Abstract
Current understanding of the activation of STATs is through binding between the SH2 domain of STATs and phosphotyrosine of tyrosine kinases. Here we demonstrate a novel role of RACK1 as an adaptor for insulin and insulin-like growth factor 1 receptor (IGF-1R)-mediated STAT3 activation specifically. Intracellular association of RACK1 via its N-terminal WD domains 1 to 4 (WD1-4) with insulin receptor (IR)/IGF-1R is augmented upon respective ligand stimulation, whereas association with STAT3 is constitutive. Purified RACK1 or RACK1 WD1-4 associates directly with purified IR, IGF-1R, and STAT3 in vitro. Insulin induces multiprotein complex formation of RACK1, IR, and STAT3. Overexpression or downregulation of RACK1 greatly enhances or decreases, respectively, IR/IGF-1R-mediated activation of STAT3 and its target gene expression. Site-specific mutants of IR and IGF-1R impaired in RACK1 binding are ineffective in mediating recruitment and activation of STAT3 as well as in insulin- or IGF-1-induced protection of cells from anoikis. RACK1-mediated STAT3 activation is important for insulin and IGF-1-induced anchorage-independent growth in certain ovarian cancer cells. We conclude that RACK1 mediates recruitment of STAT3 to IR and IGF-1R specifically for activation, suggesting a general paradigm for the need of an adaptor in mediating activation of STATs by receptor protein tyrosine kinases.
Insulin receptor (IR) and insulin like growth factor-1 receptor (IGF-1R) are structurally related and composed of two extracellular α-subunits and two transmembrane β-subunits (33, 57). IR mediates prenatal growth in response to IGF-II (22) and postnatal homeostasis of glucose metabolism in response to insulin (33), while IGF-1R mediates IGF-1 and IGF-II action on prenatal growth and IGF-1 action on postnatal growth, as well as a variety of physiological functions (1, 40). Dysregulation of IR- or IGF-1R-mediated signaling has been linked to oncogenic properties of transformed and cancer cells (11, 24, 27, 39, 56). Activation of IR and IGF-1R upon cognate ligand binding results in activation of different downstream signaling pathways including those of mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) through adaptors like insulin receptor substrate (IRS), Grb2, and Shc family proteins (5, 7, 13, 20, 21, 48, 49, 50, 56, 59, 60). Other substrates of IR and IGF-1R include phospholipase C proteins and signal transducers and activators of transcription (STATs) (15, 34, 51, 63). Furthermore, IR- and IGF-1R-activated signaling cross-talks via mediators such as IRS1, Shc, PI3K, and receptor for activated C kinase 1 (RACK1) with that of integrin, resulting in so-called inside-out signaling (56).
STATs comprise a family of transcriptional factors and are important in embryonic development, organ genesis, and innate and adaptive immune function, as well as regulation of cell growth, differentiation, and apoptosis (10). A large number of cytokines and growth factors, including insulin and IGF-1, can trigger STAT activation. Constitutive or elevated activation of STAT3 and/or STAT5 has been implicated in development of a variety of tumors (6, 9, 10). STAT5 and STAT3 can be activated through IR and IGF-1R (15, 17, 51, 63). JAK family kinases are involved in IR-mediated STAT5 and IGF-1R-mediated STAT3 activation (38, 63). However, the question whether STATs directly bind to the receptor protein tyrosine kinases (RPTKs) or are mediated via adaptors for their recruitment to the receptors remains open. The IR mutant unable to bind and activate STAT5 (38) was still able to activate STAT3 (our unpublished observations). No consensus STAT3 binding site was found in IGF-1R. Therefore, it suggested to us that IR/IGF-1R might mediate activation of STAT3 via an adaptor.
RACK1 was found to associate with STAT1 constitutively, enhancing interferon-mediated antiviral functions through interaction with JAK family kinases (28, 37, 54); thus, it may be a candidate for IR/IGF-1R-mediated STAT3 binding and activation. RACK1 was originally identified as a receptor for activated C kinases and is a member of the family of seven WD40-motif-containing proteins (45). RACK1 is predicted to be a scaffold protein with three dozen or so known interacting partners and has been implicated in regulating diverse biological functions (44). RACK1 is involved in regulating tyrosine kinase-mediated signaling, including Src (12, 43), IR and IGF-1R (30, 36), and JAKs (28). RACK1 was also upregulated in lung, colon, and breast carcinoma cells (2). We observed that a significant fraction of RACK1 is relocated from cytoplasm to membrane in different oncogene-transformed mammalian and avian cells (30). All these observations suggest that RACK1 regulates signaling pathways involved in transformation.
We hypothesized that RACK1 may function as an adaptor protein in the IR/IGF-1R-mediated STAT3 signaling pathway. We found that RACK1 regulated IR/IGF-1R-mediated STAT3 activation specifically by recruiting STAT3 to the receptors, which plays an important role in the colony forming ability of certain ovarian cancer cells.
MATERIALS AND METHODS
Cells and DNA transfection.
Human embryonic kidney (HEK) 293T, PA-1, SW626, SKOV3, and Mewo stable cell lines were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (Sigma). NIH 3T3, 3T3/IR (IR-overexpressed NIH 3T3 cell lines [62]), and EB69 and ES2 (two stable NIH 3T3 cell lines expressing epidermal growth factor receptor [EGFR]-Ros chimeras [61]) were maintained in DMEM with 10% bovine serum. SW626, NIH 3T3/IR, EB69, and ES2 were transfected with Lipofectamine 2000; PA-1 and SKOV3 were transfected with Lipofectamine Plus, and HEK 293T cells were transfected by calcium phosphate coprecipitation methods (14, 26).
Plasmids and their construction.
phEF-RACK1-HA (where EF is elongation factor and HA is hemagglutinin) (30), phEF-IGFR (63), phEF-IR (38), EGFR, and EGFR-Ros (61) chimeras have been described previously. The pCMV-STAT3 and pCMV-dnSTAT3 were kindly provided by James Darnell, Jr., and Curt Horvath and have been described elsewhere (31). phEF-HA-WD1-4 and phEF-HA-WD5-7, containing the WD domains 1 to 4 (residues 1 to 180) and 5 to 7 (residues 180 to 317), respectively, of RACK1, were generated by PCR amplification of the corresponding fragments and cloned into the BamHI-NotI sites of the phEF-Neo plasmid. IGF-1R S1248A and IR S1275A were generated by using a QuikChange site-directed mutagenesis kit (Stratagene) and were confirmed by DNA sequencing. RACK1, WD1-4, and WD5-7 were subcloned into bacteria-expressing vector pTAT with a six-His tag for Ni-nitrilotriacetic acid (NTA) purification. STAT3 and IR/IGF-1R cytoplasmic domains were cloned into the SalI-NotI sites of pGEX6p-1 for glutathione transferase (GST) fusion protein preparation and purification.
Antibodies.
Anti-STAT3pY705, anti-pErk1/2, anti-Erk1/2, anti-pAkt, and anti-Akt antibodies were purchased from Cell Signaling. Anti-STAT3 was purchased from Santa Cruz. Anti-RACK1, anti-pY20HRPO (where HRPO is horseradish peroxidase), goat anti-rabbit HRPO, and goat anti-mouse HPRO were purchased from Transduction Laboratories. anti-EGFR antibody was purchased from Oncogene Science. Antitubulin, anti-His, rabbit anti-mouse secondary antibody, and anti-c-Myc antibodies were purchased from Sigma. Anti-IR (15), anti-IGFR (41), and anti-Ros (32) were described previously. Anti-HA epitope was purchased from Hybridoma Center of Mount Sinai. Anti-snRNP was a gift from Serafin Pinol-Roma.
Protein purification and in vitro binding assay.
