Abstract
The Ras family GTPases RalA and RalB have been defined as central components of the regulatory machinery supporting tumor initiation and progression. Although it is known that Ral proteins mediate oncogenic Ras signaling and physically and functionally interact with vesicle trafficking machinery, their mechanistic contribution to oncogenic transformation is unknown. Here, we have directly evaluated the relative contribution of Ral proteins and Ral effector pathways to cell motility and directional migration. Through loss-of-function analysis, we find that RalA is not limiting for cell migration in normal mammalian epithelial cells. In contrast, RalB and the Sec6/8 complex or exocyst, an immediate downstream Ral effector complex, are required for vectorial cell motility. RalB expression is required for promoting both exocyst assembly and localization to the leading edge of moving cells. We propose that RalB regulation of exocyst function is required for the coordinated delivery of secretory vesicles to the sites of dynamic plasma membrane expansion that specify directional movement.
The Ral GTPases are essential components of the Ras regulatory network and make a major contribution to Ras-induced oncogenesis (6, 9). Importantly, genetic ablation of RalGDS, the Ral guanine nucleotide exchange factor connecting Ras to Ral activation, inhibits tumorigenesis in a multistage skin carcinogenesis model and transformation by Ras in tissue culture (11). Loss-of-function analysis in human cells has revealed discrete but interlocking contributions of RalA and RalB to anchorage-independent proliferation and cancer cell survival (7, 16), and Ral activation has been suggested to play a critical role in the development of tumor metastasis (28, 31, 33).
Perhaps related to their role(s) in tumor metastasis, Ral GTPases have been functionally implicated in the regulation of cell motility (2, 10, 20, 30). However, the mechanistic basis of this contribution is unknown. Ral has two well-documented effectors; RLIP76/RalBP1, a Rac/CDC42 Gap that presumably participates in the functional assembly of endocytosis machinery (13, 19), and the Sec6/8 or exocyst complex, which facilitates regulated exocytosis to regions of rapid membrane expansion (5, 17, 18, 23, 29).
To define the mechanistic contribution of Ral GTPases to cell motility and migration, we have employed small interfering RNA (siRNA)-mediated loss-of-function analysis to directly evaluate the relative contribution of RalA, RalB, and Ral effector pathways to support of this system. Neither RalA nor the RLIP76/RalBP1 effector pathway is limiting for cell motility. In contrast, RalB and the exocyst are both required. Depletion of either RalB or any of several exocyst subunits abolishes cell migration into a wounded monolayer or across a porous membrane. The mechanistic connection between RalB and exocyst function in this biological system is highlighted by the observation that RalB expression is required both to stabilize the assembly of the full heteroocomeric exocyst complex and to localize functional exocyst complexes to the leading edge of migrating cells.
MATERIALS AND METHODS
Cell culture and wound assay.
Normal rat kidney (NRK) cells were cultivated in Dulbecco's modified Eagle medium and 10% fetal calf serum under 5% CO2 on Falcon plastic dishes. Wounds were inflicted by scratching the surface with a plastic pipette tip. Images were recorded using a Nikon TE300 microscope and a Nikon Coolpix 950 camera. Adhesion assays were performed as previously described (30).
Boyden chamber assay.
The Boyden chamber assay was performed using transwell chambers with 8-μm-pore-size membranes (Biocat Cell Culture Inserts; Becton Dickinson). The chambers were inserted into 24-well culture plates containing culture medium with either with 0.5% or 10% serum. The cells (10 × 104) were loaded into the upper compartments of the Boyden chambers. Nonmigrated cells were removed with a cotton swab, and migrating cells were fixed and stained with 0.2% crystal violet in 20% ethanol for 30 min. Cells were lysed with 30% acetic acid, and absorbance was measured at 540 nm. The intensity of the color was directly proportional to the density of cells.
Immunofluorescence.
Classic methods were used with cells being fixed in 3% paraformaldehyde, permeabilized in 1% Triton X-100, and mounted using Mowiol. Primary antibodies were obtained from Stressgen (Sec6), Transduction Laboratory (Sec8), Cell Signaling (phospho-extracellular signal-regulated kinase 1/2 [ERK1/2]) and UBI (ERK1/2), Molecular Probes (Cy3- or fluorescein isothiocyanate [FITC]-coupled phalloidin), and Sigma (α-tubulin). Secondary antibodies were from Jackson Laboratories and were coupled to FITC, Cy3, or Cy5. Anti-Sec5 sera were generated by immunization of rabbits with the Ral-binding domain of Sec5 fused to gluthathione S-transferase (25). Polyclonal antibodies against Sec10 were generated against a synthetic peptide derived from human Sec10 (positions 6 to 20) (25)
Biochemistry, immunoprecipitation, and immunodetection.
For whole-cell extracts, cells were lysed directly on plates in hot Laemmli buffer. For immunoprecipitation, cells were lysed in 20 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1 mM MgCl2, 0.1 mM dithiothreitol, 1% Triton X-100, and 10% glycerol; antibodies were used at the concentrations recommended by suppliers for immunoprecipitation or for immunodetection on membranes. Proteins were visualized on membranes with a chemoluminescent detection system (ECL; Amersham). Quantifications were performed using the Las-1000plus Luminescent Image analyzer (Fuji) and Image Gauge software.
