Abstract
Thapsigargin (THG) is an inhibitor of the endoplasmic reticulum Ca2+-ATPase that induces caspase 3 activation and apoptosis in HCT116 cells through a Bax-dependent pathway. In Bax-deficient HCT116 cells, however, THG specifically generates two additional species of caspase 3, termed p40 and p64, with molecular masses of approximately 40 and 64 kDa, respectively, through unknown mechanisms. Here, we report that the Ca2+-dependent protein cross-linking enzyme tissue transglutaminase (tTGase) is involved in THG-induced p40 and p64 formation by catalyzing caspase 3 cross-linking reactions, thereby inactivating caspase 3 and apoptosis in Bax-deficient cells. Overexpression of tTGase increases p40 and p64 in THG-treated cells, and purified tTGase catalyzes procaspase 3 cross-linking in vitro. Inhibition of tTGase activity by either the tTGase inhibitor monodansylcadaverine or short-hairpin RNA reduces the cross-linked species p40 and p64 and restores caspase 3 activation in response to THG treatment. Moreover, prolonged exposure to THG results in a decrease in protein levels of XIAP and cIAP-1, which is subsequently followed by an increase in tTGase protein expression and activity. Expression of cytosolic Smac sensitizes Bax-deficient cells to THG-induced apoptosis; however, this effect is diminished by coexpression of tTGase. Taken together, these results suggest a novel role for tTGase as a new type of caspase 3 inhibitor in THG-mediated apoptosis.
Tissue transglutaminase (tTGase) is a unique member of the transglutaminase family that serves as a bifunctional enzyme for catalyzing both Ca2+-dependent protein cross-linking and Ca2+-independent GTP and ATP hydrolyses (5, 20). In the presence of Ca2+, tTGase catalyzes an acyl transfer reaction between the γ-carboxamide group of a protein-bound glutamine residue and the primary amine group of either a protein-bound lysine residue or other polyamine molecules (5). The reaction results in a posttranslational modification, which is highly stable and resistant to mechanical and proteolytic breakdown, through either the formation of an irreversible protein cross-link or the incorporation of a polyamine into an acyl donor protein (9). These irreversible protein cross-links have been implicated in the following diverse physiological processes: cell growth and differentiation, receptor-mediated endocytosis, cell adhesion, and regulation of apoptosis (25, 30, 35, 38). In numerous experimental models, tTGase has been shown to be induced and activated during apoptosis (10, 27, 31, 44). However, since tTGase exhibits both anti- and proapoptotic features in different experimental systems, the precise role of tTGase in apoptosis remains elusive.
Apoptosis is executed by caspases, a family of cysteine proteases whose activation is controlled by two major pathways in mammalian cells: the intrinsic and extrinsic pathways (6). In the intrinsic pathway, various cytotoxic stimuli cause the release of cytochrome c from the mitochondria into the cytosol (39). Once it is released from mitochondria, cytochrome c forms complexes with procaspase 9 and Apaf-1 in the presence of dATP or ATP, resulting in activation of this initiator caspase (19). On the other hand, the extrinsic pathway utilizes death receptors, such as Fas, TNFR1, DR3, DR4, and DR5, for the activation of caspases. Binding of ligand to these cell surface receptors recruits adaptor proteins, such as FADD, to the cytoplasmic domain of the receptors, which in turn recruits the initiator procaspase 8 to form the death-inducing signaling complex that induces caspase 8 activation (3, 26). Once activated, these upstream initiator caspases cleave and activate downstream executioner caspases, such as caspase 3, caspase 6, and caspase 7 (7). While the IAP family proteins block caspase 3 and caspase 9 activation, Smac and Omi promote caspase activation by antagonizing IAPs when they are released from mitochondria (37). The antiapoptotic Bcl-2 family proteins inhibit the release of these proapoptotic factors from mitochondria, whereas the proapoptotic Bcl-2 family members induce those events (11, 24, 29). The extrinsic and intrinsic pathways cross-talk through caspase 8 cleavage of the BH3-only protein Bid, which activates the intrinsic pathway to amplify the extrinsic apoptosis signals (18, 21).
