Abstract
We studied the evolution of human immunodeficiency virus type 1 (HIV-1) in a cohort of long-term survivors infected with an attenuated strain of HIV-1 acquired from a single source. Although the cohort members experienced differing clinical courses, we demonstrate similar evolution of HIV-1 nef/long-terminal repeat (LTR) sequences, characterized by progressive sequence deletions tending toward a minimal nef/LTR structure that retains only sequence elements required for viral replication. The in vivo pathogenicity of attenuated HIV-1 is therefore dictated by viral and/or host factors other than those that impose a unidirectional selection pressure on the nef/LTR region of the HIV-1 genome.
The nef gene is a major determinant of virulence in primate lentiviruses. Mutations in nef attenuate replication capacity and pathogenicity of simian immunodeficiency viruses (SIV) (3, 7, 10, 11, 13, 19) and may promote long-term survival of human immunodeficiency virus type 1 (HIV-1) infection in humans (6, 14, 15, 18, 20).
The largest described cohort of long-term survivors is the Sydney Blood Bank Cohort (SBBC), which consists of multiple individuals who became infected with an attenuated strain of HIV-1 via contaminated blood products from a common blood donor between 1981 and 1984 (6, 16, 17). Viral attenuation has been attributed to gross deletions in the nef/long terminal repeat (LTR) region of the HIV-1 genome (6). Despite being infected from a single source, SBBC members comprise slow progressors (SP) and long-term nonprogressors (LTNP) (2, 4, 17). The SBBC therefore provides an unprecedented opportunity to study the pathogenesis of nef/LTR-deleted HIV-1 infection in a naturally occurring human setting.
Subjects.
We undertook a longitudinal study of SBBC SP and LTNP. The clinical history of the study subjects from infection to 1998 has been described (17). Subjects C54, C98, C49, C64, and C135 were referred to previously as recipients 13, 7, 12, 9, and 4, respectively (17). Subjects C54 and C98 have since died from causes unrelated to HIV-1 infection. An update on the results of laboratory studies and clinical history of these two subjects and of the surviving SBBC members studied in this report is summarized in Table 1 and detailed below.
TABLE 1.
Subjects, laboratory studies, and clinical history
| Subject, sex, and date of Birtha | Date transfuseda | Date of blood samplea | Yr since infection | CD4+ T-cell count (cells/μl)b | Viral load (RNA copies/ml)c | Antiretroviral drugsd | Clinical historye |
|---|---|---|---|---|---|---|---|
| D36, male, 6/4/1958 | Not applicable; infected with HIV-1 via sexual transmission 12/1980 (18) | 2/1996 | 15.2 | 609 | 1,100 | ABC, AZT, NVP (1/1999-9/2004) (4, 5); ABC, NVP, 3TC (9/2004-present) | SP; diagnosed with HIVD 12/1998 (4) |
| 4/1996 | 15.3 | 504 | 7,800 | ||||
| 10/1997 | 16.8 | 336 | 4,400 | ||||
| 1/1999 | 18.1 | 210 | 9,900 | ||||
| 9/2000 | 19.8 | 391 | BD | ||||
| 4/2001 | 20.3 | 476 | BD | ||||
| 2/2003 | 22.2 | 624 | BD | ||||
| 2/2004 | 23.2 | 638 | BD | ||||
| C49, female, 6/9/1954 | 6/11/1984 | 2/1994 | 9.7 | 1,045 | BD | None | LTNP; diagnosed with age-onset diabetes in 2004, managed by diet; chronic alcoholism |
| 5/1994 | 9.8 | 1,458 | BD | ||||
| 10/1996 | 12.1 | 1,134 | BD | ||||
| 12/1997 | 13.5 | 918 | BD | ||||
| 6/1999 | 15.0 | 605 | BD | ||||
| 11/2001 | 17.4 | 624 | BD | ||||
| 8/2002 | 18.2 | 468 | BD | ||||
| 3/2004 | 19.7 | 874 | BD | ||||