His-tagged TAT-RACK1 and mutants were purified with Ni-NTA agarose (QIAGEN). GST-STAT3, GST-IRKD (where KD is kinase domain), and GST-IGFRKD were purified with glutathione-Sepharose beads (Amersham). An in vitro binding assay was performed in NP-40 lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 1% NP-40, 10% glycerol, 1 mM EDTA, pH 8.0, 1 mM phenylmethylsulfonyl fluoride, 1% aprotinin). Indicated amounts of proteins were combined in NP-40 lysis buffer and gently rotated at 4°C overnight, followed by the addition of 20 μl of Ni-NTA agarose or 10 μl of glutathione-Sepharose beads for 1 h. Beads were recovered by gentle centrifugation at 3,000 × g for 3 min and washed twice with NP-40 lysis buffer. Samples were boiled in sodium dodecyl sulfate (SDS)-loading buffer.
Preparation of cell lysates, immunoprecipitation, SDS-PAGE and immunoblotting.
For direct Western analysis, cell lysates were prepared with ice-cold radioimmunoprecipitation assay buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 5 mM EDTA, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.4 mM phenylarsine oxide, and 25 mM NaF). For coimmunoprecipitation, cell lysates were extracted with NP-40 lysis buffer as described above. Cell lysates or immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with indicated antibodies.
Velocity sedimentation on glycerol gradient.
HEK 293T cells were transiently transfected with 600 ng of IR and STAT3 in 10-cm dishes, followed by serum starvation for 24 h and insulin stimulation for 15 min. Cell lysates were prepared and loaded onto a 20 to 40% glycerol gradient in NP-40 lysis buffer. Centrifugation was done in an SW40 Ti rotor (Beckman) at 38,000 rpm for 66 h, and fractions were collected from the bottom. Proteins from gradient fractions or immunoprecipitates from the selected fractions with anti-RACK1 antibody were separated by SDS-PAGE, followed by immunoblotting with indicated antibodies.
RNA interference.
Templates of small interfering RNA (siRNA) against RACK1 were designed according to the instructions in the Silencer siRNA Construction kit (Ambion Inc.) and synthesized (Invitrogen). In vitro transcription and formation of double-strand siRNA were performed according to the protocol provided with the kit. Sequences of the siRNA1 templates are 5′-AAGCAAGAAGTTATCAGTACCCCTGTCTC (sense) and 5′-AAGGTACTGATAACTTCTTGCCCTGTCTC (antisense). Sequences for the scrambled control for siRNA1 are 5′-CACAAGTCGAAACGTAAATGTCCTGTCTC (sense) and 5′-ACATTTACGTTTCGACTTGTGCCTGTCTC (antisense). Sequences of the siRNA2 templates are 5′-AACTATGGAATTCCACAGCGTCCTGTCTC (sense) and 5′-AAACGCTGTGGAATTCCATAGCCTGTCTC (antisense). pRS-shRNA, containing the RACK1 sequence as a template for shRNA construction, has been previously described (42).
Reporter assay.
HEK 293T cells were transfected with 150 ng of phEF-IR, 50 ng of pCMV-STAT3, and 250 ng of phEF-Neo or RACK1, along with 50 ng of pRL-TK and 50 ng of TKS3 (53) (STAT3 reporter construct); NIH 3T3/IR cells were transfected with 50 ng of pRL-TK, and 150 ng of 3×Ly6E (31) (a STAT3 reporter construct containing three Ly6E sites), together with 1 μg of phEF-Neo or RACK1 or together with siRNA or scrambled control (10 nM). After 24 h (for RACK1 or phEF-neo) or 96 h (for siRNA or scrambled control), cells were serum starved for 12 h and then stimulated with insulin for 6 h. Luciferase activity was measured by a dual-luciferase reporter assay kit from Promega.
Colony formation assay, anoikis assay, and migration and invasion assays.
At 48 h posttransfection, transfected PA-1, SW626, and SKOV3 cells were trypsinized, counted, and resuspended. A total of 2.5 × 105 PA-1, 105 SW626, and 105 SKOV3 cells were used for colony formation assays with or without the addition of insulin and IGF-1 as previously reported (30); 105 cells were used for the migration and invasion assays (3). At 24 h posttransfection, NIH 3T3 cells were starved overnight, followed by insulin or IGF-1 treatment for 10 h. Anoikis assays were carried out as described previously (55).
RESULTS
RACK1 regulates IR/IGF-1R-mediated STAT3 activation specifically.
RACK1 was required for STAT1 activation via alpha interferon (IFN-α) receptor (37, 54) and shown to associate with IR and IGF-1R and to regulate IR- and IGF-1R-mediated signaling functions (30, 35, 36). To investigate whether RACK1 is involved in IR- and IGF-1R-mediated signaling pathways, HEK 293T cells were transiently transfected with phEF-IR (Fig. 1A and E) or phEF-IGF-1R (Fig. 1D), STAT3, and phEF-neo or RACK1. Overexpression of RACK1 in HEK 293T cells significantly enhanced insulin- and IGF-1-mediated STAT3 phosphorylation (Fig. 1A, D, and E), suggesting that RACK1 played an important role in IR/IGF-1R-STAT3 activation, which appeared to be receptor specific since RACK1 failed to enhance fetal calf serum-induced STAT3 activation (Fig. 1A). Receptor specificity was further confirmed since overexpression of RACK1 could not enhance STAT3 activation mediated by EGFR (see Fig. S1A in the supplemental material) or EGFR-Ros chimeras (see Fig. S1B and C in the supplemental material) (61). In agreement with the above results, the overexpression of RACK1 in NIH 3T3/IR cells, murine embryonic fibroblasts stably overexpressing human IR (Fig. 1B and F), or the introduction of TAT-his-RACK1 (TAT is a positively charged peptide from human immunodeficiency virus type 1 that can facilitate protein entry into living cells[29]) into Mewo cells, a human melanoma cell line (Fig. 1C), also enhanced insulin-induced endogenous STAT3 activation. RACK1-mediated STAT3 activation through IR was specific since RACK1 could not affect insulin-induced phosphorylation levels of IR, Akt, and MAPK in HEK 293T and NIH 3T3/IR cells (Fig. 1A, B, E, and F).
FIG. 1.
RACK1 enhances IR/IGF-1R-mediated STAT3 tyrosine phosphorylation specifically. HEK 293T cells were transfected with 150 ng of IR (A and E) or 150 ng of IGF-1R (D), 150 ng of STAT3, and 250 ng of phEF-RACK1-HA or phEF-Neo at 60% confluence. (B and F) NIH 3T3/IR cells were transfected with 1 μg of phEF-RACK1-HA or phEF-Neo. After 24 h, cells were serum starved overnight and then were stimulated with insulin (50 nM), IGF-1 (50 ng/ml), or 10% fetal calf serum-DMEM, as indicated, for 15 min or were left unstimulated. (C) Mewo cells were starved overnight, followed by introduction of TAT-RACK1 in serum-free DMEM containing 10% methanol for 30 min and then stimulated with insulin for 15 min. Protein was extracted using NP-40 lysis buffer. Twenty micrograms of cell lysate or immunoprecipitate from 500 μg of total cell lysate was resolved in SDS-PAGE and subjected to Western blotting with the indicated antibodies. Results represented five and three independent experiments of HEK 293T and NIH 3T3/IR cells, respectively. In all the experiments described hereafter, protein densitometry was measured by Image J, and the activation levels were normalized to their corresponding protein levels. Ins, insulin.
To assess the importance of endogenous RACK1 in IR/IGF-1R-STAT3 signaling, transient transfection of in vitro synthesized double-strand siRNA in NIH 3T3/IR cells, but not the scrambled control RNA, resulted in more than a70% decrease in RACK1 expression; accordingly, insulin-induced phosphorylation of STAT3 was reduced to 38% (Fig. 2A). Mewo cells stably transfected with the pRetroSuper (pRS) control plasmid or pRS-shRNA against RACK1 (42) were examined for IR-mediated STAT3 activation. Expression of pRS-shRNA in Mewo cells resulted in up to 40% downregulation of the RACK1 protein without affecting unrelated proteins such as β-tubulin, Erk1/2, Akt, or IR (Fig. 2B). Insulin-induced phosphorylation of STAT3 was reduced to 36% in the pRS-shRNA-transfected Mewo cells in comparison to the control (Fig. 2B). To check the effect of RACK1 expression on IGF-1-induced STAT3 phosphorylation, two independent siRNAs of RACK1 were transiently transfected into SKOV3 cells (ovarian cancer cell line with a high level of IGF-1R expression), resulting in a decrease of RACK1 protein expression to about 20% (Fig. 2C); IGF-1-induced phosphorylation of STAT3 was also significantly reduced to about 50% by both siRNAs. By contrast, insulin- or IGF-1-induced phosphorylation of Erk1/2, Akt, or receptors was not affected by downregulation of the RACK1 expression (Fig. 2B and C). The above observations indicate that endogenous RACK1 plays an important role in mediating STAT3 activation through IR/IGF-1R specifically and neither overexpression nor downregulation of RACK1 affects other IR/IGF-1R-mediated signaling including Erk1/2 and PI3K. Moreover, the RACK1-mediated insulin and IGF-1-induced STAT3 phosphorylation is IR/IGF-1R specific.
