Abstract
The lesions caused by maedi-visna virus (MVV) are known to be immune mediated with a presumed contribution by the response to viral antigens. However, very little is known about the T-cell response to individual viral proteins. We have therefore expressed the three individual gag antigens of MVV strain EV1 (p16, p25, and p14) in a bacterial expression system and used the purified recombinant proteins to analyze the antibody and CD4+ T-cell response to MVV. Plasma samples were taken from sheep after 1 year of infection with MVV. The titers for antibodies in these samples were determined by indirect enzyme-linked immunosorbent assays and were as follows: anti-p25 antibody, 1:400 to >1:3,200; anti-p16 antibody, 1:400 to 1:3,200; and anti-p14 antibody, 1:<100 to 1:3,200. When the induction of antibodies was followed over time postinfection (p.i.), samples positive for anti-p25 were seen by day 24 p.i., followed by anti-p16 by day 45 p.i., and lastly anti-p14 by day 100 p.i. T-cell proliferative responses to all three gag antigens were detected in persistently infected sheep peripheral blood lymphocytes. The antigens were therefore used to raise T-cell lines from persistently infected sheep. These T-cell lines were shown to be specific for the recombinant gag antigens and for viral antigen expressed on infected macrophages. The proliferative response was restricted to major histocompatibility complex class II HLA-DR and so was due to CD4+ T lymphocytes. All three gag antigens may therefore play a role in immune-mediated lesion formation in MVV disease by presentation on infected macrophages in lesions.
Lentiviruses are a subfamily of the Retroviridae. They cause slow, chronic disease and eventually, death of the infected animal. The group comprises the immunodeficiency viruses (human, simian, feline, and bovine immunodeficiency viruses), equine infectious anemia virus, and the small-ruminant lentiviruses, maedi-visna virus (MVV) and caprine arthritis encephalitis virus (CAEV). All the viruses infect accessory cells of the immune system (macrophages and dendritic cells) with the immunodeficiency viruses all having an extended tropism for lymphocytes (15).
The macrophage and dendritic cell infection which is seen in the small-ruminant lentiviruses (40, 54) causes immune-mediated lesions to develop in a variety of organs. The lesions are caused by lymphocyte accumulations, which often organize into follicular structures. Other organ-specific changes, such as smooth muscle hyperplasia in the lung or demyelination in the central nervous system, also occur (6). Virus is usually cell associated, with only low frequencies of infected cells being detected even in lesions that are well developed (8, 35, 47). How the virus persists has been under investigation for many years with both latent or restricted infection and infection of myeloid precursors put forward as strategies which may aid the virus to survive in the host (19, 20, 44). Immune evasion has also been suggested as a reason for virus persistence. This comes from data where the antibody response was found to be weak and poorly neutralizing, and only transient T-cell immune responses were seen after infection (30, 39, 47, 57, 59, 64). However, when studied closely, both T-cell proliferative and cytotoxic T-lymphocyte responses are detected, but the virus epitopes stimulating these responses are unknown (5, 52).
We therefore expressed the matrix (p16 gag), core (p25 gag), and nucleocapsid (p14 gag) antigens of MVV in a bacterial expression system using a histidine tag to purify all the proteins, and the proteins were then used to analyze the immune response to the individual antigens. We analyzed the antibody and T-cell proliferative responses to the antigens and also determined whether virally infected macrophages could present antigen to T-cell lines raised to the antigens. The results show that all three gag antigens induced antibody and T-cell proliferative responses after infection with MVV. Infected macrophages were able to present these antigens to gag-specific T cells. Persistence of the virus does not appear to be caused by the lack of an immune response to viral gag antigens. However, accessory cells infected with MVV will present viral antigen to CD4 T cells, increasing lymphoproliferation and lesion formation around infected cells. Cytokines released by activated CD4 T cells may provide a further stimulus for MVV replication by enhancing the differentiation of monocytes to macrophages and so enhancing continued lesion formation.
MATERIALS AND METHODS
Sheep.
Adult Finnish Dorset crossed sheep (MVV-free flock from the Moredun Research Institute, Edinburgh, United Kingdom) were uninfected or infected with 5 × 105 50% tissue culture infectious doses (TCID50) MVV strain EV1 (55) subcutaneously. Persistently infected sheep used to generate antigen-specific T-cell lines were infected for greater than 3 years and did not show clinical signs of disease. All sheep were used in accordance with procedures laid out in the Animals (Scientific Procedures) Act 1986 of the United Kingdom.
Virus.
MVV strain EV1 (55) was grown in sheep skin cell lines as previously described (51).
PCR.
Low-molecular-weight viral DNA was prepared by a method similar to the method of Clements et al. (12) from cells infected at a low multiplicity and then harvested when monolayer syncytial formation was greater than 70%. The DNA concentration was measured by absorbance at 260 nm. This material contains unintegrated proviral DNA, and 1 μg was used as the template with 0.1 nmol primers in standard PCRs. Primers were as follows: for p16, 449H (5′-GACCCGGGATCCAGACATGGCGAAGCAAGGCTCAAG) and 450H (5′-TCCCGGGAATTCAATTTAGTACACTTCTCTATGTTT); for p25, 769F (5′-CCCGGGATCCGAGAAATGGACCCTATTGTAAATCTGC) and 768F (5′-AACCCGGGAATTCTTTACCCTTCTGATCCTACATCTC); for p14, 771F (5′-CATCCCGGGATCCAGGAATGGAAGGGTTTAAAATGCAAC) and 770F (5′-TTTCCCGGGAATTCTGTTACAACATAGGAGG). The positions of SmaI sites are indicated in italic type in the sequences. BamHI or EcoRI sites are shown in bold type, and the ATG or TAA start and stop codons are underlined in the forward and reverse primers, respectively. The PCR used Taq polymerase (Roche Diagnostics Ltd., Lewes, United Kingdom) in 6 mM MgCl2 and was carried out at a melting temperature of 95°C for 0.6 min, annealing temperature of 45°C for 0.5 min, and extension temperature of 72°C for 2.5 min for 35 cycles with a final extension of 5 min. The p25 and p14 PCR products were cloned into SmaI-cut pTZ19R (Pharmacia, Amersham Biosciences UK Ltd., Chalfont St. Giles, United Kingdom) and the p16 PCR product was cloned into pCRII (Invitrogen Ltd., Paisley, United Kingdom), and then all the PCR products were sequenced. BamHI-and-EcoRI double-digested genes were then inserted into BamHI-and-EcoRI double-digested pRSET B (p16 and p14) or C (p25) (pRSET from Invitrogen Ltd., Paisley, United Kingdom) to give in-frame translation from the pRSET start codon and to label the recombinant proteins with a nickel-binding six-histidine tag at the N termini. Correct insertion of the gene was verified by restriction enzyme digestion and sequencing (data not shown).
Expression and purification of recombinant gag antigens.