For “scratch tests,” NRK cells were brought to confluence and scratched orthogonally at least 20 times with a p20-200 yellow tip. Cells were either further incubated for 1 or 3 h or harvested immediately. Preparation of cell extracts and the Ral trap experiments were performed according to published procedures (34). Leupeptin was from Sigma, MG132 was from Calbiochem, and concanamycin B was a gift from P. Benaroch.
siRNAs.
siRNAs were transfected at 160 nM with Oligofectamine (Invitrogen) (8). SiRNA were ordered from Dharmacon, except those targeting Sec10 and Sec8 (Proligo). Target sequences were GUAGAGAGGACCAUGAUGU (RLIP76), GAGACAACUACUUCCGAAG (RalA+B), GGUUUCUGUAGAAGAGGCA (RalA), UGACGAGUUUGUAGAAGAC (RalB-2), ACGUGGACAAGGUGUUCUU (RalB-1), CGGCAGAAUGGAUGUCUGC (Sec5-1), GGUCGGAAAGACAAGGCAGAU (Sec5-2), CUGGAGGCAGAGCAUCAACAC (Sec6), ACAGUGUCCUCUUCGAGAG (Sec8), and UGAGUUUCUAGAUGGAGAA (Sec10).
RESULTS
RalB but not RalA is required for cell migration.
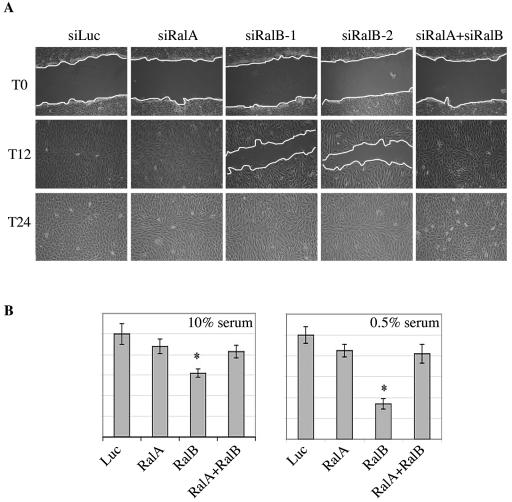
To examine the relative contributions of RalA and RalB to epithelial cell motility, we selectively depleted RalA and RalB in NRK cells with gene-specific siRNAs (Fig. 1). As shown in Fig. 2, RalA depletion had no overt consequences on the capacity of cells to repair a “wounded” monolayer. In contrast, RalB depletion with either of two independent siRNAs resulted in a severe cell migration defect, as monitored by wound healing (Fig. 2A) or migration across porous membranes (Fig. 2B). Estimates of the relative abundance of RalA and RalB proteins in NRK cells, using semiquantitative immunoblots of whole-cell lysates together with known concentrations of myc-tagged RalA and RalB, suggest there is at least fivefold-more RalB than RalA in these cells (data not shown). Given the relatively equivalent efficiency of siRNA-mediated depletion of these proteins (Fig. 1), the selective insensitivity of cells to loss of RalA is not likely to be a consequence of differences in siRNA efficiency. In fact, as has been previously observed for tumor cell survival (7), RalA depletion rescued the sensitivity of cells to loss of RalB (Fig. 2A), suggesting that these proteins may play antagonistic roles in the regulation of cell migration. Consistent with this hypothesis, activated RalA has recently been shown to activate migration and activated RalB to slow migration in two cancer cell lines (22). Similar to previous observations of other cell types (7), depletion of RalA or RalB had no measurable consequences on cell proliferation in adherent cultures (data not shown).
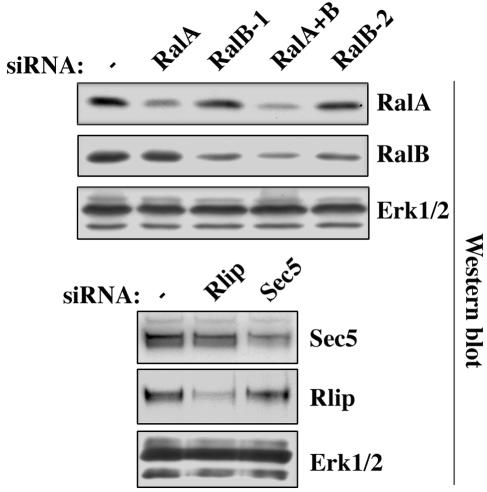
FIG. 1.
siRNA specificity and efficiency in NRK cells. NRK cells were transfected with the indicated siRNAs. After 48 h, whole-cell extracts were analyzed by immunoblotting with the indicated antibodies. ERK1/2 is shown as a loading control.
FIG. 2.
RalB is required for cell migration. (A) RalB depletion inhibits wound healing. Confluent monolayers of NRK cells transfected with the indicated siRNA were wounded 36 h posttransfection (T0), and healing was followed over time. T12 and T24 refer to 12 and 24 h postwounding. The panels shown are representative of three independent experiments. (B) Quantitative cell migration assays. NRK cells were transfected as above with the indicated siRNAs. At 48 h posttransfection, cells were delivered to Boyden chambers containing 10% (left) or 0.5% (right) serum. After 20 h, the cultures were fixed and cell migration was assessed by crystal violet staining as described in Materials and Methods. Error bars represent standard deviations from the mean from three independent experiments. Statistically significant differences are indicated by an asterisk (P < 0.05).