FIG. 2.
Downregulation of RACK1 expression attenuates endogenous IR/IGF-1R-mediated tyrosine phosphorylation of STAT3 specifically. (A) NIH 3T3/IR cells were transiently transfected with RACK1-specific siRNA1 or a scrambled control. (B) Mewo mass culture from pRS vector control or pRS/shRNA specific to human RACK1 was selected by puromycin. (C) SKOV3 cells were transiently transfected with human RACK1-specific siRNA1 and siRNA2 or a scrambled control for siRNA1 (c-siRNA). Cells were serum starved overnight and then stimulated with insulin (50 nM) or IGF-1 (50 ng/ml) for 15 min or left unstimulated. SDS-PAGE and Western blotting were the same as described in the legend to Fig. 1. Results represent three independent experiments. All the activation levels were normalized to their corresponding protein expression levels. Student t tests were performed with P values of ≤0.02 (*). ip, immunoprecipitation; ib, immunoblotting; Ins, insulin.
RACK1 associates with IR/IGF-1R and STAT3 both in vivo and in vitro.
To investigate the mechanistic role of RACK1 in the IR/IGF-1R-STAT3 pathway, coimmunoprecipitation was carried out to test the interaction of endogenous IR, IGF-1R, RACK1, and STAT3. Treatment of the starved HEK 293T cells with insulin or IGF-1 resulted in significantly enhanced coimmunoprecipitation of IR or IGF-1R with RACK1 and STAT3 beyond a basal level of association (Fig. 3A and D), confirming our previous result of IGF-1-augmented association between IGF-1R and RACK1 (30). When IR was transiently expressed in an IR null cell line called SV40, IR could also be coimmunoprecipitated with RACK1 upon insulin induction but not in SV40 cells expressing green fluorescent protein, suggesting that the interaction is specific (Fig. 3B). The same result was confirmed in NIH 3T3 cells (Fig. 2A and 3C). Detection of association between IR and RACK1 turns out to be very sensitive to the detergent and glycerol components in the buffer (see Fig. S2 in the supplemental material). The IR antibody we generated could not precipitate IR efficiently in digitonin-containing buffer for unknown reasons, which may explain why IR and RACK1 association could not be detected in our previous study (30). In contrast to the association with IR or IGF-1R, the association between RACK1 and STAT3 appeared to be constitutive and did not depend on insulin induction (Fig. 3A and C). No such interaction was detected using the isotype nonimmune mouse immunoglobulin M as a control antibody (Fig. 3A).
FIG. 3.
Association between endogenous IR/IGF-1R, RACK1, and STAT3 intracellularly. HEK 293T cells (A), SV40 IR−/− cells transiently transfected with 2 μg of green fluorescent protein or IR (B), 3T3/IR cells (C), or HEK 293T cells transfected with 200 ng of phEF-neo or IGF-1R (D) were starved overnight, either left untreated or treated with insulin (A, B, and C) or IGF-1 (D) for 15 min. Cell lysate preparation and coimmunoprecipitation were performed as described in the legend to Fig. 1 with the indicated antibodies, followed by SDS-PAGE and immunoblotting (TCL, total cell lysates). Results are from three independent experiments. ip, immunoprecipitation; ib, immunoblotting; TCL, total cell lysate; Ins, insulin.
To further explain the enhancement of IR/IGF-1R-mediated STAT3 activation by RACK1, different amounts of RACK1 were transiently transfected together with IR or IGF-1R and STAT3 into HEK 293T cells. At a low level of RACK1 expression, the association between IR (Fig. 4A) or IGF-1R (Fig. 4B) and exogenous RACK1 was found to be elevated upon respective ligand induction, with a certain basal level of constitutive association; with increasing levels of RACK1 expression (up to 250 ng of RACK1 transfection), the association was elevated but became constitutive, suggesting that the RACK1 level is very important for inducible association. The ectopic expression of RACK1 also affected the association pattern between the endogenous RACK1 and IGF-1R, inducible association of which was also dependent on the RACK1 level in that the association was elevated and inducible at a low RACK1 level and gradually became constitutive with an increasing level of RACK1 (Fig. 4B). Similar to the association between of RACK1 and IR, the association between STAT3 and IR also shifted from inducible to constitutive with increasing levels of RACK1 (Fig. 4A).
FIG. 4.
Interactions between IR/IGF-1R and RACK1 or STAT3 were dependent on the RACK1 expression level and WD1-4 of RACK1. Different amounts of RACK1 construct were transfected into HEK 293T cells, along with 150 ng of STAT3 and IR (A) or with 150 ng of STAT3 and IGF-1R (B). (C) HEK 293T cells were transfected with 150 ng of IR, 150 ng of STAT3, and 250 ng of phEF-RACK1-HA or its mutants, phEF-HA-WD1-4, phEF-HA-WD5-7, or phEF-Neo control vector. After starvation and induction with ligands, cell lysate preparation and coimmunoprecipitation were performed as described in the legend to Fig. 1 with the indicated antibodies, followed by SDS-PAGE and immunoblotting. (D) Extracts of HEK 293T cells transiently overexpressing IR and STAT3, either treated or nontreated with insulin after starvation, were fractionated by glycerol gradient sedimentation. Proteins of the selected gradient fractions were immunoprecipitated with anti-RACK1 antibody and separated by SDS-PAGE, followed by immunoblotting with the indicated antibodies. Results represent a typical one from three independent experiments. ip, immunoprecipitation; ib, immunoblotting; TCL, total cell lysate; Ins, insulin; Endo, endogenous.
To determine the importance of endogenous RACK1 for the interaction between IR and STAT3, siRNA of RACK1 was transiently transfected into in NIH 3T3/IR cells. Reduced expression of RACK1 caused a correspondingly reduced interaction between IR and STAT3 (Fig. 2A, lower panel) and diminished IR-mediated STAT3 activation (Fig. 2A, upper panel). The parallel increase and decrease of RACK1/IR and STAT3/IR association according to the RACK1 expression level suggests that RACK1 plays an important role in mediating the association of STAT3 with IR.
To determine which region of RACK1 is responsible for its interaction with STAT3 and IR/IGF-1R, truncation mutants of RACK1 were constructed. Both IR and STAT3 were coimmunoprecipitated with HA-RACK1 and the 3′ deletion mutant HA-WD1-4 (amino acids 1 to 180) but not with the 5′ deletion mutant HA-WD5-7 (amino acids 181 to 317) (Fig. 4C). The association indicates that interaction between RACK1 and IR or STAT3 is not due to nonspecific interaction by HA-tagged protein. This result suggests that the WD1-4 domain is sufficient to interact with IR and STAT3 and enhance the IR-mediated STAT3 activation (see Fig. S3 in the supplemental material).
To detect the triple complex formation of IR, STAT3, and RACK1, glycerol gradient sedimentation was performed to examine the distribution patterns of IR, STAT3, and RACK1 proteins with or without insulin induction in HEK 293T cells. The results of immunoblotting analysis revealed that STAT3 or RACK1 had broad distribution along the glycerol gradient, peaking between fractions 16 to 18, while IR only appeared in fractions 9 to 15 with a peak at fractions 13 to 14. Insulin induction did not shift the gross distribution patterns of all three proteins (see Fig. S4 in the supplemental material). However, IR was coimmunoprecipitated with RACK1 protein upon insulin induction, which further supports the results shown in Fig. 3; the IR peak shifted to the heavier sedimentation fraction 12 (Fig. 4D). STAT3 associated with RACK1 constitutively (Fig. 3A and C and 4D); however, insulin stimulation led to the accumulation of STAT3/RACK1 complexes to the faster sedimentation fractions 12 to 15, with a peak at fraction 12 (Fig. 4D), corresponding to the IR peak coimmunoprecipitated with RACK1. The result supports the insulin-inducible triple protein complex formation of IR, RACK1, and STAT3 and strengthens our hypothesis that RACK1 recruits STAT3 to IR for activation.
