Abstract
The D9 and D10 proteins of vaccinia virus are 25% identical to each other, contain a mutT motif characteristic of nudix hydrolases, and are conserved in all sequenced poxviruses. Previous studies indicated that overexpression of D10 and, to a lesser extent, D9 decreased the levels of capped mRNAs and their translation products. Here, we further characterized the D10 protein and showed that only trace amounts are associated with purified virions and that it is expressed exclusively at late times after vaccinia virus infection. A viable deletion mutant (vΔD10) produced smaller plaques and lower virus yields than either wild-type virus or a D9R deletion mutant (vΔD9). Purified vΔD10 virions appeared normal by microscopic examination and biochemical analysis but produced 6- to 10-fold-fewer plaques at the same concentration as wild-type or vΔD9 virions. When 4 PFU per cell of wild-type or vΔD9 virions or equal numbers of vΔD10 virions were used for inoculation, nearly all cells were infected in each case, but viral early and late transcription was initiated more slowly in vΔD10-infected cells than in the others. However, viral early transcripts accumulated to higher levels in vΔD10-infected cells than in cells infected with the wild type or vΔD9. In addition, viral early and late mRNAs and cellular actin mRNA persisted longer in vΔD10-infected cells than in others. Furthermore, analysis of pulse-labeled proteins indicated prolonged synthesis of cellular and viral early proteins. These results are consistent with a role for D10 in regulating RNA levels in poxvirus-infected cells.
Vaccinia virus (VACV), the most extensively studied member of the Poxviridae family, contains a large double-stranded DNA genome encoding approximately 200 proteins. Replication occurs in the cytoplasm and is orchestrated in a programmed manner, with sequential transcription of early-, intermediate-, and late-stage genes (25). Numerous positive regulatory factors have been identified, notably the proteins required for stage-specific transcription in conjunction with the viral DNA-dependent RNA polymerase (for a review, see reference 8). The changes in total viral early, intermediate, and late RNA levels, however, suggest the deployment of negative, as well as positive, regulators (3). Indeed, the speed at which RNA levels change can only be explained if viral RNAs have very short half-lives, as was proposed nearly 40 years ago (26, 31). The accelerated turnover of cellular mRNAs during VACV infection (9, 19, 20, 29) might occur by a related or identical degradation mechanism.
A putative negative regulator of gene expression was identified in a transfection-based DNA library screen that was successfully used to find activators of VACV late transcription (21, 32). The inhibitory activity was mapped primarily to the D10R gene of VACV, with the D9R gene contributing more modestly to the effect. (Note: VACV genes are designated with a capital letter that describes the HindIII fragment containing the open reading frame, followed by a number that corresponds to the position of this gene on the fragment, and either L or R, indicating the direction of transcription; L or R is omitted when referring to the protein or RNA product.) The down-regulation of reporter gene expression and RNA levels directed by D10 was independent of the promoter used but was abrogated by the presence of the encephalomyocarditis virus internal ribosome binding site, suggesting that the inhibition was specific for 5′ capped RNAs. The D9R and D10R genes are predicted to encode proteins with 25% sequence identity to each other. The D9R gene is conserved in all chordopoxviruses and the D10R gene is conserved in entomopoxviruses as well, suggesting ancient and essential functions. Interestingly, the D9 and D10 proteins each contain a mutT motif, a signature sequence characteristic of nudix hydrolases, which cleave nucleoside diphosphates linked to other moieties (5, 22). MutT proteins have a wide range of activities, but the one that seems most relevant to the present study is the RNA cap-cleaving activity of yeast and human DCP2, which accelerate mRNA degradation (16, 36).
Prior studies demonstrated that D9R is not essential for viral replication in tissue culture cells, but parallel attempts to isolate D10 deletion mutants were unsuccessful (17, 32). Similarly, the D9R homolog but not the D10R homolog could be deleted from fowlpox virus (6). Here, we demonstrate that D10 is expressed at the late stage of VACV infection but unlike the majority of late proteins is not selectively packaged into virus particles. In contrast to the previous attempts cited above, we successfully isolated a D10R deletion mutant. Thus, D10R is one of the few genes conserved in all sequenced poxviruses that have been shown to be nonessential for replication in tissue culture cells. Another example is the type 1 topoisomerase (12). Nevertheless, replication of the D10R deletion mutant virus was impaired, as it made small plaques on cell monolayers and the specific infectivity of virus particles was reduced by 6 to 10 fold. Although the observed phenotypic differences between the mutant and wild-type viruses were subtle and multiplicity of infection dependent, a notable finding was the prolongation of the time during which early viral and cellular mRNA could be detected.
MATERIALS AND METHODS
Cells and virus.
BS-C-1 (ATCC CCL-26) cells and HeLa S3 (ATCC CCL-2.2) suspension cells were grown in Eagle's minimal essential medium (EMEM) (Quality Biologicals, Inc., Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT) and MEM spinner medium (Quality Biologicals, Inc.) supplemented with 5% equine serum (HyClone), respectively. The WR strain (ATCC VR-1354) of VACV was used to derive recombinant viruses. Virus stocks were prepared as previously described (18). For all viral infections, EMEM supplemented with 2.5% FBS was used.
Recombinant PCR and plasmid construction.
To construct vD10V5, a recombinant PCR product was created containing the 3′ portion of D10R appended with a V5 epitope tag directly preceding the stop codon, the entire bacterial β-glucoronidase (gus) gene regulated by the VACV P11 late promoter, and the 5′ portion of the flanking D11L gene. The D9 knockout plasmid was constructed by ligating a portion of the D8L gene flanked by the restriction sites PstI and NotI and a segment of the D10R gene flanked with KpnI and BamHI restriction sites into the pSL1180EGFP plasmid, creating a construct containing the enhanced green fluorescent protein (EGFP) gene flanked by D8L and D10R. A similar procedure was used to generate the D10 knockout plasmid in which the D9R and D11L genes flanked EGFP regulated by the VACV P11 late promoter. The ΔD10 revertant virus (vΔD10 rev) was created by construction of a recombinant PCR fragment that contained wild-type D10R engineered with a silent mutation at amino acid 57 (threonine ACT to ACA) flanked on each side by a portion of D9R and D11R.
