Abstract
The distinct expression of R-cadherin in the induced aggregating metanephric mesenchyme suggests that it may regulate the mesenchymal-epithelial transition during kidney development. To address whether R-cadherin is required for kidney ontogeny, R-cadherin-deficient mice were generated. These mice appeared to be healthy and were fertile, demonstrating that R-cadherin is not essential for embryogenesis. The only kidney phenotype of adult mutant animals was the appearance of dilated proximal tubules, which was associated with an accumulation of large intracellular vacuoles. Morphological analysis of nephrogenesis in R-cadherin−/− mice in vivo and in vitro revealed defects in the development of both ureteric bud-derived cells and metanephric mesenchyme-derived cells. First, the morphology and organization of the proximal parts of the ureteric bud epithelium were altered. Interestingly, these morphological changes correlated with an increased rate of apoptosis and were further supported by perturbed branching and patterning of the ureteric bud epithelium during in vitro differentiation. Second, during in vitro studies of mesenchymal-epithelial conversion, significantly fewer epithelial structures developed from R-cadherin−/− kidneys than from wild-type kidneys. These data suggest that R-cadherin is functionally involved in the differentiation of both mesenchymal and epithelial components during metanephric kidney development. Finally, to investigate whether the redundant expression of other classic cadherins expressed in the kidney could explain the rather mild kidney defects in R-cadherin-deficient mice, we intercrossed R-cadherin−/− mice with cadherin-6−/−, P-cadherin−/−, and N-cadherin+/− mice. Surprisingly, however, in none of the compound knockout strains was kidney development affected to a greater extent than within the individual cadherin knockout strains.
Organogenesis involves the integration of a complex series of cellular events, including differentiation, proliferation, apoptosis, epithelial-mesenchymal interactions, cell migration, cell sorting, cell shape changes, and cell aggregation. The final outcome is a functional three-dimensional organization of cells into an organ. Differential cellular adhesion mediated by cell adhesion molecules is crucial for many of these processes (8).
A common theme during the formation of many organs is the requirement for epithelial-mesenchymal interactions. Much of the information regarding the molecular mechanisms for these processes has been derived from studies of the metanephric kidney. Therefore, the kidney is a general model for organogenesis. The metanephric kidney develops from reciprocal inductive events between the metanephric mesenchyme and the ureteric bud epithelium (9, 25, 40). Signals from the metanephric mesenchyme lead to branching morphogenesis of the ureteric epithelium, whereas signals emanating from the ureteric epithelium induce the aggregation of mesenchymal cells and their subsequent differentiation into an epithelium which forms the functional unit within the kidney, the nephron. This process is then repeated throughout organogenesis. The fact that kidney development involves several morphogenetic processes (e.g., cell aggregation, cell movement, mesenchymal-epithelial conversion, cell sorting, and cell shape changes) that members of the classic cadherin superfamily of cell adhesion molecules have been implicated in validates studies of cadherin function during kidney organogenesis.
The classic cadherins contain a highly conserved cytoplasmic domain that interacts with a family of cytoplasmic proteins called catenins: α-catenin, β-catenin, plakoglobin, and p120 (30, 31, 36, 41). The formation of the cadherin-catenin complex is required for cadherin-mediated cell adhesion, and it is thought that α-catenin, which binds to the cadherin-β-catenin or to the cadherin-plakoglobin complex, mediates binding to the actin cytoskeleton (1, 4, 14). Cadherins cluster in cell-cell contact regions called adherens junctions. These subcellular structures are important cell-cell signaling centers within cells, and cadherins are crucial for their establishment and maintenance (3, 12, 49).
Both reverse and gain-of-function genetics in invertebrates and vertebrates have shown that cadherins regulate morphogenesis of tissues and organs during embryogenesis (6, 13, 24, 34, 35, 37, 46). Furthermore, recent data strongly suggest that cadherins are involved in histogenesis (23, 38, 49); thus, in addition to their role in cell adhesion, cadherins participate more directly in a signaling pathway.
Several cadherins are expressed during nephrogenesis: E-cadherin (5, 11, 38, 48), N-cadherin (11, 22), R-cadherin (11, 22, 38), K-cadherin (cadherin-6) (5, 32, 50), cadherin-11 (5, 15, 21), and P-cadherin (11, 45). Their temporally and spatially regulated expression patterns during kidney ontogeny have implicated them in both the initial aggregation of mesenchymal cells and the complex morphogenetic events leading to formation of the nephrons. In vitro studies of tubulogenesis in the presence of functional blocking antibodies against cadherin-6 and E-cadherin demonstrated that cadherin-6 (5) is involved in the conversion of the mesenchymal cells into an epithelium but E-cadherin is not (48). Of the classic cadherin genes that have been inactivated by gene targeting (E-cadherin [24, 37], N-cadherin [35], P-cadherin [34], cadherin-11 [16], and cadherin-6 [26]), only cadherin-6 exhibits a kidney phenotype (26). Targeted inactivation of cadherin-6 results in a delay in the conversion of the induced mesenchyme into a polarized epithelium, and as a consequence, fusion of the comma-shaped body to the ureteric bud epithelium is affected (26). The functional role of E-cadherin and N-cadherin during kidney ontogeny cannot be determined, because E-cadherin and N-cadherin mutant mice die in utero prior to kidney development.
R-cadherin is a classic cadherin that has been identified in chickens, mice, rats, and humans (17, 18, 28, 44). It is expressed in restricted areas of the developing nervous system, somitic myotome, skeletal muscle, smooth muscle, thymus, and kidney (11, 18, 29, 38). Remarkably, the expression of R-cadherin in embryonic stem (ES) cells lacking E-cadherin can rescue striated muscle and epithelia (38), implicating R-cadherin in the formation of these tissues.
In the metanephric kidney, R-cadherin has been reported to be transiently expressed in the aggregating mesenchymal cells, renal vesicle, comma- and S-shaped bodies, maturing podocytes, and collecting ducts (11, 38). Furthermore, the subtle effect of cadherin-6 deletions on mesenchyme-to-epithelial conversion and the overlapping expression patterns of cadherin-6 and R-cadherin during this event imply that R-cadherin functionally compensates for the lack of cadherin-6 (26). We created an R-cadherin null mutation by homologous recombination in ES cells to study the role of R-cadherin during kidney development. Because R-cadherin-deficient mice are viable and fertile, the protein is not essential for embryogenesis. A subtle proximal tubule defect was the only phenotype observed in the adult kidney in R-cadherin-deficient mice. However, several embryonic phenotypes, including defective mesenchyme-to-epithelial conversion and branching and patterning of ureteric bud epithelium, indicate that R-cadherin is required for normal nephrogenesis. To determine whether redundant cadherin function can explain the subtle phenotype of kidney development in R-cadherin mutant mice, we intercrossed these mice with cadherin-6−/−, P-cadherin−/−, and N-cadherin+/− strains. However, no further kidney phenotypes were observed in the compound knockout mice compared to the individual cadherin knockout strains.
MATERIALS AND METHODS
Targeted disruption of R-cadherin.
Genomic DNA corresponding to the R-cadherin locus was isolated from a mouse 129/Sv genomic library by using a 5" region of mouse R-cadherin cDNA as a probe (43). A restriction map was established, and exon-intron boundaries were determined by sequence analysis. A 3.5-kb XhoI-EcoRI fragment including exon 1 and exon 2 was replaced by a neomycin (neo) expression cassette, pMC1neo (47), in the same transcriptional orientation. A herpes simplex virus thymidine kinase gene cassette (HSV-TK) was inserted at the 3" end of the construct (27). The linearized targeting construct was electroporated into R1 ES cells, and transformants were selected on G418 and ganciclovir. Southern blot analysis, using a 0.7-kb NdeI-BamHI genomic DNA fragment located 5" of the short arm of the targeting vector as a probe (Fig. 2A), was used to screen for homologous recombination events at the R-cadherin locus. Over 1,200 G418-resistant clones were screened, and only one ES clone with a mutated allele was found. This clone was injected into C57BL/6J recipient blastocysts and transferred into the uteri of pseudopregnant females to generate chimeric mice. Germ line chimeras were crossed with 129/Sv or C57BL/6J females to insert the mutation into different genetic backgrounds. The F1 heterozygous mice carrying the R-cadherin mutation were identified by Southern blot analysis and intercrossed to produce F2 homozygous offspring. The F2 mice were genotyped by PCR with DNA from tail biopsies. For genotyping, an internal primer for the R-cadherin gene (primer 1, 5"-AAC CCA GTC TCA TAC CTC TGG A-3") and a primer specific for the neo gene (primer 3, 5"-GTC GGT CTT GAC AAA AAG AAC C-3") were used to identify the mutated allele. Primer 1 and a primer specific for the R-cadherin gene 3" of the neo insertion site (primer 2, 5"-GAG TGA ATT AAG TTG CCC CAA G-3") were used to identify the wild-type allele. Samples were amplified for 35 cycles at 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min. For the genotyping of N-cadherin, cadherin-11, cadherin-6, and P-cadherin mutant mice, the primers and PCR conditions were as previously described (16, 24, 26, 34, 35, 37).