Real-time imaging of live-cell assays was used to directly examine the consequences of RalB depletion on the velocity and persistence of migrating cells. As shown in Table 1, neither RalA nor RalB depletion had significant consequences on the persistence of directional movement. However, RalB depletion inhibited the velocity of migrating cells. RalA depletion did not result in any marked change in cell velocity relative to controls, suggesting that this protein does not function to restrict migration under these conditions.
TABLE 1.
Velocity and persistence of migrating NRK cellsa
| Transfection | Velocity (mean ± SD) | Velocity difference versus Luc | Persistence (mean ± SD) | Persistence difference versus Luc |
|---|---|---|---|---|
| siLuc | 0.854 ± 0.168 | 0.687 ± 0.106 | ||
| siRalA | 0.792 ± 0.175 | − (P = 0.32) | 0.686 ± 0.090 | − (P = 0.97) |
| siRalB | 0.588 ± 0.155 | +++ (P < 0.0001) | 0.667 ± 0.121 | − (P = 0.59) |
Monolayers of control cells (transfected with a siRNA against luciferase [siLuc]) or cells depleted of RalA or RalB by siRNA (siRalA or siRalB) were wounded and filmed during 24 h, starting 1 h after the wound. Cells were tracked on films using MetaMorph and analyzed for speed and persistence; persistence was calculated as the distance between a starting point and an arrival point divided by the length of the path followed between these two points. The calculation in the table results from data collected by tracking at least 25 cells in each case. The only and extremely (P < 0.0001) significant difference observed was the decrease in velocity of cells depleted of RalB. This difference was reproduced in two other filmed experiments. Velocity is given in micrometers per minute.
The exocyst complex is a Ral effector critical for cell migration.
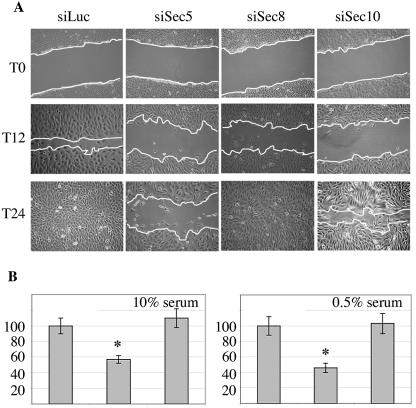
The proteins Sec5, a component of the exocyst complex, and RLIP76/RalBP1 directly interact with activated RalA and with RalB and function as effector proteins in the Ral regulatory network (6, 9). To help define the regulatory system driven by RalB to support cell motility, NRK cells were transfected with siRNAs to selectively deplete RLIP76 or Sec5 (Fig. 1). Depleting RLIP76 did not affect cell migration (Fig. 3). In contrast, depletion of Sec5, like depletion of RalB, slowed the wound healing response, which was still incomplete by 24 h (Fig. 3A), and inhibited migration across porous membranes (Fig. 3B). Live-cell imaging revealed that Sec5-depleted cells display reduced velocity (see Movie S1 in the supplemental material). Similar results were obtained with an independent siRNA targeting Sec5 (data not shown). siRNAs against two other exocyst subunits, Sec10 and Sec8, also slowed migration, suggesting that the exocyst complex as a whole supports this system (Fig. 3A).
FIG. 3.
The exocyst is required for cell migration. (A) Depletion of exocyst subunits inhibits wound healing. Confluent monolayers of NRK cells transfected with the indicated siRNA were wounded (T0), and healing was followed over time as described in the legend to Fig. 2A. Panels shown are representative of three independent experiments. (B) Quantitative cell migration assays. Following transfection with the control siRNAs (left columns) or siRNA depleting Sec5 (middle columns), or RLIP76 (right columns), NRK cell migration in Boyden chambers was assayed as described in the legend to Fig. 2B.
Migration enhances the activation of RalB and its interaction with its Sec5.
The observations described above reveal a prominent role for RalB and the exocyst for support of cell migration, implying that RalB activation may connect the exocyst to cell migration machinery. To determine if RalB is activated by migratory cues, we examined the consequences of monolayer wounding on RalA- and RalB-GTP loading and RalB/Sec5 complex formation. As shown in Fig. 4, conditions that promote cell migration also promoted RalB activation and RalB/Sec5 complex formation. In contrast, RalA activation was unaffected.
FIG. 4.