To test if RACK1 can interact with IR, IGF-1R, and STAT3 directly, in vitro binding assays were carried out with GST-STAT3, GST-IRKD (containing IR cytoplasmic domain), GST-IGFRKD (containing IGF-1R cytoplasmic domain), and His-tagged RACK1 or its truncation mutants. All the fusion proteins were expressed and purified from Escherichia coli, except that IRKD was also expressed in insect cells. His-RACK1 could pull down IR kinase domain expressed in insect cells (4) in a dose-dependent manner (Fig. 5A). His-RACK1 directly interacted with GST-STAT3, GST-IRKD, and GST-IGFRKD but not with GST (Fig. 5B). His-RACK1 and WD1-4, but not WD5-7, were able to associate with GST-STAT3, GST-IRKD, and GST-IGFRKD (Fig. 5C, D, and E). These data support the intracellular association of the proteins described above and suggest that RACK1 could interact directly with IR, IGF-1R, and STAT3.
FIG. 5.
RACK1 interacts with IRKD, IGFRKD, and STAT3 directly in vitro. (A) Two milligrams of insect cell lysate containing IR cytoplasmic domain was incubated with different amounts of purified His-RACK1. (B) Purified His-RACK1 was incubated with GST (lane 1), GST-IRKD (lane 2), GST-IGFRKD (lane 3), and GST-STAT3 (lane 4) attached to glutathione beads and incubated in NP-40 lysis buffer at 4°C overnight. (C, D, and E) The same molar quantities of different purified proteins were mixed and similarly incubated overnight. Subsequent to the incubation, the mixture was divided evenly into two tubes and incubated with either nickel (for His-RACK1) or glutathione beads (for GST-tagged proteins). (F) Input amounts of His-RACK1, His-WD1-4, and His-WD5-7 for panels C, D, and E, detected by anti-His antibody. All the samples were resolved by SDS-PAGE, followed by Pauceau S staining and/or immunoblotting with the indicated antibodies. Results represent a typical one from three independent experiments. ip, immunoprecipitation; ib, immunoblotting.
Association between IR/IGF-1R and RACK1 is critical for STAT3 recruitment and activation.
It was shown that an IGF-1R point mutation of S1248A (IGF-1R/S1248A) and double mutations of Y1250F/Y1251F (IGF-1R/YY mutant) failed to associate with RACK1 (35, 36); however, the functional consequence of the mutant in IGF-1R signaling is still unclear. The serine 1248 of IGF-1R is conserved in the IR at position 1275 (25), suggesting that S1275 could mediate RACK1 interaction with IR. IGF-1R/S1248A, IGF-1R/YY mutant, and IR/S1275A were constructed to test their ability to associate with RACK1 and activate STAT3. An equivalent amount of the wild-type or mutant receptor was transfected with STAT3 into the HEK 293T cells. The ability of IGF-1R/S1248A (Fig. 6A and C) and IR/S1275A mutants (Fig. 6B and D) to associate with the endogenous RACK1 was significantly decreased to 37% and 33%, respectively, and so was their ability to recruit STAT3 for tyrosine phosphorylation in comparison with their respective wild-type receptors. The residual activation of STAT3 by the mutant receptors could have resulted from the heterodimerization between the mutant receptors and their respective endogenous receptors in HEK 293T cells. Similar results of decreased IGF-1-induced STAT3 activation with the IGF-1R/YY mutant was observed (data not shown). The other signaling pathways, including those of MAPK, PI3K, IRS1, SHC, Grb2, phospholipase Cγ, FAK, and p130cas, were unaffected compared to the wild-type receptors (Fig. 6A to D and data not shown). Our data demonstrate that the association between RACK1 and IR/IGF-1R is important for the receptor-mediated recruitment and activation of STAT3 specifically.
FIG. 6.
Association between IR/IGF-1R and RACK1 is important for STAT3 activation. (A and C) HEK 293T cells were transfected with 150 ng of STAT3, along with 150 ng of phEF-neo, phEF-IGFR, or phEF-IGFR/S1248A. (B and D) A total of 150 ng of STAT3 was transfected into HEK 293T cells with 150 ng of either wild-type IR or mutant IR/S1275A. At 24 h after transfection, cells were serum starved overnight and then stimulated with IGF-1 (A and C) or insulin (B and D), and cell lysates were prepared as above. One milligram of lysate was used for immunoprecipitation. Lysates or immunoprecipitates were separated by SDS-PAGE and detected by immunoblotting with the indicated antibodies. (C and D) The histograms show average quantification results of the IR/IGF-1R/RACK1 association and activation of various signaling molecules from two independent experiments. Student t tests were performed with P values of ≤0.01 (*). ip, immunoprecipitation; ib, immunoblotting; Ins, insulin.
Overexpression of RACK1 enhances tyrosine-phosphorylated STAT3 in both cytoplasm and nucleus and augments transcriptional activity of STAT3.
To examine whether overexpression of RACK1 would affect the nuclear translocation and transcriptional activity of STAT3, fractionation was performed at different times after insulin stimulation. Nuclear translocation of pSTAT3 was observed within 15 min upon insulin stimulation, reaching a maximum around 2 h in both control and RACK1-overexpressing cells. The pSTAT3 level in both nuclear and cytoplasmic fractions was greatly elevated and prolonged in RACK1 overexpressing cells (see Fig. S5 in the supplemental material).
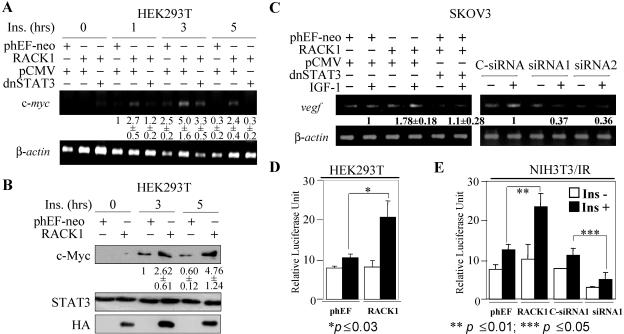
c-myc is an important STAT3 target proto-oncogene (6, 9, 10) in the regulation of cell cycle progression and proliferation; vegf is another STAT3 target gene involved in angiogenesis (46). Overexpression of RACK1 enhanced insulin-induced c-myc mRNA (Fig. 7A) and protein (Fig. 7B) in HEK 293T cells and IGF-1 induced vegf mRNA in SKOV3 cells. The enhancement was dependent on STAT3 because dominant negative STAT3 (dnSTAT3, defective in DNA binding and shown to be able to block the wild-type STAT3 activation [31]) greatly reduced the RACK1-mediated enhancement of insulin-induced c-myc mRNA (Fig. 7A) and IGF-1-induced vegf mRNA (Fig. 7C). The expression of IGF-1-induced vegf gene in SKOV3 cells was also dependent on endogenous RACK1 since downregulation of RACK1 expression by two independent siRNAs significantly reduced IGF-1-induced vegf gene expression (Fig. 7C). Overexpression of RACK1 significantly augmented insulin-induced transcription of the STAT3-specific reporter gene in both HEK 293T and NIH 3T3 cells (Fig. 7D and E). Downregulation of RACK1 by siRNA significantly decreased insulin-induced STAT3-specific reporter gene expression in NIH 3T3 cells, suggesting that RACK1 specifically regulates the IR/IGF-1R-mediated STAT3 phosphorylation and activation of its transcriptional activity and target gene expression.
FIG. 7.