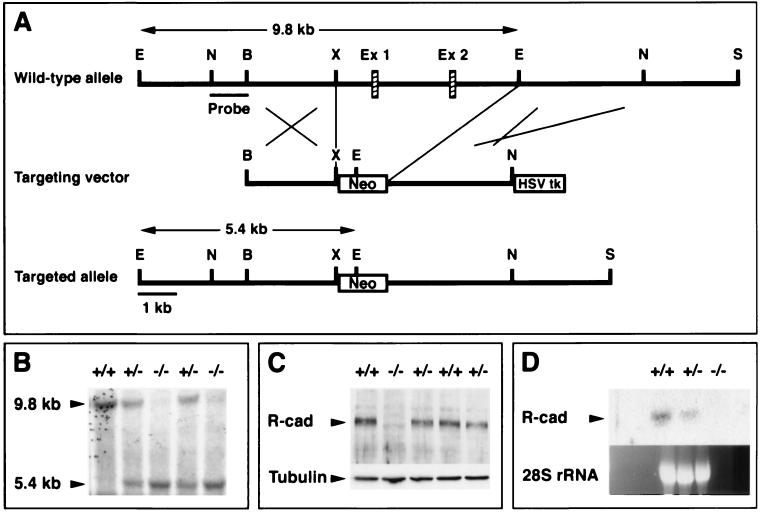
FIG. 2.
Inactivation of the R-cadherin gene. (A) Both the wild-type and the mutant alleles are shown. Exon 1, which includes the ATG, and exon 2 were replaced by pMC1neo. The 3" end of the targeting construct was flanked by HSV-TK. E, EcoRI; N, NdeI; B, BamHI; X, XhoI; Ex 1, exon 1; Ex 2, exon 2; S, SalI. (B) Southern blotting was used to characterize the R-cadherin mutant allele in mice. (C) Immunoblotting analysis of 13.5-dpc homozygous null brain extracts revealed no R-cadherin protein. The same blot was exposed to anti-R-cadherin (R-cad) and anti-α-tubulin (Tubulin) MAbs. (D) No R-cadherin mRNA (R-cad) could be detected by Northern blot analysis of 18.5-dpc homozygous kidney total RNA. The probe encompasses cDNA sequences encoding the complete cytoplasmic tail of R-cadherin. Similarly, R-cadherin mRNA was not detected when probes from the middle and 5" regions of the cDNA were used (data not shown).
Morphological and histological analysis.
For the histological analysis, adult mice were perfused for vascular fixation with 4% paraformaldehyde (PFA) in 0.1 M sodium phosphate buffer, pH 7.4. Kidneys were removed and postfixed in 4% buffered PFA at 4°C overnight, washed in phosphate-buffered saline (PBS) overnight at 4°C, dehydrated in graded alcohols, and embedded in paraffin and Epon 812. Six-micrometer (paraffin) and 2-μm (Epon 812) sections were stained with hematoxylin-eosin and by the periodic acid-Schiff reaction (PAS) and photographed under a Zeiss Axioplan light microscope. Embryonic kidneys were immersion fixed. To avoid misinterpretation of fixation artifacts, the same numbers of R-cadherin−/− and control mice from the same litter were perfused in each experiment.
Organ culture.
Kidneys were dissected from 12.5-day-postcoitum (dpc) mouse embryos in Dulbecco's phosphate-buffered saline. Kidney rudiments were cultured on Transwell filter inserts (Costar; pore size = 3.0 μm) in Dulbecco's modified Eagle’s medium supplemented with 10% fetal calf serum, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. After 48 h in culture, the numbers of ureteric bud tips and laminin-positive comma- and S-shaped structures were counted. The ratio of laminin-positive structures to bud tips was calculated. The unpaired two-tailed t test was used to compare results.
Immunoreagents.
Rat monoclonal antibodies directed against E-cadherin (ECCD-2) (42), R-cadherin (MRCD-5), N-cadherin (MNCD-2) (29), and P-cadherin (PCD-1) (Zymed Labs) were used. Rabbit polyclonal antibodies directed against cadherin-6, laminin, and WT1 were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Additional rabbit polyclonal antibodies directed against cadherin-6 were generously supplied by R. Mège, Paris, France. Rabbit polyclonal antibodies directed against PAX-2 were purchased from Zymed Labs. Cy3-coupled goat anti-rabbit, biotin-coupled anti-rat, and anti-rabbit secondary antisera were purchased from Jackson ImmunoResearch Laboratories Inc. and used at 1:500, 1:300, and 1:500 dilutions, respectively. Cy3-streptavidin was purchased from Jackson ImmunoResearch Laboratories Inc. and used at a 1:500 dilution. The Vectastain ABC kit was purchased from Vector Laboratories, Inc. (La Jolla, Calif.).
Immunohistochemistry.
For immunohistochemistry, tissues were fixed, sectioned, and stained as described previously (10). LTA lectin (Sigma) binding was used to visualize proximal tubules (5). For whole-mount immunohistochemistry of organ cultures, kidney explants were fixed in 100% methanol for 10 min and washed twice in PBS-0.1% Tween 20 (PBST) for 20 min. Explants were incubated overnight with primary antibodies at 4°C and washed repeatedly with PBST for 8 h at 4°C. They were then incubated with secondary antibodies overnight at 4°C and washed repeatedly with PBST for 8 h. Whole-mount explants were placed under coverslips in fluorescent mounting medium (DAKO) and examined by microscopy.
TUNEL assay.
The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed using terminal transferase (Boehringer) and biotin-16-dUTP (Boehringer). Sections were postfixed in 4% PFA-PBS for 15 min at room temperature, washed 3 times for 5 min in sterile PBS, and incubated in 100 μl of prereaction mix (1× terminal transferase buffer, 1.5 mM CoCl2) per slide for 10 min at room temperature. Sections were then incubated for 1 h at 37°C in a humidified chamber in 100 μl of reaction mix (prereaction mix supplied with 50 U of terminal transferase and 4 μM biotin-16-dUTP) covered by Parafilm. Following 3 washes at 5 min each in PBS and 1 h of incubation at room temperature in 10% fetal calf serum, sections were visualized with Cy-conjugated streptavidin as described above. For double immunofluorescence, the additional staining was performed as above according to the TUNEL assay.
Northern blot and RNA preparation.
Total RNA was prepared according to a protocol supplied by the manufacturer (RNeasy; QIAGEN). RNA (20 μg per lane) was separated on a 1% agarose-formaldehyde gel and then blotted onto a GeneScreen Plus membrane (NEN Life Science Products), hybridized at 62°C in QuickHyb (Stratagene), and washed at high stringency (0.1% sodium dodecyl sulfate, 0.1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) at 62°C. The cDNA probe used corresponded to the whole intracellular domain of R-cadherin (nucleotides 2314-2793 [28]).
Western immunoblotting.
Immunoblotting samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose filters (Bio-Rad). Proteins were blocked overnight and incubated in the primary antibody with TBST-Ca2+ (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM CaCl2, 0.1% Tween 20). The filters were then incubated with a conjugated secondary antibody and visualized by chemoluminescence by use of the ECL detection kit (Amersham).
Electron microscopy.
For transmission electron microscopy, the specimens were fixed overnight in 2.5% glutaraldehyde in 0.1 M phosphate buffer at 4°C, then rinsed in phosphate buffer, postfixed in 1% OsO4 in 0.1 M phosphate buffer for 2 h, dehydrated in graded alcohol solutions, and embedded in Poly/Bed (Polyscience Inc., Warrington, Pa.). Sections were cut on an LKB Ultratome. The sections were placed onto gold grids, stained with uranyl acetate and lead citrate, and examined under a JEOL 100 CX electron microscope.
Measurement of renal function.
Relative kidney function was estimated from the plasma clearance of [51Cr]EDTA. Intraperitoneal injections of [51Cr]EDTA were given at a dose of 0.3 MBq in 0.2 ml per mouse (+/+, n = 6; −/−, n = 5). Blood samples were taken 30, 60, and 90 min after injection. Results are expressed as residual activity (percentage of the injected dose) per ml of plasma. This provides a quantitative measure of the functional status of the kidney and is related to the glomerular filtration rate (GFR) (in milliliters of plasma/minute), although GFR cannot be directly estimated from these data. To further assess kidney function, glucose, protein, creatinine, and urea were measured in the blood and urine of wild-type and null mutant animals that had been deprived of food overnight. Statistic analyses were performed using the two-tailed t test.
