Abstract
Cellular N-Ras provides a steady-state antiapoptotic signal, at least partially through the regulation of phosphorylated Akt and Bad levels. Fibroblasts lacking c-N-Ras expression are highly sensitive to the induction of apoptosis by a variety of agents. Reduction of pBad and pAkt levels using a phosphatidylinositol 3-kinase inhibitor was not sufficient to sensitize the control cell population to the high level of apoptosis observed in the N-Ras knockout cell lines, suggesting that c-N-Ras provides at least one other antiapoptotic signal. Stimulation of the control cells with apoptotic agents results in a transient increase in Jun N-terminal protein kinase (JNK)/p38 activity that decreased to baseline levels during the time course of the experiments. In all cases, however, sustained JNK/p38 activity was observed in cells lacking c-N-Ras expression. This correlated with sustained levels of phosphorylated MKK4 and MKK3/6, upstream activators of JNK and p38, respectively. Mimicking the sustained activation of JNK in the control cells did result in increasing their sensitivity to apoptotic agents, suggesting that prolonged JNK activity is a proapoptotic event. We also examined the potential downstream c-N-Ras targets that might be involved in regulating the duration of the JNK/p38 signal. Only the RalGDS 37G-N-Ras protein protected the N-Ras knockout cells from apoptosis and restored transient rather than sustained JNK activation. These data suggest that cellular N-Ras provides an antiapoptotic signal through at least two distinct mechanisms, one which regulates steady-state pBad and pAkt levels and one which regulates the duration of JNK/p38 activity following an apoptotic challenge.
The immediate family of Ras proteins consists of four isoforms: Harvey (Ha), N, and two splice variants of the Kirsten (K) gene, K(A) and K(B). These proteins are highly homologous within the N-terminal 165 amino acids (85, 106). The Ras proteins have no sequence similarity in their C-terminal hypervariable regions, comprising residues 166 to 185 or 186 (3, 10, 69, 106). Ras proteins function as molecular switches cycling between an inactive GDP-bound state and an active GTP-bound state (10, 69, 106). Two regions of sequence identity include the switch 1 (amino acids 32 to 40) and switch 2 (amino acids 60 to 72) regions (68, 128). The switch 1 and switch 2 regions undergo a large conformational change when Ras binds GTP, forming the effector-binding domain (68, 69, 128). It is through this effector-binding domain that Ras-GTP interacts with downstream targets.
Ras-GTP transmits its signal through interactions with a large number of target proteins. These include the Raf kinases (Raf-1, B-Raf, and A-Raf) (77, 78, 118, 120, 124, 136), phosphatidylinositol 3-kinase (PI3-kinase) (96, 97), and RalGDS family members (44, 58, 109, 130). Other candidate Ras effector proteins have been identified and include AF-6, Canoe, Rin-1, Nore-1, PKCζ, and phospholipase Cɛ (PLCɛ) (10, 18, 54, 69, 106).
Studies using recombinant proteins suggested that all the Ras isoforms are capable of binding the same effectors with similar affinities (41). Mounting evidence, however, suggests that this is not the case in whole cells (37, 69, 129, 133). One study reported that oncogenic K-Ras more potently activated Raf-1 than G12V-Ha-Ras in transfected COS cells (133). Our laboratory has demonstrated that Raf-1 preferentially binds to c-N-Ras in G12V-Ha-Ras-transformed C3H10T1/2 fibroblasts (37). We have also observed that c-N-Ras promotes cell survival through an Akt-dependent pathway whereas neither c-K(A)-Ras nor c-K(B)-Ras could substitute for this function (129).
Through interactions with downstream effectors, Ras proteins perform functional roles in a large number of biological processes including cell proliferation, transformation, cell cycle progression, migration, differentiation, immune responses, apoptosis, and cell survival (10, 26, 28, 69, 129). Because Ras is positioned as a central molecular switch in the coordinated regulation of multiple biological outcomes, it must interact with a variety of downstream targets to exert its effects through cellular signaling pathways that ultimately influence the cell fate. In fact, the use of selective effector-binding mutant Ras proteins demonstrated that Ras must interact with multiple downstream targets to generate a transformed phenotype (129).
The role of Ras and Ras-dependent signaling pathways in apoptosis and cell survival is the subject of intense investigation. Many of these studies have used ectopic expression of oncogenic Ras to examine whether Ras promotes or inhibits apoptosis. A few studies have reported that oncogenic Ras acts to induce apoptosis (12, 66, 67, 81, 83, 116). In contrast, a growing body of evidence suggests that oncogenic Ras prevents apoptosis and promotes cell survival (70, 89, 91, 93, 98, 103, 112). The actual outcome of oncogenic Ras signaling in promoting or inhibiting apoptosis may be influenced by the level of Ras expression, the specific cell system, and/or the presence or absence of survival or other factors (28).
The effects of Ras on cell survival and apoptosis may be mediated by signaling through different pathways. Signaling through Raf-1/MEK/ERK is proapoptotic in some circumstances (32, 51). However, it has also been reported that the Ras/Raf/MEK/ERK pathway also functions in preventing apoptosis (6, 31, 53, 76, 105). Recent reports suggest that additional functions of Raf-1, independent of MEK and ERK activities, are involved in counteracting the induction of apoptosis (47, 73).
A large body of evidence supports a survival function for Ras by its signaling through the PI3-kinase/Akt pathway (86, 99, 129). Akt is thought to promote cell survival, in part, through phosphorylation and inactivation of proapoptotic Bad and caspase-9 (19, 28, 56). It has been reported that in some circumstances, promotion of cell survival by Ras proceeds through both the Raf-1/MEK/ERK and PI3-kinase pathways (42, 132). A recent report also indicates that oncogenic Ras inhibits anoikis-induced cell death through stabilization of BclxL expression and that this effect was independent of either ERK or PI3-kinase (98). This suggests that, as with transformation (125), Ras may mediate cell survival through multiple pathways.
In an effort to dissect distinct Ras isoform-specific functions, we have previously demonstrated that c-N-Ras promotes cell survival through an Akt-dependent pathway. In contrast to control cells (N+/+), N-Ras knockout fibroblasts (N−/−) possess low levels of steady-state phosphorylated Bad and are highly sensitive to the induction of apoptosis. Ectopic expression of c-N-Ras in the N-Ras knockout cells restores pBad levels to those observed in control cells and rescues the apoptotically sensitive phenotype (129). Ectopic expression of either c-K(A)- or c-K(B)-Ras could not reverse the heightened apoptotic sensitivity of the parental N-Ras knockout cells. Experiments with inhibitors of PI3-kinase, which also inhibit Akt signaling, suggested that c-N-Ras performs additional, Akt-independent functions that promote cell survival.
The present study is aimed at elucidating other mechanisms by which c-N-Ras promotes cell survival. Using wild-type and selective effector-binding domain mutants of N-Ras, we have found that c-N-Ras provides protection from apoptosis by attenuation of Jun N-terminal protein kinase (JNK) activity. This survival function operates independently of signaling through either Raf-1/MEK/ERK or PI3-kinase (p110α or p110β).
MATERIALS AND METHODS
Antibodies and reagents.
Phosphospecific Bad polyclonal (Ser136), phosphospecific Akt rabbit polyclonal (Ser473), phospho-p38 polyclonal, p38 polyclonal (for Western blots), phospho-MKK4 polyclonal, phospho-MKK3/6 polyclonal, and MKK3 polyclonal antibodies were from Cell Signaling Technology (Beverly, Mass.). Phosphospecific c-Jun (Ser73) polyclonal antibody, glutathione S-transferase (GST)-tagged ATF2, full-length inactive His6-tagged JNK1α1, and full-length inactive GST-tagged p38α were from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Phosphospecific c-Jun (Ser63) monoclonal, c-Jun monoclonal, phospho-JNK monoclonal, phospho-Erk monoclonal, N-Ras monoclonal, Erk1 polyclonal, Erk2 polyclonal, MKK4 polyclonal (MEK-4), and JNK1 polyclonal antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Hamster anti-mouse Fas receptor antibody (clone Jo2) was from BD Pharmingen (San Diego, Calif.). Anti-rabbit secondary antibody conjugated to horseradish peroxidase (HRP) was from BD Transduction Laboratories (Lexington, Ky.). Anti-mouse antibody conjugated to HRP was from Kirkegaard & Perry Laboratories (Gaithersburg, Md.). Protein G and β-estradiol were from Sigma-Aldrich Chemical Co (St. Louis, Mo.). Nonimmune rabbit immunoglobulin G (IgG) was from Pierce Chemical Co. (Rockford, Ill.), and nonimmune rabbit serum was from Biodesign International (Saco, Maine). Anti-p38 rabbit serum (for immunoprecipitation) was a generous gift from Andrew C. Larner (Cleveland Clinic Foundation, Lerner Research Institute). The Akt kinase assay kit was from Cell Signaling Technology.
Cell culture.
N-Ras knockout (N−/−) and control N+/+ mouse embryo fibroblasts were a generous gift from R. Kucherlapati (Albert Einstein College of Medicine) (117). The fibroblasts were immortalized as previously described (129). To avoid any cell-specific changes arising from immortalization, multiple independently isolated cell lines were used throughout these studies. Independently derived N-Ras knockout cell lines are designated N−/−(#), where the number indicates a different cell line. N+/+ control cell lines are also designated with numbers to indicate independently immortalized cell lines [e.g., N+/+(1)]. Cells were grown in complete medium consisting of Dulbecco's modified Eagle's medium (DMEM) containing 12% fetal bovine serum (Atlanta Biologicals, Norcross, Ga.), 1× antibiotic/antimycotic, and 1× nonessential amino acids (Life Technologies, Inc.-Invitrogen, Rockville, Md.). Cells stably expressing an estrogen-responsive MEKK3 construct (MEKK3:ER) were grown in phenol red-free DMEM (Life Technologies, Inc.-Invitrogen) containing sodium bicarbonate, 1 mM sodium pyruvate (Sigma), 1× antibiotic/antimycotic, 1× nonessential amino acids, 10% charcoal-stripped fetal bovine serum (Atlanta Biologicals), and 100 μg of G418 per ml. The cells were kept in complete medium for all experiments unless otherwise stated. Serum starvation was performed by rinsing cells with phosphate-buffered saline (PBS) (20 mM Na2HPO4, 120 mM NaCl [pH 7.4]) and incubating them in DMEM containing nonessential amino acids and antibiotic/antimycotic solution.
Pharmacological treatments.
Recombinant murine tumor necrosis factor alpha (TNF-α) (Calbiochem-Novabiochem Corp., La Jolla, Calif.) was dissolved in 0.2-μm-pore-size filtered PBS containing 0.1% bovine serum albumin (Sigma-Aldrich) and stored in aliquots at −80°C. The Fas receptor was activated by incubation of cells for the indicated times in complete medium containing 1 μg of anti-Fas receptor antibody (clone Jo2, form NA/LE; BD Pharmingen) per ml and 0.5 μg of protein G (Sigma-Aldrich) per ml. LY294002 was from Calbiochem-Novabiochem and was used at a final concentration of 20 μM.
Cloning and transfections.
c-N-Ras/pIBW3 containing the c-N-Ras gene under the control of the thymidine kinase promoter was a generous gift from Angel Pellicer (New York University). N-Ras knockout cells ectopically expressing c-N-Ras at control N+/+ cell levels were prepared as described previously and maintained in complete medium containing 200 μg of G418 per ml (129). N-Ras knockout cells ectopically expressing c-K(A)-Ras or c-K(B)-Ras were generated by transfection of these constructs in a pTargeT vector containing a cytomegalovirus promoter (Promega, Madison, Wis.) and maintenance of stable clones in 200 μg of G418 per ml (129).
The MEKK3:ER/pcDNA3 construct was a generous gift from Ulrich Siebenlist (National Institutes of Health). N-Ras knockout and control N+/+ cells were transfected with 200 ng of MEKK3:ER/pcDNA3 using Lipofectamine-Plus (Invitrogen/Life Technologies), and stable clones were selected in 450 μg of G418 per ml. Positive clones were detected by immunoblotting with an anti-estrogen receptor (ER) antibody (data not shown). Stable clones were maintained in phenol red-free DMEM as described above.
(i) Generation of N-Ras(61K) effector domain mutant constructs.
We introduced missense mutations into the appropriate regions of cDNA to generate the 35S, 37G, and 40C N-Ras effector domain mutants. cDNA sequences encoding the effector domain mutants were generated with the QuikChange site-directed mutagenesis kit (Stratagene) and the cDNA sequence for N-Ras(61K) in the pBluescript II KS(+)-N-ras(61K) plasmid as a template for mutagenesis. The mutations at position 35, 37, and 40 were created by changing a threonine to serine, a glutamic acid to glycine, and a tyrosine to cysteine at these amino acid locations, respectively. The pBluescript II KS(+)-N-ras(61K) template was constructed by inserting the cDNA encoding N-Ras(61K) into the BamHI site of pBluescript II KS(+) (Stratagene). The N-ras(61K) cDNA was obtained by digesting the previously generated and characterized pCMVneo-N-ras(61K) with BamHI (92). After mutagenesis, all constructs were confirmed by sequencing. The BamHI fragment from each effector domain mutant was inserted into the BamHI site of the pZIP-NeoSV(x)1 retrovirus mammalian expression vector (11a). The orientation was verified by sequencing.
(ii) Establishment of N-Ras knockout fibroblasts expressing effector domain mutants in N-Ras knockout cells.
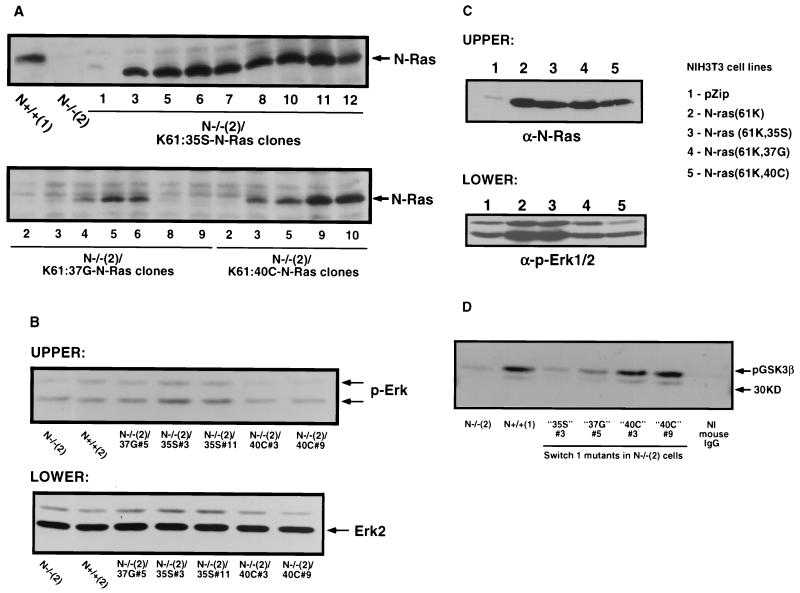
N-Ras knockout cells were transfected as described above with 300 ng of each of the N-Ras effector domain mutant constructs, and stable clones were selected in 450 μg of G418 per ml. Individual clones were maintained in DMEM containing 12% fetal bovine serum, 1× antibiotic/antimycotic, and 200 μg of G418 per ml. Levels of expression were confirmed by Western analysis (see Fig. 6).