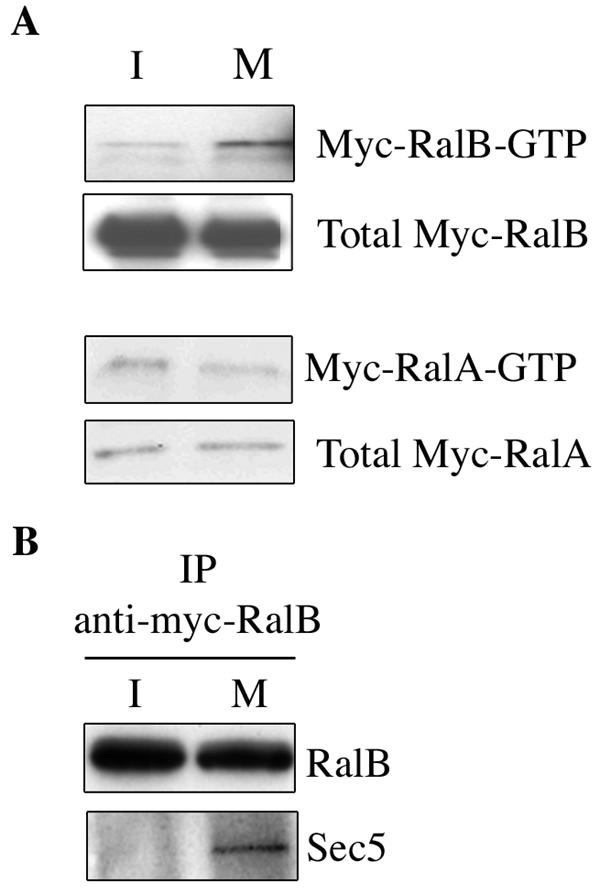
RalB is activated upon release of cell-cell contacts and forms a complex with Sec5. (A) Exponentially growing NRK cells were transfected with myc-tagged RalB or myc-tagged-RalA. Forty-eight hours later, confluent monolayers were scratched extensively to generate a large number of cell edges no longer involved in cell-cell contacts. Cell extracts were prepared either immediately (for simplicity, they are labeled I, for immobile) or 1 h later (M, migrating) and assayed for relative amounts of activated RalA and RalB with immobilized gluthathione S-Sepharose-Ral-binding domain (34). (B) Myc-RalB was immunoprecipitated from the same cell extracts derived in the experiment shown in panel A and evaluated for coprecipitation of endogenous Sec5.
Cell migration stimulates RalB-dependent exocyst complex assembly.
The exocyst is composed of eight proteins, which associate and dissociate dynamically. The balance of exocyst assembly and dissociation may be a key attribute allowing coordinated regulation of exocyst function, together with dynamic cell biological events (3). In HeLa cells, depletion of RalA destabilizes protein-protein interactions within the exocyst (17), and Ral was suggested to regulate the assembly interface of a full octameric exocyst complex from subcomplexes containing two independent exocyst subunits that are direct Ral effector proteins, Sec5 and Exo84 (18).
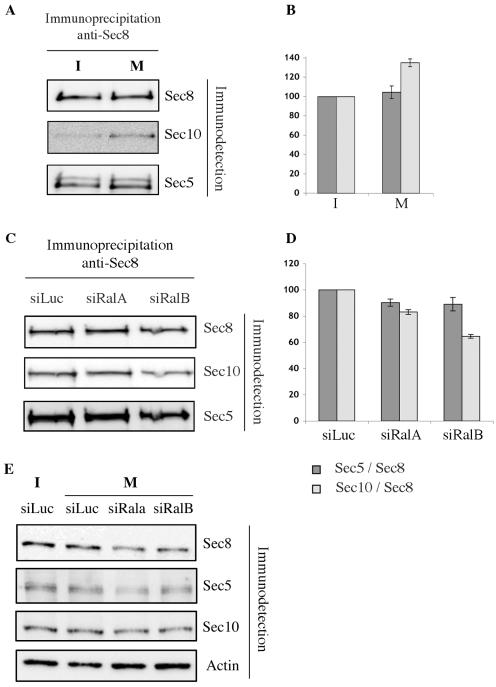
To examine if cell migratory cues impact the stability of the exocyst complex, a monolayer of confluent NRK cells was extensively scratched to maximize the number of “free edges” where cell-cell contacts were released. Given that Sec5 and Sec8 are found in the same membrane fraction in resting cells while Sec10 is in another (18), we examined the consequences of cell migration on Sec8/Sec5 and Sec8/Sec10 complex assembly. As shown in Fig. 5 and consistent with expectations from the results of Moskalenko et al. (17, 18), Sec8/Sec5 complex formation was unaffected by conditions promoting cell migration, while Sec8/Sec10 complex formation was enhanced.
FIG. 5.
Migration triggers RalB-dependent stabilization of the exocyst complex. (A) Migration promotes the exocyst assembly. Monolayers of NRK cells were scratched to liberate cell edges as shown in Fig. 4. Endogenous Sec8 was immunoprecipitated from cell extracts prepared immediately (I, for immobile) or 3 h after wounding (M, migrating) and assessed for coprecipitation of endogenous Sec5 and Sec10. A representative experiment of three independent experiments is shown. (B) The amount of Sec10 and Sec5 recovered in the immunoprecipitates was quantified with a Luminescent Image analyzer and normalized for the amount of Sec8. Each experiment was repeated three times. The amount of Sec5 and Sec10 associated with Sec8 in immobile cells is defined as 100%. (C) Migration-induced exocyst assembly is RalB dependent. NRK cells were transfected with control siRNAs (targeting luciferase; siLuc), or siRNA against RalA or RalB (siRalA or siRalB), and exocyst assembly was evaluated as in described in the legend to panel A. (D) Quantitative analysis of exocyst assembly was performed as described for panel B. The amount of Sec5 and Sec10 associated with Sec8 in siLuc-transfected cells is defined as 100%. (E) Exocyst complex components were immunodetected in lysates of cells depleted of RalA or RalB by siRNA. siLuc was used as a siRNA control. Actin was used as a loading control.