The gag gene containing pRSET vectors were transformed into Escherichia coli BL21(DE3) (Invitrogen Ltd., Paisley, United Kingdom). Protein expression in log-phase cultures was induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Test experiments determined the optimal time of induction of the proteins of interest (3 to 5 h for p25 and 4 to 5 h for p14). When no p16 expression was detected in E. coli BL21(DE3), this plasmid was transferred to the BL21(DE3) bacterial strain carrying pLysS (optimum time of induction 5 to 6 h). BL21(DE3) containing pRSET C was used to produce the control pRSET C histidine tag peptide. Purification was performed following the manufacturer's instructions (Invitrogen Ltd., Paisley, United Kingdom) on nickel chelated Probond resin using 20 mM sodium phosphate and 500 mM sodium chloride (native buffer as all the proteins were soluble in this system). Fractions containing protein were pooled, concentrated, and sterile filtered, and then the protein concentration was measured using Bio-Rad protein assay (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom). Purified proteins were aliquoted and stored at −70°C.
SDS-PAGE and Western blotting.
Bacterial protein preparations or purified proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) run under reducing conditions and Western blotting by the method of Reyburn et al. (51). Total proteins were detected by staining gels with a rapid silver staining kit (Sigma-Aldrich Co. Ltd., Poole, United Kingdom). Immunoblots were probed overnight with sheep serum before or after infection (diluted 1/100 in phosphate-buffered saline containing 5% nonfat dried milk) at 4°C, washed, and then incubated with anti-sheep immunoglobulin conjugated to alkaline phosphatase (1:1,000; Sigma-Aldrich Co. Ltd., Poole, United Kingdom) at room temperature for 1 h before the blots were developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate.
Indirect anti-gag ELISAs.
The wells on flexible polyvinyl chloride assay plates (Falcon 353912; BD Biosciences, Cowley, United Kingdom) were coated with purified gag antigen or pRSET C peptide at 2.5 μg/ml in 100 μl 0.1 M carbonate-bicarbonate buffer pH 9.6 overnight at 4°C. Enzyme-linked immunosorbent assays (ELISAs) were performed by the method of Bird et al. (4) using serum dilutions starting at 1/100 and then anti-sheep immunoglobulin conjugated to horseradish peroxidase (DakoCytomation Ltd., Ely, United Kingdom). The end point of a serum dilution series was taken as the last dilution that gave an optical density greater than two times the background (pRSET C-coated wells). The reciprocal of this dilution was taken as the serum titer. Serum titers for the gag antigens were compared by Mann-Whitney nonparametric statistics. Where assays were performed with 1 dilution of serum, the sample was considered positive for a specific antibody if the optical density was greater than two times the background as described above.
Generation of antigen-specific T-cell lines.
Antigen-specific T-cell lines were generated in a manner similar to the method of Bujdoso et al. (9) by antigen and then interleukin-2 (IL-2) stimulation. Briefly, peripheral blood mononuclear cells (PBMC) from persistently MVV-infected sheep were stimulated with 50 μg purified gag antigen per ml of RPMI 1640 with 5 mM glutamine, 100 U penicillin/ml, 200 μg streptomycin/ml, and 50 μM β-mercaptoethanol (RPMI) and 10% fetal calf serum (FCS) (10% RPMI) in 24-well plates (3 × 106 cells/ml/well). Cells were incubated at 37°C and 5% CO2 for 6 to 7 days, harvested, and centrifuged over a Ficoll-Hypaque gradient (Lymphoprep; Nycomed, Oslo, Norway) to remove dead cells. Interphase cells were washed two times in RPMI with 2% FCS and then plated out at 5 × 105 viable lymphocytes/ml in 10% RPMI with 25 U recombinant human IL-2 (National Institute for Biological Standards and Control) per ml for a further 6 to 7 days. Fresh recombinant human IL-2 was added after 3 to 4 days in 0.5 ml medium. Viable cells were once again harvested as described above and put into a second round of antigen stimulation using 2 × 106 irradiated (30 cGy) autologous PBMC per well with 106 lymphocytes per well. Again these cells were taken after antigen stimulation and given a cycle of IL-2 stimulation as described above. Lines were harvested and stored under liquid nitrogen. This protocol typically gave T-cell lines that contained approximately 65% CD4+, 20% CD8+, and 2% B lymphocytes.
T-cell proliferation assays. (i) PBMC assay (52).
PBMC from persistently infected or uninfected sheep were cultured at 105 cells per well with purified gag antigen or pRSET C peptide (12 to 50 μg/ml) in a final volume of 200 μl 10% RPMI for 5 days. Proliferation was measured by [3H]thymidine incorporation (1 μCi per well) for the last 5 h of the assay. Cells were harvested using an automatic cell harvester (Tomtec Harvester 96), and thymidine incorporation was measured by liquid scintillation counting on a Wallac 1450 microbeta counter. Cultures were set up in triplicate. Stimulation indices (SIs) with gag antigens were calculated using SI = cpm with recombinant gag antigen/cpm with pRSET C peptide. Differences in SIs between gag antigens were analyzed by Mann-Whitney nonparametric statistics with results from six infected sheep (assays with all antigens performed at the same time).
(ii) Assay with T-cell lines.
Antigen-presenting cells for assays with the T-cell lines were either autologous monocyte-derived macrophages (MDM) or irradiated PBMC. Autologous monocytes were isolated by adherence on tissue culture plastic (2 h) from PBMC (33). Adherent monocytes were cultured in 10% RPMI with 10% lamb serum for 5 days until they matured into MDM. Cells were infected or mock infected with MVV (1 to 2 TCID50 per cell) in RPMI with 2% FCS for 2 h, the virus inoculum was removed, and the medium was replaced with 10% RPMI. This was changed every day for 4 days. The medium of one set of macrophages was changed and replaced with heat-inactivated (56°C, 30 min) medium from MVV-infected macrophages. MDM were harvested with 5 mM EDTA in phosphate-buffered saline and plated in 96-well flat-bottomed plates at the stated numbers for T-cell assays. PBMC were irradiated with 30 cGy and used at 2 × 105 cells per well as antigen-presenting cells in assays.
Antigen-specific T-cell lines (described above) were used at 5 × 104 cells per well in RPMI with 10% lamb serum. Lymphocytes and MDM or irradiated PBMC with soluble purified gag antigen or pRSET C peptide were cultured together for 5 days and labeled and harvested as described above. T-cell cultures with MDM were set up in quadruplicate and with irradiated PBMC in triplicate. Concanavalin A (5 μg/ml) was used as a positive control in T-cell assays.
RESULTS
Production of recombinant fusion proteins.