FIG. 6.
Expression of switch 1 effector domain mutant N-Ras constructs in the N-Ras knockout cells and NIH 3T3 cells. (A) N-Ras knockout cells were transfected with three different switch 1 mutant N-Ras constructs (K61:35S, K61:37G, and K61:40C) and stable clones were selected in G418 as described in Materials and Methods. A 100-μg portion of each lysate (including the parental N-Ras knockout cells and control N+/+ cells) was electrophoresed on SDS-13% polyacrylamide gels and transferred to PVDF. The blot was developed with anti-N-Ras monoclonal antibody and anti-mouse secondary antibody-HRP. Detection was performed by standard ECL techniques. (B) N-Ras knockout and control N+/+ cells and N-Ras knockout cells stably expressing the switch 1 mutant N-Ras proteins were subcultured in serum-containing medium and left untreated. At 48 h after passage, the cells were harvested and lysates were prepared as described in Materials and Methods. A 20-μg portion of each lysate was electrophoresed on two separate 13% polyacrylamide gels and transferred to PVDF. The upper blot was developed with anti-phospho-Erk (pErk) and anti-mouse antibody-HRP, and the lower blot was developed with anti-Erk2 polyclonal antibody and anti-rabbit-HRP for total Erk2. Bands were visualized by standard ECL techniques. (C), NIH 3T3 cells were transfected with the indicated constructs, including the effector domain mutant N-Ras constructs, as described in Materials and Methods. (Upper panel) Expression levels of ectopic N-Ras proteins in NIH 3T3 cells was determined by Western analysis. Cultures were grown to approximately 80% confluency and then lysed in a detergent buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% NP-40, 50 mM NaF, 1 mM NaVO3, 1 mM dithiothreitol, 100 μM phenylmethylsulfonyl fluoride, 1 μg of aprotinin per ml, 1 μg of leupeptin per ml). Protein concentrations were determined with the bicinchoninic acid kit (Pierce). SDS-PAGE was used to resolve 40 μg of total protein from each sample on a 15% polyacrylamide gel. Proteins were transferred to Immobilon-P membranes (Millipore), and the blot was developed with anti-N-Ras monoclonal antibody and anti-mouse secondary antibody-HRP. Bands were visualized by standard ECL (Amersham) techniques. (Lower panel) Lysates prepared as described for the upper panel were used to determine the steady-state levels of Erk expression and activation. Following electrophoresis and transfer, the blot was developed with phospho-p44/42 MAP kinase (T202/Y204) E10 antibody (1:1,000; Cell Signaling Technology). Detection was performed by ECL (Amersham) following incubation with HRP-conjugated secondary anti-mouse IgG antibodies. (D) N-Ras knockout and control N+/+ cells and N-Ras knockout cells expressing the switch 1 effector domain mutant N-Ras constructs were grown to approximately 70% confluence in complete serum-containing medium for 2 days. The cells were harvested, and lysates were prepared in the lysis buffer supplied by the manufacturer. Protein concentrations were determined as described in Materials and Methods. Immobilized Akt 1G1 monoclonal antibody (20 μl of slurry) was used to immunoprecipitate Akt from 0.5 mg of each lysate for 3 h at 4°C. The immunocomplexes were washed and used in a kinase assay as specified by the manufacturer, except that 2 μg of the substrate GSK-3 fusion protein was used per reaction. The supernatants were removed, the pellets were washed with 30 μl of buffer, and the supernatants were combined and electrophoresed on SDS-12% polyacrylamide gels. Following transfer to PVDF, the blot was developed with anti-phsopho-GSK3α/β (pGSK-3β) polyclonal antibody and goat-anti-rabbit-HRP secondary antibody followed by donkey anti-goat antibody-HRP tertiary amplification. Detection was performed using standard ECL techniques.
(iii) Establishment and characterization of NIH 3T3 cells expressing N-Ras effector domain mutants.
To establish NIH 3T3 cells stably expressing the N-Ras(61K) effector domain mutants, cultures of NIH 3T3 cells were transfected with the empty pZIP-NeoSV(x)1 vector or pZIP-NeoSV(x)1 vector containing cDNA sequences encoding N-Ras(61K) and the 35S, 37G, and 40C N-Ras(61K) effector domain mutants. The NIH 3T3 cells were transfected by calcium phosphate precipitation as described previously (16) and selected for 14 days in DMEM supplemented with 10% calf serum and 400 μg of Geneticin (G418; Invitrogen/Life Technologies) per ml. Multiple drug-resistant colonies were pooled to establish mass populations.
Preparation of cell lysates.
All lysis buffers contained the phosphatase inhibitors 30 mM β-glycerophosphate, 5 mM p-nitrophenyl phosphate, 1 mM each phosphoserine and phosphothreonine, 0.2 mM phosphotyrosine, and 100 μM sodium orthovanadate and the protease inhibitors 25 μg each of aprotinin, leupeptin, and pepstatin A per ml and 1 mM phenylmethylsulfonyl fluoride. For Western analyses or immunoprecipitations, the cells were harvested by being scraped into PBS. The pelleted cells were resuspended in p21 buffer (20 mM MOPS (3-[N-morpholino]propanesulfonic acid), 5 mM MgCl2, 0.1 mM EDTA, 200 mM sucrose [pH 7.4]) containing 1% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate; U.S. Biochemical Corp.). The cells were lysed for 20 min on ice and centrifuged at 13,000 × g for 10 min, and the supernatant was retained for further experiments. The protein concentration was determined by the method of Bradford (7). For Western analysis of phospho-Bad, cells were harvested and combined with their medium and centrifuged at 1,000 × g for 10 min. The cells were washed once in TBS (Tris-buffered saline; 20 mM Tris, 140 mM NaCl[pH 7.4]) and solubilized in TBS containing 1% NP-40 NP-40 (Igepal; Sigma) and phosphatase and protease inhibitors as described above. After 20 min on ice, the lysate was centrifuged at 13,000 × g, and the supernatant was retained for protein measurements and Western analysis.
Western analysis.
Lysates containing equal amounts of protein were loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and following electrophoresis the gel was transferred to a polyvinylidene difluoride membrane (PVDF) (Hybond P; Amersham/Pharmacia Biotech.). Blocking was performed for 45 min using Blocker Casein in PBS (Pierce Chemical Co.) containing 5% newborn calf serum (Life Technologies, Inc.-Invitrogen). The blots were incubated with primary antibodies for 2 to 3 h at room temperature or overnight at 4°C. Following washing in TBS-0.1% Tween, the blots were incubated with secondary antibodies (1:1,000) for 1 h at room temperature. After being washed, the blots were developed, as indicated, by enhanced chemiluminescence (ECL) or ECL-Plus (Amersham-Pharmacia Biotech.) and exposure to film (Hyperfilm ECL; Amersham-Pharmacia Biotech.). The immunoblots were quantitated using a Microtek scanner and NIH Image 1.60b7. The bands were quantitated by normalization with respect to untreated controls.
Immunoprecipitations.
JNK was immunoprecipitated from 50 μg of whole-cell lysate by using 1.5 μg of anti-JNK1 polyclonal antibody (Santa Cruz Biotechnology; sc-571) for 1.5 h at 4°C followed by the addition of protein A-Sepharose (Repligen) for 1 h at 4°C. The immunoprecipitates were washed three times with p21 buffer containing 1% CHAPS and then once with p21 buffer prior to the kinase assay. p38 was immunoprecipitated using 3 μl of anti-p38 polyclonal antiserum by the same method as that used for immunoprecipitation of JNK.
Kinase Assays. (i) JNK/p38 assays.
Immunoprecipitates of JNK or p38 were incubated for 20 min at 37°C in kinase buffer consisting of p21 buffer containing protease and phosphatase inhibitors, 10 μM ATP, 5 μg of GST-ATF2 per reaction, and 25 μCi of [γ-32P]ATP (6,000 Ci/mmol; New England Nuclear) per reaction. The reactions were terminated by centrifugation at 13,000 × g at 4°C and removal of the supernatant solution. The pellets were washed with p21 buffer, the supernatant solutions were combined, and 5× Laemmli sample buffer was added. The supernatant solutions were loaded onto SDS-12% polyacrylamide gels, and following electrophoresis the gel was transferred to PVDF. Signals were detected using a Molecular Dynamics PhosphorImager.
(ii) Upstream activator double kinase assays.
A 2-μg portion of inactive His6-tagged JNK1α1 was incubated with 12 μg of untreated or treated cell lysate for 20 min at 30°C in the presence of 50 μM unlabeled ATP, and the His6-tagged JNK was isolated using nickel resin (ProBond; Invitrogen). The isolated JNK was washed with p21 buffer and incubated in kinase buffer with 5 μg of GST-ATF2 at 37°C for 20 min. The supernatant solution was removed, the pellets were washed, and the supernatant solutions were combined, electrophoresed, and transferred as described above. A similar method was used to examine the activation of p38, except that the GST-p38 was isolated after the first kinase reaction with lysate by using glutathione-agarose (Sigma-Aldrich). The second kinase reaction to measure the activity of the isolated GST-p38 was performed as described above using 5 μg of GST-ATF2 (per reaction) as the substrate.
(iii) Akt assays.
Akt assays were performed using an Akt kinase assay kit (Cell Signaling Technology). A 20-μl volume of the anti-Akt slurry was used to immunoprecipitate Akt from 500 μg of cell lysate prepared in the lysis buffer provided in the kit. After being washed, the immunoprecipitates were used in a kinase assay containing ATP and 2 μg (rather than the 1 μg suggested by the manufacturer) of GSK-3β fusion protein substrate per reaction. The reaction mixtures were incubated for 30 min at 30°C, and the reactions were terminated by the addition of sample buffer. The pellets were washed once with sample buffer, and the supernatants were combined and mixed with Laemmli sample buffer and subjected to SDS-PAGE (12% polyacrylamide). Following transfer to PVDF, the blots were developed with anti-phospho-GSK-3α/β. Bands were visualized using goat anti-mouse antibody-HRP followed by donkey anti-goat antibody-HRP and standard ECL techniques. A nonimmune control immunoprecipitation was performed using mouse IgG and protein G-Sepharose.
Kinase assay products were quantitated using a Microtek scanner and NIH Image 1.60b7. Bands were quantitated by normalization with respect to untreated controls.
Apoptosis assay.
Untreated or treated cells in 12-well cluster plates were scraped in their medium and centrifuged at 500 × g for 5 min. The cell pellet was resuspended in 200 μl of lysis buffer supplied by the manufacturer (Cell Death Detection ELISA Plus kit; Roche Molecular Biochemicals). Then 20-μl aliquots were used in the analysis that measures the appearance and relative amounts of cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes), with detection by a microtiter plate reader at 405 nm, as specified by the manufacturer. Incubation was performed overnight at 4°C instead of the 2 to 3 h at room temperature as suggested by the manufacturer. The reading from the negative control (buffer only) supplied by the manufacturer was subtracted from all sample values. All experiments were performed in triplicate, and error bars indicate the sample standard deviation of the mean (σn − 1).
RESULTS
Endogenous c-N-Ras provides more than one survival signal.
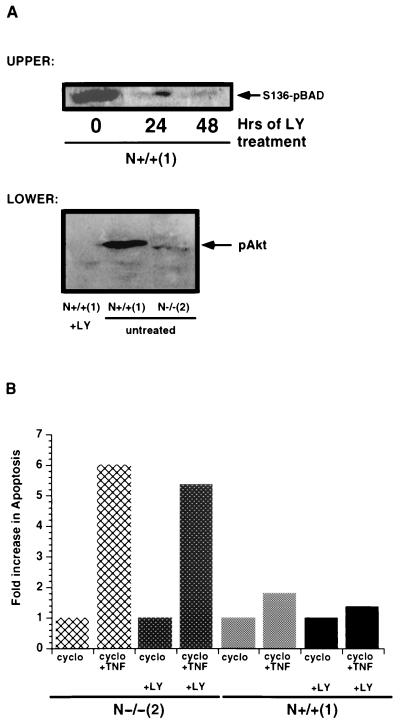
Akt plays a role in cell survival in response to cellular stress and certain apoptotic stimuli (20, 28, 57, 99, 100). We have previously demonstrated that c-N-Ras possesses a steady-state survival function that is dependent on Akt activity. N-Ras knockout fibroblasts express barely detectable levels of phosphorylated Bad (pBad) and are highly sensitive to the induction of apoptosis by a variety of treatments (129). Ectopic expression of c-N-Ras in the N-Ras knockout fibroblasts, at levels similar to those observed for endogenous c-N-Ras in control N+/+ cells (hereafter referred to as c-N-Ras reconstituted cells), restores pBad levels to near-control levels and partially relieves their apoptotic sensitivity. These data prompted us to ask whether this was the only function of c-N-Ras in promoting cell survival. We would predict that control cells might become as susceptible to the induction of apoptosis as the N-Ras knockout cells if signaling through Akt was diminished through the use of PI3-kinase inhibitors. Treatment of N+/+ cells with 20 μM LY294002, a PI3-kinase inhibitor, was sufficient to decrease the steady-state levels of pBad-Ser136 compared to that in untreated control cells (Fig. 1A, upper panel). Similar to pBad, the level of Ser473 phosphorylated Akt (pAkt) was also significantly decreased in the LY294002-treated control N+/+ cells. This level of pAkt is similar to that observed in untreated N-Ras knockout cells (Fig. 1A, lower panel). The total abundances of both Bad and Akt are unchanged between untreated and treated N-Ras knockout or control cells (reference 129 and data not shown). Pretreatment of control N+/+ cells with LY294002 did not, however, result in increased levels of apoptosis in response to TNF-α and cycloheximide (Fig. 1B). We have found little (<5%) difference in the apoptosis measurements over the 4-h treatment period between untreated and cycloheximide-only-treated cells (reference 129 and data not shown). The N-Ras knockout cells underwent a five- to sixfold increase in apoptosis after 4 h of treatment with TNF-α plus cycloheximide. Since the N-Ras knockout cells possess very low levels of pBad even at steady state, treatment with LY294002 does not cause a further increase in their level of apoptosis in response to TNF-α and cycloheximide. These data suggested that c-N-Ras may be providing more than one survival signal and that one of its survival functions is Akt independent.