RESULTS
Expression of R-cadherin in the developing metanephric kidney.
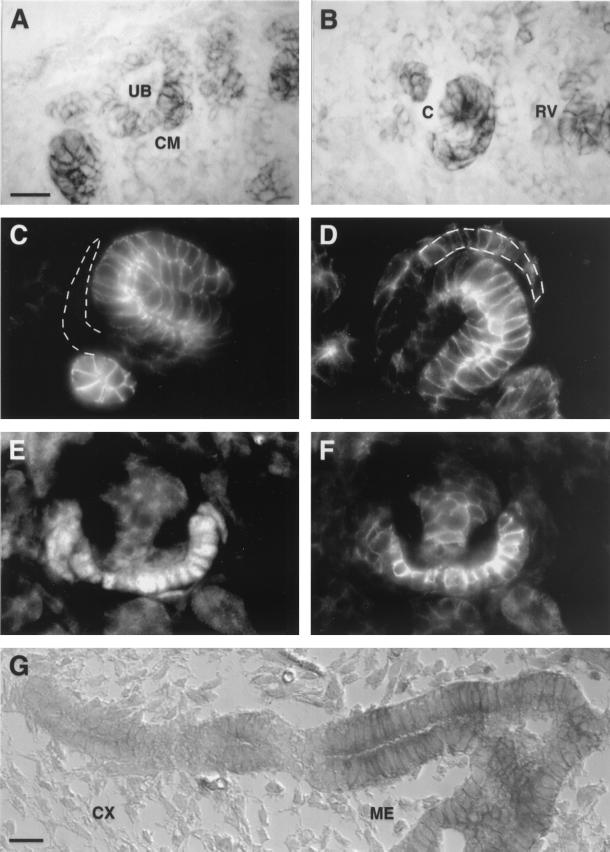
Although R-cadherin has previously been reported to be expressed in the kidney (11, 38), we characterized this in more detail in this study. R-cadherin was initially expressed at low levels in the aggregating mesenchymal cells surrounding the epithelial cells of the ureteric bud tips (Fig. 1A) but not in the ureteric epithelium (Fig. 1A). R-cadherin protein levels gradually increased during the subsequent maturation stages, i.e., in the renal vesicle and comma-shaped body (Fig. 1B). Interestingly, R-cadherin expression was patterned in the S-shaped body, suggesting its involvement in the formation of specific parts of the tubule system. Whereas the distal part of the S-shaped body forms the distal tubule, the proximal part gives rise to the proximal tubule and the glomerulus. E-cadherin serves as a marker for the putative distal tubule cells, because it is expressed in the distal but not proximal tubule epithelial cells (Fig. 1C) (5, 11, 38, 48). WT1 is specifically expressed in the developing podocytes and therefore serves as a marker for these cells (Fig. 1E) (2, 33). By using these markers, we determined that expression of R-cadherin in the S-shaped body is patterned, with the highest levels seen in the E-cadherin-expressing cells in the distal part and in the most proximal part, including the glomerular cleft (Fig. 1D). In the glomerular cleft, R-cadherin is specifically expressed in the developing podocytes but not in the neighboring cells, which become Bowman's capsule cells (Fig. 1D). Whereas R-cadherin was expressed in maturing podocytes (Fig. 1F), it was undetectable in mature glomeruli (data not shown). Although R-cadherin was not expressed in the distal parts of the ureteric bud epithelium, it was transiently expressed in the proximal parts of the ureteric bud epithelium (Fig. 1G). In summary, the expression pattern of R-cadherin during nephrogenesis suggests that it is involved in glomerulogenesis and in the formation of the tubule system.
FIG. 1.
R-cadherin expression during nephrogenesis. (A and B) Immunoperoxidase detection of R-cadherin on sagittal sections of 17.5-dpc kidneys with anti-R-cadherin monoclonal antibody (MAb). R-cadherin is expressed in the condensing mesenchyme (CM), renal vesicle (RV), and comma-shaped body (C) but not in the ureteric bud tips (UB). (C and D) Immunofluorescence staining of sagittal sections of 17.5-dpc kidney with anti-E-cadherin (C) and anti-R-cadherin (D) MAbs. E-cadherin and R-cadherin are coexpressed in the distal part of the S-shaped body. R-cadherin, but not E-cadherin, is also expressed in the most proximal part, specifically in podocyte progenitors within the glomerular cleft (indicated by a broken line). (E and F) Double immunofluorescence stainings of sagittal sections of 17.5-dpc kidney with anti-WT1 polyclonal antibody (PAb) (E) and anti-R-cadherin MAb (F). R-cadherin is expressed in podocytes of immature glomeruli. (G) Immunoperoxidase detection of R-cadherin on sagittal sections of 15.5-dpc kidney with anti-R-cadherin MAb. R-cadherin is expressed in the proximal parts of the ureteric bud epithelium which lie within the medulla (ME), whereas distal parts within the cortex (CX) lack R-cadherin. Bars, 20 μm (A and G) (bar length in panel A also applies to panels B to F).
R-cadherin-deficient mice are viable and fertile.
R-cadherin-deficient mice were created to study R-cadherin function during organogenesis of the kidney. To inactivate the R-cadherin gene, the first two exons, including the ATG and signal peptide and part of the propeptide, were replaced with the neomycin gene under the control of the TK promoter by homologous recombination in ES cells (Fig. 2A). This mutation was carried through the germ line of chimeric mice from one ES clone (Fig. 2B). Neither R-cadherin mRNA nor protein was detected in homozygous null mutant mice, confirming that we have indeed created a null mutation in the R-cadherin gene (Fig. 2C and D). Analysis of the genotypes of newborn litters from heterozygous intercrosses showed that mice with the different genotypes were born with the expected Mendelian frequencies (data not shown), demonstrating that R-cadherin is not essential for embryogenesis. This result was the same after four generations of 129/Sv and C57BL/6J backcrossing, suggesting that the result is independent of the genetic background.