DNA clones of p16, p25, and p14 gag were generated by PCR on low-molecular-weight DNA from MVV strain EV1-infected skin cells. The predicted peptide sequences of the recombinant proteins are shown in Fig. 1A. Recombinant p16 (rp16) was predicted to be a 177-amino-acid (aa) polypeptide: in the p16 region there was one amino acid deletion and three amino acid substitutions compared to the predicted sequence of EV1 p16. This is unlikely to be caused by a PCR artifact, as the p16 region of an independently derived full-length gag clone from EV1 has one conservative amino acid substitution compared to the p16 clone used here (data not shown). Similarly, although only 93% homologous to the predicted 1514 Icelandic strain p16 region (58), the clone used here has no insertions or deletions in comparison to this viral sequence. Recombinant p25 (rp25) was predicted to be a 258-aa polypeptide: in the p25 region there were three amino acid substitutions compared to the predicted sequence of EV1 p25. Recombinant p14 (rp14) was predicted to be a 122-aa polypeptide: in the p14 region there were three amino acid substitutions compared to the predicted EV1 p14 sequence (55). The predicted sizes of the recombinant proteins were 20.5 (rp16), 29.0 (rp25), and 13.6 kDa (rp14) while the predicted sizes of the EV1 peptides are 16.8, 24.7, and 9.6 kDa, respectively (55).
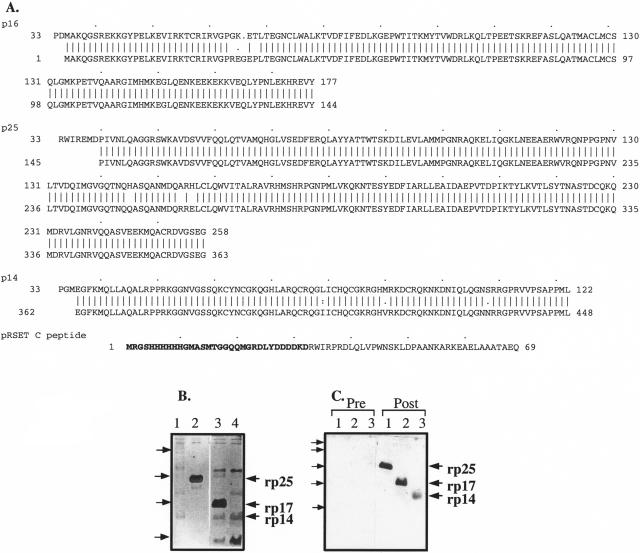
FIG. 1.
Recombinant MVV gag proteins. (A) The predicted amino acid sequences of the fusion proteins used are shown on the top lines. The recombinant proteins are compared to the predicted EV1 MVV gag sequence (bottom lines). Recombinant p16 was derived from EV1 amino acid residue (aa) 1 to 144 (nucleotides 506 to 937), rp25 from aa 145 to 363 (nucleotides 938 to 1594), and rp14 from aa 362 to 448 (nucleotides 1595 to 1849) (positions from reference 55). The predicted amino acid sequence of the pRSET C peptide is also shown with the 32 amino acids that are present at the N termini of all the recombinant proteins (shown in bold type). Recombinant protein (0.5 μg per track) purified on ProBond nickel columns was run on 15% SDS-polyacrylamide gels. The gels were silver stained (B) or electroblotted onto a nitrocellulose membrane (C). (B) Track 1, pRSET C peptide; track 2, rp25; track 3, rp16; track 4, rp14. The pRSET C peptide had run off the bottom of the gel, but the contaminant bands are clearly shown. (C) Tracks 1, rp25; tracks 2, rp16; tracks 3, rp14. The blots were developed with pre- (Pre) and postinfected (Post) sheep plasma samples (from the same sheep) as indicated above the lanes.
Recombinant protein-containing fractions from columns were pooled, and the purity of the fractions was examined by silver staining SDS-polyacrylamide gels (Fig. 1B). Recombinant p25 gave the greatest yield of all the polypeptides (15.0 to 20.0 mg/liter) and the cleanest protein preparations, although contaminating bacterial protein bands were detectable (Fig. 1B). This recombinant protein runs as a doublet, suggesting loss by degradation of some of the C-terminal portion (the N-terminal His tag was still functional [data not shown]). The major band was approximately 30 kDa by Western blotting (Fig. 1C and also with monoclonal antibody VPM70 [51], which is anti-p25 specific [data not shown]). Recombinant p16 and rp14 preparations were less clean, with a higher percentage of contaminating bands (Fig. 1B, rp16 and rp14 yields were 1.5 to 2.0 and 1.0 to 1.5 mg/liter, respectively). When these proteins were repurified on the nickel-chelated column, the contaminating bands remained, although there was a total loss of proteins (data not shown). Polypeptides purified once on the Probond column were used in immunological assays. Recombinant p16 gave a detectable band at approximately 20 kDa, while rp14 ran at approximately 17 kDa after Western blotting (Fig. 1C and with an anti-His tag monoclonal antibody [data not shown]). Recombinant p14 is a basic protein which may account for the aberrant size seen on the gel. A control peptide from pRSET C in BL21(DE3) cells was produced for use as a control in immunological assays and is 69 aa long and has a predicted size of 7.9 kDa (Fig. 1A). This was not detectable on the silver-stained gel where it had run off the bottom (Fig. 1B), but the contaminating bands present in the preparation are visible.
The N-terminal His tag may be cleaved from the proteins using the enterokinase cleavage site (Asp4Lys) present in the pRSET vectors. Trial enterokinase digests of rp25 did not result in the expected product of 25.4 kDa but in smaller, nonspecific cleavage products (data not shown). It has been reported that enterokinase will also cleave after lysine preceded by two or three aspartates (36), but no recognizable enterokinase cleavage sites are present in the p25 sequence. Due to the failure to generate cleaved p25, all the recombinant fusion proteins were used in immunological assays with the His-tagged fusion partner attached.
The purified fusion proteins were tested for antigenic authenticity on Western blots using MVV strain EV1-infected sheep serum samples to detect the proteins (Fig. 1C). All three proteins were detected by serum from a persistently infected sheep, but no reactivity was seen in preinfected serum. Sera from rabbits immunized with the recombinant proteins recognized gag proteins produced in MVV-infected cells (data not shown).
Analysis of the antibody response to gag antigens in infected sheep.