FIG. 1.
c-N-Ras promotes cell survival by a PI3-kinase-independent mechanism. (A) (Upper panel) N+/+ control cells were left untreated or treated with 20 μM LY294002 for 24 or 48 h. At the indicated times, lysates were prepared as described in the text and their protein concentrations were determined. A 150-μg portion of lysate was loaded in each lane of an SDS-13% polyacrylamide gel, electrophoresed, and transferred to PVDF as described in Materials and Methods. The blot was incubated with anti-phospho-Bad (Ser136) polyclonal antibody at a 1:250 dilution overnight at 4°C followed by anti-rabbit-HRP secondary antibody. Detection was performed using ECL-Plus. The results are representative of three separate experiments. (Lower panel) Control N+/+(1) cells were left untreated or treated for 24 h with LY294002, and lysates were prepared. N-Ras knockout cells were left untreated and lysates were prepared as described in the text. A 100-μg portion of each lysate was loaded in each lane of an SDS-10% polyacrylamide gel, electrophoresed, and transferred to PVDF as described in the text. The blot was developed with anti-phospho-Akt (Ser473) primary antibody followed by anti-rabbit-HRP secondary antibody. Detection was performed by standard ECL techniques. The results are representative of two separate experiments. (B) N-Ras knockout and control N+/+ cells in 12-well cluster plates were left untreated or pretreated for 24 h with 20 μM LY294002. Untreated or LY294002-pretreated cells were left untreated or treated for 4 h with either 1 ng of recombinant murine TNF-α per ml in the presence of 2 μg of cycloheximide (cyclo) per ml or 2 μg of cycloheximide per ml. alone. The cells were scraped in their medium and centrifuged, and the cell pellet was resuspended in 200 μl of lysis buffer provided in the Cell Death Detection ELISA Plus kit (Roche Molecular Biochemicals). The Cell Death assay was performed as described in Materials and Methods. The data are plotted as the fold increase in apoptosis compared to that of cycloheximide-treated samples. The assay was performed in triplicate and is representative of three independent experiments.
c-N-Ras decreases the magnitude and duration of JNK activation.
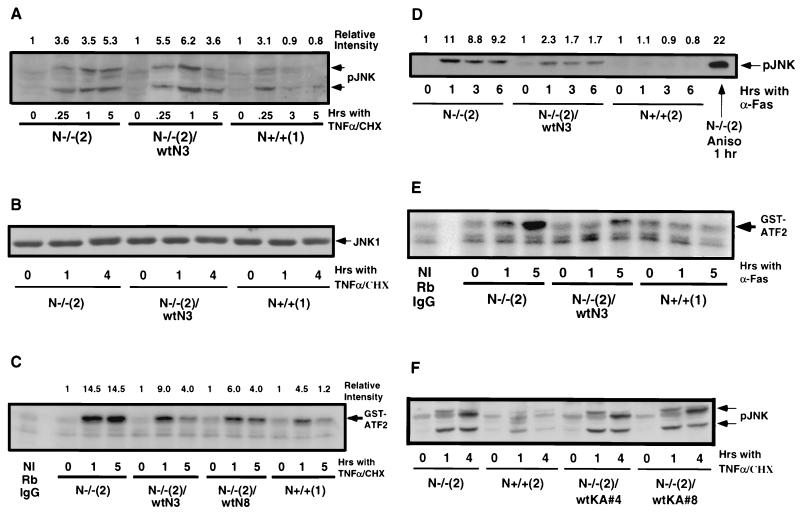
JNK activation occurs in response to a variety of cellular stresses as well as certain growth factor and cytokine stimulation (14, 21, 43, 63, 74, 131). In some systems the activation of JNK may play a role in promoting apoptosis (25, 40, 55, 64, 84, 87, 114, 134). We investigated the kinetics of JNK activation in the N-Ras knockout and control cells in response to apoptotic agonists (Fig. 2A). N-Ras knockout fibroblasts, control N+/+ cells, and c-N-Ras reconstituted cells [N−/−(2)/wtN3] were untreated or treated for various times with 1 ng of TNF-α per ml in the presence of cycloheximide. All of the cell lines expressed minimal levels of phosphorylated JNK (pJNK) at steady state (t = 0). The level of pJNK in the N-Ras knockout cells increased by 15 min, peaked at 1 h, and remained elevated by 5 h after treatment with TNF-α plus cycloheximide. Treatment of N-Ras knockout cells with low doses of TNF-α in the presence of cycloheximide for 4 to 5 h is sufficient to cause approximately 50% cell death by apoptosis (129). The level of pJNK in control N+/+ cells was moderately increased, peaking at 1 h (Fig. 6B and data not shown) and then declining to basal levels by 3 to 5 h after treatment with TNF-α plus cycloheximide. c-N-Ras reconstituted knockout cells, which express c-N-Ras at control cell levels, possessed levels of pJNK that approximated those seen in control cells, although they were not completely down to basal levels by 5 h after treatment with TNF-α plus cycloheximide. This may correlate with their ability to only partially rescue the heightened apoptotic sensitivity of the parental knockout cells in response to TNF-α plus cycloheximide (129). The differences observed in pJNK are not a result of changes in the total abundance of JNK1 protein. All of the cell lines possessed similar levels of total JNK1 in the absence or presence of TNF-α plus cycloheximide (Fig. 2B). Similar results have been observed using other independently derived N-Ras knockout and control cell lines as well as other clones of c-N-Ras reconstituted cell lines (data not shown).
FIG. 2.
c-N-Ras downregulates JNK activation and activity following treatment with apoptotic agonists. (A) N-Ras knockout fibroblasts, control N+/+ cells, and c-N-Ras reconstituted cells were left untreated (t = 0) or treated for various times with 1 ng of TNF-α per ml in the presence of 2 μg of cycloheximide (CHX) per ml. At the indicated times, the cells were harvested and lysates were prepared as described in the text. A 50-μg portion of protein was loaded in each lane of an SDS-10% polyacrylamide gel. Following electrophoresis and transfer, the blot was developed with anti-phospho-JNK (pJNK) and anti-mouse secondary antibody-HRP. Detection was performed using standard ECL techniques. The results are representative of three different experiments. (B) N-Ras knockout, control N+/+, and c-N-Ras reconstituted cells were untreated or treated with TNF-α and cycloheximide as described for panel A. A 50-μg portion of protein was loaded in each lane of an SDS-10% polyacrylamide gel. Following electrophoresis and transfer, the blot was developed with anti-JNK1 rabbit polyclonal antibody and anti-rabbit secondary antibody-HRP. Detection was performed using standard ECL techniques. The results are representative of two different experiments. (C) N-Ras knockout cells, control N+/+ cells and c-N-Ras reconstituted cells [cell lines N−/−(2)/wtN3 and N−/−(2)/wtN8] were left untreated or treated with 1 ng of TNF-α per ml in the presence of 2 μg of cycloheximide per ml. At the indicated times, the cells were harvested and lysates prepared as described in the text. JNK1 was immunoprecipitated from 50 μg of each lysate. The immunocomplexes were incubated for 20 min at 37°C in kinase buffer. The supernatants, which contain the ATF2 substrate, were loaded onto SDS-12% polyacrylamide gels, electrophoresed, and transferred to PVDF. Signals were detected using a PhosphorImager. A nonimmune (NI) control was performed using an equal amount (1.5 μg) of purified rabbit (Rb) IgG and the 1-h TNF-α-plus-cycloheximide-treated N+/+(1) cell lysate. The results are representative of three separate experiments. (D) N-Ras knockout cells, control N+/+ cells, and c-N-Ras reconstituted cells [N−/−(2)wtN3] were left untreated or treated with 1 μg of activating anti-Fas antibody per ml in the presence of 0.5 μg of protein G per ml. At the indicated times, the cells were harvested and lysates were prepared as described in the text. A 50-μg portion of each lysate was electrophoresed on an SDS-10% polyacrylamide gel and transferred to PVDF. The blot was developed with anti-pJNK monoclonal antibody and goat anti-mouse IgG-HRP as described for panel A. Detection was performed using standard ECL techniques. The positive control was N-Ras knockout cells treated for 1 h with 20 μg of anisomycin (Aniso) per ml. The results are representative of six separate experiments. (E) N-Ras knockout and control N+/+ cells and c-N-Ras reconstituted cells were left untreated or treated with anti-Fas antibody and protein G as described for panel D. At the indicated times, the cells were harvested and lysates were prepared for immunoprecipitation of JNK as described for panel C. The immunoprecipitated JNK from each sample was used in a kinase reaction as described for panel C, using GST-ATF2 as the substrate. Following electrophoresis and transfer to PVDF, the radioactivity incorporated into the GST-ATF2 substrate was detected using a PhosphorImager. The nonimmune control (NI) used rabbit IgG and the N+/+(1) cells treated for 1 h, as described for panel C. The results are representative of two separate experiments. (F) N-Ras knockout and control cells and N-Ras knockout cells ectopically overexpressing c-K(A)-Ras (clones 4 and 8) were left untreated or treated for 1 or 4 h with TNF-α plus cycloheximide as described for panel A. A 50-μg portion of protein was loaded in each lane of an SDS-10% polyacrylamide gel. Following electrophoresis and transfer, the blot was developed with anti-phospho-JNK (pJNK) and anti-mouse-HRP secondary antibody. Detection was performed using standard ECL techniques.
Analysis of JNK activity demonstrated a similar pattern to that observed with phosphorylation of JNK (Fig. 2C). N-Ras knockout, control N+/+ cells, and two different clones of c-N-Ras reconstituted cells were left untreated or treated with 1 ng of TNF-α per ml in the presence of cycloheximide. At the indicated times, all of the cells were harvested and JNK1 was immunoprecipitated from the lysates for measurement of its in vitro kinase activity. JNK activity in the N-Ras knockout cells increased approximately 14-fold and remained elevated as these cells underwent apoptosis (Fig. 2C). Control N+/+ cells exhibited a 4.5-fold increase in JNK activity that was transient in nature. The pattern of JNK activity in the c-N-Ras reconstituted cells more closely resembled that observed in the control N+/+ cells in that it was also transiently increased. Both reconstituted cell lines, however, exhibited a larger increase in JNK activity and were more delayed in returning to baseline than the control cells. As observed with the phosphorylation of JNK, this may correlate with the ability of the ectopically expressed c-N-Ras to only partially rescue the heightened apoptotic sensitivity of the parental knockout cells in response to TNF-α plus cycloheximide (129). Nevertheless, these data demonstrate that in our system, a robust and sustained activation of JNK correlates with apoptosis. The data also suggest that attenuation of the JNK response to apoptotic induction may be another mechanism by which c-N-Ras provides a cellular survival function.
We sought to determine whether the patterns of JNK phosphorylation and activity observed in the N-Ras knockout and control cells were similar by using other methods of apoptotic induction. If this were the case, it would suggest that the role of c-N-Ras in cell survival in response to apoptotic induction is global and is not restricted to a single type of stimulus. Fas is another member of the TNF receptor family which can trigger apoptosis in some cell types (1, 17, 104, 111, 123). We previously demonstrated that the N-Ras knockout fibroblasts are more sensitive to activation of the Fas receptor than are control N+/+ fibroblasts or c-N-Ras reconstituted cells (129). As observed with TNF-α plus cycloheximide, N-Ras knockout cells demonstrated a strong and sustained stimulation of JNK phosphorylation in response to Fas ligation compared to untreated cells. Control N+/+ cells did not show any significant increases in pJNK in response to anti-Fas treatment (Fig. 2D). This correlates with their failure to undergo apoptosis under these conditions (129). We observed significantly less pronounced activation of JNK in the c-N-Ras reconstituted cells under these conditions (Fig. 2D). This also correlates with the ability of ectopically expressed c-N-Ras to provide protection from Fas-mediated apoptosis in the N-Ras knockout cells (129). Differences in JNK phosphorylation did not arise from different levels of total JNK protein abundance. N-Ras knockout, control, and c-N-Ras reconstituted cells contained similar amounts of JNK1 protein in the presence or absence of anti-Fas antibody (data not shown). These results are consistent with those obtained using other independently isolated N-Ras knockout and control cell lines (data not shown). We measured JNK activity from untreated or anti-Fas treated cells by an in vitro kinase assay. We observed a slower increase in JNK activity than in phosphorylation of JNK in the N-Ras knockout cells; however, the JNK activity remained elevated as these cells underwent apoptosis (Fig. 2E). Similar to the levels of phosphorylated JNK, the c-N-Ras reconstituted knockout cells displayed less pronounced and delayed JNK activity upon anti-Fas treatment, with the control cells displaying little JNK activity during this time course. This correlates with their higher resistance to the induction of apoptosis. These data again parallel the results observed with TNF-α treatment. The activation of JNK in response to serum withdrawal also parallels the results obtained with TNF-α and Fas stimulation, although the time course is slightly different (data not shown).
We tested the specificity of the apparent regulation of JNK activation by c-N-Ras. We previously demonstrated that ectopic overexpression of c-K(A)-Ras in the N-Ras knockout cells could not substitute for wild-type levels of c-N-Ras in providing protection from TNF-α-plus-cycloheximide-induced apoptosis (129). The stable c-K(A)-Ras-expressing N-Ras knockout cells contain higher levels of c-K(A)-Ras than do the parental N-Ras knockout cells or control N+/+ cells (129). These c-K(A)-Ras-expressing cells and the parental N-Ras knockout cells and control N+/+ cells were treated with TNF-α plus cycloheximide, and the levels of pJNK were examined by Western analysis. Two different c-K(A)-Ras-expressing cell lines demonstrated sustained phosphorylation of JNK similar to that observed for the parental N-Ras knockout cells (Fig. 2F). Control cells again showed reduced and transient JNK phosphorylation under these conditions. The total abundance of JNK1 protein was unchanged between the cell lines used and under the treatment conditions employed (data not shown). Similar to c-K(A)-Ras, c-K(B)-Ras expression in the N-Ras knockout cells does not lead to a reduction in JNK phosphorylation in response to treatment with TNF-α plus cycloheximide (data not shown). Taken together, these data provide evidence that c-N-Ras generates a second survival signal in addition to up-regulation of steady-state pAkt and pBad levels. In this case, c-N-Ras specifically appears to attenuate the magnitude and duration of JNK activation in response to the induction of apoptosis.
c-N-Ras also attenuates the activation of p38 in response to apoptotic induction.