| C54, male, 2/17/1928 | 7/24/1984 | 7/1993 | 9.0 | 1,519 | N/A | None | LTNP; IDDM; surgery for colon cancer in 1995; died 8/28/2001 from myocardial infarct; death not related to HIV-1 |
| 6/1995 | 10.9 | 1,504 | 3,000 | ||||
| 3/1996 | 11.7 | 1,188 | 1,500 | ||||
| 9/1996 | 12.2 | 1,120 | 1,800 | ||||
| 5/1997 | 12.8 | 1,286 | 5,500 | ||||
| 8/1997 | 13.1 | 1,419 | 1,700 | ||||
| 3/2000 | 15.7 | 840 | 1,600 | ||||
| 5/2001 | 16.8 | 1,537 | 2,660 | ||||
| C64, female, 3/20/1926 | 5/4/1983 | 8/1996 | 13.3 | 925 | BD | None | LTNP; hypertension; hypercholesterolemia |
| 2/1997 | 13.8 | 851 | BD | ||||
| 5/1997 | 14.0 | 1,050 | BD | ||||
| 8/1997 | 14.3 | 805 | BD | ||||
| 11/1997 | 14.5 | 936 | BD | ||||
| 4/1999 | 15.9 | 1,026 | BD | ||||
| 11/1999 | 16.5 | 1,332 | BD | ||||
| 5/2000 | 17.0 | 875 | BD | ||||
| C98, male, 7/11/1937 | 1/2/1982 | 7/1993 | 11.5 | 880 | N/A | d4T, NVP, IND (11/1999-death) | SP; prednisone since 1995 for asthma; died 3/30/2001 from bronchial amyloidosis; death not related to HIV-1 |
| 10/1995 | 13.8 | 576 | 670 | ||||
| 11/1996 | 14.8 | 646 | 690 | ||||
| 5/1997 | 15.3 | 527 | 760 | ||||
| 2/1998 | 16.1 | 627 | 1,100 | ||||
| 10/1998 | 16.8 | 429 | 1,500 | ||||
| 3/2000 | 18.2 | 684 | BD | ||||
| 3/2001 | 19.6 | 324 | BD |
Dates shown are month/day/year. The dates refer to times when PBMC were collected for HIV-1 nef/LTR sequencing. The results of only those laboratory studies that correspond to these time points are shown.
CD4+ T-cell levels were measured by flow cytometry.
Plasma HIV-1 RNA was measured using COBAS AMPLICOR HIV-1 monitor version 1.0 (Roche Molecular Diagnostic Systems, Branchburg, N.J.) prior to July 1999 and version 1.5 after July 1999. HIV-1 RNA levels of <400 copies/ml (version 1) or <50 copies/ml (version 1.5) were considered below detection. BD, below detection; N/A, not available.
ABC, abacavir; AZT, zidovudine; NVP, nevirapine; 3TC, lamivudine; d4T, stavudine; IND, indinavir.
HIVD, HIV-associated dementia; IDDM, insulin-dependent diabetes melitis.
SBBC subjects with slowly progressing HIV-1 infection include the donor (D36) and transfusion recipient C98. D36 commenced highly active antiretroviral therapy (HAART) in January 1999 following HIV-associated dementia that coincided with a fall in the CD4 cell count to <200 cells/μl and the presence of high plasma and cerebrospinal fluid (CSF) HIV-1 RNA levels (4, 5). As reported previously, C98 commenced prednisone for treatment of asthma in 1995 (17). C98 was diagnosed with pulmonary amyloidosis in 1998. C98 commenced HAART in November 1999 after experiencing a steady decline in his CD4+ T-cell count and a gradual increase in HIV-1 RNA from below-detectable levels to 1,500 RNA copies/ml. During 2001 his CD4+ T-cell count declined and fluctuated between 213 and 484 cells/μl despite the continuance of HAART and a viral load below detectable levels. He died at the age of 64 in March 2002 of amyloidosis, which was not HIV-1 related.
Nonprogressing SBBC subjects include transfusion recipients C49, C54, C64, and C135; these subjects have experienced steady CD4+ T-cell counts since infection was first identified, with median values of >900, >1,000, >900, and > 500 cells/μl, respectively. HIV-1 RNA levels have remained consistently low or below detectable levels in these subjects despite being infected for up to 20 years without antiretroviral intervention. Patient C54 died aged 73 from a myocardial infarct in September 2001. C135 was not included in the present study because of the lack of consistent amplification of HIV-1 DNA.
Methods.