Generation of recombinant VACV.
To generate the recombinant viruses, BS-C-1 cells were infected with VACV WR strain (to make vD10V5, vΔD9, and vΔD10) or vΔD10 (for vΔD10rev) at 1 PFU per cell and subsequently transfected with the appropriate DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The transfected cell lysates were used to infect fresh BS-C-1 monolayers and subsequently overlaid with 1% low-melting-point agarose (Invitrogen) in 1× plaque medium (Quality Biological, Inc.) supplemented with 2.5% FBS. To identify vD10V5, a second agarose overlay was applied 2 days later that contained 0.2 mg of 5-bromo-4-chloro-3 indolyl-β-d-glucuronic acid (BD Biosciences, Palo Alto, Calif.)/ml. Blue plaques exhibiting gusA activity were picked and repeatedly plaque purified. For vΔD9 and vΔD10, plaques were identified on the basis of EGFP expression, while vΔD10rev plaques were identified by loss of EGFP expression. Recombinant viruses were plaque purified at least four times and confirmed to be correct by PCR amplification and sequencing of the insertion sequence and immediate flanking genomic region.
Purification of VACV.
Wild-type WR virus and recombinant viruses were grown in HeLa S3 cells and purified by sedimentation through a 36% sucrose cushion and banded once or twice on a 24% to 40% continuous sucrose gradient (18). The number of virus particles was determined from the optical density at 260 nm (1 U = 1.2 × 1010 particles). Using this value, the particle-to-PFU ratio of wild-type VACV was determined to be approximately 80.
Western blotting.
Proteins derived from six-well dishes of infected BS-C-1 cell lysates or purified virions were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically onto a nitrocellulose membrane (Invitrogen, Carlsbad, CA). The membrane was blocked in 5% nonfat dry milk in TTBS (100 mM Tris [pH 7.5], 150 mM NaCl, 0.05% [vol/vol] Tween-20) and subsequently incubated with the appropriate primary antibody. For the experiments analyzing D10 expression, monoclonal anti-V5 antibody (Invitrogen) was used at a 1:5,000 dilution, and this immunoblot was reprobed using polyclonal anti-G7 at a 1:1,000 dilution (35). For Western blots of SDS-dissociated virions, polyclonal antisera directed against either H4 (1), A4 (15), or L1 (2) were used at a dilution of 1:1,000. The membrane was then washed in TTBS and incubated with anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (Amersham) at a dilution of 1:2,000 or anti-rabbit IgG conjugated to horseradish peroxidase at a dilution of 1:10,000 (Amersham). The amount of IgG bound was determined by treating the membrane with either SuperSignal West Dura Extended Duration or Femto Maximum Sensitivity chemiluminescent substrate (Pierce, Rockford, IL). The BCA protein assay kit (Pierce) was used to determine the concentration of purified virions and lysates after solubilization in 2% (vol/vol) SDS.
Plaque assay.
BS-C-1 cell monolayers grown in six-well tissue culture dishes were infected with 10-fold serial dilutions of VACV. After 1 h of adsorption, the monolayers were washed and covered with EMEM containing 5% fetal bovine serum and 0.5% methylcellulose. After 2 days at 37°C, the cells were stained with crystal violet.
One-step virus growth.
BS-C-1 cells grown in 12-well plates were incubated with 5 PFU per cell of VACV for 1 h at 37°C. The infected cells were washed extensively and then incubated with medium. At multiple times after infection, cells were harvested and subjected to three successive freeze-thaw cycles to induce cell lysis. The resulting titers of the infected cell lysates were determined by plaque assay as described above.
Flow cytometry.
BS-C-1 cell monolayers grown in six-well dishes were infected with VACV. After 1 h at 37°C, the cells were washed three times, and the medium was replaced. At 4 h postinfection, the cells were washed three times with phosphate-buffered saline, pH 7.4, and subsequently fixed with 1% paraformaldehyde for 15 min. The cells were stained with monoclonal antibody TW2.3 directed against the VACV E3 protein (37) in Hanks' balanced salt solution (HBSS) containing 0.1% saponin and 0.1% bovine serum albumin (BSA) for 1 h at room temperature. Next, the cells were washed twice with HBSS-0.1% saponin-0.1% BSA and stained with Alexa Fluor 488 conjugated to goat anti-mouse antibody (Invitrogen) for 1 h at room temperature. The cells were washed several times in HBSS-0.1% saponin-0.1% BSA, followed by phosphate-buffered saline. Cell fluorescence was measured using a FACSCalibur (BD Biosciences) machine; collected data were analyzed using CellQuest software (BD Biosciences).
Northern blot analysis.
At multiple times after infection, six-well dishes of BS-C-1 cells were harvested, and total RNA was extracted with the RNaqueous kit (Ambion, Austin, TX). Total RNA was calculated by using a spectrophotometer, with 4 μg of total RNA loaded onto the gel for each RNA sample. RNA was resolved by electrophoresis, and Northern blot analysis was performed using the NorthernMax-Gly kit (Ambion) according to the manufacturer's protocol. For the CllR probe, a PCR product containing the entire open reading frame (ORF) was synthesized with [α-32P]dCTP using the Decaprime (Ambion) random prime labeling kit. An F18R (F17R in the Copenhagen strain) antisense probe was transcribed from a PCR fragment containing the F18R sequence regulated by a bacteriophage T7 promoter. The F18R riboprobe was uniformly labeled with [α-32P]UTP using the MaxiScript T7 in vitro transcription kit (Ambion). The labeled actin antisense probe was generated by in vitro transcription of the pTRI-Beta-actin-Human vector (Ambion). The probes were separated from unincorporated nucleotides by use of NucAway spin columns (Ambion). For loading controls, blots were stripped and reprobed with labeled 28S antisense rRNA that had been transcribed in vitro from the pTRI-RNA 28S vector (Ambion).
Pulse-labeling of proteins.