Prolonged activation of p38 has been associated with apoptosis in some cell types (33, 52, 62, 63, 131, 135). We examined the activation of p38 in N-Ras knockout and control cells in response to apoptotic induction. N-Ras knockout cells treated with low-dose TNF-α in the presence of cycloheximide possessed elevated and sustained phosphorylated p38 levels (Fig. 3A). We observed only minimal phosphorylation of p38 in control N+/+ cells. c-N-Ras reconstituted cells exhibited a minor basal phosphorylation of p38 that increased only a little at 1 h and declined thereafter. These c-N-Ras reconstituted cells are partially protected from TNF-α-induced apoptosis (data not shown) compared to parental N-Ras knockout cells, similar to that observed with other c-N-Ras reconstituted cell lines (129). Measurements of p38 activity correlated with the results obtained by analysis of the phosphorylation of p38. N-Ras knockout cells exhibited increasing p38 kinase activity following treatment with TNF-α plus cycloheximide, which was not observed in either control N+/+ cells or c-N-Ras reconstituted cells (Fig. 3B). We observed only transient p38 kinase activity that decreased by approximately 30 and 50% in control cells and the c-N-Ras reconstituted cells, respectively, 4 h after treatment with TNF-α plus cycloheximide. Similar to the results with JNK, the total amount of p38 protein was unchanged in all the cell lines both at steady state (t = 0) and under the treatment conditions used (Fig. 3C). This suggests that the differences observed between N-Ras knockout and control cells in p38 phosphorylation and activity do not arise from changes in p38 protein levels. We have also observed elevated and prolonged p38 phosphorylation and activity in the N-Ras knockout cells in response to Fas activation. In response to Fas ligation, activation of p38 is minimal in control cells and only transiently increased in c-N-Ras reconstituted knockout cells (data not shown).
FIG. 3.
c-N-Ras downregulates p38 activation and activity following the induction of apoptosis. (A) N-Ras knockout cells, control N+/+ cells, and c-N-Ras reconstituted cells [N−/− (2)/wtN43A] were left untreated or treated for varying times with 1 ng of TNF-α per ml in the presence of 2 μg of cycloheximide (CHX) per ml. At the indicated times, the cells were harvested and lysates prepared as described in the text. A 50-μg portion of each lysate was loaded onto an SDS-10% polyacrylamide gel, and following electrophoresis and transfer, the blot was developed with anti-phospho-p38 (pp38) and anti-rabbit secondary antibody-HRP. Detection was performed by standard ECL techniques. The results are representative of two separate experiments. (B) N-Ras knockout and control cells and c-N-Ras reconstituted cells were left untreated or treated with TNF-α and cycloheximide as in panel A. At the indicated times, cell lysates were prepared and 50 μg of each lysate was used to immunoprecipitate p38 as described in Materials and Methods. The resulting immunocomplexes were washed and used in a kinase assay as described in the text with 25 μCi of [γ-32P]ATP per reaction and GST-ATF2 as the substrate. Signals were detected with a PhosphorImager. The nonimmune control (NI) consisted of immunoprecipitation of 1 h treated N+/+(1) control cells using an equal amount of nonimmune rabbit serum. The results are representative of four separate experiments. (C) Cells were left untreated or treated with TNF-α and cycloheximide as described for panel A. At the indicated times, cell lysates were prepared and 50 μg of each lysate was used to analyze total levels of p38 using anti-p38 polyclonal antibody. The blot was developed by using anti-rabbit secondary antibody-HRP and standard ECL techniques. The results are representative of two separate experiments. (D) N-Ras knockout cells [N−/−(3) cell line], control N+/+ cells, and c-N-Ras reconstituted cells [N−/−(3)/wtN5] were left untreated in complete medium or starved of serum for various times as described in Materials and Methods. At the indicated times, cell lysates were prepared. A 50-μg portion of each lysates was loaded onto an SDS-10% polyacrylamide gel, and following electrophoresis and transfer to PVDF, the blot was developed with anti-phospho-p38 and anti-rabbit secondary antibody-HRP as described in the text. Bands were visualized by standard ECL techniques. The results are representative of two separate experiments.
Withdrawal of trophic factors can induce apoptosis in some cell lines (30, 34, 61, 62, 131). We previously demonstrated that, unexpectedly, withdrawal of serum from the N-Ras knockout cells resulted in greater than 50% cell death by apoptosis within 48 h (129). We therefore investigated p38 activation in response to serum withdrawal. N-Ras knockout cells, control N+/+ cells, and c-N-Ras reconstituted knockout cells were maintained in serum-containing medium (t = 0) or serum-free medium for the indicated times. The amount of phosphorylated p38 was determined by Western analysis. As with TNF-α (Fig. 3A) and Fas (data not shown), serum deprivation induced an elevated and sustained phosphorylation of p38 that was not observed in either control N+/+ cells or the c-N-Ras reconstituted cells (Fig. 3D). The total amount of p38 protein was unchanged between N-Ras knockout and control cells up to 48 h of serum starvation (data not shown). These data support a functional role for c-N-Ras in cell survival through attenuation of the duration and, in some cases, the magnitude of both JNK and p38 activation following apoptotic induction by several different experimental conditions. One question remaining is the mechanism through which c-N-Ras controls the duration of JNK and p38 activation. It is possible that c-N-Ras either downregulates upstream JNK/p38 activators or increases the activity of JNK/p38-specific phosphatases or some combination of the two mechanisms.
c-N-Ras downregulates the activation of upstream kinase activators of JNK and p38.
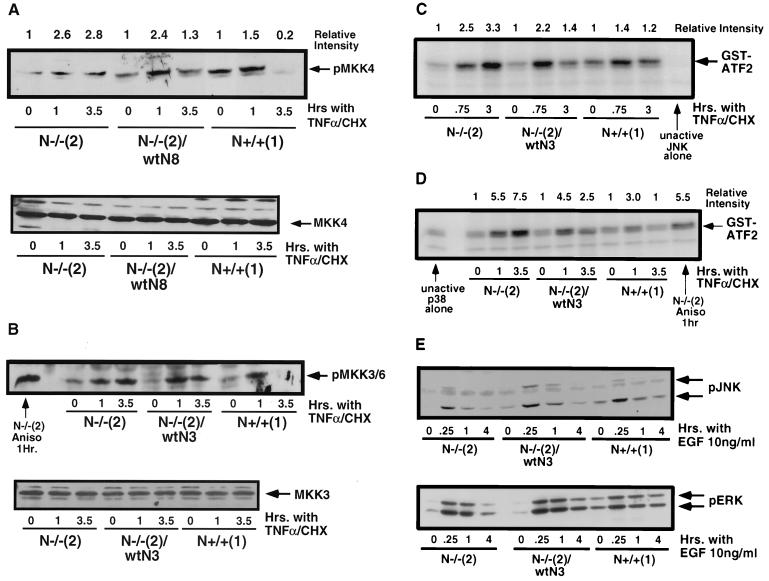
We have previously demonstrated that c-N-Ras regulates the level of pBad and that this regulation appears to be dependent on Akt activity (129). One of the major questions in the present study is the nature of the mechanism by which c-N-Ras regulates or attenuates the activation of JNK or p38. We investigated the activation of upstream kinase activators of JNK and p38 in N-Ras knockout and control N+/+ cells by using phosphospecific antibodies to these upstream kinase activators. MKK4 is one of the upstream activators of JNK (21, 48, 49, 75). In response to treatment with TNF-α plus cycloheximide, a sustained elevation of the level of phosphorylated MKK4 was observed in the N-Ras knockout cells (Fig. 4A, upper panel). Control N+/+ cells had a basal level of phosphorylated MKK4 that increased slightly by 1 h and was reduced by 3.5 h after treatment with TNF-α plus cycloheximide. We have found that the steady-state (t = 0) level of pMKK4 in the control cells is subject to variability; however, it is always reduced to minimal levels by 3 to 4 h after treatment with TNF-α plus cycloheximide (data not shown). We observed only transiently increased levels of phosphorylated MKK4 in the c-N-Ras reconstituted cells. The levels of total MKK4 protein were constant in all the cell lines both at steady state and following TNF-α treatment (Fig. 4A, lower panel). These data suggest that one mechanism by which c-N-Ras regulates JNK activation may be through regulation of MKK4 activity.
FIG. 4.
c-N-Ras downregulates the upstream kinase activators of JNK and p38. (A) (Upper panel) N-Ras knockout cells, control N+/+ cells, and c-N-Ras reconstituted cells [N−/− (2)/wtN3] were left untreated or treated with 1 ng of TNF-α per ml in the presence of 2 μg of cycloheximide (CHX) per ml. At the indicated times, cell lysates were prepared and 100 μg of protein was loaded in each lane of an SDS-10% polyacrylamide gel. Following electrophoresis and transfer to PVDF, the blot was incubated with anti-phospho-MKK4 (pMKK4) polyclonal antibody. The blot was developed by using anti-rabbit secondary antibody-HRP and standard ECL techniques. The positive control consisted of N-Ras knockout cells treated for 1 h with 20 μg of anisomycin (Aniso) per ml. The results are representative of four separate experiments. (Lower panel) A 100-μg portion of the same lysates as in the upper panel was electrophoresed, transferred, and blotted with anti-MKK4 polyclonal antibody (1:1,000 dilution) followed by anti-rabbit secondary antibody-HRP. Detection was performed by standard ECL techniques. The results are representative of two separate experiments. (B) (Upper panel) N-Ras knockout, control N+/+, and c-N-Ras reconstituted cells were left untreated or treated with TNF-α and cycloheximide as in panel A. At the indicated times, cell lysates were prepared and 100 μg of protein of each lysate was electrophoresed and transferred to PVDF. The blot was developed with anti-phospo-MKK3/6 (pMKK3/6) antibody and anti-rabbit secondary antibody-HRP. Bands were visualized by standard ECL techniques. This experiment is representative of three separate experiments. (Lower panel) Cells were left untreated or treated with TNF-α and cycloheximide as in panel A. Cell lysates (100 μg) were prepared for analysis of total MKK3 levels by using anti-MKK3 polyclonal antibody. The blot was developed by using anti-rabbit secondary antibody-HRP and standard ECL techniques. (C) N-Ras knockout and control N+/+ cells and c-N-Ras reconstituted cells were left untreated or treated with TNF-α and cycloheximide as described for panel A. Cell lysates were prepared at the indicated times, and protein concentrations determined. A 2-μg portion of unactive His6-tagged JNK1α1 was incubated with 12 μg of each lysate in the presence of 50 μM unlabeled ATP for 20 min at 30°C. The added His6-JNK1α1 was isolated by incubation with nickel resin (ProBond; Invitrogen) for 45 min at 4°C. The resin was washed with p21 buffer and used in a second kinase assay to measure the activity of the isolated, recombinant JNK1α1 by using GST-ATF2 as the substrate as described in Materials and Methods. The unactive JNK1α1 incubated in the absence of lysate had no activity. The results are representative of three separate experiments. (D) Cells were left untreated or treated with TNF-α and cycloheximide as in panel A, and at the indicated times cell lysates were prepared. A 1-μg portion of unactive GST-p38α was incubated for 20 min at 30°C, as described in the text, with 12 μg of each lysate in p21 buffer containing 50 μM ATP. The GST-p38α was isolated with glutathione-agarose, and its activity was measured in a second kinase reaction using [γ-32P]ATP with GST-ATF2 as the substrate, as described in Materials and Methods. N-Ras knockout cells were treated for 1 h with 20 μg of anisomycin per ml, and the lysate was used to activate the GST-p38α as a positive control. The unactive GST-p38α had no activity when incubated in the absence of cell lysate. (E) N-Ras knockout, control N+/+, and c-N-Ras reconstituted cells were passaged into complete medium and 48 h later were either left untreated (t = 0) or challenged with 10 ng of EGF per ml for the times indicated. Cell lysates prepared at the indicated times were electrophoresed and transferred to PVDF. The membranes were immunoblotted for phospho-JNK (upper panel, 50 μg/lane) and pErk (Santa Cruz Biotechnology; lower panel, 20 μg/lane). The results are representative of three separate experiments.
Two different upstream kinases, MKK3 and MKK6, activate p38 (24, 38, 75, 79, 95, 110, 127). The phospho-MKK3/6 antibody recognizes both phosphorylated MKK3 and phosphorylated MKK6, which have indistinguishable mobilities in SDS-PAGE (Cell Signaling Technology, personal communication). Treatment of the N-Ras knockout cells with 1 ng of TNF-α per ml in the presence of cycloheximide led to a sustained increase in the phosphorylation of MKK3/6 (Fig. 4B). In contrast, we observed only a transient increase in the level of phosphorylated MKK3/6 in the N+/+ control cells, and the level was completely down to basal levels by 3.5 h posttreatment. c-N-Ras reconstituted cells possessed levels of phosphorylated MKK3/6 that peaked at 1 h and declined by 3.5 h after treatment with TNF-α plus cycloheximide (Fig. 4B, upper panel). The levels of total MKK3 protein were similar in all the cell lines and at all the time points examined (Fig. 4B, lower panel). These data suggest that, as was observed with JNK activity, attenuation of p38 activity by c-N-Ras correlates with a decrease in the duration of the activation of the upstream kinase activators of p38. Unfortunately, we have been unable to specifically immunoprecipitate any of the MKK proteins to test for changes in their relative specific activities. These experiments await the production of antibodies that will selectively immunoprecipitate the various MKK proteins for in vitro kinase assays. Even with the available reagents, however, we consistently observed phosphorylated MKK4 and MKK3/6 at the 3.5-h time point in the N-Ras knockout cells, in contrast to the lack of signal in the N+/+ control cells.
To validate the results obtained by analyzing the phosphorylation of MKK4 and MKK3/6, we used unactive recombinant JNK and p38 in in vitro double kinase assays. These experiments allowed us to analyze the generic activity of the upstream kinase activators of JNK and p38 by using recombinant JNK and p38 as substrates for in vitro kinase assays. N-Ras knockout fibroblasts, control N+/+ cells, and c-N-Ras reconstituted cells were left untreated or treated with TNF-α plus cycloheximide and then used for lysate preparation. Unactive recombinant, His6-tagged JNK was mixed with an aliquot of each lysate and incubated in the presence of ATP. Following this first kinase reaction, the recombinant JNK was isolated using a nickel resin and incubated with [γ-32P]ATP and purified GST-ATF2. The amount of phosphorylated GST-ATF2 was quantitated by SDS-PAGE and PhosphorImager analysis. As observed by examination of the phosphorylation state of MKK4, there was increased and persistent activation of upstream JNK activators in the N-Ras knockout cells. Similarly, as was seen with endogenous JNK activity, there was elevated and sustained activation of the recombinant JNK incubated with lysates from the N-Ras knockout cells (Fig. 4C). Both the control N+/+ cells and the c-N-Ras reconstituted knockout cells exhibited only transient activation of the recombinant JNK, indicating only transient activation of the upstream kinase activators of JNK in these cells that contain c-N-Ras.
