Saos-α2 clones than in Saos-α2/α1 clones (Fig. 5A), further confirming the role of α2β1 in PP2A regulation and the generality of this observation in different cell types. Introduction of α2 or α2/α1 cDNAs into Saos cells did not alone influence serine/threonine phosphatases, since both α2- and α2/α1-expressing cells showed equal phosphatase activities when cultured on fibronectin and since the phosphatase activities in Saos-α2/α1 and Saos vector control cells were the same (Fig. 5C). In accordance with the results of the measurement done in fibroblasts, in Saos cells the α2β1-induced phosphatase activity was predominantly PP2A, since 0.2 nM okadaic acid inhibited the activity by 60% (Fig. 5B). The activation of PP2A by α2β1 integrin represents a novel integrin-mediated signaling mechanism, where an integrin α subunit specifically activates a serine/threonine phosphatase.
FIG. 5.
An integrin α2 cytoplasmic tail is needed for activation of a serine/threonine phosphatase PP2A. Saos-α2 and Saos-α2/α1 cells were serum starved, detached, and cultured inside collagen for 1.5 h (A and B) or cultured on fibronectin (C). Measurement of PP1-PP2A phosphatase activity was done from cell lysates using 32P-labeled glycogen phosphorylase as a substrate. Two different Saos-α2 and Saos-α2/α1 single-cell clones were tested. The effect of 0.1 nM okadaic acid (OA) (inhibits half maximally PP2A but has no effect on PP1) was tested in the in vitro phosphatase reaction (B). Phosphatase activity relative to cell lysate protein content is shown. If the experiment was performed with several cell clones, the clone numbers are indicated (e.g., #2).
Akt forms a complex with PP2A.
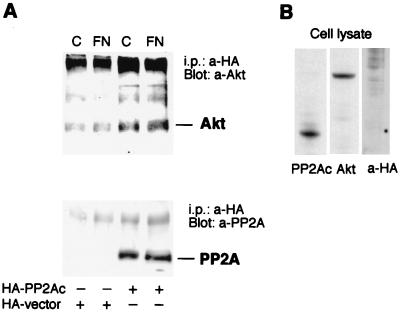
Recently, PP2A has been shown to form a complex with a number of kinases (1, 42, 57, 58). In most cases, PP2A inactivates the kinases. Here, we examined whether Akt associates physically with PP2A. Human primary fibroblasts were transfected with the endogenous catalytic subunit of PP2A (PP2Ac) containing a HA tag or with an empty HA tag vector only. The anti-HA immunoprecipitates were Western blotted with an anti-Akt antibody, and the Akt protein was found in HA-PP2Ac-transfected samples (Fig. 6A). The control samples had some Akt but much less than the samples from HA-PP2Ac-transfected cells (Fig. 6A). The matrix (fibronectin or collagen) had no effect on the coprecipitation (Fig. 6A). In another set of experiments human primary fibroblasts were infected with adeno-myrAkt to increase the level of active kinase in the cells. PP2Ac was detected in Akt immunoprecipitates. Neither Akt nor PP2A could be detected in precipitates prepared with control IgG (not shown). We also verified the interaction between Akt and PP2Ac in fibroblasts transfected with a vector encoding HA-tagged PP2Ac and infected with adeno-myrAkt (not shown). To confirm the specificity of the antibodies used, Western blotting was performed on whole-cell extracts from collagen-stimulated, nontransfected human primary fibroblasts. Anti-PP2A and anti-Akt antibodies detected a single specific band, and anti-HA showed no unspecific binding to cellular proteins (Fig. 6B).
FIG. 6.
PP2A interacts with Akt in transfected fibroblasts in vivo. (A) Human primary fibroblasts were transfected with Fugene 6 reagent and 6 μg of plasmid encoding the HA-tagged PP2A catalytic subunit (HA-PP2Ac) or with empty plasmid (HA vector). After 1 h inside collagen or on fibronectin, the cells were harvested, lysed, and immunoprecipitated (i.p.) with anti-HA antibody. The immunoprecipitates were analyzed by Western blotting with antibodies against Akt or the PP2A catalytic subunit. (B) Immunoblots from whole-cell lysates (nontransfected) using the same antibodies, indicating that the antibodies against Akt and PP2A recognize the corresponding wild-type proteins and that the anti-HA antibody shows no unspecific binding to cellular proteins.