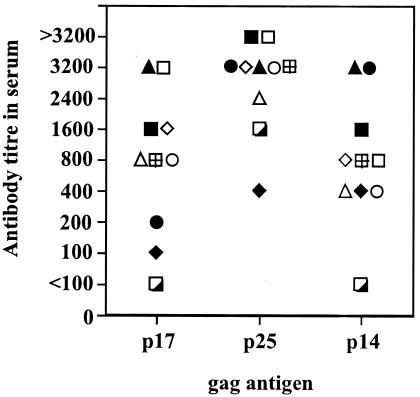
Sheep persistently infected with MVV have been reported to make antibodies reactive with virtually all MVV proteins (34), and the pattern of development of these antibodies to individual gag proteins after infection and their persistence through the course of disease have been studied by Western blotting (24, 25). In these studies, the response to p14 was often weak and transient, while the response to p25 and p16 was much clearer and consistent, although antibody to p16 was usually later to develop than that to p25 and varied in strength throughout the response. Here we have tried to quantify this more clearly using ELISAs with the recombinant gag antigens. Plasma samples from 10 sheep infected for 1 year with MVV EV1 were used to determine the titers of anti-p25, anti-p16, and anti-p14 antibodies (Fig. 2). Anti-p25 antibody titers varied from 1:400 to >1:3,200, anti-p16 antibody varied from <1:100 to 1:3,200, and anti-p14 from <1:100 to 1:3,200. The titers seen using p25 gag as the antigen were greater than either p16 or p14 gag antigen ELISAs (P < 0.01 for both by the Mann-Whitney test), but there was no statistical difference in the titer between the p16 and p14 assays. The p25 ELISA assay is therefore the most sensitive. The time course of induction of antibody after infection was also analyzed by ELISA using the fusion proteins and infected sheep plasma samples diluted 1:100. The mean absorbance values of five different sheep at different times postinfection (p.i.) are shown in Fig. 3. Using twice the background values obtained on pRSET C control peptide as the cutoff (absorbance readings for pRSET C control peptide were never greater than 0.07 [data not shown]), samples positive for anti-p25 were seen by day 24 p.i. followed by anti-p16 by day 45 p.i. and lastly anti-p14 by day 100 p.i. Antibodies were detectable consistently up to over 1 year p.i. When the ELISA results were compared to Western blot results using the same plasma sample on EV1 antigen grown in tissue culture, there was general agreement as to when the antibodies were first detectable. The ELISAs detected the antibody slightly earlier than Western blotting (data not shown).
FIG. 2.
Antibody titers against individual gag proteins in plasma samples from persistently infected sheep. Purified recombinant gag antigens were used to coat plates for ELISAs using plasma samples from sheep infected for 1 year with MVV. Antibody titer is expressed as the reciprocal of the greatest plasma dilution which gave an optical density at 490 nm twice that of background (pRSET C peptide) values. Individual sheep values are shown.
FIG. 3.
Time course of induction of antibodies to individual gag antigens. Purified recombinant gag antigens were used to coat plates for ELISAs using plasma samples from sheep infected subcutaneously with MVV (5 × 105 TCID50). Plasma samples were collected at different times postinfection and used at 1:100 dilution in the assays. The results show the mean optical density at 490 nm readings ± standard deviations (error bars) for five sheep for the individual gag antigens. Optical density readings from control pRSET C peptide ELISAs were always less than 0.07. Recombinant antigens are p16 (closed squares), p25 (closed circles), and p14 (closed triangles).
Previously published studies have never detected an immunoglobulin G2 (IgG2) antibody response to MVV antigens in infected sheep (38, 45). This was investigated with the indirect ELISAs described above using McM1 and McM3 mouse monoclonal antibodies specific for sheep IgG1 and IgG2, respectively (2) and an anti-mouse horseradish peroxidase conjugate. Only IgG1 antibodies were detectable against all three gag antigens (data not shown). No anti-gag IgG2 antibodies were detectable after infection using these indirect ELISAs. The monoclonal antibody McM3 was active, as IgG2 antibodies in serum from a sheep immunized with rp25 in complete Freund's adjuvant, which is known to induce an IgG2 response (4), were detected using the same antibody stock (data not shown).
T-lymphocyte proliferative response to gag antigens.
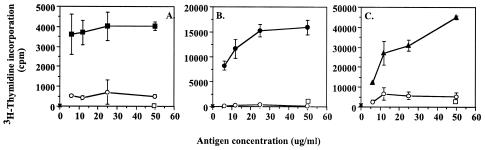
The cell-mediated immune response to the individual gag antigens was analyzed using the T-lymphocyte proliferative response of peripheral blood T cells from persistently infected (3 to 8 years) sheep to the recombinant fusion proteins. Uninfected sheep T cells were used as controls with the gag antigens, while pRSET C peptide was used as a control antigen with infected sheep. Representative results of six experiments (n = 6 infected sheep) are shown in Fig. 4. All persistently infected sheep that showed a positive T-cell proliferative response to whole EV1 antigen showed proliferative responses to all three gag proteins. These were not detected in uninfected sheep (n = 9) (Fig. 4). Proliferative responses to the pRSET C control peptide preparations were never seen. The magnitude of the proliferative response to the individual gag antigens varied among sheep, as did the hierarchy of response (sheep are not inbred), but overall, both p25 and p16 gave a greater response than p14 (stimulation indices analyzed by Mann-Whitney test [P < 0.05]). There was no significant difference between the stimulation caused by p25 and p16.
FIG. 4.
T-cell proliferative response of sheep PBMC to individual gag antigens. PBMC from a MVV-infected sheep (A) or an uninfected sheep (B) were cultured with different concentrations (in micrograms per milliliter) of rp16 (closed squares), rp25 (closed circles), rp14 (closed triangles), and pRSET C peptide (open circles) for 5 days. Cells cultured with medium alone (open squares), mock-infected cell antigen (open triangles), or viral antigen (large closed triangles) were used as controls. Lymphocyte proliferation was assessed by [3H]thymidine incorporation. Data are expressed as mean cpm ± standard deviations (error bars) from triplicate cultures.
Generation of gag-specific T-cell lines.
Peripheral blood T lymphocytes from persistently infected sheep (two sheep per gag antigen) were used to generate gag-specific T-cell lines using the recombinant proteins. Examples of one of the two cell lines generated to each of the gag antigens are shown in Fig. 5. Thymidine incorporation by T lymphocytes in response to stimulation by specific gag antigen presented by autologous irradiated PBMC is clearly greater than to control pRSET C peptide presented by autologous irradiated PBMC and increased in a dose-dependent manner as the concentration of antigen increased. The T-cell lines required antigen-presenting cells (here irradiated PBMC) to present the antigen, as T cells with antigen alone did not proliferate (Fig. 5).
FIG. 5.
Production of p16, p25, or p14 gag-specific T-cell lines. PBMC of MVV-infected sheep were used to generate gag-specific T-cell lines by two cycles of specific antigen and IL-2 stimulation. The specificity of the lines was determined using 5 × 104 T cells cultured with autologous irradiated PBMC per well in the presence of different concentrations (in micrograms per milliliter) of purified rp16 (A, closed squares), rp25 (B, closed circles), and rp14 (C, closed triangles) or pRSET C peptide (open circles) for 5 days. T cells in the presence of specific recombinant antigen but no irradiated PBMC (open squares) or T cells and irradiated PBMC with no antigen (×) were used as controls. Lymphocyte proliferation was assessed by [3H]thymidine incorporation. Data are expressed as mean cpm ± standard deviations (error bars) of triplicate cultures.
Presentation of gag antigens by MVV-infected macrophages.