A similar experiment was performed using unactive recombinant GST-tagged p38. Aliquots of lysates from untreated or TNF-α-plus-cycloheximide-treated cells were incubated with ATP and recombinant p38. The p38 protein was isolated using glutathione-agarose and incubated with [γ-32P]ATP and GST-ATF2. PhosphorImager analysis revealed a similar pattern of activation of the recombinant p38 (Fig. 4D) to that observed with recombinant JNK (Fig. 4C) or endogenous p38 (Fig. 3A). The results reflected similar differences in upstream kinase activity to those seen in the analysis of MKK3/6 phosphorylation (Fig. 4B). There was elevated and sustained activation of one or more specific upstream kinase activators of p38 in the N-Ras knockout cells compared to both control N+/+ cells and c-N-Ras reconstituted cells (Fig. 4D). The data from these generic double-kinase assays more closely resemble the data obtained with the endogenous JNK/p38 immunoprecipitation and direct kinase assays than with the phosphoblots of MKK4 and MKK3/6. This might suggest that there are other JNK or p38 upstream activators that are also downregulated through a c-N-Ras-specific function.
We next tested whether N-Ras knockout cells were able to inactivate JNK in a context outside of apoptosis. It was possible that these cells lacked a phosphatase, preventing the down-regulation of JNK. To test this possibility, we treated cells with a mitogen that does not cause apoptosis. Cells were untreated in normal medium containing serum (to prevent serum withdrawal-dependent induction of apoptosis in the N-Ras knockout cells) for 48 h and then stimulated for various times with 10 ng of epidermal growth factor per ml (EGF). At the indicated times, the cells were harvested and lysates were prepared to assess the level of phosphorylated JNK and ERK. The level of phosphorylated JNK peaked at 15 min and decreased thereafter in all of the cell lines. Similar results were observed with pERK, except that its level remained elevated at 1 h following EGF treatment and then declined (Fig. 4E). There were no significant differences in total JNK1 or total ERK2 between N-Ras knockout, control, and c-N-Ras reconstituted cells, which were unchanged in response to EGF treatment (data not shown). These data imply that the N-Ras knockout cells are fully capable of downregulating activated JNK. Furthermore, the data suggest that activation of JNK occurs in a transient fashion in response to mitogen stimulation, unlike the sustained activation observed in the N-Ras knockout cells following induction of apoptosis (Fig. 2A and B).
JNK plays a proapoptotic role in the N-Ras knockout and control cells.
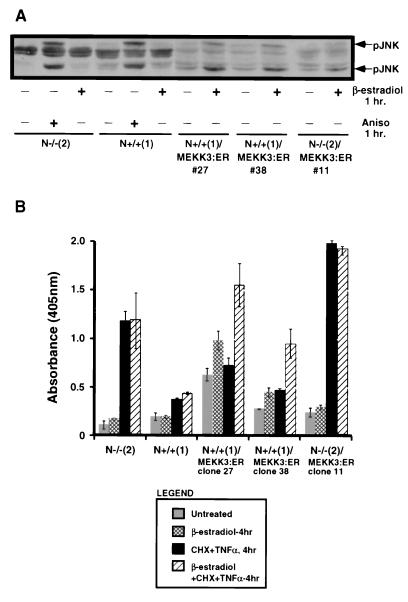
Our data suggest that persistent JNK activity, and possibly p38 activity, correlates with an apoptotically sensitive phenotype. We generated stable cell lines expressing an estrogen-regulated MEKK3 construct in an effort to determine whether persistent JNK activity is either proapoptotic or a failed protective mechanism. As a positive control for pJNK, the N-Ras knockout and control cells were treated for 1 h with 20 μg of anisomycin per ml. β-Estradiol did not activate JNK in the untransfected N-Ras knockout or control N+/+ cell lines (Fig. 5A). There was a modest increase in the level of phosphorylated JNK upon β-estradiol treatment of the MEKK3:ER-transfected cell lines, demonstrating that the MEKK3:ER was performing as expected. The increased JNK phosphorylation persisted over the time course of the experiments with continuous exposure to β-estradiol (data not shown). There were no significant differences in total JNK1 protein between the MEKK3:ER-transfected cell lines and either parental control cells or N-Ras knockout cells, and the JNK1 protein levels were unchanged by β-estradiol treatment (data not shown). There may be a small amount of leakiness of the MEKK3:ER construct, since the basal levels of pJNK in the transfected cell lines were slightly elevated compared to that observed in the parental cell lines (Fig. 5A).
FIG. 5.
Artificial elevation of JNK activation increases the level of apoptosis. (A) N-Ras knockout and control N+/+ cells stably expressing a MEKK3:ER construct and the parental cell lines were left untreated or treated for 1 h with 2 μM β-estradiol. A 50-μg portion of each cell lysate was electrophoresed, and the resultant blots were probed with anti-phospho-JNK as described in the legend to Fig. 2A. N-Ras knockout and control N+/+ cells were treated for 1 h with 20μg of anisomycin (Aniso) per ml as a positive control. The results are representative of three separate experiments. (B) N-Ras knockout and control N+/+ cells and their cognate clones that stably express the MEKK3:ER construct were left untreated or treated for 4 h with either 2 μM β-estradiol alone or a combination of β-estradiol and 1 ng of TNF-α per ml in the presence of 2 μg of cycloheximide (CHX) per ml. At 4 h of treatment, the cells were harvested, lysed, and analyzed for the level of apoptosis by a Cell Death Detection ELISA as described in Materials and Methods. Following substrate addition, readings were performed in a microtiter plate reader at λ = 405 nm. Assays were performed in triplicate, and the results are plotted with error bars representing the sample standard deviation of the mean (σn − 1). The results are representative of three separate experiments.
An apoptosis assay was performed on cells left untreated or treated with TNF-α plus cycloheximide in the presence or absence of β-estradiol. As expected, there was a five- to sixfold increase in apoptosis after 4 h of treatment with TNF-α in the N-Ras knockout cells (Fig. 5B). β-Estradiol treatment alone did not result in increased apoptosis in the untreated N-Ras knockout cells. Similarly, β-estradiol did not enhance their sensitivity to TNF-α-induced apoptosis. The control N+/+ cells remained relatively insensitive to TNF-α plus cycloheximide in the absence or presence of β-estradiol. β-Estradiol treatment alone did not lead to a substantial increase in apoptosis in either the N-Ras knockout or control cells stably expressing the MEKK3:ER construct (Fig. 5B). However, the level of apoptosis in the MEKK3:ER-transfected control cell lines was significantly increased in response to combined treatment with β-estradiol and TNF-α plus cycloheximide (Fig. 5B). Again, continuous treatment with β-estradiol in the MEKK3:ER-transfected cells lead to sustained phosphorylation of JNK (data not shown). Therefore, the results observed with the control N+/+ cells stably expressing MEKK3:ER demonstrate that sustained activation of JNK plays a proapoptotic role. These data verify our interpretation of the results obtained with the N-Ras knockout cells, suggesting that sustained JNK activation can play a proapoptotic role.
c-N-Ras regulates the duration of JNK activation through a distinct set of downstream targets.
In addition to examining which upstream JNK activators participate in sustained JNK activation, we examined which N-Ras-dependent pathway(s) might contribute to the attenuation of JNK activation. GTP-bound Ras can bind many different effectors (69, 106). Specific mutations within the switch 1 region of the effector-binding domain of Ras were found to produce Ras proteins capable of binding a limited number of effectors (125). The T-to-S mutation at amino acid 35 (35S) produces a Ras protein capable of binding only Raf-1. The Y40-to-C mutation (40C) renders the Ras-GTP protein capable of binding only PI3-kinase (p110α and β). The E37-to-G mutation (37G) produces a Ras protein capable of binding only RalGDS (125). Since these mutants were first described, the 37G mutant Ras protein has also been shown to bind Rin 1, AF-6, PLCɛ, and p110δ (18, 54, 60, 106). The failure of each of these Ras switch 1 mutations in a G12V background to transform fibroblasts suggests that Ras must activate more than one effector pathway to generate a transformed phenotype (125, 126). For the present study, the same effector domain mutations were made in a Q61K-N-Ras background. We chose to use the selective switch 1 mutants in an activated (K61) background to overcome any potential problems that might arise in the interaction of the cellular switch 1 mutants with Ras exchange factors. These mutant N-Ras proteins were transfected into N-Ras knockout cells, and stable clones were selected in G418. The level of effector domain mutant N-Ras expression was compared to the level of endogenous c-N-Ras in the control N+/+ cells (Fig. 6A).
We tested the switch 1 N-Ras-expressing cells for the predicted interactions with the appropriate downstream effectors. We first examined the ability of the 35S (Raf-1-binding) N-Ras mutants to lead to elevated steady-state Erk/mitogen-activated protein (MAP) kinase phosphorylation. N-Ras knockout and control N+/+ cells and several cell lines expressing the switch 1 mutant N-Ras proteins were subcultured in complete serum-containing medium and left untreated for 48 h. The cells were then harvested, and the level of phospho-Erk (pErk) was examined by immunoblotting. There was elevated pErk in both 35S N-Ras-expressing cell lines compared to N-Ras knockout, control N+/+, or the other switch 1 mutant-expressing cells (37G or 40C) (Fig. 6B, upper panel). The levels of total Erk protein were the same in all the cell lines examined (Fig. 6B, lower panel). We chose to verify these results by further testing the function of the 35S mutant N-Ras proteins after stable expression in NIH 3T3 cells. Stable cell lines expressing the various switch 1 mutant N-Ras proteins (Fig. 6C, upper panel) were grown in complete medium and harvested after reaching approximately 80% confluence, as described in Materials and Methods. The levels of pErk were examined by immunoblotting and compared to those in vector-transfected cells (Fig. 6C, lower panel). The results demonstrated that the level of pErk was elevated in the 35S (Raf-1-binding) N-Ras-expressing cells compared to either the 37G or the 40C mutant N-Ras-expressing cells (Fig. 6C, lower panel). The levels of expression of total Erk1 protein were identical in all the cell lines examined (data not shown).
We next tested the ability of the 40C (PI3-kinase-binding, p110α/β) mutant N-Ras-expressing cells to lead to elevated Akt activity since Akt is activated by PI3-kinase. N-Ras knockout cells, control N+/+ cells, and N-Ras knockout cells stably expressing the various switch 1 mutant N-Ras proteins were grown in complete medium for 48 h and harvested for an Akt assay by using the detergent-containing buffer supplied by the manufacturer in the Akt kinase assay kit, as described in Materials and Methods. Akt was immunoprecipitated from equal amounts of protein (0.5 mg) and used in a kinase assay with GSK-3β fusion protein as the substrate. Following electrophoresis and transfer to PVDF, the blot was developed with anti-phospho-GSK-3α/β. There was elevated steady-state Akt activity in both 40C mutant N-Ras-expressing cell lines (Fig. 6D). Similarly, there was increased steady-state Akt activity in the control cells, which compares favorably with previous results demonstrating that the control cells possess elevated phospho-Akt levels at steady state (129). The levels of total Akt were similar in all the cell lines tested (data not shown). Finally, all of the cell lines were tested using a Ral-binding domain protein to pull down Ral-GTP as a test for the function of the 37G (RalGDS, etc., binding) N-Ras mutant. Unfortunately, there was a high level of Ral-GTP in all of the cell lines including the N-Ras knockout and control cells, making it difficult to distinguish small differences resulting from expression of the 37G mutant (data not shown). All of the cell lines expressed equal amounts of both RalA and RalB (data not shown). The pulldown assay is inherently inadequate because any Ral-GTP that is bound to effector proteins would be left behind in this type of pulldown assay. Furthermore, this assay also tests the ability of the 37G mutant to bind only to RalGDS and none of the other effectors to which this mutant is reported to bind. We have, however, found that there was modestly elevated Akt activity in the 37G-expressing cells compared to either the parental N-Ras knockout cells or the 35S-expressing cells (Fig. 6D). Since the 37G mutant also binds the p110δ isoform of PI3-kinase, these data suggest that this mutant is also functional in the N-Ras knockout cells.
We sought to investigate whether expression of any of the effector domain mutant N-Ras proteins leads to attenuation of JNK activation, as observed in control or c-N-Ras reconstituted cells. N-Ras knockout cells, control N+/+ cells, c-N-Ras reconstituted cells, and N-Ras knockout cells expressing the effector domain mutant N-Ras proteins were left untreated or treated with TNF-α and cycloheximide as described above. At the indicated times, all the cells were harvested and lysates were prepared for the analysis of JNK phosphorylation. Only the 37G N-Ras protein was capable of restoring the attenuation of JNK activation, as observed with control N+/+ cells (Fig. 7A). The 37G mutant N-Ras-expressing cells demonstrated a transient increase in JNK phosphorylation, as observed with control N+/+ or c-N-Ras reconstituted cells. The 37G mutant N-Ras was as effective as wild-type c-N-Ras in restoring JNK regulation. Neither the Raf-1-binding 35S nor the PI3-kinase-binding 40C mutant N-Ras proteins were capable of restoring JNK regulation. The total levels of JNK1 protein were similar in all cell lines and under the treatment conditions used (data not shown). These data imply that attenuation of JNK activation by N-Ras does not proceed through either a Raf-1- or PI3-kinase (p110α or p110β)-dependent pathway. The data do suggest that the attenuation of JNK activation by N-Ras during apoptotic induction proceeds through a distinct set of downstream targets, which may include RalGDS, AF-6, Rin 1, PLCɛ, and/or p110δ.
FIG. 7.
c-N-Ras downregulates the activation of JNK through a distinct set of downstream targets. (A) N-Ras knockout and control N+/+ cells, c-N-Ras reconstituted cells [N−/−(2)/wtN43A], and N-Ras knockout cells stably expressing the switch 1 mutant N-Ras constructs were left untreated or treated with 1 ng of TNF-α per ml in the presence of 2 μg of cycloheximide (CHX) per ml. At the indicated times, cell lysates were prepared and 50 μg of each lysate was electrophoresed on SDS-10% polyacrylamide gels and transferred to PVDF. The blot was developed with anti-phospho-JNK monoclonal antibody and anti-mouse-HRP secondary antibody as described in the legend to Fig. 2A. Bands were visualized by standard ECL techniques. The results are representative of three separate experiments. (B) Cells (as described for panel A) were left untreated or treated with TNF-α and cycloheximide as described for panel A. A 50-μg portion of each lysate was electrophoresed and transferred as described previously. The blot was developed with anti-phospho-c-Jun (Ser63) and anti-mouse secondary antibody-HRP. Detection was performed using standard ECL techniques. The results are representative of three separate experiments. (C) Cells were left untreated or treated with TNF-α and cycloheximide for 4 h as described for panel A. At 4 h of treatment, the cells were harvested and lysed for analysis of the level of apoptosis by a Cell Death Detection ELISA Plus as described in the legend to Fig. 1B and Materials and Methods. Assays were performed in triplicate, and the results are plotted with error bars representing the sample standard deviation of the mean (σn − 1). The results are representative of two separate experiments.