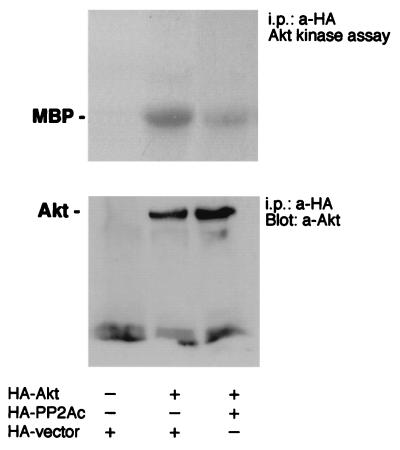
By transient cotransfections using PP2Ac together with tagged Akt, we assessed whether PP2A is directly responsible for the dephosphorylation of Akt. Cells were lysed, and Akt was immunoprecipitated in the presence of okadaic acid (1 nM) to prevent PP2A action during and after cell lysis. Overexpression of PP2Ac reduced Akt activity in an in vitro kinase assay of serum-starved Saos-α2/α1 cells that were stimulated with collagen for 1 h (Fig. 7). In three separate experiments the reduction in Akt activity was 36% ± 23%. Thus, overexpression of PP2A was able to reduce Akt phosphorylation in cells where active endogenous Akt could be observed due to the inability of the mutant integrin to activate PP2A. The data suggest a tight interrelationship between PP2A activity and Akt dephosphorylation.
FIG. 7.
Overexpression of PP2A inactivates Akt. The effect of the HA-PP2A catalytic subunit or HA vector on the kinase activity of cotransfected Akt was tested. Akt was immunoprecipitated from transfected (36 h posttransfection) and serum-starved Saos-α2/α1 cells after 1.5 h of treatment inside collagen. Akt activity was determined by the ability to phosphorylate myelin basic protein (MBP). A control blot shows that the overexpression of the PP2A catalytic subunit has no effect on the amount of Akt protein in cells.
PP2A activation by α2β1 integrin is independent of p38 and dependent on Cdc42.
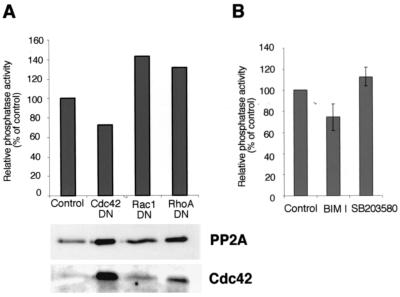
The mechanism of PP2A activation by α2β1 integrin was studied. We have previously shown that integrin α2β1 stimulates p38 kinase via small GTPase Cdc42 (25). This led us to study the possible role of Cdc42 in α2β1-mediated activation of PP2A. Saos-α2 cells were transiently transfected with a dominant-negative Cdc42 mutant (Cdc42DN). Transfection efficiency was determined and seemed to vary between 25 and 50% (not shown). Cdc42DN effectively inhibited integrin α2β1-induced activation of PP2A. In five separate experiments PP2A activity was 53% ± 13% lower in Cdc42DN-transfected cells than in vector control cells. The inhibitory effect of Cdc42DN was found to be statistically significant (Student t test; P < 0.016). Figure 8A shows a representative experiment. We also performed control assays to show that the transfections had no effect on the amount of PP2A in cells (Fig. 8A). Dominant-negative RhoA and Rac1 mutants had no effect on PP2A activity in collagen-stimulated Saos-α2 cells (Fig. 8A). In contrast, the active Cdc42 mutant increased PP2A activity to some extent (not shown).
FIG. 8.
Activation of PP2A by α2β1 integrin is dependent on Cdc42. (A) The effect of the transfected dominant-negative mutant forms of Cdc42 (Cdc42DN), Rac1 (Rac1DN), and RhoA (RhoADN) on the activation of PP2A in Saos-α2 cells. Phosphatase activity relative to cell lysate protein content is shown. Immunoblotting with antibodies against PP2A and Cdc42 was performed to show that Cdc42 was overexpressed in transfected cells and that it had no effect on the amount of PP2A protein in cells. (B) Serum-starved fibroblasts were detached and left untreated or treated with inhibitor BIM I (10 μM) or SB203580 (20 μM) and placed inside collagen for 1 h. Phosphatase activity relative to cell lysate protein content is shown (mean ± SEM). BIM I, four separate experiments; SB203580, two separate experiments (n = 3 for each).