We have reported previously that thapsigargin (THG), an inhibitor of endoplasmic reticulum (ER) Ca2+-dependent ATPase, triggers the apoptosis of HCT116 cells through a DR5 (death receptor)-initiated but Bax (mitochondria)-dependent mechanism (40, 43). In response to THG, the ER stress-activated transcription factor CHOP upregulates DR5 expression, which subsequently activates caspase 8. The activated caspase 8 cleaves Bid and procaspase 3 to produce tBid (18, 21) and the p20 (also called p24)/p12 subunits of caspase 3 (8, 32), respectively. In the presence of Bax, tBid induces the release of Smac and Omi in order to remove the inhibitory effects of IAPs, allowing caspase 3 to be fully processed to p19/p17/p12 subunits. In contrast, cells lacking Bax fail to release mitochondrial IAP inhibitors, thus preventing the full processing of caspase 3 in response to THG (40). Interestingly, two high-molecular-weight species of caspase 3, termed p40 and p64, appeared in Bax-deficient cells after prolonged exposure to THG. In this study, we investigated the underlying mechanism for the generation of p40 and p64 caspase 3 and found that tTGase is the enzyme responsible for cross-linking caspase 3 and suppressing apoptosis in THG-treated, Bax-deficient HCT116 cells.
MATERIALS AND METHODS
Reagents.
Thapsigargin, brefeldin A (BFA), 5-fluorouracil (5-FU), vinblastine (VBL), guinea pig liver tTGase, monodansylcadaverine (MDC), antitubulin and antiactin monoclonal antibodies, and z-VAD-fmk were purchased from Sigma. Anti-caspase 3 antibody was described previously (17). Anti-XIAP and cytochrome c monoclonal antibodies were purchased from BD Biosciences. Anti-cIAP-1 polyclonal antibody was purchased from R&D Systems. Anti-tTGase monoclonal antibody (CUB9402) was obtained from NeoMarkers. Anti-Myc monoclonal and polyclonal antibodies were described previously (14).
Cell culture and transfection.
HCT116 cells were maintained in McCoy's 5A medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. HeLa cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serium and 1% penicillin/streptomycin. Cell transfection was performed using Lipofectamine 2000 (Invitrogen) as described previously (41).
Plasmid.
Human tTGase and caspase 3 cDNAs were amplified by PCR and subcloned into the EcoRI and XhoI sites of the pcDNA3-Myc vector. The accuracy of the plasmids was verified by DNA sequencing. The pSHAG-MAGIC vector encoding short-hairpin RNA (shRNA) against tTGase was obtained from the Expression Arrest human shRNA collection (Open Biosystems). The green fluorescent protein (GFP)-IETD-Smac expression plasmid was described previously (13, 40).
Immunoblot and immunoprecipitation.
Whole-cell lysates were prepared in 1% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate) or 0.2% Nonidet P-40 lysis buffer as described previously (42). For the cytosolic (S-100) fraction preparation, cells were resuspended in mitochondrial lysis buffer (40) and homogenized with a Dounce homogenizer. The lysates were centrifuged at 100,000 × g for 30 min to obtain an S-100 fraction. For coimmunoprecipitation, cells were transfected with pcDNA3-Myc-tTGase and cultured in the presence or absence of THG for 24 h. The cells were washed with phosphate-buffered saline and lysed in Nonidet P-40 lysis buffer. Cell lysates containing 500 μg of total proteins were incubated with 15 μl of protein G agarose preadsorbed with anti-Myc monoclonal antibody in 500 μl of the same lysis buffer at 4°C overnight. After being washed three times with the same lysis buffer, the beads were resuspended in Laemmli sample buffer to elute proteins. The samples were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane. The membranes were then blocked and probed with an appropriate primary antibody and horseradish peroxidase (HRP)-conjugated secondary antibody. Detection was performed using SuperSignal West Pico chemiluminescence substrate (Pierce).
In vitro tTGase assay.