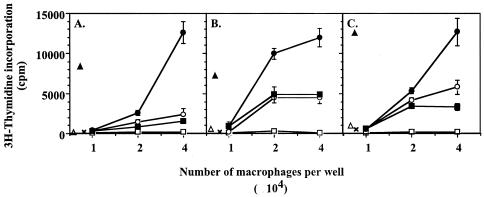
Very little is known about the ability of MVV-infected macrophages to present MVV antigens to CD4+ T cells. Initial experiments used macrophages infected in vitro to present MVV antigen to CD4+ T lymphocytes purified from PBMC of autologous persistently infected sheep. The primary isolated CD4+ T lymphocytes were stimulated to proliferate by in vitro MVV-infected macrophages in comparison to uninfected macrophages (data not shown), but this does not show which viral antigens are recognized by the CD4+ T lymphocytes. Therefore, the gag-specific T-cell lines generated above were used to determine whether MVV-infected macrophages could present gag antigens to T cells. Monocyte-derived macrophages from the same sheep as the T-cell lines were infected with MVV for 4 days in vitro before harvest and plating as antigen-presenting cells for the gag-specific T-cell lines. These macrophages caused the proliferation of T-cell lines specific for all three gag antigens (p16, p25, and p14 [Fig. 6A, B, and C, respectively]). As the monocyte-derived macrophages were from the same sheep as the T-cell lines, i.e., infected sheep, the mock-infected monocyte-derived macrophages had low levels of MVV present. This led to low levels of stimulation of the T-cell lines as may be seen from Fig. 6. However, superinfection of the macrophages with MVV in vitro increased the proliferation of the T-cell lines above this background (Fig. 6). Mock-infected macrophages also presented the recombinant antigens to the relevant cell lines (data not shown). Supernatant from the in vitro superinfected macrophages was removed every 24 h throughout the 4-day infection period, heat inactivated, and transferred to mock-infected macrophages. These macrophages were then also used as antigen-presenting cells for the T-cell lines. They gave the same levels of proliferation of the T-cell lines as the mock-infected macrophages did (Fig. 6). This suggests that viral antigen released into the supernatant from superinfected cells is not sufficient to lead to antigen presentation by macrophages and increased stimulation of T lymphocytes. Therefore, the superinfected macrophages may be presenting virus antigen produced within the cell.
FIG. 6.
Presentation of gag antigens by MVV-infected macrophages to T lymphocytes. Monocyte-derived macrophages were cultured in flasks for 5 days and then infected with 2 TCID50 MVV EV1 per cell and cultured for a further 4 days. Cells were harvested, washed three times, and plated at 104, 2 × 104, or 4 × 104 cells per well with 5 × 104 autologous cells from a p16 (A), p25 (B), or p14 (C) gag-specific T-cell line (closed circles). Mock-infected autologous MDM (open circles) or MDM that had been cultured with heat-inactivated supernatant from infected MDM (closed squares) were also used as antigen-presenting cells for the T-cell lines. T cells with MDM in the presence of VPM 54 (12 μg/ml final), an anti-MHC class II HLA-DR mouse monoclonal antibody, were used to show presentation by MHC class II (open squares). T cells with recombinant antigen (open triangles), T cells with autologous irradiated PBMC and no antigen (×), or T cells with autologous irradiated PBMC as antigen-presenting cells and the relevant recombinant antigen (50 μg/ml) (closed triangles) were used to show that the lines needed antigen-presenting cells and specific antigen to respond. Lymphocyte proliferation was assessed by [3H]thymidine incorporation after 5 days. Data are expressed as mean cpm ± standard deviations (error bars) of quadruplicate cultures.
When the proliferation assay was performed in the presence of VPM54, a monoclonal antibody specific for ovine major histocompatibility complex (MHC) class II HLA-DR alpha chain (16), all T-cell proliferation was abolished (Fig. 6). The gag antigens were therefore being presented on MHC class II HLA-DR.
DISCUSSION
The antigens used in this study were derived by PCR on low-molecular-weight DNA from infected cells. There is no infectious molecular clone available from the EV1 strain of MVV, and the virus stocks used will contain a population of related viruses. Differences in the sequences of the clones compared to the published sequence are therefore not unexpected. However, it is not possible to determine whether the mutations were introduced by PCR artifacts or were derived from defective proviral DNA products.
Most published reports on immunity to individual MVV antigens have studied the antibody response, often to help develop serological diagnostic tests. Complement fixing or neutralization assays were used in early reports examining the time at which antibody appears in the serum of sheep infected with MVV. With these assays, antibody first appears 1 to 4 months postinfection (intracerebral or intrapulmonary infection) in serum and cerebrospinal fluid (14, 23, 47, 56). Using a whole-virus ELISA and intratracheal infection, antibody is detected in serum within 6 weeks postinfection even with animals receiving low viral doses (60). The time after subcutaneous infection at which we first detected anti-gag antibodies by the indirect ELISAs is consistent with this; the anti-p25 response was detected at 24 days postinfection. When the humoral response is studied, by Western blotting serum onto whole-virus antigen, antibody to p25 also appears first (21 to 49 days p.i.), antibody to p16 appears second (35 to 59 days p.i.), and the antibody response to p14 is weak and inconsistent (24, 25). Indeed, the anti-p14 and p16 response was usually lost in sheep with gross lesions with occasional loss in the anti-p25 response, while that to envelope protein increases (24). The p14 response is more consistent in the results presented here from 5 sheep followed over approximately 18 months, although in the larger group used at 1 year postinfection, 1 sheep out of 10 did not have a detectable antibody response to p14 or p16. The antibody response detected here at 1 year postinfection reflects the ability to detect early antibody responses, with the p25 ELISA detecting the highest titers of antibody in infected sheep and also being able to detect antibody in the most dilute serum samples (data not shown). There was no evidence here at 1 year postinfection of loss of anti-gag reactivity as was seen in clinically affected sheep (24). When glutathione S-transferase fusion peptides of gag were used in Western blot assays to localize the immunodominant domains of gag, the most immunoreactive regions were mapped to the N terminus of p25 and the C terminus of p14 (29). However, there is a response to p16 in this and other studies (26, 28). These studies used field and experimentally infected sheep. All the gag antigens induce CD4 T-cell responses, and it is therefore unlikely that lack of T-cell help to B cells is affecting the antibody response (although the p14 response is the weakest T-cell proliferative response). During virus infection the gag antigens are synthesized from a single polyprotein and so should be synthesized in equimolar amounts. However, it is likely that the stability of the proteins is different. p25 is the major structural capsid protein which may self assemble into stable virus-like particles, and there is limited evidence for this with MVV capsid (50, 51). Certainly from the bacterial expression data presented here, p25 was the easiest gag antigen to express and purify. In an infected animal this may affect the amount of antigen available to stimulate an antibody response. The binding of the recombinant proteins to the ELISA plates may also vary and affect the sensitivity of the ELISAs. Recombinant p14 has a predicted pI point of 10.71, the most basic of the proteins, while p16 has a pI of 7.54 and p25 has a pI of 6.59. They will therefore have different charges (p14 will be positively charged, while both p16 and p25 will be negatively charged) at pH 9.6, which was used during binding to the plastic.