Proximal tubule morphology but not kidney function is affected in R-cadherin-deficient mice.
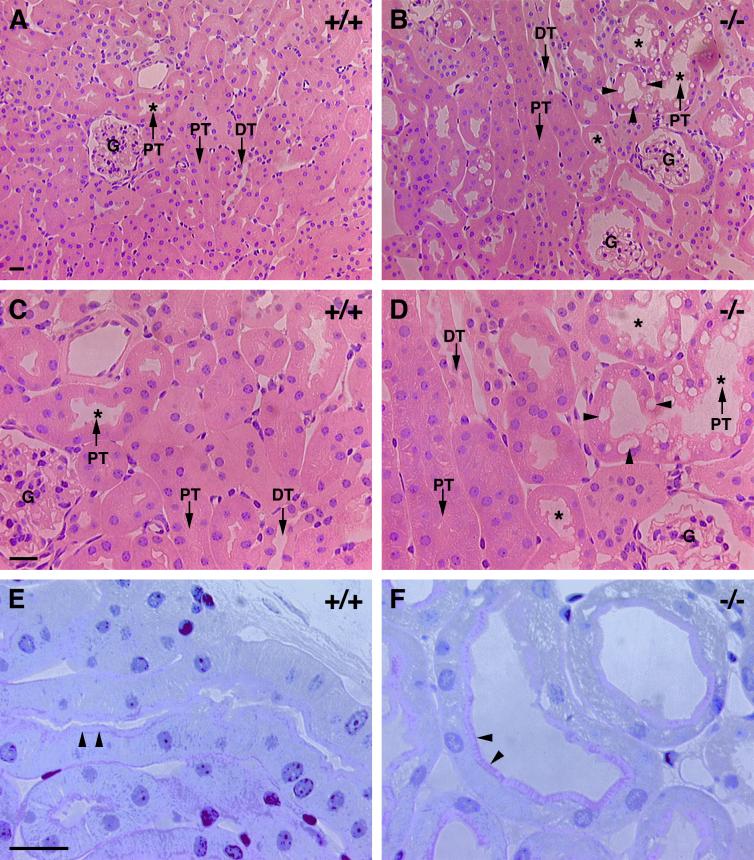
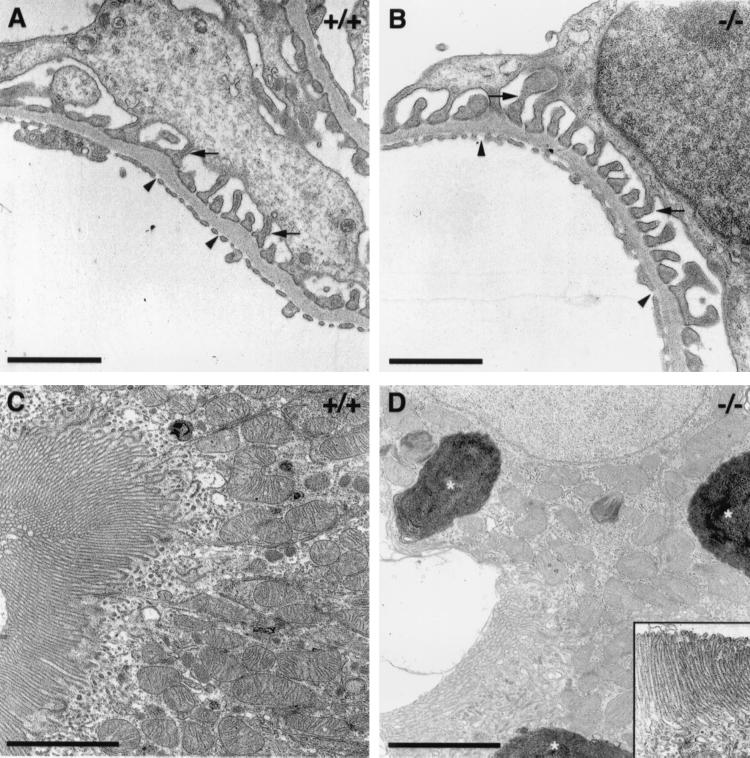
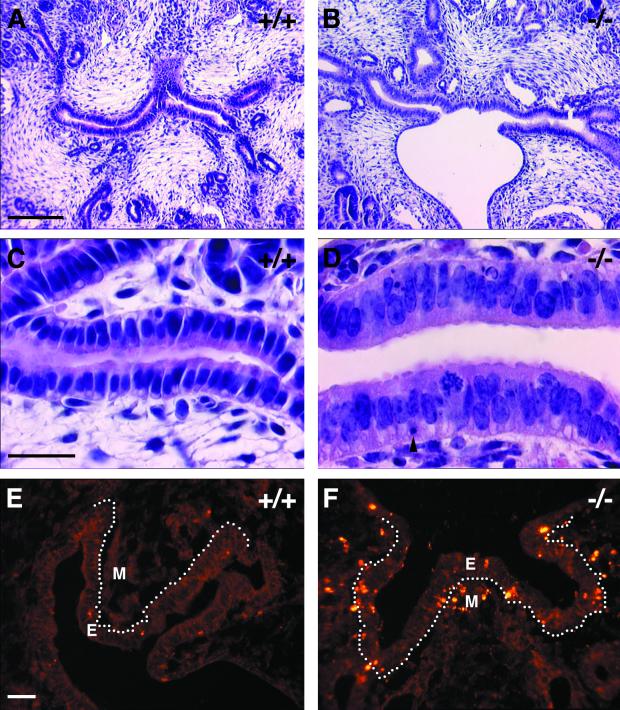
Neither the external morphology of adult kidneys, as judged by color, shape, size, and weight, nor the gross anatomy and organization and number of nephrons appeared to be affected in the R-cadherin−/− mice (data not shown). However, analysis of perfusion-fixed kidneys from adult mice revealed that certain changes of the proximal tubule system occurred reproducibly in mutant animals. All subsequent morphological analyses presented here were performed on ten wild-type and twelve mutant mice from the same litters. First, the diameter of the lumina of most of the proximal tubules (more then 50%) increased in all R-cadherin-deficient mice, whereas the phenotype was observed in less than 10% of the proximal tubule in one control (Fig. 3). PAS staining together with ultrastructural analysis demonstrated that the brush border was intact in mutant proximal tubules (Fig. 3E and F and Fig. 4C and D). Second, in certain regions of the mutant proximal tubule epithelium, extensive vacuolization was observed (Fig. 3B and D). These intracellular vacuoles appeared to be large lysosomal organelles filled with amorphous membranous material, indicating they could be autophagosomes or derived from endocytosis (Fig. 4D). The intracellular vacuoles that were occasionally observed in proximal tubule epithelial cells of control mice were much smaller.
FIG. 3.
Proximal tubule epithelium is affected in adult R-cadherin mutant mice. (A and B) Stainings of sagittal sections of 6-month-old wild-type (A, +/+) and R-cadherin-deficient (B, −/−) mice with hematoxylin-eosin. (C and D) Enlarged views of images presented in panels A and B, respectively. In the proximal tubule epithelia of mutant animals, tubule lumena are enlarged and intracellular vacuoles accumulate within the cells. Proximal tubules, distal tubules, enlarged proximal tubule lumina, and intracellular vacuoles are indicated by PT, DT, stars, and arrowheads, respectively. (E and F) Staining of sagittal sections of 6-month-old wild-type (E, +/+) and R-cadherin-deficient (F, −/−) mice with PAS. Brush borders are indicated with arrowheads. Bars, 20 μm (A, C, and E) (bar lengths also apply to panels B, D, and F, respectively).
FIG. 4.
Ultrastructural analysis of glomeruli and proximal tubules in mice lacking R-cadherin protein. (A and B) Transmission electron microscopy of wild-type (A, +/+) and null mutant (B, −/−) glomeruli. Podocytic foot processes and capillary endothelial fenestrations are indicated by arrows and arrowheads, respectively. (C and D) Transmission electron microscopy of proximal tubule epithelial cells of wild-type (C, +/+) and null mutant (D, −/−) mice. The brush border was unaffected (see inset in D); however, large lysosomal-like structures (stars) accumulated within the cytoplasm of mutant cells (D). Bars: 0.5 μm (A and B); 2.5 μm (C); 3.5 μm (D).
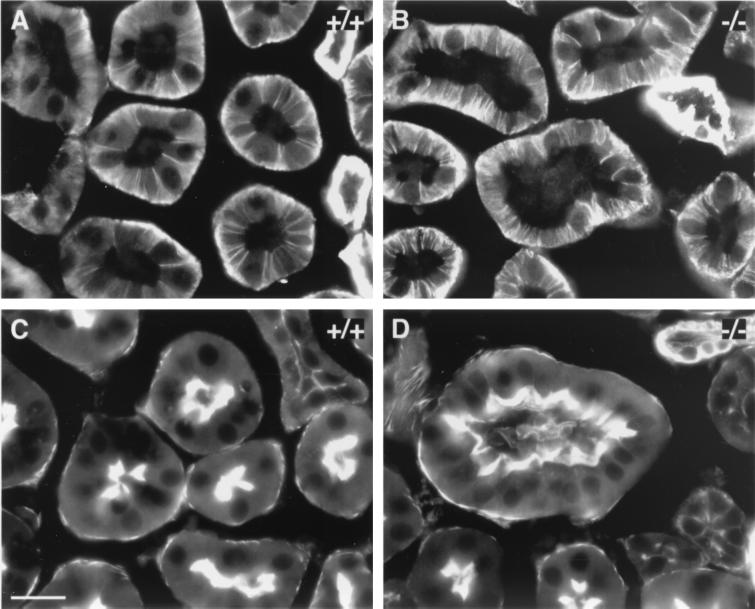
To characterize the proximal tubule phenotype further, we examined the status of cell junctional complexes and cell polarity by ultrastructural analysis and studies of the subcellular distribution of cell polarity markers, such as Na+/K+-ATPase and F-actin, which accumulate basolaterally and apically, respectively. However, electron microscopy demonstrated that the occurrence and morphology of cell junctions were unaffected in proximal tubules of mutant animals (data not shown). Correspondingly, the subcellular distributions of Na+/K+-ATPase (Fig. 5A and B) and F-actin (Fig. 5C and D) were unaffected. The ultrastructural examination also demonstrated that glomeruli were unaffected in R-cadherin-deficient mice, i.e., the branching of the capillary network, fenestration of the endothelium, and formation of foot processes from podocytes (Fig. 4A and B).
FIG. 5.
Cell polarity is unaffected in the adult proximal tubule epithelium of R-cadherin knockout mice. (A and B) Immunofluorescence stainings of sagittal sections of adult wild-type (A, +/+) and homozygous mutant (B, −/−) kidneys with anti-Na+/K+-ATPase polyclonal antibody. (C and D) Stainings of F-actin on sections of adult wild-type (C, +/+) and homozygous mutant (D, −/−) kidneys with rhodamine-phalloidin. The subcellular distributions of Na+/K+-ATPase and F-actin are unaffected. Bar: 20 μm (C) (bar length also applies to panels A, B, and D).