Peripheral blood mononuclear cells (PBMC) were obtained at each of the times indicated in Table 1 according to guidelines endorsed by the Australian Red Cross Blood Service human ethics committee. The nef/LTR region of the HIV-1 genome was amplified from PBMC by nested PCR, as described previously (4, 20). The products of six independent PCRs were pooled and cloned into pGEM-T-Easy (Promega, Madison, WI), and the nucleotide sequences of multiple independent clones were determined using a SequiTherm EXCEL II DNA sequencing kit (Epicenter Technologies, Madison, WI) and a model 4000L LI-COR DNA sequencer (LI-COR, Lincoln, NE). Nucleotide sequences were aligned and analyzed using DNAMAN software (Lynnon, Quebec, Canada).
Results.
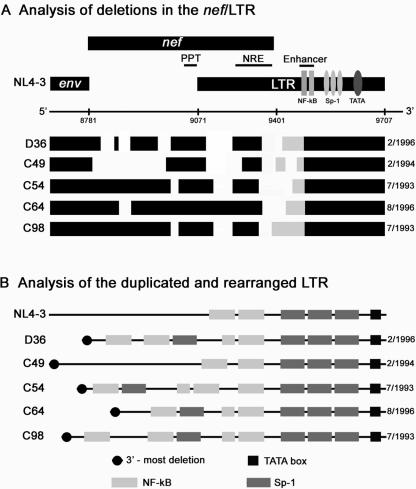
Intersubject nef/LTR sequences cloned from the earliest available PBMC samples were heterogenous but contained a number of common mutations (Fig. 1A): (i) deletions of various lengths in the amino terminus of the nef gene, (ii) at least one deletion in the nef/LTR overlap region, and (iii) duplication and/or rearrangement of nuclear factor-κB (NF-κB) and specificity factor 1 (Sp-1) binding sites in the LTR (Fig. 1B). The 3′-most deletion in the nef/LTR overlap region shared by all viruses is thought to have been present in the transmitted virus, as none of the subjects has antibodies to a peptide of the corresponding region in the Nef protein (9). None of the viruses are capable of encoding Nef, carrying either an in-phase termination codon (D36) or lacking the nef ATG. The duplicated or rearranged region of the LTR was unique to each of the cohort members and varied with respect to the number and arrangement of NF-κB and Sp-1 sites inserted (Fig. 1). Thus, despite the common origin of the viruses, the nef/LTR regions differed from subject to subject.
FIG. 1.
Analysis of SBBC nef/LTR sequence. (A) Schematic representation of nef/LTR sequence deletions of HIV-1 cloned from the earliest available PBMC samples. The data shown represent a consensus of at least 20 independent nef/LTR sequences cloned from each PBMC sample. The genomic structures are compared to wild-type HIV-1 (NL4-3). Numbers refer to nucleotide positions in NL4-3. Black blocks represent intact sequence, and gaps represent deletions. Gray blocks represent the sequence area containing duplicated and rearranged NF-κB and Sp-1 binding sites in the LTR. The dates shown represent the times when PBMC were collected for analysis. (B) More-detailed analysis of the LTR, depicting transcription factor binding sites. PPT, polypurine tract; NRE, negative regulatory element.
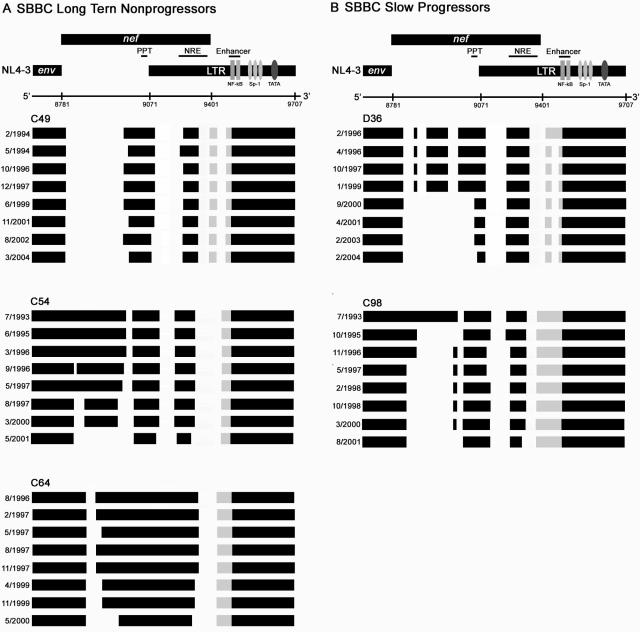
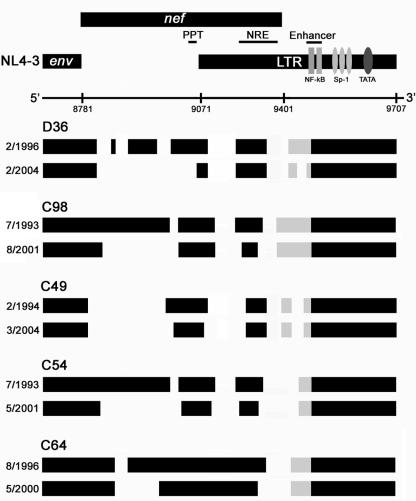
Sequential analysis of nef/LTR sequences spanning a 4- to 10-year period demonstrated a further loss of nef sequence that differed in magnitude between subjects (Fig. 2). A large deletion of 128 bp emerged in D36, effectively removing the entire nef gene with the exception of the region surrounding the nef start codon, the polypurine tract which contains terminal signals for HIV-1 integration, and a 90-bp region of the nef/LTR overlap region surrounding the negative regulatory element. The basal promoter and enhancer elements of the