The roles of other previously identified α2β1 downstream effectors, p38 kinase (25) and protein kinase C-ζ (PKC-ζ) (61), were also studied. Inhibition of p38 kinase with SB203580 did not influence PP2A activity, while PKC inhibitor BIM I, capable of inhibiting the PKC-ζ isoform at the concentration used, showed some effect (Fig. 8B). However, this inhibition was not found to be statistically significant, and the high BIM I concentration may also inhibit other kinases. The inability of p38 inhibition to influence PP2A activity indicates that α2β1 must regulate at least two distinct signaling functions, namely, the activation of PP2A and p38.
DISCUSSION
PP2A comprises a family of protein serine/threonine phosphatases. They can interact with a substantial number of proteins and contribute to the regulation of numerous signaling pathways (26). Active PP2A can inhibit the cell cycle, predominantly at the G2/M checkpoint (33), induce apoptosis (13, 48), and act as a tumor suppressor (45). In this study we demonstrate that α2β1 integrin modulates Akt and GSK3β phosphorylation in response to cell adhesion to 3D collagen by activating PP2A. In general integrin-mediated cell adhesion is known to trigger initial tyrosine phosphorylation events, such as the activation of FAK and Src family kinases, that result in the activation of downstream signaling pathways, a number of which involve the activity of various serine/threonine kinases (50). However, relatively little is known about how specific protein tyrosine and serine/threonine phosphatases function to regulate integrin-mediated signals. The suggestion that the activation of PP2A in response to collagen is specifically mediated by α2β1 integrin is based on the following facts: (i) PP2A activation could be inhibited by a function-blocking antibody to α2 integrin and (ii) human osteosarcoma cells lacking α2β1 integrin or expressing a signaling deficient, chimeric α2/α1 subunit were shown to be unable to activate PP2A in response to collagen even though these cells adhere to collagen similarly to the α2β1-expressing cells (25). Thus, our results demonstrate the association of integrin function with a protein serine/threonine phosphatase. In the previous reports, a link between integrins and protein tyrosine phosphatase SHP-2 has been recently established and the phosphatase function has been shown to be a positive regulator of integrin function (23, 39). Another example of the interrelationship between phosphatases and integrins comes from studies demonstrating that the expression of dual-specificity phosphatase PTEN is essential in maintaining the anchorage dependence of growth via negative regulation of FAK and downstream kinases (49).
The in vitro activity of PP2A can be regulated by phosphorylation (7) and methylation (31, 59). Cells also have specific PP2A inhibitor proteins (34, 35). The cellular pathways regulating PP2A activity are incompletely known, and accordingly the exact mechanism of PP2A activation by α2β1 integrin remains to be shown. Active Cdc42 seems indispensable in the stimulation of PP2A by α2β1 and collagen, since expression of dominant-negative Cdc42 blocked α2β1-mediated PP2A activation. Cdc42 may regulate the formation of a PP2A-containing adhesion and signaling complex. Indeed, a previous report indicates that PP2A can accumulate at focal contact sites due to its interaction with paxillin (24). Furthermore, our results suggest that the cytoplasmic domain of α2 integrin may also participate in the activation of PP2A.
Cdc42 may be one of the prime effectors of α2β1 signaling, since it is also required for α2β1-mediated activation of the p38 α isoform (25). p38 can activate PP2A (55), but here the inhibition of the p38 pathway had no effect on PP2A activation by α2β1 integrin. Additionally, a PKC inhibitor did not affect PP2A activation. PKC-ζ has been described as one of the signaling proteins activated by α2β1-mediated binding to collagen (60). Ceramides can concomitantly activate PKC-ζ and PP2A, suggesting that these pathways might be connected to each other (40).
Akt activity can, in general, be inhibited by either inhibition of upstream activators or by direct dephosphorylation (37). The fact that here α2β1 integrin inhibited Akt without concomitant attenuation of PI-3K led us to consider a possible link between the observed PP2A induction and Akt inactivation. PP2A has been shown to inactivate Akt in vitro, while other serine/threonine phosphatases, such as PP1, are unable to use phosphorylated Akt as a substrate (2). Calyculin A is able to prevent PP2A-dependent dephosphorylation of Akt (37), and here we show that it prevented α2β1-related Akt inactivation. Furthermore, the cotransfection experiments using the PP2A catalytic subunit with Akt demonstrated a direct relationship between PP2A and Akt kinase activity.
Recent studies indicated that PP2A associates with kinases and thereby negatively regulates their function (1, 41, 56, 57). Here we suggest that activated PP2A is the mechanism behind the modulation of Akt activity by α2β1 integrin. Furthermore, the dephosphorylation of GSK3β, one of the downstream targets of Akt, correlated with Akt deactivation. Dephosphorylation of GSK3β leads to its activation (28). Besides the Akt/GSK-3β pathway, PP2A has the potency to affect several other signaling pathways, and therefore the activation of PP2A may participate in many α2β1-mediated cellular events, including inhibition of the cell cycle (30).