Overexpression of RACK1 enhances IR IGF-1R-mediated STAT3 transcriptional activity. HEK 293T cells were transfected with IR (150 ng) and STAT3 (150 ng), together with phEF-Neo+pCMV, RACK1+pCMV, or RACK1+dnSTAT3 (phEF-neo and RACK1, 250 ng; pCMV and dnSTAT3, 500 ng). After 24 h, cells were serum starved for 12 h and then stimulated with insulin for different time periods as indicated or left unstimulated. (A) Total RNA was extracted, followed by reverse transcription-PCR, using β-actin as an internal control. (B) Protein levels were measured by SDS-PAGE and immunoblotting. The c-Myc RNA and protein levels were quantified and averaged from two independent experiments. (C) SKOV3 cells were transfected with phEF-Neo+pCMV, RACK1+pCMV, or RACK1+dnSTAT3 (phEF-neo and RACK1, 2 μg; pCMV and dnSTAT3, 1 μg) or transfected with siRNA1, siRNA2, or C-siRNA1. After 24 or 96 h (for siRNA only), cells were serum starved for 24 h and then stimulated with 50 ng/ml IGF-1 for 6 h or left unstimulated. Reverse transcription-PCR was performed as described for panel A. The RACK1 expression level in siRNA-transfected experiments was shown in Fig. 2C. Numbers below the gels are the average from two independent experiments. (D) HEK 293T cells were transfected with STAT3-specific reporter plasmid TKS3 (50 ng) or pLucTK (control), together with IR, STAT3, and RACK1 or phEF-Neo. (E) 3T3/IR cells were transfected with STAT3-specific reporter plasmid 3×Ly6E and pLucTK, together with RACK1 or phEF-Neo, or transfected together with siRNA or scrambled control. After 24 h (for RACK1 or phEF-neo) or 96 h (for siRNA1 or scrambled control), cells were serum starved for 12 h and then stimulated with insulin for 6 h. Luciferase activity was measured by a dual-luciferase reporter assay kit from Promega. Results were from three independent experiments. Student t tests were performed for data shown in panels D and E with the P values indicated. Ins, insulin.
RACK1 modulates certain oncogenic properties of cancer cells via the IR/IGF-1R-STAT3 signaling pathway.
Previous results showed that IR/IGF-1R, STAT3, and RACK1 were either up-regulated or constitutively active in certain cancer cells (see above); STAT3 was found to be important in regulating anchorage-independent growth and survival (55). These observations prompted us to investigate the role of IR/IGF-1R-RACK1-STAT3 signaling in regulating certain oncogenic properties.
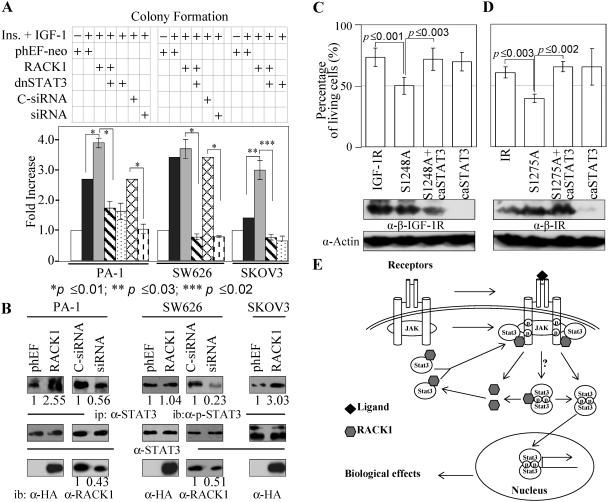
A panel of human ovarian cancer cell lines was screened for the expression of RACK1 and found to express various levels of RACK1 (data not shown). Three lines, PA-1, SW626, and SKOV3, with high and low RACK1 expression levels and with moderate to high levels of expression of IR/IGF-1R and STAT3 were chosen for further analysis (see Fig. S6A in the supplemental material). PA-1 and SW626 cells had a relatively high level of RACK1. Insulin and IGF-1 promoted a 2.5-fold increase of colony formation in PA-1 cells, which was further enhanced by about 40% upon overexpression of RACK1 (Fig. 8A). Overexpression of RACK1 had only a small effect on promoting insulin- and IGF-1-stimulated colony formation of SW626 cells (Fig. 8A). However, reducing the RACK1 level by twofold using siRNA in PA-1 and SW626 cells (Fig. 8B) greatly reduced the insulin- and IGF-1-induced colony forming ability of the cells (Fig. 8A). The control siRNA had no significant effect. Introduction of dnSTAT3 together with RACK1 into PA-1 and SW626 cells neutralized the enhancing effect of RACK1 and further reduced the insulin- and IGF-1-induced colony forming ability of the cells (Fig. 8A). SKOV3 cells had a relatively low level of RACK1 (see Fig. S6A in the supplemental material). Overexpression of RACK1 resulted in a twofold enhancement of the insulin- and IGF-1-induced colony forming ability of the cells (Fig. 8A). Similarly, introduction of the dnSTAT3 together with RACK1 into SKOV3 cells neutralized the effect of RACK1 and further reduced the colony forming ability to a level similar to that of dnSTAT3 alone (Fig. 8A). The tyrosine phosphorylation level of STAT3 was consistent with overexpression or reduction of RACK1 in the three cancer lines, with the exception of RACK1 overexpression in SW626 cells (Fig. 8B). This could be due to the fact that SW626 cells have a relatively high level of RACK1, which is more than sufficient to mediate activation of a relatively low level of STAT3 (see Fig. S6A in the supplemental material). The differential response of the three lines toward dnSTAT3 could be due to different STAT3 expression levels in the cells; SW626, with a lower expression level of STAT3, was more sensitive to dnSTAT3, while PA-1, with a higher level of STAT3 than SW626 and SKOV3 cells, was relatively more resistant to dnSTAT3 (see Fig. S6A in the supplemental material). Unlike anchorage-independent growth, RACK1 was also able to enhance the migration and invasion activity of PA-1 cells; dnSTAT3 was unable to reverse the effect of RACK1 (see Fig. S6B in the supplemental material).
FIG. 8.
RACK1 contributes to certain oncogenic properties of ovarian cancer cells through the IR/IGF-1R-STAT3 signaling pathway. PA-1, SW626, and SKOV3 cells were transfected with different plasmids (2 μg of RACK1 or phEF-neo, 1 μg of dnSTAT3, 10 nM of C-siRNA or siRNA) as indicated. (A) Colony formation assays of PA-1, SW626, and SKOV3 cells were performed 48 h posttransfection as described in Materials and Methods. All the experiments were carried out in the presence of insulin (50 nM) and IGF-1 (50 ng/ml), except where indicated. (B) Cell lysates from different transfections were collected 48 h or 96 h (for siRNA-transfected cells only) posttransfection. Protein levels were measured by SDS-PAGE and immunoblotting. Results for colony formation assays were from three independent experiments with duplication. Results for STAT3 activation levels represent one typical experiment from three independent experiments. Student t tests were performed with P values indicated. (C and D) NIH 3T3 cells were transfected with different plasmids as indicated. At 24 h posttransfection, cells were serum starved overnight, followed by IGF-1 or insulin treatment for 10 h. Anoikis assays were carried out with the presence of IGF-1 or insulin as described in Materials and Methods. Student t tests were performed with the P values indicated. (E) Model for RACK1-mediated STAT3 activation by IR and IGF-1R (see text for details). Ins, insulin; C-siRNA, scrambled control siRNA.
To test the ability of mutant receptors, IR/S1275A and IGF-1R/S1248A, in protecting cells from anoikis and thus enabling the anchorage-independent growth, NIH 3T3 cells were transiently transfected with an equivalent amount of wild-type or mutant receptor (Fig. 8C and D). IGF-1R/S1248A (Fig. 8C) and IR/S1275A mutants (Fig. 8D), which were attenuated in recruiting and activating STAT3 (Fig. 6), also had a reduced ability compared to their wild-type counterpart receptors in protecting cells from anoikis. Constitutively active STAT3 restored the protection ability from anoikis by the mutant receptors to a level similar to their corresponding wild-type receptors (Fig. 8C and D), suggesting that IR/IGF-1R-RACK1-STAT3 signaling is important for IR/IGF-1R-mediated protection from anoikis.