To examine if enhanced Sec8/Sec10 complex formation was dependent on Ral, the experiment described above was repeated with migrating cells transfected with a control siRNA or siRNAs targeting RalA or RalB. While the association between Sec5 and Sec8 was marginally sensitive at best to a depletion of RalA or RalB (Fig. 5C and D), the Sec8/Sec10 complex displayed selective sensitivity to RalB depletion. Absolute amounts of exocyst subunits were equivalent upon RalA or RalB depletion (Fig. 5E).
RalB recruits the exocyst to the leading edge of migrating cells.
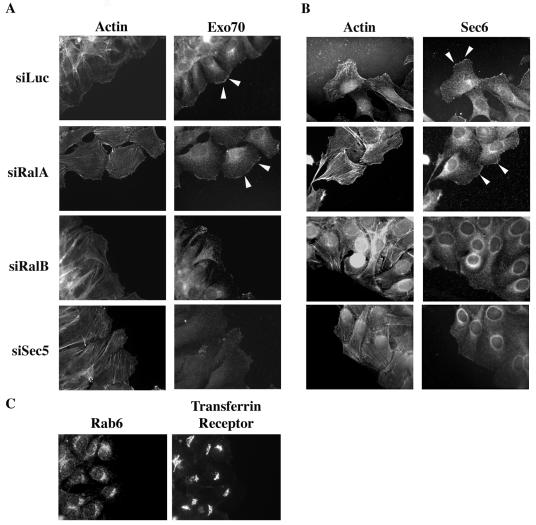
Motile cells develop a region of rapid plasma membrane expansion (leading edge) oriented toward the direction of movement. We examined the distribution of the exocyst in motile cells using monoclonal antibodies that recognize endogenous Exo70 and Sec6. As shown in Fig. 6A and B, Exo70 and Sec6 were enriched at the leading edges of plasma membranes free of cell-cell contacts at the forefront of motile cells. These proteins were not detected on plasma membranes bordering adjacent cells. In all cells, Exo70 and Sec6 were also detected on perinuclear in vesicular structures. Neither Rab6 nor the transferrin receptor displayed enrichment on the leading edge of motile cells, suggesting the localization pattern observed for Exo70 and Sec6 is not an artifact of membrane ruffling. By 3 h, the clusters of Exo70- and Sec6-positive perinuclear vesicles were oriented toward the wound in the first row of cells. Endogenous Sec10 was also enriched at the plasma membrane facing the wound (data not shown).
FIG. 6.
RalB recruits Sec6 and Exo70 to the leading edge of motile cells. Monolayers of NRK cells were wounded 36 h posttransfection with the indicated siRNAs. Cells were fixed 3 h after wounding, and the indicated proteins were detected with specific antibodies. F-actin was visualized with FITC-conjugated phalloidin. Exo70 (A) and Sec6 (B) can be observed recruited at the leading edge, as opposed to two other proteins resident on membranes, Rab6 and the transferring receptor (C). Arrowheads indicate enrichment of Exo70 or Sec6 at the leading edge of the plasma membrane.
The subcellular distribution of Exo70 and Sec6 (Fig. 6A and B) was not affected by the depletion of RalA. In contrast, depletion of either RalB or Sec5 inhibited accumulation of Exo70 and Sec6 at the leading edge of motile cells, suggesting that RalB is required for both assembly and localization of the exocyst in response to cell migratory cues. Depletion of RalA, which suppressed the effect on migration of RalB depletion (Fig. 1), also restored localization of Exo70 to the leading edge of RalB-depleted cells (data not shown).
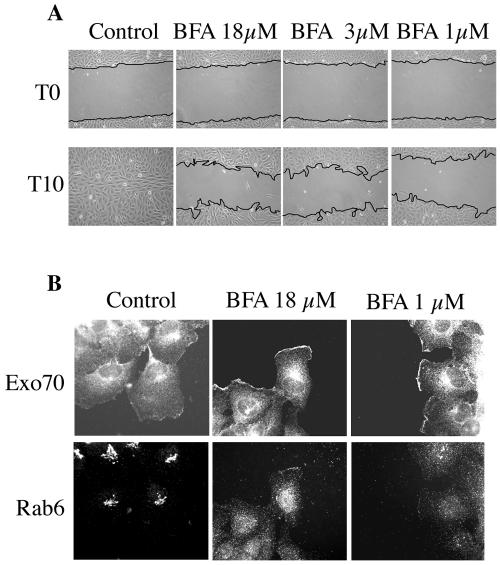
Brefeldin A (BFA)-induced disruption of the Golgi apparatus inhibits migration, presumably as a consequence of blocking resupply of secretory vesicles (1). Given that the exocyst has been observed on both the Golgi apparatus and the plasma membrane (35), we examined the consequences of BFA exposure on Exo70 recruitment to the leading edge. Under conditions where BFA clearly blocked cells migration, Exo70 was still recruited to the leading edge of cells bordering a monolayer wound (Fig. 7). This observation suggests that the capacity of RalB to recruit the exocyst is independent of an intact Golgi apparatus and is consistent with the proposed involvement of the exocyst with regulation of vesicle recycling (14, 25).