To conclude, we have introduced a novel α2β1-related signaling pathway, namely, activation of PP2A. Protein phosphatases have wider substrate specificity than protein kinases, and therefore they may affect several signaling pathways. In general, the regulation of cellular phosphatase activity is less studied than that of kinases, and there are only a few reports indicating their regulation by a specific integrin. Integrin α2β1-mediated PP2A activation provides a versatile mechanism to regulate numerous signaling mechanisms and cellular functions.
Acknowledgments
We thank K. Walsh, K. Vuori, J. C. Lacal, G. W. G. Wilkinson, and D. Brautigan for the vector constructs and adenoviruses used in this study. Technical assistance by M. Potila, M. Tuominen, and T. Heikkilä is gratefully acknowledged.
This study was financially supported by grants from the Academy of Finland, the Sigrid Jusélius Foundation, the Finnish Cancer Association, Farmos Research Foundation, the Turku University Central Hospital, and the Technology Development Center in Finland.
REFERENCES
- 1.Abraham, D., K. Podar, M. Pacher, M. Kubicek, N. Welzel, B. A. Hemmings, S. M. Dilworth, H. Mischak, W. Kolch, and M. Baccarini. 2000. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275:22300-22304. [DOI] [PubMed] [Google Scholar]
- 2.Andjelkovic, M., T. Jakubowicz, P. Cron, X. F. Ming, J. W. Han, and B. A. Hemmings. 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. USA 93:5699-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger, K. R., L. A. Serunian, S. P. Soltoff, P. Libby, and L. C. Cantley. 1989. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57:167-175. [DOI] [PubMed] [Google Scholar]
- 4.Berditchevski, F., K. F. Tolias, K. Wong, C. L. Carpenter, and M. E. Hemler. 1997. A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81), and phosphatidylinositol 4-kinase. J. Biol. Chem. 272:2595-2598. [DOI] [PubMed] [Google Scholar]
- 5.Chan, B. M., P. D. Kassner, J. A. Schiro, H. R. Byers, T. S. Kupper, and M. E. Hemler. 1992. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell 68:1051-1060. [DOI] [PubMed] [Google Scholar]
- 6.Chan, T. O., S. E. Rittenhouse, and P. N. Tsichlis. 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68:965-1014. [DOI] [PubMed] [Google Scholar]
- 7.Chen, J., B. L. Martin, and D. L. Brautigan. 1992. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science 257:1261-1264. [DOI] [PubMed] [Google Scholar]
- 8.Chung, H., and D. L. Brautigan. 1999. Protein phosphatase 2A suppresses MAP kinase signalling and ectopic protein expression. Cell. Signal. 11:575-580. [DOI] [PubMed] [Google Scholar]
- 9.Clark, E. A., and J. S. Brugge. 1995. Integrins and signal transduction pathways: the road taken. Science 268:233-239. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, P., S. Klumpp, and D. L. Schelling. 1989. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 250:596-600. [DOI] [PubMed] [Google Scholar]
- 11.Coppolino, M. G., and S. Dedhar. 2000. Bi-directional signal transduction by integrin receptors. Int. J. Biochem. Cell Biol. 32:171-188. [DOI] [PubMed] [Google Scholar]
- 12.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 13.Deng, X., T. Ito, B. Carr, M. Mumby, and W. S. May, Jr. 1998. Reversible phosphorylation of Bcl2 following interleukin 3 or bryostatin 1 is mediated by direct interaction with protein phosphatase 2A. J. Biol. Chem. 273:34157-34163. [DOI] [PubMed] [Google Scholar]
- 14.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 15.Fluck, J., C. Querfeld, A. Cremer, S. Niland, T. Krieg, and S. Sollberg. 1998. Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J. Investig. Dermatol. 110:153-157. [DOI] [PubMed] [Google Scholar]
- 16.Fornaro, M., C. A. Steger, A. M. Bennett, J. J. Wu, and L. R. Languino. 2000. Differential role of beta(1C) and beta(1A) integrin cytoplasmic variants in modulating focal adhesion kinase, protein kinase B/AKT, and Ras/mitogen-activated protein kinase pathways. Mol. Biol. Cell 11:2235-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujio, Y., K. Guo, T. Mano, Y. Mitsuuchi, J. R. Testa, and K. Walsh. 1999. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol. Cell. Biol. 19:5073-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujio, Y., and K. Walsh. 1999. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J. Biol. Chem. 274:16349-16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giancotti, F. G. 2000. Complexity and specificity of integrin signalling. Nat. Cell Biol. 2:E13-E14. [DOI] [PubMed] [Google Scholar]