At 1 h after infection, BS-C-1 cell monolayers in 12-well tissue culture dishes were washed and incubated with EMEM supplemented with 2.5% fetal bovine serum. At subsequent times, the infected cells were incubated with methionine and cysteine-free medium (Sigma-Aldrich, St. Louis, MO) containing 2.5% dialyzed fetal bovine serum (Invitrogen) for 30 min. Then the infected cells were pulsed with 100 μCi of [35S]methionine and [35S]cysteine per ml for 30 min, harvested, and washed. The cell pellets were incubated with micrococcal nuclease (0.1 μg per μl) in 10 mM Tris-HCl (pH 7.5), 10 mM NaCl, 1 mM CaCl2, 0.2% (vol/vol) NP-40, 20 mM β-mercaptoethanol, and 1× complete mini EDTA-free protease inhibitor (Roche, Indianapolis, IN) for 30 min on ice. The radiolabeled samples were resolved on 4 to 12% NuPAGE Bis Tris gels (Invitrogen) in NuPAGE MOPS (morpholinepropanesulfonic acid) running buffer (Invitrogen), dried onto Whatmann paper, and visualized by autoradiography.
RESULTS
Temporal expression of D10.
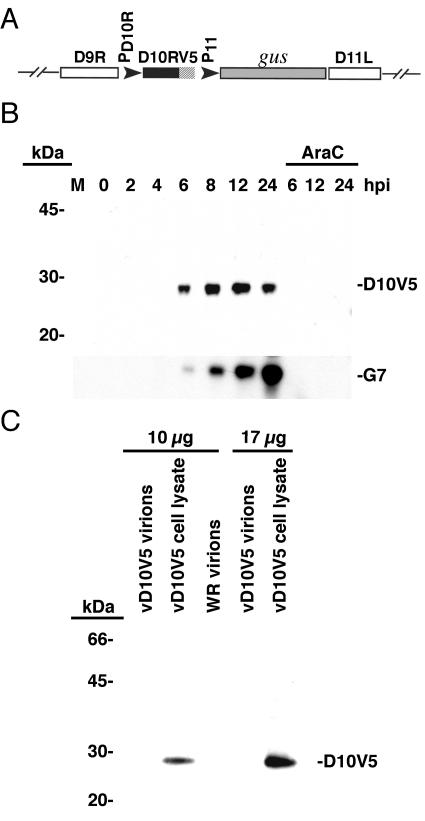
The presence of a late promoter regulating the D10R ORF was previously demonstrated by transcriptional mapping (24). Because no antibodies were available to analyze the protein product of the D10R gene, we constructed a recombinant VACV (vD10V5) in which a V5 epitope tag was added to the C terminus of the D10 protein, without altering the promoter sequence (Fig. 1A). The gus gene was coinserted downstream to facilitate recombinant virus isolation by staining plaques with 5-bromo-4-chloro-3-indoxyl-beta-d-glucuronide (10). The plaque size and growth kinetics of vD10V5 were similar to that of wild-type VACV WR, suggesting that the epitope tag was not detrimental to virus replication (data not shown). Whole-cell lysates of cells infected with vD10V5 and WR were prepared at successive times after infection in the presence or absence of the DNA replication inhibitor AraC. Proteins from the infected cell lysates were resolved by SDS-PAGE, followed by Western blotting with V5 antibody. The V5 antiserum did not react with proteins derived from WR-infected lysates, confirming its specificity (data not shown). In the vD10V5-infected cell lysates, a 29-kDa protein corresponding to the predicted size of D10 was first detected 6 h postinfection with maximal protein accumulation at 12 h, followed by a decrease in protein levels at 24 h (Fig. 1B). The consistent reduction in D10 at 24 h after infection was unusual, as VACV late proteins typically continue to increase over this time interval, as shown by reprobing the blot with antibody to the 42-kDa G7 late protein (Fig. 1B). We have not determined whether the diminution of D10 was due to decreased rates of protein synthesis, increased rates of degradation, or a combination of the two. The absence of D10 in the presence of AraC demonstrated that D10 expression requires DNA replication, in accord with other late-stage proteins (Fig. 1B).
FIG. 1.
Expression of D10. (A) Schematic illustrating the genomic organization of recombinant vD10V5 encoding a V5 epitope tag at the end of the D10R ORF. PD10R refers to the natural promoter of D10; P11 indicates the VACV promoter that normally regulates late expression of the gene encoding the 11K (F18) protein. (B) Western blot showing temporal expression of D10. BS-C-1 cells were mock infected (M) or infected with vD10V5 at a multiplicity of infection of 5 PFU per cell in the absence or presence of the DNA replication inhibitor AraC. Cell lysates were prepared at the hour postinfection (hpi) indicated, and proteins were resolved by SDS-PAGE, followed by immunoblotting with monoclonal antibody to the V5 epitope tag. The blot was subsequently reprobed with a polyclonal antibody that recognizes G7, a late VACV protein. The masses and mobilities of marker proteins are shown on the left. (C) Analysis of virions for D10. The vD10V5-infected cell lysates and purified WR and vD10V5 virions were solubilized in 2% SDS, and total protein was quantified by the bicinchoninic assay (Pierce). Equivalent amounts of protein (either 10 or 17 μg total protein) from each source were resolved by SDS-PAGE and subjected to immunoblotting using V5 antibody.
D10 is not selectively packaged in virus particles.
To determine whether D10 is packaged into virions for assembly or use early in infection, as occurs with the majority of VACV late proteins, vD10V5 virions were purified by sedimentation through a sucrose cushion and two sucrose gradients. Proteins from purified virions were resolved by SDS-PAGE and subjected to immunoblotting with V5 antibody. D10V5 could only be detected in trace amounts, even when relatively large quantities of purified virions were analyzed (not shown), despite the sensitivity of D10V5 detection in infected cell lysates as seen in Fig. 1B. To compare the amounts of D10V5 in vD10V5-infected cell lysates and purified virions, the same quantities of total protein from each source were resolved by SDS-PAGE, followed by immunoblotting with V5 antibody. The D10 signal was discernible only in the infected cell lysates (Fig. 1C). Given that infected cell lysates contain a large amount of cellular protein, true virion components should be considerably enriched in purified virions. The observation that D10V5 was much more abundant in lysates than in virions indicated that D10V5 is not selectively packaged. Efforts to localize the D10V5 protein by confocal microscopy using V5 antibody indicated diffuse cytoplasmic fluorescence (not shown).