To extend the above results, we examined the phosphorylation of c-Jun, a transcription factor that is downstream of JNK, as another measure of JNK activity. c-Jun can be phosphorylated on multiple residues including Ser63 and Ser73, which are located near the δ domain and the N-terminal transactivation domain (2, 23, 90, 94, 107, 108). Cells were treated with TNF-α and cycloheximide as described above. The level of Ser63 phosphorylation of endogenous c-Jun was analyzed using a phospho-specific antibody. We observed a large increase in the level of Ser63 phosphorylated c-Jun 4 h after treatment with TNF-α plus cycloheximide in the N-Ras knockout cells (Fig. 7B). This is a point where these cells are nearly 50% dead by apoptosis (129). The level of Ser63 c-Jun phosphorylation was only minimally elevated in the c-N-Ras reconstituted cells. Similar to the N-Ras knockout parental cells, N-Ras knockout cells stably expressing either the 35S (Raf-1-binding) or 40C (PI3-kinase-binding) switch 1 mutant N-Ras proteins exhibited elevated and sustained Ser63 c-Jun phosphorylation. We observed identical results using other clones of N-Ras knockout cells expressing either the 35S or 40C mutant N-Ras proteins (data not shown). In sharp contrast, little Ser63 c-Jun phosphorylation was observed in either clone of the N-Ras knockout cells expressing the 37G (RalGDS) effector domain mutant N-Ras protein, similar to that observed with control N+/+ cells. Similar results were observed when the Ser73 c-Jun phosphorylation site was examined (data not shown). The differences in c-Jun phosphorylation were not a result of different levels of total c-Jun protein. All of the cell lines possessed similar levels of total c-Jun protein in the presence or absence of apoptotic treatments (data not shown). These data strongly suggest that attenuation of JNK activation by c-N-Ras proceeds through a mechanism that is independent of both Raf-1 and PI3-kinase (p110α or p110β).
Because the 37G switch 1 mutant N-Ras protein expressed in the N-Ras knockout cells restores JNK regulation, similar to endogenous wild-type c-N-Ras, we determined whether this mutant could also rescue the apoptotically sensitive phenotype of the parental N-Ras knockout cells. N-Ras knockout cells, control N+/+ cells, c-N-Ras restored cells, and N-Ras knockout cells expressing different levels of the effector domain mutant N-Ras proteins were left untreated or treated with TNF-α and cycloheximide as described above. After 4 h the cells were harvested and the level of apoptosis was measured using a Cell Death Detection ELISA. The N-Ras knockout cells underwent an approximately fourfold increase in apoptosis compared to untreated cells (untreated, 0.2; treated, 0.77 [data not shown and Fig. 7C]). The control cells and the c-N-Ras reconstituted cells did not undergo significant apoptosis under these conditions. Untreated values were approximately 0.3 for the control N+/+ cells, c-N-Ras reconstituted cells, and the N-Ras knockout cells expressing the various effector domain mutant N-Ras constructs (data not shown). N-Ras knockout cells expressing either the Raf-1-selective 35S or the PI3-kinase-selective 40C mutant N-Ras proteins were not protected from undergoing TNF-α-dependent apoptosis (Fig. 7C). As observed with JNK activation, expression of the 37G mutant N-Ras protein completely reversed the apoptotic sensitivity of the parental N-Ras knockout cells. This reversion by the 37G mutant is quite significant since it occurs at much lower levels of expression (clone 4) than the levels of expression of the 35S or the 40C mutant N-Ras proteins, which are ineffective in rescuing the N-Ras knockout cells from apoptosis. Taken together, these results suggest that attenuation of JNK activation and protection from apoptosis by N-Ras occurs through a mechanism that is independent of signaling through either Raf-1 or PI3-kinase (p110α or p110β). Since the 37G mutant N-Ras protein is capable of binding RalGDS, Rin 1, AF-6, PLCɛ, and p110δ, additional studies are be needed to examine the contribution of each pathway, RalGDS, Rin 1, PLCɛ, or p110δ to the regulation of JNK activation and protection from apoptosis. Studies with PI-3 kinase inhibitors suggest that p110δ does not contribute to the attenuation of JNK activation (data not shown) (see Discussion).
DISCUSSION
Akt plays a role in cell survival through phosphorylation of Bad on Ser136 (5, 20, 22, 88). It is likely that Akt promotes cell survival through mechanisms other than phosphorylation of Bad (27, 56). For example, Akt also phosphorylates and inactivates caspase 9 and members of the family of Forkhead transcription factors (8, 11). We previously demonstrated that c-N-Ras promotes cell survival through an Akt-dependent pathway that results in the phosphorylation of Bad (129). Furthermore, we find that this c-N-Ras-dependent survival function is operational at steady state in the absence of any treatments. It was presumed that this c-N-Ras/Akt survival pathway functions through the direct interaction of c-N-Ras with PI3-kinase, the upstream activator of Akt. In fact, PI3-kinase interacts with Ras in vitro in a GTP-dependent manner (29, 96). More recently, however, a stable Ras-PI3-kinase complex has been observed in FRTL5 cell lysates only after treatment of the cells with cyclic AMP-elevating agents (15). We believe that the survival function of c-N-Ras in producing steady-state phosphorylation of Akt and Bad proceeds through one of the PI3-kinase isoforms, since these phosphorylation events can be downregulated through the use of PI3-kinase selective inhibitors (Fig. 1A). It is unclear whether this c-N-Ras-dependent survival function proceeds through a direct interaction between c-N-Ras-GTP and PI3-kinase.
Our data suggest that c-N-Ras promotes cell survival through both Akt-dependent and Akt-independent mechanisms. The possibility that JNK may play a role in apoptosis prompted us to examine whether c-N-Ras provides a survival function through the regulation of JNK activation. The biological outcome of JNK signaling and its role in apoptosis remain controversial and may depend upon the nature of the environmental stimulus as well as the cell type and the presence of other regulatory factors (48, 49, 64, 75). Emerging evidence indicates that whether the activation of JNK is pro-survival or pro-apoptosic may depend on the magnitude and duration of JNK activation. There are several reports which provide evidence indicating that persistent, rather than transient, JNK activation or activity is associated with apoptosis (13, 14, 35, 46, 82, 87, 102, 113, 119, 121). Our data are consistent with the concept that elevated and sustained phosphorylation of JNK is proapoptotic. Cells that are more resistant to the induction of apoptosis (control N+/+ and c-N-Ras reconstituted cells) display only transient JNK phosphorylation and activity. Cells that are more apoptotically sensitive (N-Ras knockout cells) demonstrate prolonged activation of JNK. In fact, artificially elevating and prolonging the duration of JNK activation resulted in increased apoptotic sensitivity [N+/+(1)/MEKK3:ER] (Fig. 5). Our data therefore suggest that c-N-Ras provides protection from apoptosis through the attenuation of the magnitude and duration of JNK activity (Fig. 2).
The activation of p38 plays a role in the apoptotic process in some cell types (9, 52, 59, 62, 72, 131, 135). As has been observed with JNK, in some cases elevated and sustained activation of p38 correlates with apoptosis (33, 52, 62, 63, 131, 135). We found similar results when we examined p38 activation. Our data suggest that, as observed with JNK, elevated and prolonged activation of p38 is associated with apoptosis and that c-N-Ras functions to promote cell survival through attenuation of the magnitude and duration of p38 activation (Fig. 3).
One of the major questions in the present study is the nature of the mechanism by which c-N-Ras regulates JNK and p38 activation. Three possibilities in answering this question exist: (i) c-N-Ras reduces the activation of upstream kinase activators of JNK and p38; (ii) c-N-Ras upregulates a JNK- or p38-specific phosphatase; or (iii) a combination of the two mechanisms. There are a limited number of upstream dual-specificity kinase activators of JNK and p38; they include MKK4 and MKK7 for JNK activation (21, 45, 49, 65, 80, 101, 115), and MKK3 and MKK6 for p38 activation (24, 39, 95, 110). MKK4 is also capable of activating p38 as well as JNK (24, 49). All four kinases activate JNK or p38, respectively, through phosphorylation of both a threonine and a tyrosine residue.
We investigated the activation kinetics of these upstream JNK/p38 activators by examining their phosphorylation state both at steady state and following treatment with apoptotic agonists. The data reveal a pattern of MKK protein phosphorylation that parallels the results obtained with JNK and p38 activation. Cells that are apoptotically sensitive (N-Ras knockout) display elevated and sustained activation of upstream kinase activators for both the JNK and p38 signaling pathways. Cells that are resistant to the induction of apoptosis (control N+/+ cells and c-N-Ras reconstituted cells) possess only transient activation of either MKK4 or MKK3/6. These results were confirmed by indirect measurement of the activities of the upstream kinase activators by using recombinant JNK and p38 in the in vitro double-kinase assays (Fig. 4). Taken together, these results indicate that c-N-Ras promotes cell survival by downregulating JNK and p38 activation through a mechanism involving c-N-Ras-dependent downregulation of the upstream dual-specificity kinase activators of both JNK and p38. These results also suggest that c-N-Ras functions at a position that is a branch point upstream of all of the MKK proteins, whereby c-N-Ras prevents sustained activation of the MKK proteins that function in both the JNK and p38 signaling pathways.
While the JNK group of MAP kinases are strongly stimulated by extracellular stress and inflammatory cytokines, they can be modestly activated by growth factors (36, 50, 64, 75). We treated cells with EGF to investigate whether the N-Ras knockout cells exhibit elevated JNK activity because they were unable to downregulate JNK. We observed transient phosphorylation of JNK in all of the cell lines (Fig. 4E). These data suggest that transient activation of JNK is not associated with apoptosis and that the N-Ras knockout cells are capable of downregulating JNK activity. We have not ruled out the possibility that different inactivation mechanisms might be involved in JNK downregulation, one of which might be deficient in the N-Ras knockout cells. However, our data clearly demonstrate a failure to inactivate JNK/p38, as well as their upstream activators, following apoptosis induction in the absence of c-N-Ras.
It therefore appears that c-N-Ras promotes cell survival through upregulation of Akt activity and downregulation of JNK. Our data also suggest that c-N-Ras functions at a branch point upstream of the MKK activators of both the JNK and p38 signaling pathways. We therefore chose to go downstream of N-Ras to determine the effector(s) through which N-Ras causes downregulation of JNK activation. We stably expressed three different switch 1 effector domain mutant N-Ras constructs in the N-Ras knockout cells. We were surprised to find that only the RalGDS-binding N-Ras mutant (37G) was capable of both restoring JNK regulation and rescuing the apoptotically sensitive phenotype of the parental N-Ras knockout cells. While these results were unexpected, experiments using the MEK-specific inhibitor U0126 have indicated that inhibition of the Erk/MAP kinase pathway does not lead to a further increase in the apoptotic sensitivity of either the N-Ras knockout or control cells (data not shown). We have also demonstrated that inhibition of PI3-kinase in the control N+/+ cells does not, by itself, increase their apoptotic sensitivity (Fig. 1B). These data suggest that at least one cellular survival mechanism is the downregulation of JNK signaling, which proceeds through a mechanism that is independent of either Raf-1 or PI3-kinase (p110α or p110β). We have also found that the 37G switch 1 mutant N-Ras restores JNK regulation in the presence of PI3-kinase inhibitors (data not shown). This, then, suggests that attenuation of JNK activity by N-Ras occurs through a unique set of downstream effectors independent of Raf-1 and PI3-kinase.
One concern was the failure of the PI3-kinase-binding N-Ras mutant to rescue the apoptotic sensitivity of the N-Ras knockout cells. A large body of evidence supports a survival role for PI3-kinase and Akt (4, 19, 28, 56, 86, 99, 129). While one of the survival functions of c-N-Ras is the restoration of Akt activity, it is unclear whether this function is the result of a direct interaction between c-N-Ras and PI3-kinase. Further, we have evidence suggesting that a direct interaction between oncogenic Ha-Ras and PI3-kinase is dispensable for G12V-Ha-Ras-dependent transformation of IEC-6 epithelial cells but not for their migration toward soluble fibronectin (49a). It is conceivable that in some cases Ras may lead to the activation of PI3-kinase and Akt by indirect mechanisms. Another possibility is that c-N-Ras provides its steady-state antiapoptotic function through the p110δ subunit of PI3-kinase. While the p110α isoform reportedly binds only to the 40C switch 1 Ras mutant, p110δ binds to the 37G and not the 40C mutant Ras (60). This, then, suggests the possibility that c-N-Ras might interact with only the p110δ catalytic subunit of PI3-kinase to provide an antiapoptotic signal. Nevertheless, our data strongly support a survival function of c-N-Ras through a direct interaction with RalGDS, AF-6, Rin 1, PLCɛ, or p110δ, which leads to the downregulation of JNK activation and cell survival.
It is of interest that the Raf-1-binding mutant 35S does not rescue the apoptotically sensitive phenotype of the parental N-Ras knockout cells at levels of expression that are significantly above that observed for the c-N-Ras in the control N+/+ cells (Fig. 6A). The PI3-kinase-binding mutant 40C does not reverse the parental N-Ras knockout cell phenotype at levels that are either below or above the level of c-N-Ras in the control N+/+ cells. In contrast, the 37G, “RalGDS/AF-6/Rin 1/PLCɛ/p110δ”-binding N-Ras mutant rescues the apoptotic sensitivity of the parental N-Ras knockout cells at levels of expression that are considerably below the level of c-N-Ras present in the control cells (Fig. 6A, 37G clones 4 and 5). Expression of c-N-Ras in the N-Ras knockout cells also rescues the apoptotic sensitivity of the parental N-Ras knockout cells at levels of expression that are nearly identical to the levels of c-N-Ras in the control N+/+ cells (129). These results suggest that if a protein expressed in cells is going to perform a particular function, it is likely that it will do so without the need for vast overexpression. In fact, some of the complexity that revolves around interpreting Ras function may result from overexpression versus expression at endogenous levels or transient expression versus stable expression (106).
In summary, our results indicate that c-N-Ras promotes cell survival through multiple mechanisms. c-N-Ras provides protection from apoptosis by the upregulation of Akt activity and downregulation of the magnitude and duration of JNK and p38 activity. Recent evidence supports the concept that multiple pathways contribute to Ras-dependent cell survival (71, 122). While we cannot rule out the contribution of other, as yet unidentified, effectors, it appears that c-N-Ras promotes cell survival through upregulation of Akt activity and through a RalGDS/AF-6/Rin 1/PLCɛ/p110δ-dependent mechanism that leads to downregulation of signaling through JNK and p38.