HeLa cells transfected with Myc-tagged caspase 3 were lysed in tTGase assay buffer containing 50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% Triton X-100, 5 mM CaCl2, 10 mM dithiothreitol, and protease inhibitors. After centrifugation at 13,000 × g for 10 min at 4°C, the resulting supernatant (500 μg of total protein) was incubated with 1 μg of bovine serum albumin or purified guinea pig liver tTGase at 37°C for 2 h, and 1/10 (50 μg) of the samples was then subjected to SDS-PAGE/immunoblot analysis with anti-Myc antibody. Alternatively, in vitro-translated Myc-tagged caspase 3 proteins were used. In brief, procaspase 3 (p32), the large subunit with prodomain (p20), and the small subunit (p12) of caspase 3 proteins were in vitro translated and labeled with [35S]methionine using pcDNA-Myc-caspase 3 plasmids and the TNT T7 coupled reticulocyte lysate system (Promega) according to the manufacturer's recommendations. One microliter of 35S-labeled proteins was incubated in 15 μl of tTGase assay buffer with or without 1 μg of purified guinea pig liver tTGase for 20 min at 37°C, and the samples were subjected to SDS-PAGE, followed by autoradiography.
In situ tTGase assay.
In situ tTGase activity was determined as described previously (31, 44). In brief, HCT116 cells were treated with THG for various periods of time and cultured in the presence of 1 mM biotinylated pentylamine for 1 h prior to the preparation of cell lysates by sonication in 50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EGTA, and 5 mM EDTA. After centrifugation at 13,000 × g for 10 min at 4°C, the resulting supernatant (50 μg of total protein) was separated by 12% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed with HRP-conjugated streptavidin.
Fluorescence microscopy.
HCT116 Bax−/− cells were transfected with GFP, GFP-IETD-Smac, or GFP-IETD-Smac plus Myc-tTGase expression plasmids and treated with 1 μM THG for 48 h. Both attached and detached cells were collected and fixed with 3.7% formaldehyde. After the cells were washed with phosphate-buffered saline, they were resuspended in mount medium containing DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories) and examined under a fluorescence microscope.
RESULTS
THG specifically induces high-molecular-weight forms of caspase 3 in Bax-deficient HCT116 cells.
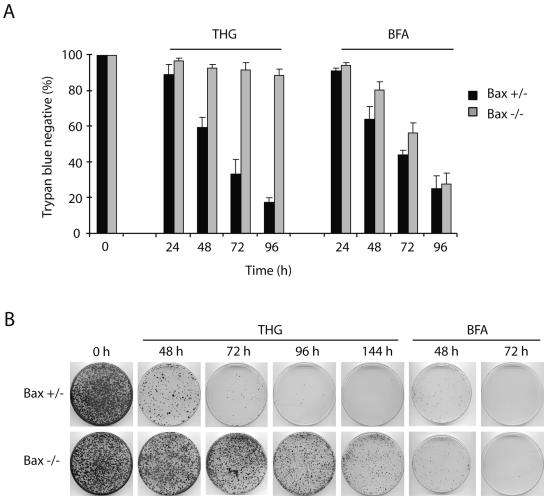
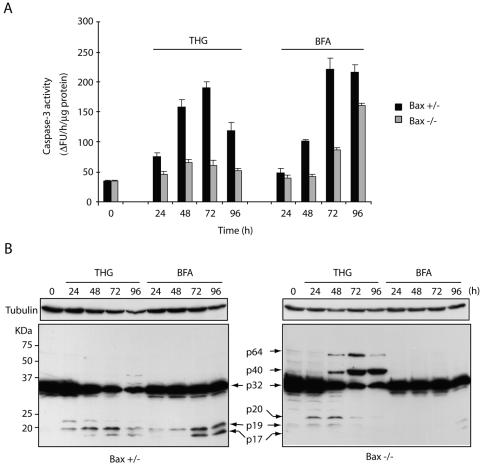
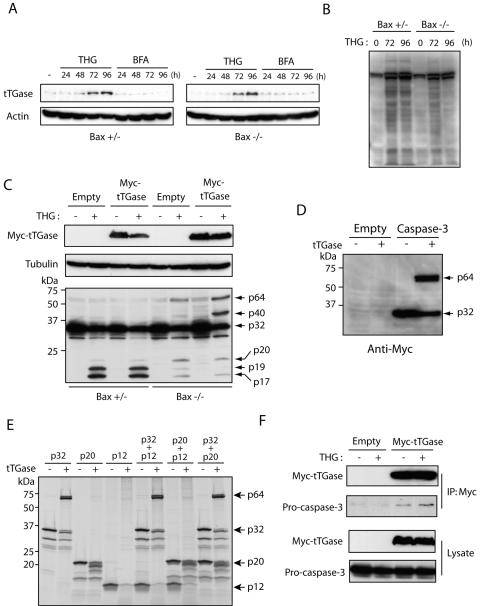
To further understand the characteristics of the high-molecular-weight species of caspase 3 observed in THG-treated, Bax-deficient HCT116 cells (40), we treated Bax+/− and Bax−/− HCT116 cells with THG as well as BFA, another type of ER stress inducer that inhibits ER-Golgi transport. We then compared the effects of these reagents on cell death, caspase 3 activation, and the formation of high-molecular-weight species of caspase 3. We used BFA at a concentration of 1 μg/ml, because at this concentration, it exhibits