Isolation of D9R and D10R deletion mutants.
Based on the high conservation of the D9R and D10R genes, one might anticipate that each would be essential for virus replication. Nevertheless, individual D9R- and D10R-inducible mutants were constructed that were viable in the absence of inducer (32). This result was subsequently explained for D9R, as a deletion mutant was isolated using a drug-selectable marker (17, 32). Using the same selection scheme, however, a D10R deletion mutant was not isolated, suggesting that D10 is essential for VACV replication (32). Although the apparent paradox could be explained if the D10-inducible mutant was slightly leaky and exceedingly low amounts of D10 were sufficient for replication, we decided to make a new attempt to isolate a D10R deletion mutant. Three plasmids were designed to delete D9R and D10R genes separately or together by flanking a VACV promoter-EGFP cassette with the corresponding genomic regions of the desired deletion (Fig. 2A). After cells were infected with wild-type VACV and transfected with the deletion plasmids, plaques exhibiting EGFP fluorescence were detected, and the recombinant virus was purified through four successive rounds of plaque isolation. Importantly, all plaques formed by the clonally purified mutants expressed EGFP, indicating that we had not isolated single-crossover mutants, which tend to undergo further recombination, resulting in both wild-type and recombinant viruses. Furthermore, several independent clones of vΔD9 and vΔD10 were shown to be missing D9 or D10 by PCR amplification and sequencing of both the insertion fragment and the surrounding genomic region. The double-deletion mutant, however, could not be isolated, leaving open the possibility that D9 and D10 may have an essential compensatory function.
FIG. 2.
Construction and replication of vΔD9 and vΔD10. (A) Diagrams depicting the genomic organization of recombinant vΔD9 and vΔD10. PD8L, PD9R, PD10R, and PD11L refer to the natural promoters of the respective genes; P11 describes the VACV promoter that normally drives late expression of the F18R gene encoding the 11K protein. (B) Plaque phenotype of vΔD9 and vΔD10. BS-C-1 monolayers grown in six-well tissue culture dishes were infected with VACV WR, vΔD9, or vΔD10. Cell monolayers were stained with crystal violet following 48 h of infection. (C) One-step growth of vΔD9 and vΔD10. BS-C-1 cell monolayers were infected with either WR, vΔD9, or vΔD10 at 5 PFU per cell. Following 1 h of adsorption at 37°C, the cell monolayers were washed extensively and overlaid with fresh medium. At various times postinfection, infected cells were harvested, and cell lysis was induced by three successive freeze-thaw cycles. The viral titers were determined by plaque assay.
Initial characterization of deletion mutants.
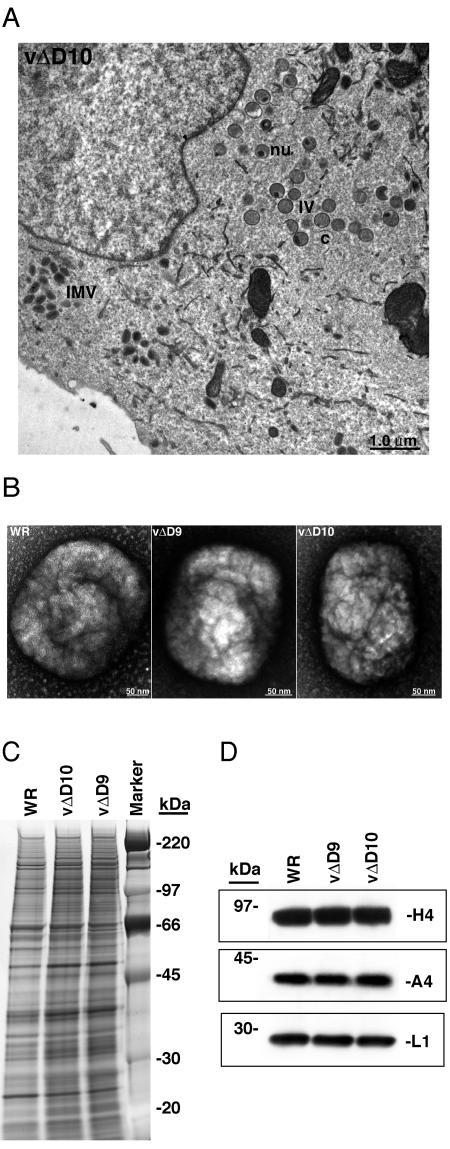
The mutants were distinguished by plaque size with WR > vΔD9 > vΔD10 (Fig. 2B). We also noted that the amplified stocks of vΔD10 consistently had lower plaque titers than those of vΔD9. One-step growth experiments in which cells were infected with a multiplicity of infection of 5 PFU per cell revealed that the kinetics of vΔD10 replication was delayed compared to that of WR or vΔD9 and that the final yields were lower (Fig. 2C). Impaired growth of vΔD10 was also observed for chicken embryo fibroblast cells and BHK cells (data not shown). To demonstrate that the growth defect was a direct consequence of the deletion of D10R, a vΔD10 revertant virus (vΔD10rev) was constructed. As expected, vΔD10rev formed plaques similar to those produced by WR and exhibited growth kinetics comparable to those of WR and vΔD9 (not shown).
The low yield of vΔD10 could be a consequence of a reduction in virus particle production or to the formation of virus particles with low infectivity. Electron microscopy of cells infected with vΔD10 showed normal virus particles and assembly intermediates (Fig. 3A). To evaluate the infectivity of the vΔD10 particles, vΔD9, vΔD10, and WR virions were purified in parallel by sedimentation through a sucrose cushion, followed by a sucrose gradient. The virus bands in the gradients were indistinguishable, and the amounts of purified particles recovered were similar, as determined from the optical density. Nevertheless, there were fewer infectious virions, determined by plaque assay, in the vΔD10 preparation than in the others. The particle-to-PFU ratios of two independent preparations of vΔD10 virions were 523 and 870, compared to 82 for both VACV WR and vΔD9. Despite the 6- to 10-fold-lower specific infectivity of the vΔD10 virions compared to the wild type, their purity and appearance were similar as determined by electron microscopy (Fig. 3B). In addition, the polypeptide pattern of vΔD10 resembled vΔD9 and VACV WR qualitatively and quantitatively, as determined by SDS-PAGE, followed by silver staining (Fig. 3C) or Western blotting for representative IMV membrane and core proteins (Fig. 3D). Furthermore, the DNA content of vΔD10 particles was similar to that of VACV WR (not shown).