FIG. 7.
Golgi disruption blocks migration but not Exocyst recruitment. (A) NRK cells were wounded in the presence of increasing concentrations of brefeldin A, and the consequences on cell migration were monitored as described in the legend to Fig. 2. Experiments were performed on collagen where migration was faster than on plastic to avoid extended exposure to BFA. (B) Cells were treated as in described for panel A. Three hours postwounding, cells were fixed and stained with the indicated antibodies.
DISCUSSION
Ral GTPase activation and function has been defined as an obligate component of oncogenic regulatory networks that promote tumor metastases (31, 33). The intimate relationship of cell motility and migration with metastasis formation, together with the known participation of Ral GTPases in the cell autonomous regulation of cell motility (10, 15, 30), suggests that at least one facet of Ral-dependent tumor metastasis may be Ral-dependent regulation of cancer cell migration. The aim of this work was to identify the molecular components and mechanisms that mediate the contribution of Ral to cell migration.
Distinct roles for RalA and RalB in cell migration.
siRNA-mediated depletion of RalA and RalB revealed that only the latter is a limiting factor for cells to maintain the capacity to migrate. To our surprise, depletion of RalA restored the loss of motility observed upon depletion of RalB. In this experiment, depletion of both GTPases together was as efficient as depletion of either alone, suggesting that reversing sensitivity to RalB siRNAs by RalA depletion is not simply a consequence of saturation of the RISC or other RNAi machinery. Suppression of RalB sensitivity in cells upon RalA depletion has also been observed in the context of cancer cell survival (7), as well as cancer cell migration (22). However, it remains to be determined if the mechanistic contribution of RalB to cell migration overlaps with its role in supporting cancer cell survival.
The exocyst is the RalB effector complex supporting cell migration.
Ral GTPases have been implicated in vesicle trafficking both in the context of endocytosis through the effector RLIP76/RalBP1 and in the context of exocytosis through the effectors Sec5 and Exo84. We have shown here that RLIP76/RalBP1 is apparently dispensable, while the exocyst is a key component immediately downstream of RalB required for cell migration. The exocyst is thought to direct targeting and tethering of subclasses of secretory vesicles to their sites of fusion with the plasma membrane; it is required, together with Ral proteins, for the asymmetric localization of plasma membrane proteins in polarized cells (17), for receptor trafficking at the postsynaptic membrane (26), and for the extension of filopods on neuronal growth cones (12).
The immunofluorescence localization analysis of the exocyst in resting versus motile cells in this study reveals that the exocyst complex is dynamically recruited to plasma membrane subdomains that have been specified for rapid expansion and that this recruitment correlates with RalB activation and requires RalB expression. In cells organized in a monolayer, the exocyst subunits Sec6 and Exo70 were primarily asymmetrically localized in the perinuclear region and enriched in the vicinity of the Golgi. In motile cells, Sec6 and Exo70 were enriched at the leading edge of the “free” plasma membrane, as opposed to membrane engaged in cell-cell contacts. This “motility-induced” alteration in exocyst localization was dependent on RalB but not on RalA. The regulated subcellular recruitment of the exocyst we observe is consistent with its presence on other dynamic membranes, such as tumor necrosis factor alpha- or interleukin-1-induced filopods (29), and the leading edge of migrating osteoblasts (24).
The current “Ral-exocyst paradigm” (18) suggests that the exocyst is in equilibrium between hemicomplexes containing discrete subunits versus the octameric holocomplex. We find that this equilibrium is shifted toward the holocomplex in migrating cells and that this shift is RalB dependent. It remains to be determined if the contribution of RalB to exocyst localization and assembly is a consequence of a single or multiple RalB-dependent regulatory interactions.
Ral GTPases have been implicated in a variety of biological systems that may be expected to impact cell motility, including filamin-dependent modulation of the actin cytoskeleton (21, 27) and exocyst-dependent microtubule polymerization (32). In the system employed here, we did not detect any contribution of RalA or RalB to global actin cytoskeletal architecture or maintenance of the microtubule network (data not shown), suggesting that such perturbations are not likely to mediate the sensitivity of cell migration to RalB expression. In addition, serum-dependent ERK1/2 activation is unaffected by RalB depletion, reflecting intact receptor-mediated signal transduction cascades (data not shown). Finally, consistent with previous evaluation of the consequences RalB depletion in matrix-attached nontransformed cells (7), we observed no cell toxicity or inhibition of proliferation during the course of the experiments reported here. Taken together, these observations support the hypothesis that impaired migration upon RalB or exocyst depletion is due to an intrinsic contribution of the exocyst to cell motility, rather than to a global perturbation of cell homeostasis.
Concluding remarks.