- 20.Henriet, P., Z. D. Zhong, P. C. Brooks, K. I. Weinberg, and Y. A. DeClerck. 2000. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc. Natl. Acad. Sci. USA 97:10026-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter, T. 1995. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80:225-236. [DOI] [PubMed] [Google Scholar]
- 22.Hynes, R. O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11-25. [DOI] [PubMed] [Google Scholar]
- 23.Inagaki, K., T. Noguchi, T. Matozaki, T. Horikawa, K. Fukunaga, M. Tsuda, M. Ichihashi, and M. Kasuga. 2000. Roles for the protein tyrosine phosphatase SHP-2 in cytoskeletal organization, cell adhesion and cell migration revealed by overexpression of a dominant negative mutant. Oncogene 19:75-84. [DOI] [PubMed] [Google Scholar]
- 24.Ito, A., T. R. Kataoka, M. Watanabe, K. Nishiyama, Y. Mazaki, H. Sabe, Y. Kitamura, and H. Nojima. 2000. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J. 19:562-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivaska, J., H. Reunanen, J. Westermarck, L. Koivisto, V. M. Kähäri, and J. Heino. 1999. Integrin alpha2beta1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the alpha2 cytoplasmic tail. J. Cell Biol. 147:401-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353:417-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khwaja, A., P. Rodriguez-Viciana, S. Wennstrom, P. H. Warne, and J. Downward. 1997. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16:2783-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, L., and A. R. Kimmel. 2000. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr. Opin. Genet. Dev. 10:508-514. [DOI] [PubMed] [Google Scholar]
- 29.Klekotka, P. A., S. A. Santoro, and M. M. Zutter. 2001. Alpha 2 integrin subunit cytoplasmic domain-dependent cellular migration requires p38 MAPK. J. Biol. Chem. 276:9503-9511. [DOI] [PubMed] [Google Scholar]
- 30.Koyama, H., E. W. Raines, K. E. Bornfeldt, J. M. Roberts, and R. Ross. 1996. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell 87:1069-1078. [DOI] [PubMed] [Google Scholar]
- 31.Lee, J., and J. Stock. 1993. Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J. Biol. Chem. 268:19192-19195. [PubMed] [Google Scholar]
- 32.Lee, J. W., and R. L. Juliano. 2000. α5β1 integrin protects intestinal epithelial cells from apoptosis through a phosphatidylinositol 3-kinase and protein kinase B-dependent pathway. Mol. Biol. Cell 11:1973-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, T. H., M. J. Solomon, M. C. Mumby, and M. W. Kirschner. 1991. INH, a negative regulator of MPF, is a form of protein phosphatase 2A. Cell 64:415-423. [DOI] [PubMed] [Google Scholar]
- 34.Li, M., H. Guo, and Z. Damuni. 1995. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry 34:1988-1996. [DOI] [PubMed] [Google Scholar]
- 35.Li, M., A. Makkinje, and Z. Damuni. 1996. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem. 271:11059-11062. [DOI] [PubMed] [Google Scholar]
- 36.Manes, S., E. Mira, C. Gomez-Mouton, Z. J. Zhao, R. A. Lacalle, and A. C. Martinez. 1999. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol. 19:3125-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier, R., M. Thelen, and B. A. Hemmings. 1998. Inactivation and dephosphorylation of protein kinase Balpha (PKBalpha) promoted by hyperosmotic stress. EMBO J. 17:7294-7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulrooney, J., K. Foley, S. Vineberg, M. Barreuther, and L. Grabel. 2000. Phosphorylation of the beta1 integrin cytoplasmic domain: toward an understanding of function and mechanism. Exp. Cell Res. 258:332-341. [DOI] [PubMed] [Google Scholar]
- 39.Oh, E. S., H. Gu, T. M. Saxton, J. F. Timms, S. Hausdorff, E. U. Frevert, B. B. Kahn, T. Pawson, B. G. Neel, and S. M. Thomas. 1999. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol. Cell. Biol. 19:3205-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry, D. K., and Y. A. Hannun. 1998. The role of ceramide in cell signaling. Biochim. Biophys. Acta 1436:233-243. [DOI] [PubMed] [Google Scholar]