The induction of T-cell immunity after infection has been followed by both T-cell proliferative assays (23, 31, 56, 59) and induction of CD8+ cytotoxic T cells (5). However, very little is known about the specific epitopes of MVV recognized by T lymphocytes during infection. One paper has shown that gag p25 stimulates T-cell proliferation (usually CD4+ lymphocytes) in PBMC from MVV-infected sheep (52). Another has shown that after vaccination of sheep with baculovirus expressed gag p55, there is a detectable T-cell proliferative response (50). We report here for the first time reactivity to gag p16 and p14 by CD4+ T cells from MVV-infected sheep. We also show that MVV-infected macrophages can present all three gag antigens to the CD4+ T cells. It is probable that the antigen is synthesized within the macrophage rather than being taken in from the surrounding fluid or dead cells, as supernatants from superinfected macrophage cultures did not cause T-cell proliferation. However, it is possible that local concentrations of newly released virus near macrophages in the superinfected cultures were high, higher than in total supernatant, allowing uptake and processing of sufficient extracellular virus in the normal manner for MHC class II presentation to T cells. We have previously shown that MVV-infected macrophages are also able to present MVV antigen to CD8+ T lymphocytes to stimulate cytotoxic T lymphocyte function and to act as targets for killing by these cells (33). Productively infected macrophages are therefore functional as antigen-presenting cells.
Monocytes/macrophages show restricted expression of MVV and CAEV when immature, but once they have matured into macrophages, they show fully productive replication with antigen expression (7, 19, 40). Viral antigen expression is seen in tissues that are targets for MVV- and CAEV-induced lesion formation (1, 21, 27, 35, 48, 49, 61). The lesions show evidence of lymphoproliferation and follicle formation (1, 43, 49, 61, 62) and have been shown to be immune mediated (41, 42). However, the role of the antiviral T-cell response is unclear. So far no one has been able to show whether T cells are protective against MVV infection (17, 18). Similar vaccination attempts against the small-ruminant lentiviruses (MVV and CAEV) have been unsuccessful at producing sterile immunity (10, 11, 13, 22, 37, 41, 46, 53) and often make the pathology seen after infection worse (37, 41, 53). Indeed, CD4+ T lymphocytes are necessary to see an efficient infection of sheep by MVV (17). After infection the level of expression of MHC is increased on microglial cells in the central nervous system in sheep (3) and is increased in the infected lung (32) and synovium (1). Infected macrophages therefore have the necessary conditions in tissues and in lesions to present viral antigen to T cells. The ability of MVV-infected accessory cells to present antigen creates an immunopathological feedback loop in MVV lesions. CD4 T cells responding to accessory cells presented antigen release cytokines, e.g., gamma interferon and granulocyte-macrophage colony-stimulating factor, which further enhance monocyte to macrophage differentiation and virus expression (63), and these activated macrophages provide the cellular microenvironment for enhanced viral replication.
Acknowledgments
Recombinant IL-2 was provided by the EU program EVA/MRC Centralised Facility for AIDS Reagents, National Institute for Biological Standards and Control, United Kingdom (grant number QLK2-CT-1999-00609 and GP828102). Inderpal Singh was support by a Nehru Cambridge Scholarship, Overseas Research Students Award, Cambridge Commonwealth Award, and Jowett Trust Award. Barbara Blacklaws was funded by Wellcome Trust Project grant 013786.
REFERENCES
- 1.Anderson, A. A., G. D. Harkiss, and N. J. Watt. 1994. Quantitative analysis of immunohistological changes in the synovial membrane of sheep infected with maedi-visna virus. Clin. Exp. Immunol. 72:21-29. [DOI] [PubMed] [Google Scholar]
- 2.Beh, K. J. 1987. Production and characterisation of monoclonal antibodies for sheep IgG subclasses IgG1 and IgG2. Vet. Immunol. Immunopathol. 14:187-196. [DOI] [PubMed] [Google Scholar]
- 3.Bergsteinsdottir, K., S. Arnadottir, S. Torsteinsdottir, G. Agnarsdottir, V. Andresdottir, G. Petursson, and G. Georgsson. 1998. Constitutive and visna virus induced expression of class I and II major histocompatibility complex antigens in the central nervous system of sheep and their role in the pathogenesis of visna lesions. Neuropathol. Appl. Neurobiol. 24:224-232. [DOI] [PubMed] [Google Scholar]
- 4.Bird, P., H. T. Reyburn, B. A. Blacklaws, D. Allen, P. Nettleton, D. L. Yirrell, N. Watt, D. Sargan, and I. McConnell. 1995. The restricted IgG1 antibody response to maedi visna virus is seen following infection but not following immunization with recombinant gag protein. Clin. Exp. Immunol. 102:274-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blacklaws, B. A., P. Bird, D. Allen, and I. McConnell. 1994. Circulating cytotoxic T lymphocyte precursors in maedi-visna virus-infected sheep. J. Gen. Virol. 75:1589-1596. [DOI] [PubMed] [Google Scholar]
- 6.Blacklaws, B. A., P. Bird, and I. McConnell. 1994. Pathogenesis and immunity in lentivirus infections of small ruminants, p. 199-212. In B. M. L. Goddeeris and W. I. Morrison (ed.), Cell-mediated immunity in ruminants. CRC Press, Boca Raton, Fla.
- 7.Brahic, M., L. Stowring, P. Ventura, and A. T. Haase. 1981. Gene expression in visna virus infection in sheep. Nature 292:240-242. [DOI] [PubMed] [Google Scholar]
- 8.Brodie, S. J., K. A. Marcom, L. D. Pearson, B. C. Anderson, A. de la Concha-Bermejillo, J. A. Ellis, and J. C. Demartini. 1992. Effects of virus load in the pathogenesis of lentivirus-induced lymphoid interstitial pneumonia. J. Infect. Dis. 166:531-541. [DOI] [PubMed] [Google Scholar]
- 9.Bujdoso, R., P. Young, G. D. Harkiss, and I. McConnell. 1989. Antigen presentation in the sheep: generation of antigen-specific T cell lines. Immunology 66:559-564. [PMC free article] [PubMed] [Google Scholar]
- 10.Cheevers, W. P., D. P. Knowles, T. C. McGuire, T. V. Baszler, and G. A. Hullinger. 1994. Caprine arthritis-encephalitis lentivirus (CAEV) challenge of goats immunized with recombinant vaccinia virus expressing CAEV surface and transmembrane envelope glycoproteins. Vet. Immunol. Immunopathol. 42:237-251. [DOI] [PubMed] [Google Scholar]
- 11.Cheevers, W. P., K. R. Snekvik, J. D. Trujillo, N. M. Kumpula-McWhirter, K. J. Pretty On Top, and D. P. Knowles. 2003. Prime-boost vaccination with plasmid DNA encoding caprine-arthritis encephalitis lentivirus env and viral SU suppresses challenge virus and development of arthritis. Virology 306:116-125. [DOI] [PubMed] [Google Scholar]
- 12.Clements, J. E., O. Narayan, D. E. Griffin, and R. T. Johnson. 1979. The synthesis and structure of visna virus DNA. Virology 93:377-386. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip, R. C., H. D. Lehmkuhl, K. A. Brogden, and M. J. F. Schmerr. 1987. Failure of experimental vaccines to protect against infection with ovine progressive pneumonia (maedi-visna) virus. Vet. Microbiol. 13:201-204. [DOI] [PubMed] [Google Scholar]