almost the same cytotoxicity and kinetics of cell death as does 1 μM THG in Bax-positive HCT116 cells (Fig. 1A). In contrast to THG, which kills HCT116 cells in a Bax-dependent manner, BFA was able to induce cell death of both Bax-positive and -negative cells at essentially the same kinetics (Fig. 1A). These results were confirmed by clonogenic assay. As shown in Fig. 1B, the number of clonogenic cells was dramatically decreased in Bax-positive HCT116 cells after 48 h of exposure to THG. In contrast, a significant portion of Bax−/− cells could be recovered to form colonies even after 144 h of treatment with THG. Consistent with the trypan blue dye exclusion assay, BFA treatment suppressed colony formation in both Bax+/− and Bax−/− HCT116 cells. However, BFA-induced caspase 3 activation was delayed in Bax-negative cells compared to that in Bax-positive cells (Fig. 2A), suggesting that other mechanisms may contribute to BFA-induced cell death as well. Indeed, BFA has been shown to induce autophagy (28). Interestingly, immunoblot analysis showed that the p20 large subunit containing the prodomain of caspase 3 was accumulated in THG-treated, Bax-deficient cells at early time points and decreased after 48 h, and two additional bands with high molecular masses (∼40 kDa and ∼64 kDa), termed p40 and p64, respectively, appeared simultaneously. This suggests that a highly stable caspase 3 complex formation (resistant to SDS and 2-mercaptoethanol) occurred in Bax-deficient cells after prolonged exposure to THG. In contrast, BFA caused full processing of caspase 3 and increased its enzymatic activity even in Bax−/− cells after long-term exposure, and the high-molecular-weight species of caspase 3 were not observed in BFA-treated cells (Fig. 2). These results suggest the possibility that THG fails to induce cell death of Bax-deficient cells due to the inactivation of caspase 3 by the generation of high-molecular-weight caspase 3 species.
FIG. 1.
Bax-deficient HCT116 cells are selectively resistant to THG. (A) HCT116 Bax+/− and Bax−/− cells were treated with 1 μM THG or 1 μg/ml BFA for the indicated periods of time and subjected to trypan blue dye exclusion assay. (B) HCT116 Bax+/− and Bax−/− cells were treated with 1 μM THG or 1 μg/ml BFA for the indicated periods of time, and 1 × 104 cells were reseeded on 100-mm plates in normal growth medium. Ten days later, colonies were visualized with hematoxylin-eosin staining.
FIG. 2.
BFA activates caspase 3 independently of Bax while THG generates high-molecular-mass species of caspase 3. HCT116 Bax+/− or Bax−/− cells were treated with 1 μM THG or 1 μg/ml BFA for the indicated periods and subjected to caspase 3 activity assay (A) or immunoblot analysis (B) with anti-caspase 3 rabbit antiserum or anti-α-tubulin monoclonal antibody. ΔFU, difference in fluorescence units.
The formation of high-molecular-weight species of caspase 3 is suppressed under conditions favorable for caspase 3 proteolytic cleavage.