FIG. 3.
Electron microscopy and protein composition of virions with deleted D10R gene. (A) Transmission electron microscopy of infected cells. BS-C-1 cells were infected with vΔD10 for 18 h and examined by electron microscopy. Representative immature virions (IV), immature virions with nucleoids (nu), and mature virus particles (IMV) are labeled. (B) Negative-stained images of sucrose gradient-purified WR, vΔD9, and vΔD10 virions. Virus particles were placed on grids, washed, and stained with 7% uranyl acetate and 50% ethanol for 30 s. (C) SDS-PAGE analysis of sucrose gradient-purified WR, vΔD9, and vΔD10. Equal numbers of particles were solubilized in SDS-PAGE loading buffer and resolved by SDS-PAGE, followed by silver staining. (D) Western blot analysis of purified virions. Equal numbers of purified WR, vΔD9, and vΔD10 virions were solubilized, and the proteins were separated by SDS-PAGE, followed by immunoblotting with antibody directed against H4 (an early transcription factor associated with RNA polymerase), A4 (an IMV core protein), or L1 (an IMV membrane protein). Masses of marker proteins are shown on the left.
It is relevant to point out that the one-step growth curves shown in Fig. 2C were carried out with equal PFU of WR, vΔD9, and vΔD10. Therefore, the virus particle multiplicity of infection used for vΔD10 was sixfold higher than for the other viruses. For some types of mutations, defects can be partially or even completely overcome by increasing the number of infecting particles; such an effect may have enhanced the replication of vΔD10.
Effect of the D10R deletion on early transcription.
To determine the stage in the viral life cycle perturbed by the absence of D10, monolayers were inoculated with 4 PFU per cell of VACV WR or vΔD9 and the equivalent number of vΔD10 particles. We noted in each culture that essentially all cells were rounded by 4 h, suggesting that virus entry and early gene expression were not grossly impaired. Flow cytometry was used to quantitatively corroborate this impression. At 4 h, >90% of cells infected with each of the viruses expressed E3, a viral early protein for which a monoclonal antibody was available (Fig. 4A). However, the mean fluorescence intensity was reduced in cells infected with vΔD10 compared to WR and vΔD9 (Fig. 4A), suggesting lower expression of E3 at this time.
FIG. 4.
Viral early gene expression. (A) Flow cytometry. BS-C-1 cells were infected with approximately 4 PFU per cell of purified VACV WR or vΔD9 or with an equivalent number of vΔD10 particles. After 4 h, the cells were fixed and stained with a monoclonal antibody to the VACV E3 early protein, followed by Alexa Fluor 488 conjugated to goat anti-mouse antibody. The numbers of fluorescent cells and the mean fluorescence intensities (MFI) were determined by flow cytometry. (B) Northern blot analysis of early transcripts from cells infected with VACV WR, vΔD9, or vΔD10. BS-C-1 cells were infected with equal amounts of purified virions as described in the legend to panel A. At the indicated hours after infection (hpi), cells were harvested for RNA extraction. Total RNA was resolved by electrophoresis, followed by Northern blotting. The resulting blot was probed with radioactively labeled C11R, a VACV gene expressed early in infection. The electrophoretic mobilities of marker RNAs of indicated length are shown on the left, and the position of the C11R transcript is shown on the right.
To further examine early gene expression, cells were infected as above and RNA was isolated at various times for gel electrophoresis and Northern blot analysis. The latter was carried out using a radioactively labeled probe complementary to the VACV early transcript encoded by the C11R gene. The C11R transcript was detected at 2 h in each case but the intensity was highest in the RNA from WR and lowest in RNA from vΔD10 (Fig. 4B). At 4 h, the intensity of the C11R transcript increased in all three samples but the difference in relative amounts remained. However, in cells infected with WR or vΔD9, the amount of C11R RNA was decreased by 6 h and was undetectable at 12 h, whereas the C11R mRNA persisted in cells infected with vΔD10 infection (Fig. 4B).
Effect of the D10R deletion on viral DNA replication and late transcription.
To analyze viral DNA replication, cells were infected with the same numbers of VACV WR, vΔD9, and vΔD10 virions as above and harvested at various times. DNA accumulation as determined by dot blot analysis was delayed slightly for vΔD10 compared to vΔD9 and VACV WR, as expected from the kinetics of early transcription. However, after 6 h, there was little difference between the samples (not shown).
Northern blot analysis was performed with antisense-labeled F18R RNA (F17R in VACV Copenhagen strain). The F18R late RNA was chosen because it undergoes posttranscriptional cleavage, generating a discrete band, rather than the heterogeneous smear due to read-through of most late VACV mRNAs (14). The F18R transcript was detected at 6 h, but the intensity was WR > vΔD9 > vΔD10 (Fig. 5). At 9 and 12 h, however, the intensity of the vΔD10-expressed F18R RNA was higher than that of either WR or vΔD9 (Fig. 5).
FIG. 5.
Northern blot analysis of a VACV late transcript. BS-C-1 monolayers grown in six-well dishes were infected with 4 PFU of purified VACV WR and the same number of purified vΔD9 and vΔD10 virus particles. At the hours postinfection (hpi) indicated, total RNA was extracted and resolved by electrophoresis, followed by Northern blotting. The Northern blot was hybridized with a radioactively labeled F18R riboprobe. The electrophoretic mobilities of marker RNAs of indicated length are shown on the left, and the position of the F18R transcript is shown on the right.
Effect of the D10R deletion on viral and cellular protein synthesis.