N-Ras provides a steady-state survival signal in a nonclassical signaling environment. Our previous work and the data described in this report support the concept that Ras proteins, or at least c-N-Ras, possess functions other than to regulate acute proliferative signaling. The best illustration of tonic Ras-dependent signaling is the reaction to serum withdrawal. Removal of serum, which is usually associated with inactivation of Ras proteins, results in increased apoptosis in the N-Ras knockouts but not in control N+/+ cells. This, then, implies that c-N-Ras, in the absence of serum, is providing a functional antiapoptotic signal. This suggests the possibility of a novel Ras paradigm that is distinct from the control of acute, proliferative signal transduction.
Acknowledgments
We thank Joseph A. DiDonato, Andrew C. Larner, Xiaoxia Li, Mark Hamilton, Maria Karasarides, Thomas E. Patterson, Jinhui Liao, and Sarah M. Planchon for helpful discussions and/or critical review of the manuscript. We thank Christiana Obiaya for technical assistance.
This work was supported by NIH grant GM62644 to A.W. and NIH grant RO1 CA42978-14 to C.D.
REFERENCES
- 1.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 2.Baker, S. J., T. K. Kerppola, D. Luk, M. T. Vandenberg, D. R. Marshak, T. Curran, and C. Abate. 1992. Jun is phosphorylated by several protein kinases at the same sites that are modified in serum-stimulated fibroblasts. Mol. Cell. Biol. 12:4694-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbacid, M. 1987. ras genes. Annu. Rev. Biochem. 56:779-827. [DOI] [PubMed] [Google Scholar]
- 4.Benistant, C., H. Chapuis, and S. Roche. 2000. A specific function for phosphatidylinositol 3-kinase alpha (p85alpha- p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene 19:5083-5090. [DOI] [PubMed] [Google Scholar]
- 5.Blume-Jensen, P., R. Janknecht, and T. Hunter. 1998. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 8:779-782. [DOI] [PubMed] [Google Scholar]
- 6.Bonni, A., A. Brunet, A. E. West, S. R. Datta, M. A. Takasu, and M. E. Greenberg. 1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358-1362. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 9.Bulavin, D. V., S. Saito, M. C. Hollander, K. Sakaguchi, C. W. Anderson, E. Appella, and A. J. Fornace, Jr. 1999. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18:6845-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 11.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 11a.Cepko, C. L., B. E. Roberts, and R. C. Mulligan. 1984. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell 37:1053-1062. [DOI] [PubMed] [Google Scholar]
- 12.Chang, M. Y., S. J. Won, B. C. Yang, M. S. Jan, and H. S. Liu. 1999. Selective activation of Ha-ras(val12) oncogene increases susceptibility of NIH/3T3 cells to TNF-alpha. Exp Cell Res 248:589-98. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Y. R., C. F. Meyer, and T. H. Tan. 1996. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J. Biol. Chem. 271:631-634. [DOI] [PubMed] [Google Scholar]
- 14.Chen, Y. R., X. Wang, D. Templeton, R. J. Davis, and T. H. Tan. 1996. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 271:31929-31936. [DOI] [PubMed] [Google Scholar]
- 15.Ciullo, I., G. Diez-Roux, M. Di Domenico, A. Migliaccio, and E. V. Avvedimento. 2001. cAMP signaling selectively influences Ras effectors pathways. Oncogene 20:1186-1192. [DOI] [PubMed] [Google Scholar]
- 16.Clark, G. J., A. D. Cox, S. M. Graham, and C. J. Der. 1995. Biological assays for Ras transformation. Methods Enzymol. 255:395-412. [DOI] [PubMed] [Google Scholar]
- 17.Cleveland, J. L., and J. N. Ihle. 1995. Contenders in FasL/TNF death signaling. Cell 81:479-482. [DOI] [PubMed] [Google Scholar]
- 18.Cullen, P. J. 2001. Ras effectors: buying shares in Ras plc. Curr. Biol. 11:R342-R344. [DOI] [PubMed] [Google Scholar]
- 19.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 20.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 21.Davis, R. J. 1999. Signal transduction by the c-Jun N-terminal kinase. Biochem. Soc. Symp. 64:1-12. [DOI] [PubMed] [Google Scholar]
- 22.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687-689. [DOI] [PubMed] [Google Scholar]
- 23.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 24.Derijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 25.Desbois-Mouthon, C., A. Cadoret, M. J. Blivet-Van Eggelpoel, F. Bertrand, M. Caron, A. Atfi, G. Cherqui, and J. Capeau. 2000. Insulin-mediated cell proliferation and survival involve inhibition of c-Jun N-terminal kinases through a phosphatidylinositol 3-kinase- and mitogen-activated protein kinase phosphatase-1-dependent pathway. Endocrinology 141:922-931. [DOI] [PubMed] [Google Scholar]
- 26.Downward, J. 1997. Cell cycle: routine role for Ras. Curr. Biol. 7:R258-R260. [DOI] [PubMed] [Google Scholar]
- 27.Downward, J. 1999. How BAD phosphorylation is good for survival. Nat. Cell Biol. 1:E33-E35. [DOI] [PubMed] [Google Scholar]
- 28.Downward, J. 1998. Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 8:49-54. [DOI] [PubMed] [Google Scholar]
- 29.Downward, J. 1997. Role of phosphoinositide-3-OH kinase in Ras signaling. Adv. Second Messenger Phosphoprotein Res. 31:1-10. [DOI] [PubMed] [Google Scholar]
- 30.Dragovich, T., C. M. Rudin, and C. B. Thompson. 1998. Signal transduction pathways that regulate cell survival and cell death. Oncogene 17:3207-3213. [DOI] [PubMed] [Google Scholar]
- 31.Fang, X., S. Yu, A. Eder, M. Mao, R. C. Bast, Jr., D. Boyd, and G. B. Mills. 1999. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 18:6635-6640. [DOI] [PubMed] [Google Scholar]
- 32.Fukasawa, K., and G. F. Vande Woude. 1997. Synergy between the Mos/mitogen-activated protein kinase pathway and loss of p53 function in transformation and chromosome instability. Mol. Cell. Biol. 17:506-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghatan, S., S. Larner, Y. Kinoshita, M. Hetman, L. Patel, Z. Xia, R. J. Youle, and R. S. Morrison. 2000. p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J. Cell Biol. 150:335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gleichmann, M., M. Weller, and J. B. Schulz. 2000. Insulin-like growth factor-1-mediated protection from neuronal apoptosis is linked to phosphorylation of the pro-apoptotic protein BAD but not to inhibition of cytochrome c translocation in rat cerebellar neurons. Neurosci. Lett. 282:69-72. [DOI] [PubMed] [Google Scholar]
- 35.Guo, Y. L., K. Baysal, B. Kang, L. J. Yang, and J. R. Williamson. 1998. Correlation between sustained c-Jun N-terminal protein kinase activation and apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J. Biol. Chem. 273:4027-4034. [DOI] [PubMed] [Google Scholar]
- 36.Gupta, S., T. Barrett, A. J. Whitmarsh, J. Cavanagh, H. K. Sluss, B. Derijard, and R. J. Davis. 1996. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 15:2760-2770. [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton, M., and A. Wolfman. 1998. Ha-ras and N-ras regulate MAPK activity by distinct mechanisms in vivo. Oncogene 16:1417-1428. [DOI] [PubMed] [Google Scholar]
- 38.Han, J., J. D. Lee, Y. Jiang, Z. Li, L. Feng, and R. J. Ulevitch. 1996. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem. 271:2886-2891. [DOI] [PubMed] [Google Scholar]
- 39.Han, J. H., J. D. Lee, Y. Jiang, Z. G. Li, L. L. Feng, and R. J. Ulevitch. 1996. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem. 271:2886-2891. [DOI] [PubMed] [Google Scholar]
- 40.Herr, I., D. Wilhelm, E. Meyer, I. Jeremias, P. Angel, and K. M. Debatin. 1999. JNK/SAPK activity contributes to TRAIL-induced apoptosis. Cell Death Differ. 6:130-135. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann, C., G. A. Martin, and A. Wittinghofer. 1995. Quantitative analysis of the complex between p21ras and the Ras-binding domain of the human Raf-1 protein kinase. J. Biol. Chem. 270:2901-2905. [DOI] [PubMed] [Google Scholar]
- 42.Hetman, M., K. Kanning, J. E. Cavanaugh, and Z. Xia. 1999. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J. Biol. Chem. 274:22569-22580. [DOI] [PubMed] [Google Scholar]
- 43.Hocevar, B. A., T. L. Brown, and P. H. Howe. 1999. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 18:1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofer, F., S. Fields, C. Schneider, and G. S. Martin. 1994. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc. Natl. Acad. Sci. USA 91:11089-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holland, P. M., M. Suzanne, J. S. Campbell, S. Noselli, and J. A. Cooper. 1997. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J. Biol. Chem. 272:24994-24998. [DOI] [PubMed] [Google Scholar]
- 46.Hu, Y. L., S. Li, J. Y. Shyy, and S. Chien. 1999. Sustained JNK activation induces endothelial apoptosis: studies with colchicine and shear stress. Am. J. Physiol. 277:H1593-H1599. [DOI] [PubMed] [Google Scholar]
- 47.Huser, M., J. Luckett, A. Chiloeches, K. Mercer, M. Iwobi, S. Giblett, X. M. Sun, J. Brown, R. Marais, and C. Pritchard. 2001. MEK kinase activity is not necessary for Raf-1 function. EMBO J. 20:1940-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichijo, H. 1999. From receptors to stress-activated MAP kinases. Oncogene 18:6087-6093. [DOI] [PubMed] [Google Scholar]
- 49.Ip, Y. T., and R. J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol. 10:205-219. [DOI] [PubMed] [Google Scholar]
- 49a.Karasarides, M., B. Anand-Apte, and A. Wolfman. 2001. A direct interaction between oncogenic Ha-Ras and phosphatidylinositol 3-kinase is not required for the Ha-Ras-dependent transformation of epithelial cells. J. Biol. Chem. 276:39755-39764. [DOI] [PubMed] [Google Scholar]
- 50.Karin, M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483-16486. [DOI] [PubMed] [Google Scholar]
- 51.Kauffman-Zeh, A., P. Rodriguez-Viciana, E. Ulrich, C. Gilbert, P. Coffer, J. Downward, and G. Evan. 1997. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385:544-548. [DOI] [PubMed] [Google Scholar]
- 52.Kawasaki, H., T. Morooka, S. Shimohama, J. Kimura, T. Hirano, Y. Gotoh, and E. Nishida. 1997. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J. Biol. Chem. 272:18518-18521. [DOI] [PubMed] [Google Scholar]
- 53.Kazama, H., and S. Yonehara. 2000. Oncogenic K-Ras and basic fibroblast growth factor prevent Fas-mediated apoptosis in fibroblasts through activation of mitogen-activated protein kinase. J. Cell Biol. 148:557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelley, G. G., S. E. Reks, J. M. Ondrako, and A. V. Smreka. 2001. Phospholipase C(epsilon): a novel Ras effector. EMBO J. 20:743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kharbanda, S., S. Saxena, K. Yoshida, P. Pandey, M. Kaneki, Q. Wang, K. Cheng, Y. N. Chen, A. Campbell, T. Sudha, Z. M. Yuan, J. Narula, R. Weichselbaum, C. Nalin, and D. Kufe. 2000. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J. Biol. Chem. 275:322-327. [DOI] [PubMed] [Google Scholar]
- 56.Khwaja, A. 1999. Akt is more than just a Bad kinase. Nature 401:33-34. [DOI] [PubMed] [Google Scholar]
- 57.Khwaja, A., P. Rodriguez-Viciana, S. Wennström, P. H. Warne, and J. Downward. 1997. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16:2783-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kikuchi, A., S. D. Demo, Z.-H. Ye, Y.-W. Chen, and L. T. Williams. 1994. ralGDS family members interact with the effector loop of ras p21. Mol. Cell. Biol. 14:7483-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimura, N., R. Matsuo, H. Shibuya, K. Nakashima, and T. Taga. 2000. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J. Biol. Chem. 275:17647-17652. [DOI] [PubMed] [Google Scholar]
- 60.Kinashi, T., K. Katagiri, S. Watanabe, B. Vanhaesebroeck, J. Downward, and K. Takatsu. 2000. Distinct mechanisms of alpha 5beta 1 integrin activation by Ha-Ras and R-Ras. J. Biol. Chem. 275:22590-22596. [DOI] [PubMed] [Google Scholar]
- 61.Kinoshita, T., T. Yokota, K. Arai, and A. Miyajima. 1995. Suppression of apoptotic death in hematopoietic cells by signalling through the IL-3/GM-CSF receptors. EMBO J. 14:266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kummer, J. L., P. K. Rao, and K. A. Heidenreich. 1997. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J. Biol. Chem. 272:20490-20494. [DOI] [PubMed] [Google Scholar]
- 63.Le-Niculescu, H., E. Bonfoco, Y. Kasuya, F. X. Claret, D. R. Green, and M. Karin. 1999. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol. Cell. Biol. 19:751-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leppa, S., and D. Bohmann. 1999. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene 18:6158-6162. [DOI] [PubMed] [Google Scholar]
- 65.Lin, A., A. Minden, H. Martinetto, F.-X. Claret, C. Lange-Carter, F. Mercurio, G. L. Johnson, and M. Karin. 1995. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science 268:286-290. [DOI] [PubMed] [Google Scholar]
- 66.Liou, J. S., C. Y. Chen, J. S. Chen, and D. V. Faller. 2000. Oncogenic ras mediates apoptosis in response to protein kinase C inhibition through the generation of reactive oxygen species. J. Biol. Chem. 275:39001-39011. [DOI] [PubMed] [Google Scholar]
- 67.Liu, H. S., C. Y. Chen, C. H. Lee, and Y. I. Chou. 1998. Selective activation of oncogenic Ha-ras-induced apoptosis in NIH/3T3 cells. Br. J. Cancer 77:1777-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma, J. P., and M. Karplus. 1997. Molecular switch in signal transduction: reaction paths of the conformational changes in ras p21. Proc. Natl. Acad. Sci. USA 94:11905-11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malumbres, M., and A. Pellicer. 1998. RAS pathways to cell cycle control and cell transformation. Front. Biosci. 3:d887-d912. [DOI] [PubMed]
- 70.Martin, J. L., and R. C. Baxter. 1999. Oncogenic ras causes resistance to the growth inhibitor insulin-like growth factor binding protein-3 (IGFBP-3) in breast cancer cells. J. Biol. Chem. 274:16407-16411. [DOI] [PubMed] [Google Scholar]