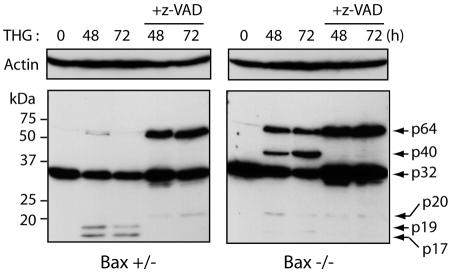
Next, to determine whether the lack of high-molecular-weight species of caspase 3 in Bax-positive cells in response to THG is due to the full processing of this caspase, we treated the cells with THG in the presence of the caspase inhibitor z-VAD-fmk. As expected, z-VAD-fmk blocked caspase 3 processing and resulted in the appearance of p64 species in Bax+/− HCT116 cells (Fig. 3). This result indicates that the high-molecular-weight species of caspase 3 induced by THG is inhibited under conditions favorable for caspase 3 proteolytic cleavage. Moreover, the p20 and p40 bands of caspase 3 disappeared, and only the p64 band was observed in Bax−/− HCT116 cells cotreated with THG and z-VAD-fmk, suggesting that the p40 band originates from the cleaved products of caspase 3 and that the p64 band is derived from the cross-linking of procaspase 3 (p32).
FIG. 3.
THG induces the p64 species of caspase 3 in HCT116 cells in the presence of z-VAD-fmk (+z-VAD). Bax+/− and Bax−/− cells were treated with 1 μM THG in the presence or absence of 50 μM z-VAD-fmk for the indicated periods and subjected to immunoblot analysis with anti-caspase 3 rabbit antiserum or anti-β-actin monoclonal antibody.
MDC inhibits high-molecular-weight caspase 3 formation and sensitizes Bax-deficient cells to THG.
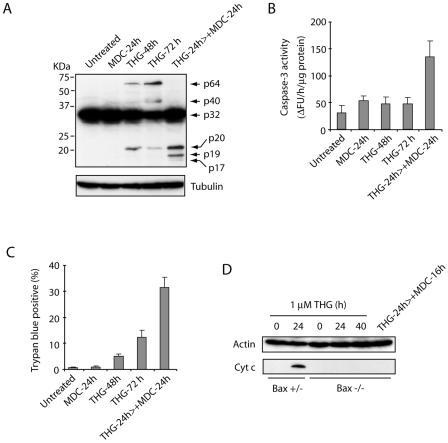
It has been shown that tTGase, a family member of the transglutaminase enzymes that catalyze the posttranslational modification of proteins by Ca2+-dependent cross-linking reactions, induces oligomerization of a variety of proteins (12, 15, 16, 23). Since tTGase is induced and activated in response to increased intracellular Ca2+ concentration and THG causes an intracellular Ca2+ increase by inhibiting the Ca2+-ATPase pump on the ER, it is plausible that tTGase is responsible for THG-induced high-molecular-weight caspase 3 formation. To investigate this possibility, we measured the effects of treating Bax-deficient HCT116 cells with THG for 24 h, followed by the addition of MDC for another 24 h. The cells were subjected to trypan blue dye exclusion assay, and the lysates were used for SDS-PAGE/immunoblot analysis and caspase 3 assay. As expected, treating cells with MDC diminished the formation of p40 and p64 bands of caspase 3 (Fig. 4A), restored the full processing of caspase 3 into p19/p17 active forms (Fig. 4A), enhanced caspase 3 activity (Fig. 4B), and induced cell death (Fig. 4C). To determine whether this restoration of caspase 3 activation by MDC occurs through the mitochondrial pathway, we examined cytochrome c release in HCT116 cells treated with THG in the presence or absence of MDC. Consistent with our previous results (40), THG treatment caused cytochrome c release in Bax-positive but not Bax-deficient cells (Fig. 4D). Importantly, the addition of MDC to THG-treated, Bax-deficient cells did not induce cytochrome c release, suggesting that MDC permits the activation of caspase 3 by inhibiting caspase 3 cross-links rather than by releasing mitochondrial apoptogenic factors.
FIG. 4.