Blood and urine tests were conducted to determine whether the proximal tubule phenotypes of R-cadherin-deficient mice correlate with impaired kidney function. Levels of urea (7 ± 0.4 mmol/liter [−/−] versus 7.2 ± 1.1 mmol/liter [+/+] [means ± standard errors of the means]), creatinine (20 ± 1 μmol/liter [−/−] versus 18 ± 1.9 μmol/liter [+/+]), and glucose (6.6 ± 0.9 mmol/liter [−/−] versus 6.7 ± 0.7 mmol/liter [+/+]) in serum were normal. Normal levels of creatinine (6.2 ± 1.7 μmol/ml [−/−] versus 5.1 ± 0.8 μmol/ml [+/+]), protein (3.2 ± 0.6 mg/ml [−/− females] versus 3.3 ± 0.8 mg/ml [+/+ females]; 7.3 ± 0.7 mg/ml [−/− males] versus 6.4 ± 0.3 mg/ml [+/+ males]), and glucose (data not shown) in urine were also found. Moreover, GFR measurements, according to the clearance of [51Cr]EDTA from the circulation (20), revealed no changes (0.26 ± 0.02 ml/min [−/−] versus 0.28 ± 0.04 ml/min [+/+]).
In summary, the only consequence of R-cadherin deficiency in the adult kidney was the dilation of the proximal tubules concomitant with the appearance of intracellular vacuoles. As expected, this subtle phenotype leads to no discernible functional consequences.
Defective differentiation of metanephric mesenchyme-derived epithelia in R-cadherin−/− kidneys.
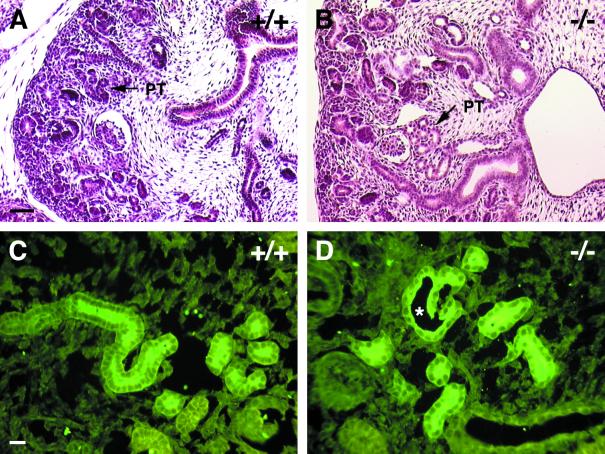
That we were unable to detect R-cadherin in adult proximal tubule epithelium and R-cadherin is transiently expressed in proximal tubule precursor cells suggested that the adult phenotype can be attributed to a developmental defect during nephrogenesis. Indeed, at 15.5 dpc, dilated proximal tubules were already present (Fig. 6A and B). The increased distance between similar numbers of proximal tubule epithelial cells per cross-sectioned tubule suggests that dilation and not an increased number of cells is the underlying cause. In parallel experiments, these tubules were shown to be proximal tubules by binding of the LTA lectin (Fig. 6C and D). Altogether, these observations suggest that R-cadherin is necessary for the normal formation and/or maintenance of the proximal tubule epithelium.
FIG. 6.
Proximal tubules are affected in developing R-cadherin-deficient kidneys. (A and B) Staining of sagittal sections of 15.5-dpc wild-type (A, +/+) and R-cadherin-deficient (B, −/−) kidneys with hematoxylin-eosin. PT indicates proximal tubules. (C and D) LTA stainings of 15.5-dpc wild-type (C, +/+) and R-cadherin-deficient (D, −/−) kidneys. The star indicates the dilated proximal tubule lumen. Bars: 50 μm (A) (bar length also applies to panel B); 20 μm (C) (bar length also applies to panel D).
The primary goal of generating R-cadherin-deficient mice was to examine whether R-cadherin is required for the conversion of the metanephric mesenchyme into an epithelium by condensing the induced mesenchymal cells. However, the fact that R-cadherin−/− kidneys have a normal number of glomeruli suggested that R-cadherin is not required for mesenchyme-to-epithelium conversion. Nevertheless, histological analysis suggested that R-cadherin plays a functional role in these events, because disorganized aggregating mesenchymal cells were consistently found near the ureteric bud tips in developing R-cadherin−/− kidneys (Fig. 7A and B). However, the induction of the mesenchyme as measured by Pax-2 expression and the number of glomeruli appeared to be unaffected (Fig. 7C and D and data not shown). We were not able to answer this question because the phenotype was subtle and appropriate statistical analyses for these phenotypes are not available. However, an alternative way to substantiate this finding was to examine whether suboptimal growth conditions during in vitro differentiation of R-cadherin null embryonic kidneys would unveil similar or even greater defects during mesenchyme-to-epithelial conversion. Indeed, the ratio of epithelial structures (including comma- and S-shaped bodies) to ureteric bud tips from in vitro-cultured kidneys was 35% lower in R-cadherin null kidneys (0.49 ± 0.09 [mean ± standard deviation of the mean] [+/+ +/−] [n = 17], 0.32 ± 0.09 [−/−] [n = 8]) (Fig. 7E and F). The unchanged number of ureteric bud tips (data not shown) indicated that the phenotype is due to a defective mesenchyme-to-epithelial conversion.
FIG. 7.
Mesenchyme-to-epithelial conversion is affected in R-cadherin mutant kidneys. (A and B) Stainings of sagittal sections of 15.5-dpc wild-type (A, +/+) and R-cadherin-deficient (B, −/−) kidneys with hematoxylin-eosin. Aggregating mesenchymal cells and ureteric bud tips are indicated by arrowheads and UB, respectively. (C and D) Immunofluorescence stainings of sagittal sections of 15.5-dpc wild-type (C, +/+) and homozygous mutant (D, −/−) kidneys with anti-Pax2 polyclonal antibody. (E and F) Whole-mount immunofluorescence stainings of organ cultures of wild-type (E, +/+) and homozygous mutant (F, −/−) kidneys with anti-laminin polyclonal antibody. Arrowheads, stars, and arrows indicate ureteric bud tips, S-shaped bodies, and perturbed ureteric bud branching, respectively. Bars: 20 μm (A) (bar length also applies to panel B); 50 μm (C) (bar length also applies to panels D to F).
Thus, analysis of kidney morphogenesis in vivo and in vitro demonstrated that R-cadherin is functionally involved in the conversion of the metanephric mesenchyme into an epithelium.
Defective morphogenesis and survival of ureteric bud epithelium.
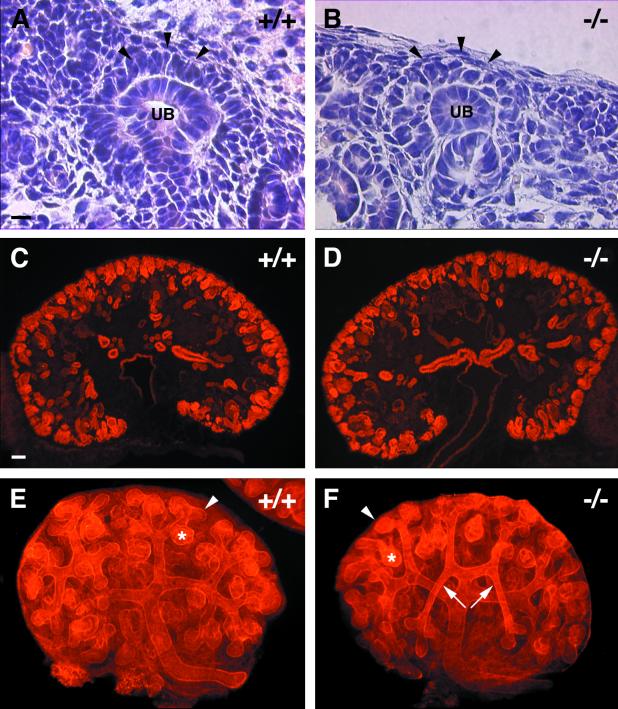
R-cadherin is also transiently expressed within selective regions of the ureteric bud epithelium, specifically within the proximal parts (Fig. 1G). In the absence of R-cadherin, the morphology and organization of this epithelium were clearly altered (Fig. 8A to D). This epithelium normally consists of tightly packed columnar epithelial cells organized as a simple pseudostratified epithelium at 15.5 dpc (Fig. 8A and C). However, in R-cadherin-deficient kidneys at 15.5 dpc, these epithelial cells lost their organized appearance, as seen by less-apparent cell-cell borders, lower nuclear-to-cytoplasmic ratios, and loss of the basal localization of the nuclei (Fig. 8B and D). Furthermore, the occasional fragmented nuclei within R-cadherin-deficient ureteric epithelia suggested an increase in programmed cell death (Fig. 8D). This finding was corroborated by TUNEL assays, which revealed increased apoptosis within the ureteric bud epithelium that normally expresses R-cadherin (Fig. 8E and F). Moreover, apoptosis was significantly increased within the mesenchyme closely associated with the epithelium (Fig. 8F). It was difficult to determine whether these morphological and cell survival changes affected the morphogenetic behavior of the ureteric bud epithelium by histological analysis of 15.5-dpc kidneys. However, analysis of whole-mount laminin-immunostained in vitro-cultured kidneys revealed consistent changes in the branching and patterning of the epithelium (Fig. 7E and F). The analysis of 20 kidney explants from wild-type and R-cadherin-deficient embryos revealed that only 1 (5%) wild-type and 19 (95%) R-cadherin mutant explants exhibited this phenotype. The phenotype can best be described as having the appearance of long, slender, unbranched, and often wrongly oriented major ureteric bud branches (Fig. 7E and F).