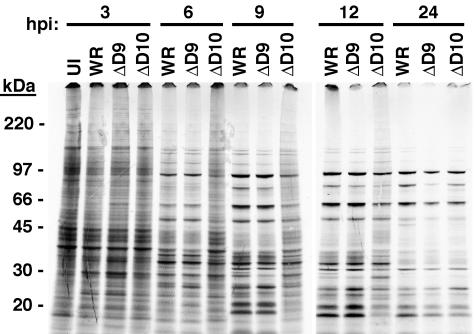
During a normal VACV infection, the onset of viral late protein synthesis and the shutdown of cell protein synthesis occur at approximately 6 h. Cells were mock infected or infected with 4 PFU per cell of VACV WR or vΔD9 or the equivalent number of vΔD10 particles. Fluorescence microscopy indicated that all of the cells infected with vΔD9 or vΔD10 expressed EGFP, which was regulated by a viral late promoter in the recombinant viruses (Fig. 2A). The cells were pulse-labeled with radioactive methionine and cysteine at various times. The infected cells were harvested, and the proteins were resolved by SDS-PAGE, followed by autoradiography. At 3 h, the pattern of labeled proteins in infected cells was still similar to that of uninfected cells, although some new bands could be seen (Fig. 6). In cells infected with WR and vΔD9, viral late proteins were detected at 6 h; the transition from host protein synthesis to viral protein synthesis was nearly complete at 9 h (Fig. 6). In contrast, in cells infected with vΔD10, the pattern was unchanged at 6 h, and the transition was incomplete at 9 h and 12 h (Fig. 6). The delayed switch from host to viral protein synthesis, however, could be overcome by a 10-fold increase in the number of ΔD10 particles used for infection, which equalized the number of PFU to that of WR (not shown).
FIG. 6.
Analysis of pulse-labeled proteins. BS-C-1 cell monolayers were infected with 4 PFU of purified VACV WR or vΔD9 or with the same number of vΔD10 virus particles. The infected cells were labeled for 30 min with [35S]methionine and [35S]cysteine at the indicated hours postinfection (hpi). The harvested cells were lysed, and proteins were resolved by SDS-PAGE and visualized by autoradiography. The positions and masses of marker proteins are indicated on the left.
Effect of the D10R deletion on the stability of cellular RNAs.
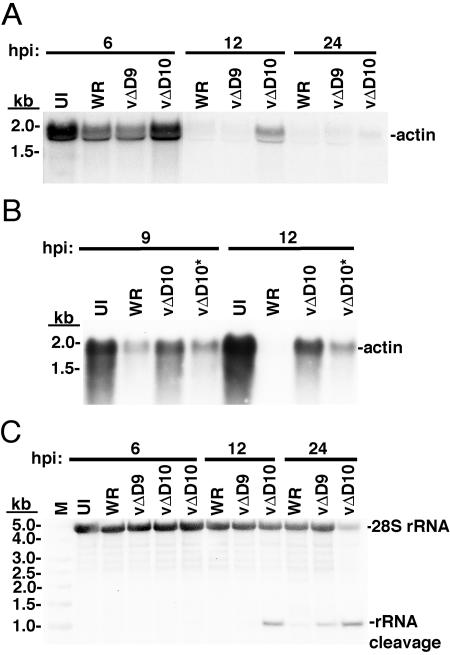
Infection with VACV causes a rapid decrease in the amounts of cellular mRNA, which must contribute to the cessation of host protein synthesis. For example, Rice and Roberts (29) showed by Northern blotting that about 50% of actin mRNA was degraded within 3 h after VACV infection of mouse L cells. We examined the fate of actin mRNA in BS-C-1 cells infected with 4 PFU per cell of VACV WR or vΔD9 or with the equivalent number of vΔD10 particles. In both WR- and vΔD9-infected cells, cellular actin mRNA was severely reduced by 6 h and was almost completely gone by 12 h postinfection (Fig. 7A). In cells infected with vΔD10, however, actin mRNA levels were not reduced at 6 h, and significant amounts persisted for at least 12 h (Fig. 7A). When the amount of vΔD10 was increased 10 fold over that used for WR, there was greater degradation of actin mRNA, but the levels were still higher than in cells infected with WR at 9 and 12 h (Fig. 7B).
FIG. 7.
Cellular mRNA and rRNA levels during vΔD9 and vΔD10 infection. (A) Steady-state levels of actin mRNA were determined following infection with 4 PFU per cell of purified VACV WR or vΔD9 or with the same number of vΔD10 virus particles. BS-C-1 cells were harvested at the indicated times after infection, and extracted RNA was subjected to Northern blot analysis. The membrane was hybridized with a radioactively labeled riboprobe that recognized cellular actin transcripts. (B) Steady-state levels of actin mRNA were determined following infection with equal numbers of purified virus particles per cell, as described in the legend to panel A, or with 10 times more vΔD10, indicated by the asterisk. Northern blot analysis was performed as described in the legend to panel A. (C) The Northern blot shown in panel A was stripped and hybridized with a probe to a 115-nucleotide conserved fragment of 28S rRNA. The electrophoretic mobilities of marker RNAs of indicated lengths are shown on the left.
We also examined rRNA by gel electrophoresis and Northern blotting using a probe to a 115-nucleotide conserved fragment of 28S RNA. In contrast to the results with actin mRNA, the 28S RNA was less stable in cells infected with vΔD10 than with WR or vΔD9, and a degradation product was seen at 12 and 24 h (Fig. 7C). A faint breakdown product of 28S RNA was also seen in cells infected with vΔD9 at 24 h (Fig. 7C).
DISCUSSION
Both D9 and D10 have been highly conserved during poxvirus evolution, suggestive of important roles in virus replication. Nevertheless, D9 was previously found to be dispensable for VACV replication in tissue culture cells, although the phenotype had not been described (17). In the latter study, the neomycin resistance gene was used to replace the D9R gene, and mutants were isolated in the presence of Geneticin. A parallel attempt to create a D10 deletion mutant, however, was unsuccessful (32). We considered that if the putative D10 deletion mutant was replication-impaired, then the toxic effect of Geniticin may have prevented the formation of visible plaques in previous attempts. A similar problem in isolating a VACV topoisomerase mutant was overcome by avoiding drug selection and using EGFP as an insertion marker (12). When we tried this alternative strategy, we were able to find small green fluorescent plaques formed by the D10R deletion mutant. In the same manner, we isolated a new D9R deletion mutant expressing EGFP but still could not isolate a combined D9R-D10R deletion mutant. The latter failure suggests that the double mutant is more impaired than either a D9R or D10R mutant, possibly because the two proteins have overlapping functions related to their 25% sequence identities. Their different times of expression, namely, early for D9 and late for D10 (23, 24), may partly explain the need for conserving two related genes. D10R is one of only a few genes conserved in all sequenced poxviruses that have been shown to be nonessential for replication in tissue culture cells. Another nonessential conserved gene is the type 1 topoisomerase (12). Although present in all chordopoxviruses, D9R is absent from entomopoxviruses.