Cell migration requires de novo plasma membrane addition at the leading edge, and a driving role for vesicle trafficking in directional cell motility has been suggested (4). The observations described here help establish a mechanistic framework for the regulation of dynamic membrane expansion in motile cells. We propose that, upon local activation by the release of plasma membranes from cell-cell contacts, RalB acts to specify and constrain vesicle trafficking for directional cell movement through mobilization of the exocyst. This task is likely coordinated with the Rab-dependent and SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor)-dependent machinery contributing to secretory vesicle dynamics, as well as other (macro)molecular machines involved in cell migration such as the protein kinase C zeta/Par3/Par6 complex. Potential molecular links between the exocyst and these systems are currently under investigation.
This conclusion does not exclude other eventually unidentified effectors of Ral as actors of migration but shows that the exocyst complex is definitively required.
Acknowledgments
This work was supported by grant CT-99-00875 from the EU and grant 5440 from Association de Recherche sur le Cancer (ARC) to J.C., by a grant Equipe labelisée from the Ligue Nationale contre le Cancer to P.C., a grant from the Association Christelle Bouillot to Inserm U528, and grant I-1414 from the Robert Welch Foundation to M.A.W. C.R. was supported by a fellowship from the Ministère de la Recherche, by ARC, and by the Fondation de la Recherche Médicale (FRM).
We are grateful to S. Hsu for providing anti-Exo70 monoclonal antibodies and to L. Feig, C. Marshall, P. Frankel, L. Quilliam, and M. Katan for reagents used during this work. We thank Dominique Morineau for his expert artwork, Chloé Camonis for film editing, and Nathalie Brandon, Marie Bourgeois, and Carole Gomez for skillful technical help. Fanny Momboisse was an efficient intern during the course of this work. We are very indebted to Franck Perez for stimulating discussions and suggestions, as well as to Jean de Gunzburg and to Julie Plastino for critical reading of the manuscript.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bershadsky, A. D., and A. H. Futerman. 1994. Disruption of the Golgi apparatus by brefeldin A blocks cell polarization and inhibits directed cell migration. Proc. Natl. Acad. Sci. USA 91:5686-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya, M., P. H. Anborgh, A. V. Babwah, L. B. Dale, T. Dobransky, J. L. Benovic, R. D. Feldman, J. M. Verdi, R. J. Rylett, and S. S. Ferguson. 2002. Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat. Cell Biol. 4:547-555. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, C., T. Hughes, M. Pypaert, and P. Novick. 2004. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 167:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bretscher, M. S., and C. Aguado-Velasco. 1998. Membrane traffic during cell locomotion. Curr. Opin. Cell Biol. 10:537-541. [DOI] [PubMed] [Google Scholar]
- 5.Brymora, A., V. A. Valova, M. R. Larsen, B. D. Roufogalis, and P. J. Robinson. 2001. The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J. Biol. Chem. 276:29792-29797. [DOI] [PubMed] [Google Scholar]
- 6.Camonis, J. H., and M. A. White. 2005. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 15:327-332. [DOI] [PubMed] [Google Scholar]
- 7.Chien, Y., and M. A. White. 2003. RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep. 4:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 9.Feig, L. A. 2003. Ral-GTPases: approaching their 15 minutes of fame. Trends Cell Biol. 13:419-425. [DOI] [PubMed] [Google Scholar]
- 10.Gildea, J. J., M. A. Harding, M. J. Seraj, K. M. Gulding, and D. Theodorescu. 2002. The role of Ral A in epidermal growth factor receptor-regulated cell motility. Cancer Res. 62:982-985. [PubMed] [Google Scholar]
- 11.Gonzalez-Garcia, A., C. A. Pritchard, H. F. Paterson, G. Mavria, G. Stamp, and C. J. Marshall. 2005. RalGDS is required for tumor formation in a model of skin carcinogenesis. Cancer Cell 7:219-226. [DOI] [PubMed] [Google Scholar]
- 12.Hazuka, C. D., D. L. Foletti, S. C. Hsu, Y. Kee, F. W. Hopf, and R. H. Scheller. 1999. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. 19:1324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jullien-Flores, V., Y. Mahe, G. Mirey, C. Leprince, B. Meunier-Bisceuil, A. Sorkin, and J. H. Camonis. 2000. RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J. Cell Sci. 113:2837-2844. [DOI] [PubMed] [Google Scholar]
- 14.Langevin, J., M. J. Morgan, C. Rossé, V. Racine, J. B. Sibarita, S. Aresta, M. Murthy, T. Schwarz, J. Camonis, and Y. Bellaïche. 2005. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9:355-376. [DOI] [PubMed] [Google Scholar]
- 15.Lee, T., L. Feig, and D. J. Montell. 1996. Two distinct roles for Ras in a developmentally regulated cell migration. Development 122:409-418. [DOI] [PubMed] [Google Scholar]