- 71.Marushige, K., and Y. Marushige. 1999. Changes in the mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling associated with the induction of apoptosis. Anticancer Res. 19:3865-3871. [PubMed] [Google Scholar]
- 72.Merritt, C., H. Enslen, N. Diehl, D. Conze, R. J. Davis, and M. Rincon. 2000. Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8+ but not CD4+ T cells. Mol. Cell. Biol. 20:936-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mikula, M., M. Schreiber, Z. Husak, L. Kucerova, J. Ruth, R. Wieser, K. Zatloukal, H. Beug, E. F. Wagner, and M. Baccarini. 2001. Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J. 20:1952-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller, B. S., U. T. Shankavaram, M. J. Horney, A. C. Gore, D. T. Kurtz, and S. A. rosenzweig. 1996. Activation of cJun NH2-terminal kinase/stress-activated protein kinase by insulin. Biochemistry 35:8769-8775. [DOI] [PubMed] [Google Scholar]
- 75.Minden, A., and M. Karin. 1997. Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys. Acta Rev. Cancer 1333:F85-F104. [DOI] [PubMed] [Google Scholar]
- 76.Miyamoto, T., and J. C. Fox. 2000. Autocrine signaling through Ras prevents apoptosis in vascular smooth muscle cells in vitro. J. Biol. Chem. 275:2825-2830. [DOI] [PubMed] [Google Scholar]
- 77.Moodie, S. A., M. J. Paris, W. Kolch, and A. Wolfman. 1994. Association of MEK1 with p21ras · GMPPNP is dependent on B-Raf. Mol. Cell. Biol. 14:7153-7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moodie, S. A., B. M. Willumsen, M. J. Weber, and A. Wolfman. 1993. Complexes of Ras · GTP with Raf-1 and mitogen-activated protein kinase kinase. Science 260:1658-1661. [DOI] [PubMed] [Google Scholar]
- 79.Moriguchi, T., F. Toyoshima, Y. Gotoh, A. Iwamatsu, K. Irie, E. Mori, N. Kuroyanagi, M. Hagiwara, K. Matsumoto, and E. Nishida. 1996. Purification and identification of a major activator for p38 from osmotically shocked cells—activation of mitogen-activated protein kinase 6 by osmotic shock, tumor necrosis factor-α, and H2O2. J. Biol. Chem. 271:26981-26988. [DOI] [PubMed] [Google Scholar]
- 80.Moriguchi, T., F. Toyoshima, N. Masuyama, H. Hanafusa, Y. Gotoh, and E. Nishida. 1997. A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stresses. EMBO J. 16:7045-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nalca, A., S. G. Qiu, N. El-Guendy, S. Krishnan, and V. M. Rangnekar. 1999. Oncogenic Ras sensitizes cells to apoptosis by Par-4. J. Biol. Chem. 274:29976-29983. [DOI] [PubMed] [Google Scholar]
- 82.Namgung, U., and Z. Xia. 2000. Arsenite-induced apoptosis in cortical neurons is mediated by c-Jun N-terminal protein kinase 3 and p38 mitogen-activated protein kinase. J. Neurosci. 20:6442-6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Navarro, P., A. M. Valverde, M. Benito, and M. Lorenzo. 1999. Activated Ha-ras induces apoptosis by association with phosphorylated Bcl-2 in a mitogen-activated protein kinase-independent manner. J. Biol. Chem. 274:18857-18863. [DOI] [PubMed] [Google Scholar]
- 84.Neo, S. Y., Y. Zhang, L. P. Yaw, P. Li, and S. C. Lin. 2000. Axin-induced apoptosis depends on the extent of its JNK activation and its ability to down-regulate beta-catenin levels. Biochem. Biophys. Res. Commun. 272:144-150. [DOI] [PubMed] [Google Scholar]
- 85.Olson, M. F., and R. Marais. 2000. Ras protein signalling. Semin. Immunol. 12:63-73. [DOI] [PubMed] [Google Scholar]
- 86.Park, D., S. K. Pandey, E. Maksimova, S. Kole, and M. Bernier. 2000. Akt-dependent antiapoptotic action of insulin is sensitive to farnesyltransferase inhibitor. Biochemistry 39:12513-12521. [DOI] [PubMed] [Google Scholar]
- 87.Park, D. S., L. Stefanis, C. Y. Yan, S. E. Farinelli, and L. A. Greene. 1996. Ordering the cell death pathway. Differential effects of BCL2, an interleukin-1-converting enzyme family protease inhibitor, and other survival agents on JNK activation in serum/nerve growth factor-deprived PC12 cells. J. Biol. Chem. 271:21898-21905. [DOI] [PubMed] [Google Scholar]
- 88.Pastorino, J. G., M. Tafani, and J. L. Farber. 1999. Tumor necrosis factor induces phosphorylation and translocation of BAD through a phosphatidylinositide-3-OH kinase-dependent pathway. J. Biol. Chem. 274:19411-19416. [DOI] [PubMed] [Google Scholar]
- 89.Peli, J., M. Schroter, C. Rudaz, M. Hahne, C. Meyer, E. Reichmann, and J. Tschopp. 1999. Oncogenic Ras inhibits Fas ligand-mediated apoptosis by downregulating the expression of Fas. EMBO J. 18:1824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng, J., G. T. Bowden, and F. E. Domann. 1997. Activation of AP-1 by okadaic acid in mouse keratinocytes associated with hyperphosphorylation of c-jun. Mol. Carcinog. 18:37-43. [DOI] [PubMed] [Google Scholar]
- 91.Perugini, R. A., T. P. McDade, F. J. Vittimberga, Jr., and M. P. Callery. 2000. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J. Surg. Res. 90:39-44. [DOI] [PubMed] [Google Scholar]
- 92.Plattner, R., M. J. Anderson, K. Y. Sato, C. L. Fasching, C. J. Der, and E. J. Stanbridge. 1996. Loss of oncogenic ras expression does not correlate with loss of tumorigenicity in human cells. Proc. Natl. Acad. Sci. USA 93:6665-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Polunovsky, V. A., A. C. Gingras, N. Sonenberg, M. Peterson, A. Tan, J. B. Rubins, J. C. Manivel, and P. B. Bitterman. 2000. Translational control of the antiapoptotic function of Ras. J. Biol. Chem. 275:24776-24780. [DOI] [PubMed] [Google Scholar]
- 94.Pulverer, B. J., J. M. Kyriakis, J. Avruch, E. Nikolakaki, and J. R. Woodgett. 1991. Phosphorylation of c-jun mediated by MAP kinases. Nature 353:670-674. [DOI] [PubMed] [Google Scholar]
- 95.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Dérijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodriguez-Viciana, P., P. H. Warne, R. Dhand, B. Vanhaesebroeck, I. Gout, M. J. Fry, M. D. Waterfield, and J. Downward. 1994. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370:527-532. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez-Viciana, P., P. H. Warne, B. Vanhaesebroeck, M. D. Waterfield, and J. Downward. 1996. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 15:2442-2451. [PMC free article] [PubMed] [Google Scholar]
- 98.Rosen, K., J. Rak, T. Leung, N. M. Dean, R. S. Kerbel, and J. Filmus. 2000. Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J. Cell Biol. 149:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rytomaa, M., K. Lehmann, and J. Downward. 2000. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: inhibition by PKB/Akt but not Raf signalling. Oncogene 19:4461-4468. [DOI] [PubMed] [Google Scholar]
- 100.Sabbatini, P., and F. McCormick. 1999. Phosphoinositide 3-OH kinase (PI3K) and PKB/Akt delay the onset of p53- mediated, transcriptionally dependent apoptosis. J. Biol. Chem. 274:24263-24269. [DOI] [PubMed] [Google Scholar]
- 101.Sanchez, I., R. T. Hughes, B. J. Mayer, K. Yee, J. R. Woodgett, J. Avruch, J. M. Kyriakis, and L. I. Zon. 1994. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 372:794-798. [DOI] [PubMed] [Google Scholar]
- 102.Sanchez-Perez, I., M. Martinez-Gomariz, D. Williams, S. M. Keyse, and R. Perona. 2000. CL100/MKP-1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene 19:5142-5152. [DOI] [PubMed] [Google Scholar]
- 103.Sawamoto, K., A. Taguchi, Y. Hirota, C. Yamada, M. H. Jin, and H. Okano. 1998. Argos induces programmed cell death in the developing Drosophila eye by inhibition of the Ras pathway. Cell Death Differ. 5:262-270. [DOI] [PubMed] [Google Scholar]
- 104.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signalling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scheid, M. P., K. M. Schubert, and V. Duronio. 1999. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 274:31108-31113. [DOI] [PubMed] [Google Scholar]
- 106.Shields, J. M., K. Pruitt, A. McFall, A. Shaub, and C. J. Der. 2000. Understanding Ras: "it ain't over 'til it's over'. Trends Cell Biol. 10:147-154. [DOI] [PubMed] [Google Scholar]
- 107.Sluss, H. K., T. Barrett, B. Dérijard, and R. J. Davis. 1994. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol. Cell. Biol. 14:8376-8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smeal, T., B. Binetruy, D. Mercola, A. Grover-Bardwick, G. Heidecker, U. R. Rapp, and M. Karin. 1992. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol. Cell. Biol. 12:3507-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spaargaren, M., and J. R. Bischoff. 1994. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc. Natl. Acad. Sci. USA 91:12609-12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stein, B., H. Brady, M. X. Yang, D. B. Young, and M. S. Barbosa. 1996. Cloning and characterization of MEK6, a novel member of the mitogen-activatedprotein kinase kinase cascade. J. Biol. Chem. 271:11427-11433. [DOI] [PubMed] [Google Scholar]
- 111.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem 69:217-245. [DOI] [PubMed] [Google Scholar]
- 112.Terada, K., Y. Kaziro, and T. Satoh. 2000. Analysis of Ras-dependent signals that prevent caspase-3 activation and apoptosis induced by cytokine deprivation in hematopoietic cells. Biochem. Biophys. Res. Commun. 267:449-455. [DOI] [PubMed] [Google Scholar]
- 113.Tobiume, K., A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda, and H. Ichijo. 2001. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870-874. [DOI] [PubMed] [Google Scholar]
- 115.Tournier, C., A. J. Whitmarsh, J. Cavanagh, T. Barrett, and R. J. Davis. 1997. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc. Natl. Acad. Sci. USA 94:7337-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trent, J. C., Jr., D. J. McConkey, S. M. Loughlin, M. T. Harbison, A. Fernandez, and H. N. Ananthaswamy. 1996. Ras signaling in tumor necrosis factor-induced apoptosis. EMBO J. 15:4497-4505. [PMC free article] [PubMed] [Google Scholar]
- 117.Umanoff, H., W. Edelmann, A. Pellicer, and R. Kucherlapati. 1995. The murine N-Ras gene is not essential for growth and development. Proc. Natl. Acad. Sci. USA 92:1709-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Van Aelst, L., M. Barr, S. Marcus, A. Polverino, and M. Wigler. 1993. Complex formation between RAS and RAF and other protein kinases. Proc. Natl. Acad. Sci. USA 90:6213-6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Villunger, A., D. C. Huang, N. Holler, J. Tschopp, and A. Strasser. 2000. Fas ligand-induced c-Jun kinase activation in lymphoid cells requires extensive receptor aggregation but is independent of DAXX, and Fas- mediated cell death does not involve DAXX, RIP, or RAIDD. J. Immunol. 165:1337-1343. [DOI] [PubMed] [Google Scholar]
- 120.Vojtek, A. B., S. M. Hollenberg, and J. A. Cooper. 1993. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]
- 121.Volloch, V., V. L. Gabai, S. Rits, T. Force, and M. Y. Sherman. 2000. HSP72 can protect cells from heat-induced apoptosis by accelerating the inactivation of stress kinase JNK. Cell Stress Chaperones 5:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.von Gise, A., P. Lorenz, C. Wellbrock, B. Hemmings, F. Berberich-Siebelt, U. R. Rapp, and J. Troppmair. 2001. Apoptosis suppression by Raf-1 and MEK1 requires MEK- and phosphatidylinositol 3-kinase-dependent signals. Mol. Cell. Biol. 21:2324-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walczak, H., and P. H. Krammer. 2000. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp. Cell Res. 256:58-66. [DOI] [PubMed] [Google Scholar]
- 124.Warne, P. H., P. R. Viciana, and J. Downward. 1993. Direct interaction of ras and the amino-terminal region of Raf-1 in vitro. Nature 364:352-355. [DOI] [PubMed] [Google Scholar]
- 125.White, M. A., C. Nicolette, A. Minden, A. Polverino, L. Van Aelst, M. Karin, and M. H. Wigler. 1995. Multiple Ras functions can contribute to mammalian cell transformation: Cell 80:533-541. [DOI] [PubMed] [Google Scholar]
- 126.White, M. A., T. Vale, J. H. Camonis, E. Schaefer, and M. H. Wigler. 1996. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J. Biol. Chem. 271:16439-16442. [DOI] [PubMed] [Google Scholar]
- 127.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 128.Wittinghofer, A., and E. F. Pai. 1991. The structure of ras protein: a model for a universal molecular switch. Trends Biochem. Sci. 16:382-387. [DOI] [PubMed] [Google Scholar]
- 129.Wolfman, J. C., and A. Wolfman. 2000. Endogenous c-N-Ras provides a steady-state anti-apoptotic signal. J. Biol. Chem. 275:19315-19323. [DOI] [PubMed] [Google Scholar]
- 130.Wolthuis, R. M. F., B. Bauer, L. J. Van't Veer, A. M. M. De Vries-Smits, R. H. Cool, M. Spaargaren, A. Wittinghofer, B. M. T. Burgering, and J. L. Bos. 1996. RalGDS-like factor (Rlf) is a novel Ras and Rap 1A-associating protein. Oncogene 13:353-362. [PubMed] [Google Scholar]
- 131.Xia, Z. G., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 132.Xue, L., J. H. Murray, and A. M. Tolkovsky. 2000. The Ras/phosphatidylinositol 3-kinase and Ras/ERK pathways function as independent survival modules each of which inhibits a distinct apoptotic signaling pathway in sympathetic neurons. J Biol Chem 275:8817-24. [DOI] [PubMed] [Google Scholar]
- 133.Yan, J., S. Roy, A. Apolloni, A. Lane, and J. F. Hancock. 1998. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 273:24052-24056. [DOI] [PubMed] [Google Scholar]
- 134.Yang, X., R. Khosravi-Far, H. Y. Chang, and D. Baltimore. 1997. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang, C. C., and D. J. Shapiro. 2000. Activation of the p38 mitogen-activated protein kinase pathway by estrogen or by 4-hydroxytamoxifen is coupled to estrogen receptor-induced apoptosis. J. Biol. Chem. 275:479-486. [DOI] [PubMed] [Google Scholar]
- 136.Zhang, Z., J. Settleman, J. M. Kyriakis, E. Takeuchi-Suzuki, S. J. Elledge, M. S. Marshall, J. T. Bruder, U. R. Rapp, and J. Avruch. 1993. Normal and oncogenic p21ras proteins bind to the amino-terminal domain of c-Raf-1. Nature 364:308-313. [DOI] [PubMed] [Google Scholar]