The tTGase inhibitor MDC restores caspase 3 activation and cell death in THG-treated, Bax-deficient cells. (A to C) HCT116 Bax−/− cells were left untreated or treated with 1 mM MDC for 24 h, 1 μM THG for 24, 48, and 72 h, or 1 μM THG for 24 h, followed by the addition of 1 mM MDC for a further 24 h. The cells were harvested and subjected to immunoblot analysis with anti-caspase 3 rabbit antiserum or anti-α-tubulin monoclonal antibody (A), caspase 3 activity assay (B), and trypan blue dye exclusion assay (C). (D) HCT116 cells were treated with 1 μM THG for 0, 24, and 40 h or 1 μM THG for 24 h, followed by the addition of 1 mM MDC for a further 16 h. The cells were subjected to subcellular fractionation, and the resulting cytosolic (S-100) fraction was analyzed by SDS-PAGE/immunoblotting with monoclonal antibodies specific for β-actin or cytochrome c. ΔFU, difference in fluorescence units; Cyt c, cytochrome c.
Caspase 3 serves as a substrate for tTGase in vitro.
To further determine the potential role of tTGase in THG-induced cell death, we next asked whether tTGase is induced and activated in HCT116 cells exposed to THG. To this end, HCT116 Bax+/− and Bax−/− cells were treated with THG or BFA as a control for various periods and subjected to immunoblot analysis with anti-tTGase antibody. As shown in Fig. 5A, tTGase expression was significantly enhanced by THG but not by BFA treatment in both Bax-positive and -negative cells. In addition, we examined the in situ transamidation activity of tTGase by detecting the incorporation of biotinylated polyamine into proteins (31, 44). Consistent with its expression, tTGase activity was also increased after long-term exposure to THG (Fig. 5B).
FIG. 5.
Caspase 3 cross-linking is catalyzed by tTGase. (A) HCT116 Bax+/− and Bax−/− cells were treated with 1 μM THG or 1 μg/ml BFA for the indicated times and subjected to immunoblot analysis with anti-tTGase or β-actin monoclonal antibody. (B) HCT116 Bax+/− and Bax−/− cells were treated with 1 μM THG for 72 and 96 h or left untreated. Biotinylated pentylamine (1 mM) was added to the culture 1 h prior to harvest, and the incorporation of biotinylated pentylamine into proteins was determined by immunoblot analysis with HRP-conjugated streptavidin. (C) HCT116 Bax+/− and Bax−/− cells were transfected with Myc-tagged tTGase or empty plasmid, cultured in the presence or absence of 1 μM THG for 48 h, and subjected to immunoblot analysis with anti-caspase 3 rabbit antiserum or anti-Myc and anti-β-actin monoclonal antibodies. (D) HeLa cells were transfected with Myc-tagged caspase 3 or empty plasmid, and cell lysates were prepared, incubated with purified tTGase for 2 h, and analyzed by SDS-PAGE/immunoblot analysis with anti-Myc monoclonal antibody. (E) The in vitro-translated 35S-labeled procaspase 3 (p32), the p20 large subunit, the p12 small subunit, and their combinations were incubated with or without purified tTGase for 20 min and subjected to SDS-PAGE/autoradiography. (F) HCT116 Bax−/− cells were transfected with pcDNA3-Myc-tTGase or control empty vector and cultured for 24 h in the presence or absence of 1 μM THG. The cells were then subjected to immunoprecipitation with anti-Myc monoclonal antibody, followed by immunoblot analysis with anti-caspase 3 or Myc polyclonal antibody.