- 14.De Boer, G. F. 1970. Antibody formation in zwoegerziekte, a slow infection in sheep. J. Immunol. 104:414-422. [PubMed] [Google Scholar]
- 15.Desrosiers, R. C. 2001. Nonhuman lentiviruses, p. 2095-2121. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 16.Dutia, B. M., J. Hopkins, M. P. Allington, R. Bujdoso, and I. McConnell. 1990. Characterization of monoclonal antibodies specific for alpha-chains and beta-chains of sheep MHC class-II. Immunology 70:27-32. [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson, K., E. McInnes, S. Ryan, P. Tonks, I. McConnell, and B. Blacklaws. 1999. CD4+ T-cells are required for the establishment of maedi-visna virus infection in macrophages but not dendritic cells in vivo. Virology 258:355-364. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson, K., E. McInnes, S. Ryan, P. Tonks, I. McConnell, and B. Blacklaws. 1999. In vivo depletion of CD8+ cells does not affect primary maedi visna virus infection in sheep. Vet. Immunol. Immunopathol. 70:173-187. [DOI] [PubMed] [Google Scholar]
- 19.Gendelman, H. E., O. Narayan, S. Kennedy-Stoskopf, P. G. E. Kennedy, Z. Ghotbi, J. E. Clements, J. Stanley, and G. Pezeshkpour. 1986. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J. Virol. 58:67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gendelman, H. E., O. Narayan, S. Molineaux, J. E. Clements, and Z. Ghotbi. 1985. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc. Natl. Acad. Sci. USA 82:7086-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgsson, G., D. J. Houwers, P. A. Palsson, and G. Petursson. 1989. Expression of viral antigens in the central nervous system of visna-infected sheep: an immunohistochemical study on experimental visna induced by virus strains of increased neurovirulence. Acta Neuropathol. 77:299-306. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez, B., R. Reina, I. Garcia, S. Andres, I. Glaria, M. Alzueta, M. I. Mora, B. M. Jugo, I. Arrieta-Aguirre, J. M. de la Lastra, D. Rodriguez, J. R. Rodriguez, M. Esteban, M. J. Grillo, B. A. Blacklaws, G. D. Harkiss, Y. Chebloune, L. Lujan, D. de Andres, and B. Amorena. 2005. Mucosal immunization of sheep with a Maedi-Visna virus (MVV) env DNA vaccine protects against early MVV productive infection. Vaccine 23:4342-4352. [DOI] [PubMed] [Google Scholar]
- 23.Griffin, D. E., O. Narayan, and R. J. Adams. 1978. Early immune responses in visna, a slow viral disease of sheep. J. Infect. Dis. 138:340-350. [DOI] [PubMed] [Google Scholar]
- 24.Houwers, D. J., and I. M. Nauta. 1989. Immunoblot analysis of the antibody response to ovine lentivirus infections. Vet. Microbiol. 19:127-139. [DOI] [PubMed] [Google Scholar]
- 25.Kajikawa, O., M. D. Lairmore, and J. C. DeMartini. 1990. Analysis of antibody responses to phenotypically distinct lentiviruses. J. Clin. Microbiol. 28:764-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keen, J., J. Kwang, and S. Rosati. 1995. Comparison of ovine lentivirus detection by conventional and recombinant serological methods. Vet. Immunol. Immunopathol. 47:295-309. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy-Stoskopf, S., O. Narayan, and J. D. Strandberg. 1985. The mammary gland as a target organ for infection with caprine arthritis-encephalitis virus. J. Comp. Pathol. 95:609-617. [DOI] [PubMed] [Google Scholar]
- 28.Kwang, J., and R. Cutlip. 1992. Analysis of antibody response to ovine lentivirus by using viral gene products expressed in a prokaryotic system. Biochem. Biophys. Res. Commun. 188:20-27. [DOI] [PubMed] [Google Scholar]
- 29.Kwang, J., S. Rosati, S. Yang, R. A. Juste, and A. de la Concha-Bermejillo. 1996. Recognition of ovine lentivirus gag gene products by serum from infected sheep. Vet. Immunol. Immunopathol. 55:107-114. [DOI] [PubMed] [Google Scholar]
- 30.Larsen, H. J., B. Hyllseth, and J. Krogsrud. 1982. Experimental maedi virus infection in sheep: cellular and humoral immune response during three years following intranasal inoculation. Am. J. Vet. Res. 43:384-389. [PubMed] [Google Scholar]
- 31.Larsen, H. J., B. Hyllseth, and J. Krogsrud. 1982. Experimental maedi virus infection in sheep: early cellular and humoral immune response following parenteral inoculation. Am. J. Vet. Res. 43:379-383. [PubMed] [Google Scholar]
- 32.Lee, W. C., P. Bird, I. McConnell, N. J. Watt, and B. A. Blacklaws. 1996. The phenotype and phagocytic activity of macrophages during maedi-visna virus infection. Vet. Immunol. Immunopathol. 51:113-126. [DOI] [PubMed] [Google Scholar]
- 33.Lee, W. C., I. McConnell, and B. A. Blacklaws. 1994. Cytotoxic activity against maedi-visna virus-infected macrophages. J. Virol. 68:8331-8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, F. H., and H. Thormar. 1979. Precipitation of visna viral proteins by immune sera of rabbits and sheep. J. Virol. 29:536-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lujan, L., I. Begara, D. D. S. Collie, and N. J. Watt. 1994. Ovine lentivirus (maedi-visna virus) protein expression in sheep alveolar macrophages. Vet. Pathol. 31:695-703. [DOI] [PubMed] [Google Scholar]
- 36.Maroux, S., J. Baratti, and P. Desnuelle. 1971. Purification and specificity of porcine enterokinase. J. Biol. Chem. 246:5031-5039. [PubMed] [Google Scholar]
- 37.McGuire, T. C., D. S. Adams, G. C. Johnson, P. Klevjer-Anderson, D. D. Barbee, and J. R. Gorham. 1986. Acute arthritis in caprine arthritis-encephalitis virus challenge exposure of vaccinated or persistently infected goats. Am. J. Vet. Res. 47:537-540. [PubMed] [Google Scholar]
- 38.Mehta, P. D., and H. Thormar. 1974. Neutralizing activity in isolated serum antibody fractions from visna-infected sheep. Infect. Immun. 10:678-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narayan, O., and J. E. Clements. 1989. Biology and pathogenesis of lentiviruses. J. Gen. Virol. 70:1617-1639. [DOI] [PubMed] [Google Scholar]
- 40.Narayan, O., J. S. Wolinsky, J. E. Clements, J. D. Strandberg, D. E. Griffin, and L. C. Cork. 1982. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J. Gen. Virol. 59:345-356. [DOI] [PubMed] [Google Scholar]
- 41.Nathanson, N., J. R. Martin, G. Georgsson, P. A. Palsson, R. E. Lutley, and G. Petursson. 1981. The effect of post-infection immunization on the severity of experimental visna. J. Comp. Pathol. 91:185-191. [DOI] [PubMed] [Google Scholar]
- 42.Nathanson, N., H. Panitch, P. A. Palsson, G. Petursson, and G. Georgsson. 1976. Pathogenesis of visna. II. Effect of immunosuppression upon early central nervous system lesions. Lab. Investig. 35:444-451. [PubMed] [Google Scholar]
- 43.Palfi, V., and R. Glavits. 1996. Interstitial mastitis in maedi-visna virus infected ewes. Magy. Allatorv. Lapja 51:586-589. [Google Scholar]
- 44.Peluso, R., A. Haase, L. Stowring, M. Edwards, and P. Ventura. 1985. A Trojan horse mechanism for the spread of visna virus in monocytes. Virology 147:231-236. [DOI] [PubMed] [Google Scholar]
- 45.Petursson, G., B. M. Douglas, and R. Lutley. 1985. Immunoglobulin subclass distribution and restriction of antibody response in visna, p. 211-216. In J. M. Sharp and R. Hoff-Jorgensen (ed.), Slow viruses in sheep, goats and cattle. Commission of European Communities, Directorate General for Agriculture, Coordination of Agricultural Research, Luxembourg, Luxembourg.