The above results are consistent with the notion that RACK1 functions upstream of STAT3 in the IR/IGF-1R-STAT3 signaling pathway, which appears to be more important for anchorage-independent growth and survival than for migration and invasion under those conditions.
DISCUSSION
STAT3 is one of the downstream effectors of IR and IGF-1R (17, 63), but the mechanism of how STAT3 is recruited to the receptors remains unresolved. RACK1 was shown to interact with IGF-1R and IR (30, 35, 36) and regulate IGF-1R-mediated mitogenic signaling. Our present data strongly suggest that RACK1 facilitates the association of STAT3 with IR and IGF-1R for phosphorylation, most likely by recruiting STAT3 to the receptors (Fig. 8E). RACK1 appears to be an important mediator since decreasing the abundance of RACK1 or impairing its binding to IR and IGF-1R greatly diminished the binding of STAT3 with the receptors and subsequent activation of STAT3. Our findings suggest that facilitated recruitment of STATs via an adaptor to RPTKs plays an important role in the activation of STATs by these receptors (Fig. 8E). We hypothesize that both IR/IGF-1R and the associated JAKs (38, 63) are involved in the phosphorylation of STAT3. However, it is not clear when RACK1 dissociates from the phosphorylated STAT3 (Fig. 8E).
RACK1 regulates IR/IGF-1R-mediated STAT3 activation in a receptor-specific and pathway-specific manner. The basis for the specificity could be due to specific interaction between RACK1 and IR or IGF-1R. Apparently, EGFR and Ros are able to activate STAT3 independent of RACK1. However, we cannot rule out the possibility that RACK1 may mediate STAT3 activation in certain other RPTKs. The RACK1 expression level did not affect other IR/IGF-1R-mediated signaling pathways in HEK 293T (Fig. 1A and E), NIH 3T3/IR (Fig. 1B and F), Mewo (Fig. 2B), SKOV3 (Fig. 2C), and SW626 (data not shown) cells, although Kiely et al. reported that overexpression of RACK1 in an IGF-1R reconstituted mouse cell line (R+) and in the human breast cancer line MCF7 cells resulted in enhanced IGF-1-induced MAPK activation but decreased Akt activation (36). We partially confirmed their results concerning the effect of RACK1 on IGF-1-induced activation of MAPK by either overexpression or repression of RACK1 in MCF7 cells but failed to see the change in Akt activation. The reason for the paradox between the observation of Kiely et al. and ours is still unclear; however, it may be cell type dependent or relevant to the IGF-1R and RACK1 expression levels in the MCF7 cells. The observed augmenting effect of RACK1 on IGF-1-induced MAPK activation (36) could be due to an indirect mechanism, for example, mediated by RACK1/protein kinase C (PKC). Nevertheless, among the six independent cell lines tested, we only observed a partial effect of RACK1 on IGF-1-induced MAPK activation in MCF7 cells (data not shown).
Our current data confirm the interaction of RACK1 and IR reported by Kiely et al. (36). The failure to observe such interaction in our previous study (30) was apparently due to the nonoptimal combination of detergent and the antibody used, as demonstrated in Fig. S2 in the supplemental material. However, failure to detect the IR/RACK1 interaction in a yeast two-hybrid assay previously (30) remains puzzling. One possibility is that the cytoplasmic domain of IR used as bait in that assay could inadvertently harbor a mutation affecting RACK1 binding but not that of STAT5 used as a positive control (30).
In MCF7 and R+ cells, Kiely et al. reported that association of RACK1 with IR and IGF-1R was constitutive (36). Although we also observed a basal level of association, our data show that association of RACK1 with IR and IGF-1R is significantly augmented by ligand stimulation at a low level of RACK1 expression and that the association becomes constitutive beyond a certain threshold of RACK1 expression, in agreement with our previous observation (30). The expression level of RACK1 seems to be critical for the pattern of interaction between IR or IGF-1R and RACK1, as well as for the interaction between STAT3 and IR, suggesting that the concentration of RACK1 around the receptors is a determining factor of the association. The binding of endogenous RACK1 to IGF-1R in HEK 293T cells shifted from being inducible to constitutive upon increasing the level of HA-RACK1 (Fig. 4B), which may be explained by heterodimerization between HA-RACK1 and endogenous RACK1 (52) or by competition for cytosolic localization. The interaction between IR and STAT3 is dramatically enhanced by overexpression of RACK1 and decreased by downregulation of RACK1 expression (Fig. 2A and 4A). These observations and the constitutive association between STAT3 and RACK1 (Fig. 3A and C and 4D) support our hypothesis that RACK1 forms a constitutive complex with STAT3 in cytosol, which is recruited to the IR/IGF-1R specifically, subsequent to the activation of receptors (Fig. 8E).
WD1-4 of RACK1 is responsible for the IGF-1R/IR and STAT3 binding. Based on the amino acid similarity and Gβ crystal structure (58), RACK1 was predicted as a seven-bladed propeller Gβ-like structure with significant differences in the sixth and seventh WD domains, which are required for interaction with different proteins including PKCβ, PDE4D5, Src, and β-integrin (44); by contrast, IR, IGF-1R, and STAT3 seem to interact with WD1-4 of RACK1. The predicted structure of WD1-4 is quite similar to that of the Gβ subunit. The finding that Gβ can also bind to IGF-1R and RACK1 (16, 19) is consistent with our result. It would be interesting to see if Gβ plays a role similar to that of RACK1 in recruiting other signaling effectors of IGF-1R.
RACK1 may form a platform in IR/IGF-1R-mediated STAT3 signaling. Triple protein complexes of IR, STAT3, and RACK1 were formed upon insulin stimulation of IR as shown in a glycerol sedimentation assay. It remains a possibility that RACK1 could recruit other molecules involved in STAT3 activation including JAK family kinases and Ser/Thr kinases. We have shown that JAKs play a positive role in IR-mediated STAT5 and STAT3 activation and IGF-1R-mediated STAT3 activation (38, 63 and data not shown). JAKs were reported to interact with RACK1, contributing to IFN-α-mediated STAT1 activation (28). It is possible that RACK1 could also recruit JAKs for IR/IGF-1R-mediated STAT3 activation. Other STAT3 regulators including PKC family kinases and MAPKs have been reported to be involved in the phosphorylation and activation processes of STAT3 upon different stimuli (23). We did find that serine 727 phosphorylation of STAT3 was also enhanced by overexpression of RACK1 (data not shown), but the mechanism for this enhanced phosphorylation is not clear at the present time. In this regard, our previous finding that IGF-1 induces increased association of IGF-1R with PKCδ and PKCμ as well as association of RACK1 and PKCμ is interesting. It will be worthwhile to further investigate the role of RACK1 in the IGF-1-induced interaction between IGF-1R and PKCs.
The IR/IGF-1R-RACK1-STAT3 pathway regulates oncogenic properties of certain ovarian cancer cells. STAT3 was found to be important in regulating anchorage-independent growth (55). The function of RACK1 in IGF-1R-mediated mitogenic effect appears to depend on the type of cells (30, 36). Overexpression of RACK1 was able to significantly enhance the insulin- and IGF-1-promoted anchorage-independent growth of PA-1 and SKOV3 cells (Fig. 8A). It was suggested that the reasons for the diverse effect of RACK1 on the growth in different cell systems could be due to the possibility that fibroblasts and transformed epithelial cells differ significantly with regard to integration and cross talk between adhesion molecule-mediated and growth factor receptor-mediated signaling (47). Another possible reason for the differential effect of RACK1 on cell growth may be the cellular localization of RACK1. RACK1 was redistributed to the membrane in several oncogene-transformed (including oncogenic IGF-1R and IR) mammalian and avian cells (30), which may allow RACK1 to regulate mitogenic signaling more effectively from the plasma membrane. In mouse fibroblasts, RACK1 is located mostly to the cytoskeleton with a certain amount in the cytoplasm (30); thus, it may sequester certain molecules from reaching the membrane. The role of RACK1 in regulating various oncogenic properties of cancer cells may be the sum of the multifaceted effect of RACK1 in various signaling pathways including the IR/IGF-1R-STAT3 signaling demonstrated previously (30, 35, 36) and in the present study as well as the PKC/JNK pathway mediated by RACK1 (42). Overexpression of RACK1 enhances stress fibers, focal adhesions, and membrane protrusion, and the opposite is true by its depletion (30). Accordingly, RACK1 is expected to play a role in regulating cell migration. However, the limited reports on the effect of RACK1 on migration are controversial (3, 8, 18). The reason for the different effects of RACK1 on migration in those cells is currently unknown.