LTR were retained, but a 44-bp deletion appeared in the rearranged or duplicated NF-κB/Sp-1 motif. The pattern of nef sequence loss in C98 was remarkably similar to that which occurred in D36. The pattern of nef sequence loss in C54 was also similar but was less extensive than that observed in D36 and C98. However, the additional loss of nef sequence in C64 was comparatively minimal. Thus, viruses harbored by D36, C54, C98, and C64 appear to be evolving in a convergent fashion toward a highly deleted, minimal nef/LTR structure containing only sequence elements that are absolutely essential for HIV-1 replication. The convergent nature of the nef/LTR evolution is further illustrated in Fig. 3, where sequences from the earliest and most recent nef/LTR clones from each subject are compared. The convergent nature of the nef/LTR sequence changes implies the presence of strong selection pressures that maintain the ability of defective HIV-1 genomes to persist in vivo.
FIG. 2.
Evolution of SBBC nef/LTR sequence deletions. Schematic representation of nef/LTR sequence deletions of HIV-1 cloned from longitudinally collected PBMC samples from SBBC long-term nonprogressors (A) and slow progressors (B). The data shown represent a consensus of at least 20 independent nef/LTR sequences cloned from each PBMC sample. The genomic structures are compared to wild-type HIV-1 (NL4-3). Numbers refer to nucleotide positions in NL4-3. Black blocks represent intact sequence, and gaps represent deletions. Gray blocks represent the sequence area containing duplicated and rearranged NF-κB and Sp-1 binding sites in the LTR. The dates shown represent the times when PBMC were collected for analysis. PPT, polypurine tract; NRE, negative regulatory element.
FIG. 3.
Convergent evolution of SBBC nef/LTR sequences to a minimal nef/LTR structure. Comparisons of the genomic structures of nef/LTR sequences cloned from the earliest available and most recently obtained PBMC samples are shown. The genomic structures are compared to those of wild-type HIV-1 (NL4-3). Numbers refer to nucleotide positions in NL4-3. Black blocks represent intact sequence, and gaps represent deletions. Gray blocks represent the sequence area containing duplicated and rearranged NF-κB and Sp-1 binding sites in the LTR. The dates shown represent the times when PBMC were collected for analysis. PPT, polypurine tract; NRE, negative regulatory element.
The highly evolved nef/LTR sequences harbored by D36, C54, and C98 are strikingly similar to those that have remained dominant in C49 for at least 10 years (Fig. 2). The presence of Nef antibodies directed against peptides spanning the entire Nef protein (with the exception of the common, 3′-most deletion in the nef/LTR overlap region) in C49 (9) suggests that prior to 1994 a near-complete nef sequence existed and that the bulk of nef/LTR evolution occurred during the first 10 years after infection. C49 has had below-detectable HIV-1 RNA levels since February 1994 (Table 1) and persistent HIV-1 cytotoxic T-cell responses since monitoring of the SBBC began in 1992 (2, 8, 17). Thus, our findings suggest that the highly evolved nef/LTR structure is stable and, in the case of C49, does not increase pathogenicity.
Discussion.