- 46.Petursson, G., S. Matthiasdottir, V. Svansson, V. Andresdottir, G. Georgsson, A. H. Martin, G. Agnarsdottir, E. Gisladottir, S. Arnadottir, S. Hognadottir, S. R. Jonsson, O. S. Andresson, and S. Torsteinsdottir. 2005. Mucosal vaccination with an attenuated maedi-visna virus clone. Vaccine 23:3223-3228. [DOI] [PubMed] [Google Scholar]
- 47.Petursson, G., N. Nathanson, G. Georgsson, H. Panitch, and P. A. Palsson. 1976. Pathogenesis of visna. I. Sequential virologic, serologic, and pathologic studies. Lab. Investig. 35:402-412. [PubMed] [Google Scholar]
- 48.Preziuso, S., G. Renzoni, T. E. Allen, E. Taccini, G. Rossi, J. C. DeMartini, and G. Braca. 2004. Colostral transmission of maedi visna virus: sites of viral entry in lambs born from experimentally infected ewes. Vet. Microbiol. 104:157-164. [DOI] [PubMed] [Google Scholar]
- 49.Preziuso, S., E. Taccini, G. Rossi, G. Renzoni, and G. Braca. 2003. Experimental maedi visna virus infection in sheep: a morphological, immunohistochemical and PCR study after three years of infection. Eur. J. Histochem. 47:373-378. [PubMed] [Google Scholar]
- 50.Rafnar, B., G. J. Tobin, K. Nagashima, M. A. Gonda, E. Gunnarsson, O. S. Andresson, G. Georgsson, and S. Torsteinsdottir. 1998. Immune response to recombinant visna virus Gag and Env precursor proteins synthesized in insect cells. Virus Res. 53:107-120. [DOI] [PubMed] [Google Scholar]
- 51.Reyburn, H. T., D. J. Roy, B. A. Blacklaws, D. R. Sargan, and I. McConnell. 1992. Expression of maedi-visna virus major core protein, p25: development of a sensitive p25 antigen detection assay. J. Virol. Methods 37:305-320. [DOI] [PubMed] [Google Scholar]
- 52.Reyburn, H. T., D. J. Roy, B. A. Blacklaws, D. R. Sargan, N. J. Watt, and I. McConnell. 1992. Characteristics of the T cell-mediated immune response to maedi-visna virus. Virology 191:1009-1012. [DOI] [PubMed] [Google Scholar]
- 53.Russo, P., C. Vitu, J. J. Fontaine, and M. Vignoni. 1993. Arthrite-encephalite caprine: essai d'une preparation vaccinale adjuvee. I. Etude clinique et virologique. Comp. Immunol. Microbiol. Infect. Dis. 16:131-136. [DOI] [PubMed] [Google Scholar]
- 54.Ryan, S., L. Tiley, I. McConnell, and B. Blacklaws. 2000. Infection of dendritic cells by the maedi-visna lentivirus. J. Virol. 74:10096-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sargan, D. R., I. D. Bennet, C. Cousens, D. J. Roy, B. A. Blacklaws, R. G. Dalziel, N. J. Watt, and I. McConnell. 1991. Nucleotide sequence of EV1, a British isolate of maedi-visna virus. J. Gen. Virol. 72:1893-1903. [DOI] [PubMed] [Google Scholar]
- 56.Sihvonen, L. 1981. Early immune responses in experimental maedi. Res. Vet. Sci. 30:217-222. [PubMed] [Google Scholar]
- 57.Sihvonen, L. 1984. Late immune responses in experimental maedi. Vet. Microbiol. 9:205-213. [DOI] [PubMed] [Google Scholar]
- 58.Sonigo, P., M. Alizon, K. Staskus, D. Klatzmann, S. Cole, O. Danos, E. Retzel, P. Tiollais, A. Haase, and S. Wain-Hobson. 1985. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell 42:369-382. [DOI] [PubMed] [Google Scholar]
- 59.Torsteinsdottir, S., G. Georgsson, E. Gisladottir, B. Rafnar, P. A. Palsson, and G. Petursson. 1992. Pathogenesis of central nervous system lesions in visna: cell-mediated immunity and lymphocyte subsets in blood, brain and cerebrospinal fluid. J. Neuroimmunol. 41:149-158. [DOI] [PubMed] [Google Scholar]
- 60.Torsteinsdottir, S., S. Matthiasdottir, N. Vidarsdottir, V. Svansson, and G. Petursson. 2003. Intratracheal inoculation as an efficient route of experimental infection with maedi-visna virus. Res. Vet. Sci. 75:245-247. [DOI] [PubMed] [Google Scholar]
- 61.Watt, N. J., T. J. King, D. Collie, N. McIntyre, D. Sargan, and I. McConnell. 1992. Clinicopathological investigation of primary, uncomplicated maedi-visna virus infection. Vet. Rec. 131:455-461. [DOI] [PubMed] [Google Scholar]
- 62.Watt, N. J., N. MacIntyre, D. Collie, D. Sargan, and I. McConnell. 1992. Phenotypic analysis of lymphocyte populations in the lungs and regional lymphoid tissue of sheep naturally infected with maedi-visna virus. Clin. Exp. Immunol. 90:204-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang, Z., G. D. Harkiss, J. Hopkins, and C. J. Woodall. 2002. Granulocyte macrophage colony stimulating factor is elevated in alveolar macrophages from sheep naturally infected with maedi-visna virus and stimulates maedi-visna virus replication in macrophages in vitro. Clin. Exp. Immunol. 129:240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zink, M. C., O. Narayan, P. G. E. Kennedy, and J. E. Clements. 1987. Pathogenesis of visna/maedi and caprine arthritis-encephalitis: new leads on the mechanism of restricted virus replication and persistent inflammation. Vet. Immunol. Immunopathol. 15:167-180. [DOI] [PubMed] [Google Scholar]