FIG. 8.
Morphogenesis of the ureteric bud is affected in R-cadherin-deficient mice. (A and B) Stainings of sagittal sections of 15.5-dpc wild-type (A, +/+) and R-cadherin-deficient (B, −/−) kidneys with hematoxylin-eosin. (C and D) Higher magnification of images presented in panels A and B, respectively. An apoptotic cell is indicated by an arrowhead. (E and F) TUNEL stainings of sagittal sections of 15.5-dpc wild-type (E, +/+) and R-cadherin-deficient (F, −/−) kidneys. Sections from wild-type and mutant kidneys were from the same region, i.e., the central part of the medulla visualizing the proximal parts of the ureteric tubules. Epithelium, mesenchyme, and border between epithelium and mesenchyme are indicated by E, M, and dotted line, respectively. Bars: 100 μm (A) (bar length also applies to panel B); 20 μm (C) (bar length also applies to panel D); 20 μm (E) (bar length also applies to panel F).
Thus, in conclusion, these data suggest that R-cadherin is required for normal differentiation and morphogenesis of the ureteric bud epithelium.
Can other classic cadherins functionally compensate for the lack of R-cadherin?
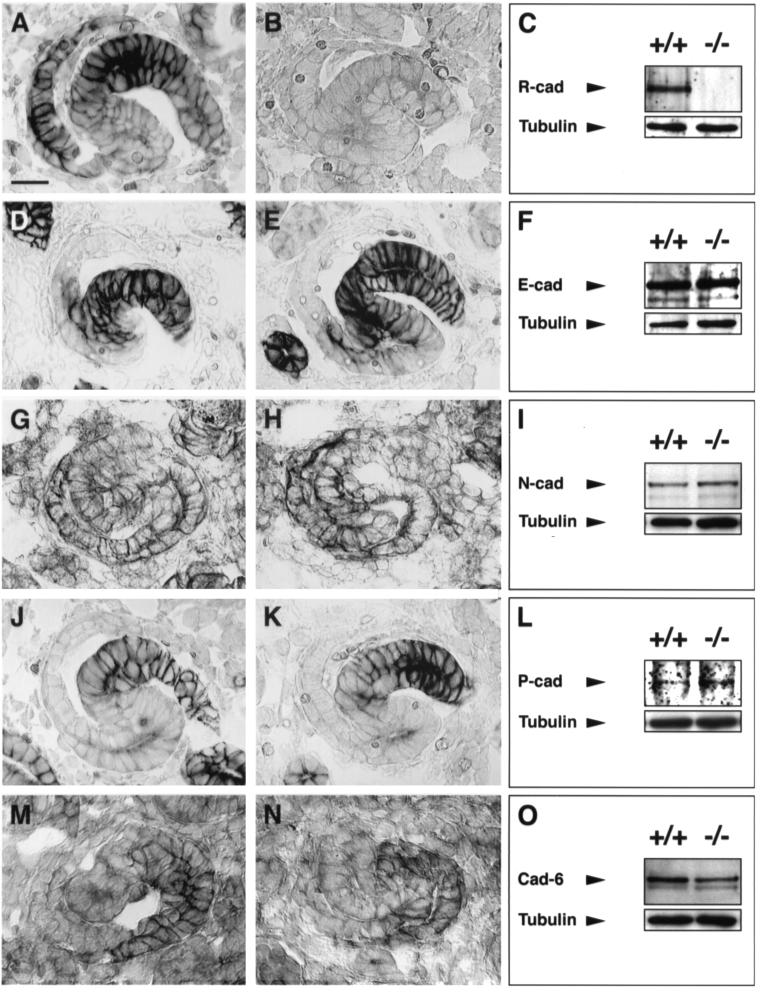
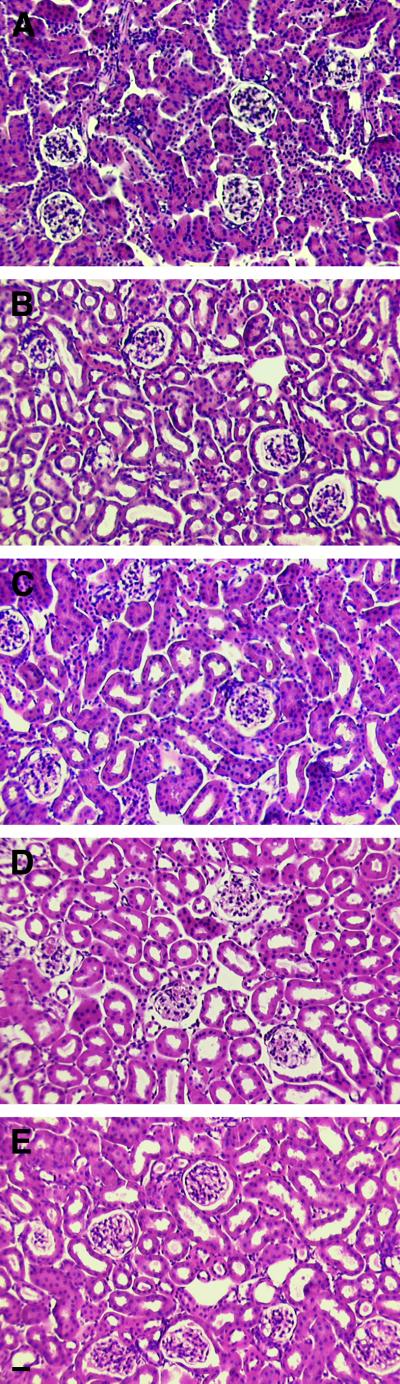
The fact that R-cadherin-deficient mice survive and have functional kidneys despite mild but distinct developmental defects during kidney formation suggests that other cadherins may have overlapping functions or may functionally compensate for the lack of R-cadherin. In the developing kidney, the expression patterns of at least five other classic cadherins (E-cadherin, N-cadherin, P-cadherin, cadherin-6, and cadherin-11) partially overlap. To confirm whether the subtle kidney phenotype in R-cadherin-deficient mice could be explained by functional compensation by some of these cadherins, we analyzed the expression levels and distribution of these cadherins, except cadherin-11, in developing R-cadherin−/− kidneys. The absence of R-cadherin did not appear to influence the expression of any of the examined cadherins (Fig. 9). To test whether the functions of these cadherins overlap with those of R-cadherin or whether these cadherins functionally compensate for R-cadherin, we generated R-cadherin−/− cadherin-6−/−, R-cadherin−/− P-cadherin−/−, and R-cadherin−/− N-cadherin+/− mice. As N-cadherin−/− mice die before the initiation of tubulogenesis (9.5 to 10 dpc), we were unable to study kidney development in R-cadherin−/− N-cadherin−/− mice. All compound knockout strains survived, and to our surprise, kidney development was not affected to a greater extent than within the individual cadherin knockout strains (Fig. 10).
FIG. 9.
Expression of cadherins in R-cadherin-deficient kidneys. Immunoperoxidase and immunoblotting analyses of R-cadherin (A to C), E-cadherin (D to F), N-cadherin (G to I), P-cadherin (J to L), and cadherin-6 (M to O) in 15.5-dpc wild-type (+/+ and panels A, D, G, J, and M) and R-cadherin-deficient (−/− and panels B, E, H, K, and N) kidneys. Immunoperoxidase images represent S-shaped bodies. S-shaped bodies are similarly oriented in all images, i.e., with the distal and proximal parts to the right and left, respectively. Tubulin antibodies were used as loading controls. Bar, 20 μm (A) (bar length also applies to panels B, D, E, G, H, J, K, M, and N).
FIG.10.
Analysis of kidney development in compound cadherin knockout strains. (A to E) Stainings of sagittal sections of 3-month-old wild-type (A), R-cadherin−/− (B), R-cadherin−/− N-cadherin+/− (C), R-cadherin−/− cadherin-6−/− (D), and R-cadherin−/− P-cadherin−/− (E) mice with hematoxylin-eosin. Bar, 20 μm (E) (bar length also applies to panels A to D).