In conclusion, RACK1 acts as an adaptor protein to facilitate the association of STAT3 with IR/IGF-1R and subsequent phosphorylation and activation of STAT3 specifically. In this fashion, RACK1 plays an important role in IR/IGF-1R-mediated promotion of anchorage-independent growth of certain ovarian cancer cells.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants CA29339 and CA55054.
We thank Steven Dowdy for providing pTAT vector; Ronald Kohanski for the gift of the IRKD-expressing insect cells; C. Horvath, J. Darnell, and R. Jove for the dnSTAT3 mutant and reporter plasmids; and Joseph Chan for discussion.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Baker, J., J. P. Liu, E. J. Robertson, and A. Efstratiadis. 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73-82. [PubMed] [Google Scholar]
- 2.Berns, H., R. Humar, B. Hengerer, F. N. Kiefer, and E. J. Battegay. 2000. RACK1 is up-regulated in angiogenesis and human carcinomas. FASEB J. 14:2549-2558. [DOI] [PubMed] [Google Scholar]
- 3.Besson, A., T. L. Wilson, and V. W. Yong. 2002. The anchoring protein RACK1 links protein kinase Cε to integrin β chains. Requirements for adhesion and motility. J. Biol. Chem. 277:22073-22084. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, S. M., J. B. Ross, and R. A. Kohnaski. 1999. Autophosphorylation dependent destabilization of the insulin receptor kinase domain: trytophan-1175 reports changes in the catalytic cleft. Biochemistry 38:3079-3089. [DOI] [PubMed] [Google Scholar]
- 5.Blenis, J. 1993. Signal transduction via the MAP kinases: proceed at your own RSK. Proc. Natl. Acad. Sci. USA 90:5889-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman, T., R. Garcia, J. Turkson, and R. Jove. 2000. STATs in oncogenesis. Oncogene 19:2474-2488. [DOI] [PubMed] [Google Scholar]
- 7.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 8.Buensuceso, C. S., D. Woodside, J. L. Huff, G. E. Plopper, and T. E. O'Toole. 2001. The WD protein Rack1 mediates protein kinase C and integrin-dependent cell migration. J. Cell Sci. 114:1691-1698. [DOI] [PubMed] [Google Scholar]
- 9.Buettner, R., L. B. Mora, and R. Jove. 2002. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 8:945-954. [PubMed] [Google Scholar]
- 10.Calo, V., M. Migliavacca, V. Bazan, M. Macaluso, M. Buscemi, N. Gebbia, and A. Russo. 2003. STAT proteins: from normal control of cellular events to tumorigenesis. J. Cell. Physiol. 197:157-168. [DOI] [PubMed] [Google Scholar]
- 11.Cantley, L. C., K. R. Auger, C. Carpenter, B. Duckworth, A. Graziani, R. Kapeller, and S. Soltoff. 1991. Oncogenes and signal transduction. Cell. 64:281-302. [DOI] [PubMed] [Google Scholar]
- 12.Chang, B. Y., R. A. Harte, and C. A. Cartwright. 2002. RACK1: a novel substrate for the Src protein-tyrosine kinase. Oncogene 21:7619-7629. (Erratum, 22:1748.) [DOI] [PubMed] [Google Scholar]
- 13.Cheatham, B., C. J. Vlahos, L. Cheatham, L.Wang, J. Blenis, and C. R. Kahn. 1994. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. 14:4902-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, C. A., and H. Okayama. 1988. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. BioTechniques 6:632-638. [PubMed] [Google Scholar]
- 15.Chen, J., H. B. Sadowski, R. A. Kohanski, and L. H. Wang. 1997. Stat5 is a physiological substrate of the insulin receptor. Proc. Natl. Acad. Sci. USA 94:2295-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, S., B. D. Spiegelberg, F. Lin, E. J. Dell, and H. E. Hamm. 2004. Interaction of Gβγ with RACK1 and other WD40 repeat proteins. J. Mol. Cell Cardiol. 37:399-406. [DOI] [PubMed] [Google Scholar]
- 17.Coffer, P. J., A. van Puijenbroek, B. M. Burgering, M. Klop-de Jonge, L. Koenderman, J. L. Bos, and W. Kruijer. 1997. Insulin activates Stat3 independently of p21ras-ERK and PI-3K signal transduction. Oncogene 15:2529-2539. [DOI] [PubMed] [Google Scholar]
- 18.Cox, E. A., D. Bennin, A. T. Doan, T. O'Toole, A. Huttenlocher. 2003. RACK1 regulates integrin-mediated adhesion, protrusion, and chemotactic cell migration via its Src-binding site. Mol. Biol. Cell 14:658-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalle, S., W. Ricketts, T. Imamura, P., Vollenweider, and J. M. Olefsky. 2001. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J. Biol. Chem. 276:15688-15695. [DOI] [PubMed] [Google Scholar]
- 20.Daris, R. J. 1993. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268:14553-14556. [PubMed] [Google Scholar]
- 21.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 22.DeChiara, T. M., E. J. Robertson, and A. Efstratiadis. 1991. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64:849-859. [DOI] [PubMed] [Google Scholar]
- 23.Decker, T., and P. Kovarik. 2000. Serine phosphorylation of STATs. Oncogene 19:2628-2637. [DOI] [PubMed] [Google Scholar]
- 24.Denley, A., J. C. Wallace, L. J. Cosgrove, and B. E. Forbes. 2003. The insulin receptor isoform exon 11− (IR-A) in cancer and other diseases: a review. Horm. Metab. Res. 35:778-785. [DOI] [PubMed] [Google Scholar]
- 25.Ebina, Y., L. Ellis, K. Jarnagin, M. Edery, L. Graf, E. Clauser, J. H. Ou, F. Masiarz, Y. W. Kan, and I. D. Goldfine. 1985. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signaling. Cell 40:747-758. [DOI] [PubMed] [Google Scholar]
- 26.Graham, F. L., and A. J. Van Der Eb. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456-457. [DOI] [PubMed] [Google Scholar]
- 27.Gray, S. G., I. Stenfeldt Mathiasen, and P. De Meyts. 2003. The insulin-like growth factors and insulin-signalling systems: an appealing target for breast cancer therapy? Horm. Metab. Res. 35:857-871. [DOI] [PubMed] [Google Scholar]
- 28.Haro, T., K.Shimoda, H. Kakumitsu, K. Kamezaki, A. Numata, F. Ishikawa, Y. Sekine, R. Muromoto, T. Matsuda, and M. Harada. 2004. Tyrosine kinase 2 interacts with and phosphorylates receptor for activated C kinase-1, a WD motif-containing protein. J. Immunol. 173:1151-1157. [DOI] [PubMed] [Google Scholar]
- 29.He, D. Y., A. J. Vagts, R. Yaka, and D. Ron. 2002. Ethanol induces gene expression via nuclear compartmentalization of receptor for activated C kinase 1. Mol. Pharmacol. 62:272-280. [DOI] [PubMed] [Google Scholar]
- 30.Hermanto, U., C. S. Zong, W. Li, and L. H. Wang. 2002. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol. Cell. Biol. 22:2345-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horvath, C. M., Z. Wen, and J. E. Darnell. 1995. A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 9:984-994. [DOI] [PubMed] [Google Scholar]
- 32.Jong, S.-M. J., and L.-H. Wang. 1987. The transforming protein P68gag-ros of avian sarcoma virus UR2 is a transmembrane protein with the gag portion protruding extracellularly. Oncogene Res. 1:7-21. [PubMed] [Google Scholar]
- 33.Kahn, C. R., and M. F. White,. 1988. The insulin receptor and the molecular mechanism of insulin action. J. Clin. Investig. 82:151-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kayali, A. G., J. Eichhorn, T. Haruta, A. J. Morris, J. G. Nelson, P. Vollenweider, J. M. Olefsky, and N. J. Webster. 1998. Association of the insulin receptor with phospholipase C-γ (PLCγ) in 3T3-L1 adipocytes suggests a role for PLCγ in metabolic signaling by insulin. J. Biol. Chem. 273:13808-13818. [DOI] [PubMed] [Google Scholar]