The D9R deletion mutant replicated as well as wild-type virus, except for a slightly smaller plaque size. However, the considerably smaller plaque size and lower titers of the D10R mutant indicated that it was more severely impaired in replication. Yet, the decrease in virus yield in one-step growth experiments was <1 log compared to that of wild-type virus, and initial SDS-PAGE analysis of infected cells pulse-labeled with radioactive amino acids looked normal. These first experiments were carried out with equal PFU of mutant and wild-type virus. It was not until we purified the virus and determined that the particle/PFU ratio of vΔD10 was 6- to 10-fold-higher than that of vΔD9 or wild-type virus did we realize that infection with equal PFU might obscure the phenotype. One reason could be that the level of early transcription is proportionate to virus particle multiplicity of infection, presumably because each virion contains its own transcription system (13). Since D9 is an early gene with a possible function overlapping that of D10, its overexpression could mitigate the D10 defect. The majority of further experiments were carried out using equal numbers of purified virus particles of wild-type and mutant viruses so that we could more properly discern the phenotype. For biochemical analyses, however, we had to be sure that all cells were infected under these conditions. We found that this occurred when cells were inoculated with about 300 particles of each virus type. Under these conditions, there were 4 PFU per cell of wild-type and vΔD9 but only about 0.4 PFU per cell of vΔD10. As a control, we also tried reducing wild-type virus to 0.4 PFU per cell; unlike the situation with vΔD10, however, not all of the cells were infected because of the low particle number. When cells were infected with equal numbers of particles, the earliest defect of vΔD10 was a relative delay in the onset of early transcription, a surprising result since we showed that D10 is a late protein and not selectively packaged into virions. Nevertheless, the levels of early RNA soon increased and in fact were higher and persisted longer in cells infected with vΔD10 than cells infected with wild-type virus or vΔD9. Similarly, the onset of late transcription was delayed, but eventually the late RNA levels in cells infected with vΔD10 exceeded those in cells infected with the other viruses. Equally striking was the persistence of cellular actin mRNA, which was still detectable at 12 h after infection with vΔD10. Delayed shutoff of host protein synthesis was also noted, consistent with the persistence of cellular mRNAs. Lastly, we noted degradation of rRNA, which could result from excess viral late mRNAs that are known to form intermolecular duplexes (7) capable of activating the 2-5A pathway, as seen with some other VACV mutants (4).
The present studies, showing that viral and cellular mRNAs persisted longer in the absence of D10 than in its presence, complemented a previous study that found greatly accelerated mRNA turnover when D10 was overexpressed (32). There are several reasons why regulating mRNA turnover might be advantageous to viruses. The most obvious is that degradation of cellular mRNA removes competition for the protein synthesizing machinery. A second is that rapid turnover of viral RNAs promotes sharp transitions between the early and late stages of viral replication. Other viruses also encode proteins that enhance degradation of viral and host mRNAs. A good example is the Vhs protein, which is conserved in all alphaherpesviruses (27). Despite its conservation, the Vhs protein is not essential, and deletion mutants exhibit only a 5- to 10-fold or less decreased virus yield in cultured cells, although they are highly attenuated in vivo (28, 33, 34). Indeed, there are similarities between the phenotypes of vhs and D10 deletion mutants. However, the herpesvirus Vhs protein is a tegument component, whereas D10 is not selectively packaged into VACV particles, although trace amounts can be detected by Western blotting. It is possible that the absence of this very small amount of D10 is responsible for the decreased infectivity of vΔD10 virions and the delayed onset of early viral mRNA synthesis. However, we are inclined to think that the mutant virions have more subtle assembly defects, due to poorly regulated gene expression.
As already pointed out, both D9 and D10 contain a mutT motif, a signature sequence characteristic of enzymes involved in hydrolysis of nucleoside pyrophosphates. Iridoviruses and African swine fever virus each contain a protein with a mutT motif, although they otherwise have little sequence similarity with D9 or D10 (22, 30). The African swine fever virus nudix hydrolase can cleave a wide range of nucleotide substrates, with a preference for diphosphoinositol polyphosphates, but is unable to cleave RNA cap analogs (11). A subset of cellular nudix hydrolases has been shown to mediate mRNA decapping, leading to the subsequent degradation of the cleaved transcript (16, 36). We speculate that D10 and, to a lesser extent, D9 destabilize mRNAs by a related mechanism. Biochemical studies to test this hypothesis are planned.
Acknowledgments
We thank Norman Cooper for tissue culture cells, Andrea Weisberg for electron microscopy, Erica Brown and Nir Netzer for help with flow cytometry, Carisa Zampieri for plasmid construction, and Teri Shors, Wolfgang Resch, Patricia Szajner, Alan Townsley, and Christiana Fogg for helpful discussions.
This research was supported by the Intramural Research Program of the NIAID, NIH.