DISCUSSION
Previously, many transcription factors, cell-cell signaling molecules, and growth factor receptors have been shown to be required for the reciprocal inductive events between the metanephric mesenchyme and ureteric bud epithelium (7, 25, 39). However, few of their targets, which presumably perform the complex morphogenetic events involved in tubulogenesis, have been identified. These morphogenetic events include the aggregation of mesenchymal cells, mesenchymal-epithelial transition, cell shape changes, and cell migration, which are all processes the classic cadherins have been implicated in. Several classic cadherins exhibit a restricted spatial and temporal expression pattern during kidney development. We have studied the significance of several of these cadherins in kidney organogenesis by analyzing mice lacking either one or two of these cadherins. In summary, our data demonstrate that R-cadherin is functionally involved in the development of both mesenchymal and epithelial components during metanephric kidney development. Furthermore, intercrossing R-cadherin knockout mice with knockout strains for cadherins that are coexpressed with R-cadherin during nephrogenesis resulted in no additional kidney phenotype, indicating that these cadherins are not functionally redundant during kidney development.
To study the functional role of R-cadherin during tissue formation and organogenesis, we generated mice lacking R-cadherin by gene targeting. Here we report that R-cadherin plays subtle but distinct roles during kidney development. Other tissues that R-cadherin function has been implicated in include the endocrine pancreas (pancreatic β cells), eye, and brain. Detailed histological analysis of pancreatic development, together with measurements of blood glucose levels in R-cadherin-deficient mice, indicates that neither pancreatic ontogeny nor β-cell functions require R-cadherin function. Regarding R-cadherin's potential role in formation of the eye and brain, preliminary histological and behavioral analysis exposed no obvious phenotypes consistent with a function in these tissues (data not shown).
In the kidney of adult R-cadherin-deficient mice we observed a dilation of the proximal tubule lumina and a concomitant accumulation of large lysosomes or autophagosomes. The fact that this phenotype was also observed during nephrogenesis suggests that the adult phenotype can be attributed to the functional role of R-cadherin during differentiation of the proximal tubule epithelium from the induced metanephric mesenchyme. This is also consistent with the transient expression of R-cadherin within the parts of the S-shaped bodies that subsequently develop into the proximal tubule epithelium. It is well known that the dilation of the proximal tubule system is often associated with glomerular disease. However, an array of tests to assess the functional state of the kidney (serum and urine analysis and measurement of GFR) demonstrated that R-cadherin-deficient mice do not suffer from kidney insufficiencies.
Studies on the potential role of R-cadherin in the earlier events during the transition of the mesenchyme into an epithelium revealed minor but consistent disturbances in the organization of induced metanephric mesenchymal cells in the close vicinity of the ureteric bud tips. Likewise, the formation of epithelial intermediates (comma- and S-shaped bodies) during nephrogenesis was significantly inhibited during in vitro differentiation of R-cadherin−/− kidneys. Taken together, these findings indicate that R-cadherin is functionally involved in the mesenchyme-to-epithelial transition. As tubulogenesis does not appear to be blocked at any specific step in R-cadherin-deficient kidneys, we can assume that either the initial condensation of the induced mesenchyme is compromised or the whole conversion process into an epithelium is slowed down. The manifestation of a more obvious phenotype during in vitro differentiation of R-cadherin-deficient kidneys suggested that when organogenesis is jeopardized, e.g., by limited availability of growth factors, the phenotype could be enhanced.
R-cadherin is transiently expressed within derivatives of induced metanephric mesenchyme and within the ureteric bud epithelium. However, its expression is restricted to proximal parts of the ureteric bud epithelium, more specifically to the region in which the ureteric bud undergoes its initial branching. Analysis of ureteric bud development in R-cadherin-deficient kidneys revealed altered morphology and organization and decreased cell survival of the epithelium in vivo and perturbed branching and patterning in vitro. These findings imply that R-cadherin is functionally involved in the differentiation and morphogenesis of the ureteric bud epithelium. The lack of detectable changes within derivatives of the ureteric bud epithelium, such as the collecting ducts, indicates that these changes are compensated for after the completion of kidney formation.
Our data led us to ask why kidney organogenesis was not affected to a greater extent in R-cadherin-deficient mice. Given the overlapping expression patterns of several cadherins with R-cadherin during kidney ontogeny (E-cadherin [5, 11, 38, 48], N-cadherin [11, 22], K-cadherin [cadherin 6] [5, 32, 50], cadherin-11 [5, 15, 21], and P-cadherin [11, 45]), it is reasonable to assume that redundancy occurs among cadherins. It is, however, difficult to assess whether this redundancy is caused by compensation or by overlapping function. The functions of cadherin-6 during kidney development were recently studied by using gene targeting (26). Cadherin-6-deficient mice exhibit a subtle developmental kidney phenotype, which may be due to compensation by R-cadherin. However, in our R-cadherin−/− cadherin-6−/− mice, kidney morphogenesis occurred in the absence of both of these cadherins. This result does not exclude the possibility that yet another cadherin does the same job as R-cadherin. For example, P-cadherin and R-cadherin are coexpressed in the proximal portion of the distal region of the S-shaped body, which gives rise to the distal tubule epithelial cells. However, R-cadherin−/− P-cadherin−/− mice did not exhibit any additional phenotype compared to R-cadherin−/− mice. N-cadherin is more likely to be expressed in the same cells as R-cadherin than the other classic cadherins. Based on sequence homology and cell aggregation properties, N-cadherin is the closest cadherin relative to R-cadherin. Furthermore, N-cadherin has been reported to interact heterophilically with R-cadherin (28). As N-cadherin−/− mice die prior to nephrogenesis, we were unable to test directly whether N-cadherin compensates for R-cadherin by generating mice lacking both R-cadherin and N-cadherin. However, it has been reported that the phenotype of a single gene knockout can be enhanced if one allele of a closely related gene family member is inactivated (19). Therefore, we generated R-cadherin−/− N-cadherin+/− mice and analyzed kidney development. These mice were, however, also indistinguishable from single R-cadherin knockout mice.
It is noteworthy that we were unable to identify cells completely lacking classic cadherins in any of the compound cadherin knockouts generated in this study. Thus, we were not able to determine whether functional redundancy exists among classic cadherins during kidney morphogenesis, and future studies should analyze additional combinatorial cadherin knockouts, including triple knockouts.
Acknowledgments
We thank R. Mège for providing the anti-cadherin-6 antibodies; P. Hörstedt for ultrastructural analysis; M.-L. Sentman for serum and urine analyses; K. Karp and M. Gref for GFR measurements; and I. Berglund, I. Fransson, and G. Pettersson for expert technical assistance.
This work was supported by research grants from the Swedish Cancer Society, the Swedish Medical and Natural Science Research Councils, Foundation for Strategic Research, Kempestiftelserna, and M. Bergvalls Stiftelse.