- 35.Kiely, P. A., M. Leahy, D. O'Gorman, and R. O'Connor. 2005. RACK1-mediated integration of adhesion and insulin-like growth factor I (IGF-I) signaling and cell migration are defective in cells expressing an IGF-I receptor mutated at tyrosines 1250 and 1251. J. Biol. Chem. 280:7624-7633. [DOI] [PubMed] [Google Scholar]
- 36.Kiely, P. A., A. Sant, and R. O'Connor. 2002. RACK1 is an insulin-like growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. J. Biol. Chem. 277:22581-22589. [DOI] [PubMed] [Google Scholar]
- 37.Kubota, T., N. Yokosawa, S. Yokota, and N. Fujii. 2002. Association of mumps virus V protein with RACK1 results in dissociation of STAT-1 from the alpha interferon receptor complex. J. Virol. 76:12676-12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le, M. N., R. A. Kohanski, L. H. Wang, and H. B. Sadowski. 2002. Dual mechanism of signal transducer and activator of transcription 5 activation by the insulin receptor. Mol. Endocrinol. 16:2764-2779. [DOI] [PubMed] [Google Scholar]
- 39.LeRoith, D., and C. T. Roberts, Jr. 2003. The insulin-like growth factor system and cancer. Cancer Lett. 95:127-137. [DOI] [PubMed] [Google Scholar]
- 40.Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson, and A. Efstratiadis. 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59-72. [PubMed] [Google Scholar]
- 41.Liu, D., C. S. Zong, and L. H Wang. 1993. Distinctive effects of the carboxyl-terminal sequence of the insulin-like growth factor I receptor on its signaling functions. J. Virol. 67:6835-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.López-Bergami P., H. Habelhah, A. Bhoumik, W. Zhang, L. H. Wang, and Z. Ronai. Receptor for activated C kinase 1 (RACK1) mediates activation of Jun N-terminal Kinase (JNK) by protein kinase C. Mol. Cell 19:309-320. (Erratum, 19:578-579.) [DOI] [PMC free article] [PubMed]
- 43.Mamidipudi, V., J. Zhang, K. C. Lee, and C. A. Cartwright. 2004. RACK1 regulates G1/S progression by suppressing Src kinase activity. Mol. Cell. Biol. 24:6788-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCahill, A., J. Warwicker, G. B. Bolger, M. D. Houslay, and S. J. Yarwood. 2002. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol. Pharmacol. 62:1261-1273. [DOI] [PubMed] [Google Scholar]
- 45.Mochly-Rosen, D., B. L. Smith, C. H. Chen, M. H. Disatnik, and D. Ron. 1995. Interaction of protein kinase C with RACK1, a receptor for activated C-kinase: a role in beta protein kinase C mediated signal transduction. Biochem. Soc. Trans. 23:596-600. [DOI] [PubMed] [Google Scholar]
- 46.Niu, G., K. L. Wright, M. Huang, L. Song, E. Haura, J. Turkson, S. Zhang, T. Wang, D. Sinibaldi, D. Coppola, R. Heller, L. M. Ellis, J. Karras, J. Bromberg, D. Pardoll, R. Jove, and H. Yu. 2002. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21:2000-2008. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor, R. 2003. Regulation of IGF-I receptor signaling in tumor cells. Horm. Metab. Res. 35:771-777. [DOI] [PubMed] [Google Scholar]
- 48.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 49.Scimeca, J. C., R. Ballotti, T. T. Nguyen, C. Filloux, and E. Van Obberghen. 1991. Tyrosine and threonine phosphorylation of an immunoaffinity-purified 44-kDa MAP kinase. Biochemistry 30:9313-9319. [DOI] [PubMed] [Google Scholar]
- 50.Skolnik, E. Y., A. Batzer, N. Li, C. H. Lee, E. Lowenstein, M. Mohammadi, B. Margolis, and J. Schlessinger. 1993. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science 260:1953-1955. [DOI] [PubMed] [Google Scholar]
- 51.Storz, P., H. Doppler, K. Pfizenmaier, and G. Muller. 1999. Insulin selectively activates STAT5b, but not STAT5a, via a JAK2-independent signalling pathway in Kym-1 rhabdomyosarcoma cells. FEBS Lett. 464:159-163. [DOI] [PubMed] [Google Scholar]
- 52.Thornton, C., K. C. Tang, K. Phamluong, K. Luong, A. Vagts, D. Nikanjam, R. Yaka, and D. Ron. 2004. Spatial and temporal regulation of RACK1 function and N-methyl-d-aspartate receptor activity through WD40 motif-mediated dimerization. J. Biol. Chem. 279:31357-31364. [DOI] [PubMed] [Google Scholar]
- 53.Turkson, J., T. Bowman, R. Garcia, E. Caldenhoven, R. P. De Groot, and R. Jove. 1998. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 18:2545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Usacheva, A., R. Smith, R. Minshall, G. Baida, S. Seng, E. Croze, and O. Colamonici. 2001. The WD motif-containing protein receptor for activated protein kinase C (RACK1) is required for recruitment and activation of signal transducer and activator of transcription 1 through the type I interferon receptor. J. Biol. Chem. 276:22948-22953. [DOI] [PubMed] [Google Scholar]
- 55.Uttamsingh, S., C. S. Zong, and L. H. Wang. 2003. Matrix-independent activation of phosphatidylinositol 3-kinase, Stat3, and cyclin A-associated Cdk2 is essential for anchorage-independent growth of v-Ros-transformed chicken embryo fibroblasts. J. Biol. Chem. 278:18798-18810. [DOI] [PubMed] [Google Scholar]
- 56.Van Obberghen, E., V. Baron, L. Delahaye, B. Emanuelli, N. Filippa, S. Giorgetti-Peraldi, P. Lebrun, I. Mothe-Satney, P. Peraldi, S. Rocchi, D. Sawka-Verhelle, S. Tartare-Deckert, and J. Giudicelli. 2001. Surfing the insulin signaling web. Eur. J. Clin. Investig. 31:966-977. [DOI] [PubMed] [Google Scholar]
- 57.Van Obberghen, E. 1994. Signalling through the insulin receptor and the insulin-like growth factor-I receptor. Diabetologia. Suppl. 2:S125-S134. [DOI] [PubMed] [Google Scholar]
- 58.Wall, M. A., D. E. Coleman, E. Lee, J. A. Iniguez-Lluhi, B. A. Posner, A. G. Gilman, and S. R. Sprang. 1995. The structure of the G protein heterotrimer Giα1β1χ2. Cell 83:1047-1058. [DOI] [PubMed] [Google Scholar]
- 59.Wang, Q., R. Somwar, P. J. Bilan, Z. Liu, J. Jin, J. R. Woodgett, and A. Klip. 1999. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 19:4008-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilden, P. A., and C. R. Kahn. 1994. The level of insulin receptor tyrosine kinase activity modulates the activities of phosphatidylinositol 3-kinase, microtubule-associated protein, and S6 kinases. Mol. Endocrinol. 8:558-567. [DOI] [PubMed] [Google Scholar]
- 61.Xiong, Q., J. L.-K. Chan, C. Zong, and L.-H. Wang. 1996. Two chimeric receptors of epidermal growth factor receptor and c-Ros that differ in their transmembrane domains have opposite effects on cell growth. Mol. Cell. Biol. 16:1509-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng, L., P. Sachdev, L. Yan, J. L. Chan, T. Trenkle, M. McClelland, J. Welsh, and L. H. Wang. 2000. Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol. Cell. Biol. 20:9212-9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zong, C. S., J. Chan, D. E. Levy, C. Horvath, H. B. Sadowski, and L. H. Wang. 2000. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J. Biol. Chem. 275:15099-15105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.