In this study, we demonstrated a progressive loss of nef/LTR sequence in an epidemiologically-linked cohort of long-term survivors who have been infected with attenuated HIV-1 for up to 25 years. The intersubject evolution of nef/LTR sequence loss appears convergent, tending toward retention of only those sequence elements that are absolutely required for HIV-1 replication. The highly evolved, minimal nef/LTR structure appears stable, being present as the dominant strain in C49 for at least 10 years. Its persistence in this subject, who has consistently had a below-detectable viral load in the absence of HAART (Table 1), initially suggested that evolution to the minimal nef/LTR sequence may result in a further-attenuated, even less-pathogenic virus. However, we found no association between evolution toward the minimal nef/LTR sequence and the clinical status of the subject. For example, both progressing (D36, C98) and nonprogressing (C54, C49) subjects harbored highly evolved forms of nef/LTR, and a nonprogressing subject (C64) harbored a significantly less evolved nef/LTR. Thus, other viral and/or host factors are likely to contribute to modulating the in vivo pathogenicity of HIV-1 strains with nef/LTR deleted.
Reversion to pathogenicity by SIV with nef deleted has been associated with restoration of a truncated Nef protein (21), acquisition of further deletions in the nef/LTR overlap region (1), and/or duplications of NF-κB binding sites in the LTR (1). In contrast to the SIV studies, the in vivo evolution of HIV-1 with nef/LTR was unidirectional toward a smaller nef/LTR sequence and the majority of the additional sequence loss was within the nef-alone region. Furthermore, none of the clones were capable of encoding Nef. In addition, the presence of duplicated NF-κB binding sites in the LTR was not associated with the clinical status of the SBBC subjects. Therefore, it is likely that any viral factors that modulate the in vivo pathogenicity of HIV-1 with nef/LTR deleted will be distinct from those in SIV with nef deleted. Interestingly, the unidirectional evolution toward the minimal nef/LTR sequence observed in SBBC members is strikingly similar to the pattern of evolution in a slow progressor infected with a nef/LTR deletion variant of HIV-1 circulating recombinant form 01_AE (15). The convergent pattern of nef/LTR evolution among viruses harbored by SBBC members is therefore unlikely to be due to a unique property of the infecting strain but rather likely reflects an intrinsic instability of HIV-1 with nef/LTR defects that is common across clades.
The molecular mechanisms underlying the increased or acquired pathogenicity of nef/LTR-deleted HIV-1 harbored by slow progressors D36 and C98 remain to be determined, but possibilities include changes in LTR or Env function that may contribute to increasing replicative capacity or cytopathicity, respectively. In support of this hypothesis, enhanced transcriptional activity of LTR clones isolated from CSF of D36 was shown to contribute to high CSF HIV-1 RNA levels and the development of HIV-associated dementia (4). Moreover, enhanced coreceptor usage by HIV-1 isolated from D36 when CD4+ cell counts fell below 200/μl contributed to enhanced cell killing in ex vivo human tissue cultures (12). Further studies on the function of sequential LTR and Env clones from SBBC subjects are in progress to elucidate their role in the pathogenesis of nef/LTR-deleted HIV-1 infection.
In conclusion, while our studies affirm that nef is important for HIV-1 pathogenesis, convergent evolution of HIV-1 in vivo toward a minimal and apparently stable nef/LTR structure via extensive loss of nef sequence was not associated with HIV-1 progression. Factors other than nef sequence therefore contribute significantly to HIV-1 progression in the SBBC of long-term survivors.
Nucleotide sequence accession numbers.
The nef/LTR nucleotide sequences reported here have been assigned GenBank accession numbers DQ287272 to DQ287311.
Acknowledgments
We thank Dale McPhee for helpful comments.
This study was supported by grants from the Australian National Center for HIV Virology Research to M.J.C. and N.J.D. and grants from the Australian National Health and Medical Research Council (NHMRC) (251520) and National Institutes of Health and the National Institute of Allergy and Infectious Diseases (1-R21-AI054207-01-A1) to P.R.G. P.R.G. is a recipient of an NHMRC R. Douglas Wright Biomedical Career Development Award.