- 41.Peterson, R. T., B. N. Desai, J. S. Hardwick, and S. L. Schreiber. 1999. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin-associated protein. Proc. Natl. Acad. Sci. USA 96:4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravanti, L., J. Heino, C. Lopez-Otin, and V. M. Kähäri. 1999. Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J. Biol. Chem. 274:2446-2455. [DOI] [PubMed] [Google Scholar]
- 43.Riikonen, T., L. Koivisto, P. Vihinen, and J. Heino. 1995. Transforming growth factor-beta regulates collagen gel contraction by increasing alpha 2 beta 1 integrin expression in osteogenic cells. J. Biol. Chem. 270:376-382. [DOI] [PubMed] [Google Scholar]
- 44.Riikonen, T., J. Westermarck, L. Koivisto, A. Broberg, V. M. Kähäri, and J. Heino. 1995. Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J. Biol. Chem. 270:13548-13552. [DOI] [PubMed] [Google Scholar]
- 45.Scheidtmann, K. H., M. C. Mumby, K. Rundell, and G. Walter. 1991. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small t antigen. Mol. Cell. Biol. 11:1996-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz, M. A., and V. Baron. 1999. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11:197-202. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz, M. A., M. D. Schaller, and M. H. Ginsberg. 1995. Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 11:549-599. [DOI] [PubMed] [Google Scholar]
- 48.Song, Q., and M. F. Lavin. 1993. Calyculin A, a potent inhibitor of phosphatases-1 and -2A, prevents apoptosis. Biochem. Biophys. Res. Commun. 190:47-55. [DOI] [PubMed] [Google Scholar]
- 49.Tamura, M., J. Gu, E. H. Danen, T. Takino, S. Miyamoto, and K. M. Yamada. 1999. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 274:20693-20703. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 51.Tsuda, M., T. Matozaki, K. Fukunaga, Y. Fujioka, A. Imamoto, T. Noguchi, T. Takada, T. Yamao, H. Takeda, F. Ochi, T. Yamamoto, and M. Kasuga. 1998. Integrin-mediated tyrosine phosphorylation of SHPS-1 and its association with SHP-2. Roles of Fak and Src family kinases. J. Biol. Chem. 273:13223-13229. [DOI] [PubMed] [Google Scholar]
- 52.Virshup, D. M. 2000. Protein phosphatase 2A: a panoply of enzymes. Curr. Opin. Cell Biol. 12:180-185. [DOI] [PubMed] [Google Scholar]
- 53.Wary, K. K., F. Mainiero, S. J. Isakoff, E. E. Marcantonio, and F. G. Giancotti. 1996. The adapter protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87:733-743. [DOI] [PubMed] [Google Scholar]
- 54.Wary, K. K., A. Mariotti, C. Zurzolo, and F. G. Giancotti. 1998. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94:625-634. [DOI] [PubMed] [Google Scholar]
- 55.Westermarck, J., S. P. Li, T. Kallunki, J. Han, and V. M. Kähäri. 2001. p38 mitogen-activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression. Mol. Cell. Biol. 21:2373-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westphal, R. S., K. A. Anderson, A. R. Means, and B. E. Wadzinski. 1998. A signaling complex of Ca2+-calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science 280:1258-1261. [DOI] [PubMed] [Google Scholar]
- 57.Westphal, R. S., R. L. Coffee, Jr., A. Marotta, S. L. Pelech, and B. E. Wadzinski. 1999. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J. Biol. Chem. 274:687-692. [DOI] [PubMed] [Google Scholar]
- 58.Whitman, M., D. R. Kaplan, B. Schaffhausen, L. Cantley, and T. M. Roberts. 1985. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature 315:239-242. [DOI] [PubMed] [Google Scholar]
- 59.Xie, H., and S. Clarke. 1994. Protein phosphatase 2A is reversibly modified by methyl esterification at its C-terminal leucine residue in bovine brain. J. Biol. Chem. 269:1981-1984. [PubMed] [Google Scholar]
- 60.Xu, J., and R. A. Clark. 1997. A three-dimensional collagen lattice induces protein kinase C-zeta activity: role in alpha2 integrin and collagenase mRNA expression. J. Cell Biol. 136:473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, J., M. M. Zutter, S. A. Santoro, and R. A. Clark. 1998. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J. Cell Biol. 140:709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, D. H., C. K. Qu, O. Henegariu, X. Lu, and G. S. Feng. 1998. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 273:21125-21131. [DOI] [PubMed] [Google Scholar]