REFERENCES
- 1.Ahn, B.-Y., and B. Moss. 1992. RNA polymerase-associated transcription specificity factor encoded by vaccinia virus. Proc. Natl. Acad. Sci. USA 89:3536-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 2005. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 341:59-71. [DOI] [PubMed] [Google Scholar]
- 3.Baldick, C. J., Jr., and B. Moss. 1993. Characterization and temporal regulation of mRNAs encoded by vaccinia virus intermediate stage genes. J. Virol. 67:3515-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayliss, C. D., and R. C. Condit. 1993. Temperature-sensitive mutants in the vaccinia virus A18R gene increases double-stranded RNA synthesis as a result of aberrant viral transcription. Virology 194:254-262. [DOI] [PubMed] [Google Scholar]
- 5.Bessman, M. J., D. N. Frick, and S. F. O'Handley. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 271:25059-25062. [DOI] [PubMed] [Google Scholar]
- 6.Binns, M. M., B. S. Britton, C. Mason, and M. E. G. Boursnell. 1990. Analysis of the fowlpox virus genome region corresponding to the vaccinia virus D6 to A1 region—location of, and variation in, non-essential genes in poxviruses. J. Gen. Virol. 71:2873-2881. [DOI] [PubMed] [Google Scholar]
- 7.Boone, R. F., R. P. Parr, and B. Moss. 1979. Intermolecular duplexes formed from polyadenylated vaccinia virus RNA. J. Virol. 30:365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broyles, S. S. 2003. Vaccinia virus transcription. J. Gen. Virol. 84:2293-2303. [DOI] [PubMed] [Google Scholar]
- 9.Brum, L. M., M. C. Lopez, J. C. Varela, H. V. Baker, and R. W. Moyer. 2003. Microarray analysis of A549 cells infected with rabbitpox virus (RPV): a comparison of wild-type RPV and RPV deleted for the host range gene, SPI-1. Virology 315:322-334. [DOI] [PubMed] [Google Scholar]
- 10.Carroll, M. W., and B. Moss. 1995. E. coli b-glucuronidase (GUS) as a marker for recombinant vaccinia viruses. BioTechniques 19:352-355. [PubMed] [Google Scholar]
- 11.Cartwright, J. L., S. T. Safrany, L. K. Dixon, E. Darzynkiewicz, J. Stepinski, R. Burke, and A. G. McLennan. 2002. The g5R (D250) gene of African swine fever virus encodes a nudix hydrolase that preferentially degrades diphosphoinositol polyphosphates. J. Virol. 76:1415-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Fonseca, F., and B. Moss. 2003. Poxvirus DNA topoisomerase knockout mutant exhibits decreased infectivity associated with reduced early transcription. Proc. Natl. Acad. Sci. USA 100:11291-11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davison, A. J., and B. Moss. 1989. The structure of vaccinia virus early promoters. J. Mol. Biol. 210:749-769. [DOI] [PubMed] [Google Scholar]
- 14.D'Costa, S. M., J. B. Antczak, D. J. Pickup, and R. C. Condit. 2004. Post-transcription cleavage generates the 3′ end of F17R transcripts in vaccinia virus. Virology 319:1-11. [DOI] [PubMed] [Google Scholar]
- 15.Demkowicz, W. E., J. S. Maa, and M. Esteban. 1992. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J. Virol. 66:386-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunckley, T., and R. Parker. 1999. The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J. 18:5411-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dvoracek, B., and T. Shors. 2003. Construction of a novel set of transfer vectors to study vaccinia virus replication and foreign gene expression. Plasmid 49:9-17. [DOI] [PubMed] [Google Scholar]
- 18.Earl, P. L., N. Cooper, S. Wyatt, B. Moss, and M. W. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.3. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. John Wiley and Sons, New York, N.Y. [Google Scholar]
- 19.Guerra, S., L. A. Lopez-Fernandez, R. Conde, A. Pascual-Montano, K. Harshman, and M. Esteban. 2004. Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. J. Virol. 78:5820-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jindal, S., and R. A. Young. 1992. Vaccinia virus infection induces a stress response that leads to association of hsp70 with viral proteins. J. Virology 66:5357-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keck, J. G., C. J. Baldick, and B. Moss. 1990. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late transactivator genes. Cell 61:801-809. [DOI] [PubMed] [Google Scholar]
- 22.Koonin, E. V. 1993. A highly conserved sequence motif defining the family of MutT-related proteins from eubacteria, eukaryotes and viruses. Nucleic Acids Res. 21:4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee-Chen, G. J., N. Bourgeois, K. Davidson, R. C. Condit, and E. G. Niles. 1988. Structure of the transcription initiation and termination sequences of seven early genes in the vaccinia virus HindIII D fragment. Virology 163:64-79. [DOI] [PubMed] [Google Scholar]
- 24.Lee-Chen, G. J., and E. G. Niles. 1988. Map positions of the 5′ ends of eight mRNAs synthesized from the late genes in the vaccinia virus HindIII D fragment. Virology 163:80-92. [DOI] [PubMed] [Google Scholar]
- 25.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 26.Oda, K., and W. K. Joklik. 1967. Hybridization and sedimentation studies on “early” and “late” vaccinia messenger RNA. J. Mol. Biol. 27:395-419. [DOI] [PubMed] [Google Scholar]
- 27.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read, G. S., B. M. Karr, and K. Knight. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 67:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice, A. P., and B. E. Roberts. 1983. Vaccinia virus induces cellular mRNA degradation. J. Virol. 47:529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnitzler, P., M. Hug, M. Handermann, W. Janssen, E. V. Koonin, H. Delius, and C. Darai. 1994. Identification of genes encoding zinc finger proteins, non-histone chromosomal HMG protein homologue, and a putative GTP phosphohydrolase in the genome of Chilo iridescent virus. Nucleic Acids Res. 22:158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebring, E. D., and N. P. Salzman. 1967. Metabolic properties of early and late vaccinia messenger ribonucleic acid. J. Virol. 1:550-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shors, T., J. G. Keck, and B. Moss. 1999. Down regulation of gene expression by the vaccinia virus D10 protein. J. Virol. 73:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smibert, C. A., and J. R. Smiley. 1990. Differential regulation of endogenous and transduced β-globin genes during infection of erythroid cells with a herpes simplex virus type 1 recombinant. J. Virol. 64:3882-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szajner, P., H. Jaffe, A. S. Weisberg, and B. Moss. 2003. Vaccinia virus G7L protein interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions. J. Virol. 77:3418-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Dijk, E., N. Cougot, S. Meyer, S. Babajko, E. Wahle, and B. Seraphin. 2002. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 21:6915-6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuwen, H., J. H. Cox, J. W. Yewdell, J. R. Bennink, and B. Moss. 1993. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3l gene. Virology 195:732-744. [DOI] [PubMed] [Google Scholar]