- 16.Lim, K. H., A. T. Baines, J. J. Fiordalisi, M. Shipitsin, L. A. Feig, A. D. Cox, C. J. Der, and C. M. Counter. 2005. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 7:533-545. [DOI] [PubMed] [Google Scholar]
- 17.Moskalenko, S., D. O. Henry, C. Rosse, G. Mirey, J. H. Camonis, and M. A. White. 2002. The exocyst is a Ral effector complex. Nat. Cell Biol. 4:66-72. [DOI] [PubMed] [Google Scholar]
- 18.Moskalenko, S., C. Tong, C. Rosse, G. Mirey, E. Formstecher, L. Daviet, J. Camonis, and M. A. White. 2003. Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 278:51743-51748. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima, S., K. Morinaka, S. Koyama, M. Ikeda, M. Kishida, K. Okawa, A. Iwamatsu, S. Kishida, and A. Kikuchi. 1999. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 18:3629-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuhaus, P., S. Oustanina, T. Loch, M. Krüger, E. Bober, R. Dono, R. Zeller, and T. Braun. 2003. Reduced mobility of fibroblast growth factor (FGF)-deficient myoblasts might contribute to dystrophic changes in the musculature of FGF2/FGF6/mdx triple-mutant mice. Mol. Cell. Biol. 23:6037-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta, Y., N. Suzuki, S. Nakamura, J. H. Hartwig, and T. P. Stossel. 1999. The small GTPase RalA targets filamin to induce filopodia. Proc. Natl. Acad. Sci. USA 96:2122-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxford, G., C. R. Owens, B. J. Titus, T. L. Foreman, M. C. Herlevsen, S. C. Smith, and D. Theodorescu. 2005. RalA and RalB: antagonistic relatives in cancer cell migration. Cancer Res. 65:7111-7120. [DOI] [PubMed] [Google Scholar]
- 23.Polzin, A., M. Shipitsin, T. Goi, L. A. Feig, and T. J. Turner. 2002. Ral-GTPase influences the regulation of the readily releasable pool of synaptic vesicles. Mol. Cell. Biol. 22:1714-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prele, C. M., M. A. Horton, P. Caterina, and G. Stenbeck. 2003. Identification of the molecular mechanisms contributing to polarized trafficking in osteoblasts. Exp. Cell Res. 282:24-34. [DOI] [PubMed] [Google Scholar]
- 25.Prigent, M., T. Dubois, G. Raposo, V. Derrien, D. Tenza, C. Rosse, J. Camonis, and P. Chavrier. 2003. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J. Cell Biol. 163:1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sans, N., K. Prybylowski, R. S. Petralia, K. Chang, Y. X. Wang, C. Racca, S. Vicini, and R. J. Wenthold. 2003. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. 5:520-530. [DOI] [PubMed] [Google Scholar]
- 27.Sawamoto, K., P. Winge, S. Koyama, Y. Hirota, C. Yamada, S. Miyao, S. Yoshikawa, M.-H. Jin, A. Kikuchi, and H. Okano. 1999. The Drosophila Ral GTPase regulates developmental cell shape changes through the Jun NH2-terminal kinase pathway. J. Cell Biol. 146:361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sriuranpong, V., A. Mutirangura, J. W. Gillespie, V. Patel, P. Amornphimoltham, A. A. Molinolo, V. Kerekhanjanarong, S. Supanakorn, P. Supiyaphun, S. Rangdaeng, N. Voravud, and J. S. Gutkind. 2004. Global gene expression profile of nasopharyngeal carcinoma by laser capture microdissection and complementary DNA microarrays. Clin. Cancer Res. 10:4944-4958. [DOI] [PubMed] [Google Scholar]
- 29.Sugihara, K., S. Asano, K. Tanaka, A. Iwamatsu, K. Okawa, and Y. Ohta. 2001. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat. Cell Biol. 17:17. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki, J., Y. Yamazaki, G. Li, Y. Kaziro, and H. Koide. 2000. Involvement of Ras and Ral in chemotactic migration of skeletal myoblasts. Mol. Cell. Biol. 20:4658-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchevkina, E., L. Agapova, N. Dyakova, A. Martinjuk, A. Komelkov, and A. Tatosyan. 2005. The small G-protein RalA stimulates metastasis of transformed cells. Oncogene 24:329-335. [DOI] [PubMed] [Google Scholar]
- 32.Wang, S., Y. Liu, C. L. Adamson, G. Valdez, W. Guo, and S. C. Hsu. 2004. The mammalian exocyst, a complex required for exocytosis, inhibits tubulin polymerization. J. Biol. Chem. 279:35958-35966. [DOI] [PubMed] [Google Scholar]
- 33.Ward, Y., W. Wang, E. Woodhouse, I. Linnoila, L. Liotta, and K. Kelly. 2001. Signal pathways which promote invasion and metastasis: critical and distinct contributions of extracellular signal-regulated kinase and Ral-specific guanine exchange factor pathways. Mol. Cell. Biol. 21:5958-5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolthuis, R. M., B. Franke, M. van Triest, B. Bauer, R. H. Cool, J. H. Camonis, J. W. Akkerman, and J. L. Bos. 1998. Activation of the small GTPase Ral in platelets. Mol. Cell. Biol. 18:2486-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeaman, C., K. K. Grindstaff, J. R. Wright, and W. J. Nelson. 2001. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J. Cell Biol. 155:593-604. [DOI] [PMC free article] [PubMed] [Google Scholar]