REFERENCES
- 1.Alexander, L., P. O. Illyinskii, S. M. Lang, R. E. Means, J. Lifson, K. Mansfield, and R. C. Desrosiers. 2003. Determinants of increased replicative capacity of serially passaged simian immunodeficiency virus with nef deleted in rhesus monkeys. J. Virol. 77:6823-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birch, M. R., J. C. Learmont, W. B. Dyer, N. J. Deacon, J. J. Zaunders, N. Saksena, A. L. Cunningham, J. Mills, and J. S. Sullivan. 2001. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC). J. Clin. Virol. 22:263-270. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti, L., V. Baptiste, E. Khatissian, M. C. Cumont, A. M. Aubertin, L. Montagnier, and B. Hurtrel. 1995. Limited viral spread and rapid immune response in lymph nodes of macaques inoculated with attenuated simian immunodeficiency virus. Virology 213:535-548. [DOI] [PubMed] [Google Scholar]
- 4.Churchill, M., J. Sterjovski, L. Gray, D. Cowley, C. Chatfield, J. Learmont, J. S. Sullivan, S. M. Crowe, J. Mills, B. J. Brew, S. L. Wesselingh, D. A. McPhee, and P. R. Gorry. 2004. Longitudinal analysis of nef/long terminal repeat-deleted HIV-1 in blood and cerebrospinal fluid of a long-term survivor who developed HIV-associated dementia. J. Infect. Dis. 190:2181-2186. [DOI] [PubMed] [Google Scholar]
- 5.Crowe, S. M., D. D. Ho, D. Marriott, B. Brew, P. R. Gorry, J. S. Sullivan, J. Learmont, and J. Mills. 2005. In vivo replication kinetics of a nef-deleted strain of HIV-1. AIDS 19:842-843. [DOI] [PubMed] [Google Scholar]
- 6.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 7.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyer, W. B., G. S. Ogg, M.-A. Demoitie, X. Jin, A. F. Geczy, S. L. Rowland-Jones, A. J. McMichael, D. F. Nixon, and J. S. Sullivan. 1999. Strong human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte activity in Sydney Blood Bank Cohort patients infected with nef-defective HIV type 1. J. Virol. 73:436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenway, A. L., J. Mills, D. Rhodes, N. J. Deacon, and D. A. McPhee. 1998. Serological detection of attenuated HIV-1 variants with nef gene deletions. AIDS 12:555-561. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann-Lehmann, R., J. Vlasak, A. L. Williams, A. L. Chenine, H. M. McClure, D. C. Anderson, S. O'Neil, and R. M. Ruprecht. 2003. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS 17:157-166. [DOI] [PubMed] [Google Scholar]
- 11.Iafrate, A. J., S. Carl, S. Bronson, C. Stahl-Hennig, T. Swigut, J. Skowronski, and F. Kirchhoff. 2000. Disrupting surfaces of nef required for downregulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J. Virol. 74:9836-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jekle, A., B. Schramm, P. Jayakumar, V. Trautner, D. Schols, E. De Clercq, J. Mills, S. M. Crowe, and M. A. Goldsmith. 2002. Coreceptor phenotype of natural human immunodeficiency virus with nef deleted evolves in vivo, leading to increased virulence. J. Virol. 76:6966-6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 15.Kondo, M., T. Shima, M. Nishizawa, K. Sudo, S. Iwamuro, T. Okabe, Y. Takebe, and M. Imai. 2005. Identification of attenuated variants of HIV-1 circulating recombinant form 01_AE that are associated with slow disease progression due to gross genetic alterations in the nef/long terminal repeat sequences. J. Infect. Dis. 192:56-61. [DOI] [PubMed] [Google Scholar]
- 16.Learmont, J., B. Tindall, L. Evans, A. Cunningham, P. Cunningham, J. Wells, R. Penny, J. Kaldor, and D. A. Cooper. 1992. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet 340:863-867. [DOI] [PubMed] [Google Scholar]
- 17.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 18.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messmer, D., R. Ignatius, C. Santisteban, R. M. Steinman, and M. Pope. 2000. The decreased replicative capacity of simian immunodeficiency virus SIVmac239Δnef is manifest in cultures of immature dendritic cells and T cells. J. Virol. 74:2406-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhodes, D. I., L. Ashton, A. Solomon, A. Carr, D. Cooper, J. Kaldor, and N. Deacon for the Australian Long-Term Nonprogressor Study Group. 2000. Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. J. Virol. 74:10581-10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawai, E. T., M. S. Hamza, M. Ye, K. E. Shaw, and P. A. Luciw. 2000. Pathogenic conversion of live attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J. Virol. 74:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]