REFERENCES
- 1.Aberle, H., S. Butz, J. Stappert, H. Weissig, R. Kemler, and H. Hoschuetzky. 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107:3655-3663. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, J. F., K. Pritchard-Jones, W. A. Bickmore, N. D. Hastie, and J. B. L. Bard. 1992. The expression of the Wilm's tumor gene, WT1, in the developing mammalian embryo. Mech. Dev. 40:85-97. [DOI] [PubMed] [Google Scholar]
- 3.Barth, A. I. M., I. S. Näthke, and W. J. Nelson. 1997. Cadherins, catenins, and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr. Opin. Cell Biol. 9:683-690. [DOI] [PubMed] [Google Scholar]
- 4.Butz, S., and R. Kemler. 1994. Distinct cadherin-catenin complexes in Ca2+-dependent cell-cell adhesion. FEBS Lett. 355:195-200. [DOI] [PubMed] [Google Scholar]
- 5.Cho, E. A., L. T. Patterson, W. T. Brookhiser, S. Mah, C. Kintner, and G. R. Dressler. 1998. Differential expression and function of cadherin-6 during renal epithelium development. Development 125:803-812. [DOI] [PubMed] [Google Scholar]
- 6.Dahl, U., A. Sjödin, and H. Semb. 1996. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development 122:2895-2902. [DOI] [PubMed] [Google Scholar]
- 7.Davies, J. A., and J. B. L. Bard. 1996. Inductive interactions between the mesenchyme and the ureteric bud. Exp. Nephrol. 4:77-85. [PubMed] [Google Scholar]
- 8.Edelman, G. M. 1986. Cell-adhesion molecules in the regulation of animal form and tissue pattern. Annu. Rev. Cell Biol. 2:81-116. [DOI] [PubMed] [Google Scholar]
- 9.Ekblom, P. 1989. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 3:2141-2150. [DOI] [PubMed] [Google Scholar]
- 10.Esni, F., I.-B. Täljedahl, A.-K. Perl, H. Cremer, G. Christofori, and H. Semb. 1999. Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets. J. Cell Biol. 144:325-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto, S., E. Yaoita, H. Matsunami, D. Kondo, T. Yamamoto, K. Kawasaki, M. Arakawa, and I. Kihara. 1998. Involvement of R-cadherin in the early stage of glomerulogenesis. J. Am. Soc. Nephrol. 9:1234-1241. [DOI] [PubMed] [Google Scholar]
- 12.Gumbiner, B. M. 1996. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345-357. [DOI] [PubMed] [Google Scholar]
- 13.Herminston, M. L., and J. I. Gordon. 1995. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J. Cell Biol. 129:489-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinck, L., I. S. Näthke, J. Papkoff, and W. J. Nelson. 1994. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J. Cell Biol. 125:1327-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann, I., and R. Balling. 1995. Cloning and expression analysis of a novel mesodermally expressed cadherin. Dev. Biol. 169:337-346. [DOI] [PubMed] [Google Scholar]
- 16.Horikawa, K., G. Radice, M. Takeichi, and O. Chisaka. 1999. Adhesive subdivisions intrinsic to the epithelial somites. Dev. Biol. 215:182-189. [DOI] [PubMed] [Google Scholar]
- 17.Hutton, J. C., G. Christofori, W. Y. Chi, U. Edman, P. C. Guest, D. Hanahan, and R. B. Kelly. 1993. Molecular cloning of mouse pancreatic islet R-cadherin: differential expression in endocrine and exocrine tissue. Mol. Endocrinol. 7:1151-1160. [DOI] [PubMed] [Google Scholar]
- 18.Inuzuka, H., S. Miyatani, and M. Takeichi. 1991. R-cadherin: a novel Ca2+-dependent cell-cell adhesion molecule expressed in the retina. Neuron 7:69-79. [DOI] [PubMed] [Google Scholar]
- 19.Joyner, A. L. 1996. Engrailed, Wnt and Pax genes regulate midbrain-hindbrain development. Trends Genet. 12:15-20. [DOI] [PubMed] [Google Scholar]
- 20.Judas, L., S. M. Bentzen, and F. A. Stewart. 1997. Progression rate of radiation damage to the mouse kidney: a quantitative analysis of experimental data using a simple mathematical model of the nephron. Int. J. Radiat. Biol. 72: 461-473. [DOI] [PubMed] [Google Scholar]
- 21.Kimura, Y., H. Matsunami, T. Inoue, K. Shimamura, N. Uchida, T. Ueno, T. Miyazaki, and M. Takeichi. 1995. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev. Biol. 169:347-358. [DOI] [PubMed] [Google Scholar]
- 22.Klein, G., M. Langegger, C. Goridis, and P. Ekblom. 1988. Neural cell adhesion molecules during embryonic induction and development of the kidney. Development 102:749-761. [DOI] [PubMed] [Google Scholar]
- 23.Larue, L., C. Antos, S. Butz, O. Huber, V. Delmas, M. Dominis, and R. Kemler. 1996. Cadherins can direct tissue formation. Development 122:3185-3194. [DOI] [PubMed] [Google Scholar]
- 24.Larue, L., M. Ohsugi, J. Hirchenhain, and R. Kemler. 1994. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. USA 91:8263-8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechner, M. S., and G. R. Dressler. 1997. The molecular basis of embryonic kidney development. Mech. Dev. 62:105-120. [DOI] [PubMed] [Google Scholar]
- 26.Mah, S. P., H. Saueressig, M. Goulding, C. Kintner, and G. R. Dressler. 2000. Kidney development in cadherin-6 mutants: delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev. Biol. 223:38-53. [DOI] [PubMed] [Google Scholar]
- 27.Mansour, S. L., K. R. Thomas, and M. R. Capecchi. 1988. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336:348-352. [DOI] [PubMed] [Google Scholar]
- 28.Matsunami, H., S. Miyatani, T. Inoue, N. G. Copeland, D. J. Gilbert, N. A. Jemkins, and M. Takeichi. 1993. Cell binding specificity of mouse R-cadherin and chromosomal mapping of the gene. J. Cell Sci. 106:401-409. [DOI] [PubMed] [Google Scholar]
- 29.Matsunami, H., and M. Takeichi. 1995. Fetal brain subdivisions defined by R- and E-cadherin expressions: evidence for the role of cadherin activity in region-specific, cell-cell adhesion. Dev. Biol. 172:466-478. [DOI] [PubMed] [Google Scholar]
- 30.Nagafuchi, A., and M. Takeichi. 1988. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 7:3679-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozawa, M., H. Baribault, and R. Kemler. 1989. The cytoplasmic domain of the cell adhesion molecule ovumorulin associated with three independent proteins structurally related in different species. EMBO J. 8:1711-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul, R., C. M. Ewing, J. C. Robinson, F. F. Marchall, K. R. Johnson, M. J. Wheelock, and W. B. Isaacs. 1997. Cadherin-6, a cell adhesion molecule specifically expressed in the proximal renal tubule and renal cell carcinoma. Cancer Res. 57:2741-2748. [PubMed] [Google Scholar]
- 33.Pritchard-Jones, K., S. Fleming, D. Davidson, W. Bickmore, D. Porteous, C. Gosden, J. Bard, A. Buckler, J. Pelletier, D. Housman, V. van Heyningen, and N. Hastie. 1990. The candidate Wilm's tumor gene is involved in genitourinary development. Nature 346:194-197. [DOI] [PubMed] [Google Scholar]
- 34.Radice, G. L., M. C. Ferreira-Cornwell, S. D. Robinson, H. Rayburn, L. A. Chodosh, M. Takeichi, and R. O. Hynes. 1997. Precocious mammary gland development in P-cadherin-deficient mice. J. Cell Biol. 139:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radice, G. L., H. Rayburn, H. Matsunami, K. A. Knudsen, M. Takeichi, and R. O. Hynes. 1997. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 181:64-78. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds, A. B., J. Daniel, P. D. McCrea, M. J. Wheelock, J. Wu, and Z. Zhang. 1994. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol. Cell. Biol. 14:8333-8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riethmacher, D., V. Brinkmann, and C. Birchmeier. 1995. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc. Natl. Acad. Sci. USA 92:855-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg, P., F. Esni, A. Sjödin, L. Larue, L. Carlsson, D. Gullberg, M. Takeichi, R. Kemler, and H. Semb. 1997. A potential role of R-cadherin in striated muscle formation. Dev. Biol. 187:55-70. [DOI] [PubMed] [Google Scholar]
- 39.Sariola, H. 1996. Does the kidney express redundant or important molecules during development. Exp. Nephrol. 4:70-76. [PubMed] [Google Scholar]
- 40.Saxen, L. 1987. Organogenesis of the kidney. In P. W. Barlow, P. B. Green, and C. C. White (ed.), Developmental and cell biology series 19. Cambridge University Press, Cambridge, United Kingdom.
- 41.Shibamoto, S., M. Hayakawa, K. Takeuchi, T. Hori, K. Miyazama, N. Kitamura, K. R. Johnson, M. J. Wheelock, N. Matsuyoshi, M. Takeichi, and F. Ito. 1995. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J. Cell Biol. 128:949-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirayoshi, Y., A. Nose, K. Iwasaki, and M. Takeichi. 1986. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct. Funct. 11:245-252. [DOI] [PubMed] [Google Scholar]
- 43.Sjödin, A., U. Dahl, and H. Semb. 1995. Mouse R-cadherin: expression during the organogenesis of pancreas and gastrointestinal tract. Exp. Cell Res. 221:413-425. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, S., K. Sano, and H. Tanihara. 1991. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul. 2:261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tassin, M., A. Beziau, M. Gubler, and B. Boyer. 1994. Spatiotemporal expression of molecules associated with junctional complexes during the in vivo maturation of renal podicytes. Int. J. Dev. Biol. 38:45-54. [PubMed] [Google Scholar]
- 46.Tepass, U., K. Truong, D. Godt, M. Ikura, and M. Peifer. 2000. Cadherins in embryonic and neural morphogenesis. Nat. Rev. Mol. Cell Biol. 1:91-100. [DOI] [PubMed] [Google Scholar]
- 47.Thomas, K. R., and M. R. Capecchi. 1987. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51:503-512. [DOI] [PubMed] [Google Scholar]
- 48.Vestweber, D., R. Kemler, and P. Ekblom. 1985. Cell-adhesion molecule uvomorulin during kidney development. Dev. Biol. 122:213-221. [DOI] [PubMed] [Google Scholar]
- 49.Vleminckx, K., and R. Kemler. 1999. Cadherins and tissue formation: integrating adhesion and signaling. BioEssays 21:211-220. [DOI] [PubMed] [Google Scholar]
- 50.Xiang, Y. Y., M. Tanaka, M. Suzuki, H. Igarashi, E. Kiyokawa, Y. Naito, Y. Ohtawara, Q. Shen, H. Sugimura, and I. Kino. 1994. Isolation of complementary DNA encoding K-cadherin, a novel rat cadherin preferentially expressed in fetal kidney and kidney carcinoma. Cancer Res. 54:3034-3041. [PubMed] [Google Scholar]