To confirm whether caspase 3 is a substrate of tTGase, Bax+/− and Bax−/− HCT116 cells were transfected with Myc-tagged tTGase expression plasmid or empty control vector, treated with THG for 48 h or left untreated, and subjected to immunoblot analysis with anti-caspase 3 antibodies. The results indicated that the formation of p40 and p64 species was enhanced by tTGase overexpression in Bax knockout cells (Fig. 5C). In contrast, neither p40 nor p64 was observed in Bax+/− cells, suggesting that this complex formation does not occur under conditions favorable for its proteolytic cleavage (Fig. 5C). To further determine whether caspase 3 can be a substrate of tTGase, we examined the ability of tTGase to cross-link caspase 3 in vitro. First, we transfected Myc-tagged noncleavable procaspase 3 mutant or control empty vector in HeLa cells and prepared cell lysates in 1% Triton X-100 lysis buffer. The cell lysates were incubated with purified guinea pig tTGase and subjected to immunoblot analysis with anti-Myc antibody (Fig. 5D). Next, we prepared 35S-labeled in vitro-translated proteins, including noncleavable procaspase 3 (p32), the p20 large subunit, and the p12 small subunit; each protein and the combinations were incubated with purified tTGase; and specific signals were detected by SDS-PAGE/autoradiography (Fig. 5E). As shown in Fig. 5D and E, tTGase catalyzed the cross-linking of procaspase 3 and generated the highly stable p64 species in vitro, even though the distinct p40 species was not produced by in vitro assay using either p32, p20, p12, or their combinations (Fig. 5E).
Finally, we performed coimmunoprecipitation to determine whether caspase 3 interacts with tTGase. HCT116 Bax−/− cells transfected with Myc-tagged tTGase or empty plasmid were treated with or without THG for 24 h and subjected to immunoprecipitation with anti-Myc antibody. As shown in Fig. 5F, endogenous caspase 3 could be coprecipitated with tTGase, and this association was enhanced by THG treatment.
Taken together, these results suggest that caspase 3 is a substrate of tTGase, which catalyzes caspase 3 cross-linking in response to THG treatment.
Suppression of tTGase expression by shRNA restores caspase 3 activation in Bax-deficient cells treated with THG.
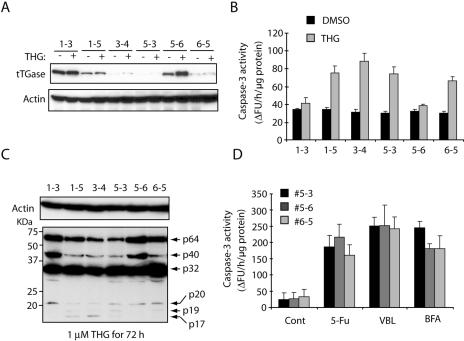
To further demonstrate the role of tTGase in caspase 3 activation, we used shRNA to decrease tTGase expression and examined its effect on THG-induced caspase 3 cross-links. The shRNA expression vector against human tTGase was transfected into HCT116 Bax-deficient cells, and stable transfectants were obtained by puromycin selection. We isolated six independent clones with different expression levels of tTGase (Fig. 6A). These stable clones were treated with THG for 72 h, or left untreated, and subjected to caspase 3 activity assay and immunoblot analysis with anti-caspase 3 antibody. Consistent with MDC treatment, knockdown of tTGase sensitized Bax-deficient HCT116 cells to THG-induced caspase 3 activation (Fig. 6B). More importantly, the formation of high-molecular-weight caspase 3 species p40 and p64 was decreased in cells expressing low levels of tTGase (Fig. 6C). In contrast, knockdown of tTGase did not affect caspase 3 activation induced by 5-FU, VBL, or BFA (Fig. 6D). Therefore, these results suggest that tTGase plays a role in the suppression of THG-induced caspase 3 activation by cross-linking this effector caspase.
FIG. 6.
Knockdown of tTGase sensitizes Bax-deficient cells to THG but not to BFA, VBL, or 5-FU. The shRNA expression plasmid against tTGase was stably transfected into HCT116 Bax−/− cells, and six independent clones were obtained by puromycin selection. The stable clones were cultured with or without 1 μM THG for 72 h and subjected to immunoblot analysis with anti-tTGase or β-actin monoclonal antibody (A) or caspase 3 activity assay (B). (C) The stable clones were treated with 1 μM THG for 72 h and subjected to SDS-PAGE/immunoblot analysis with anti-caspase 3 rabbit antiserum or anti-β-actin monoclonal antibody. (D) The stable clones were treated with 500 μM 5-FU, 20 nM VBL, or 1 μg/ml BFA for 24, 48, or 96 h, respectively, and subjected to caspase 3 activity assay. ΔFU, difference in fluorescence units; DMSO, dimethyl sulfoxide; Cont, control.
tTGase compensates for IAP function at the late stage of THG treatment.
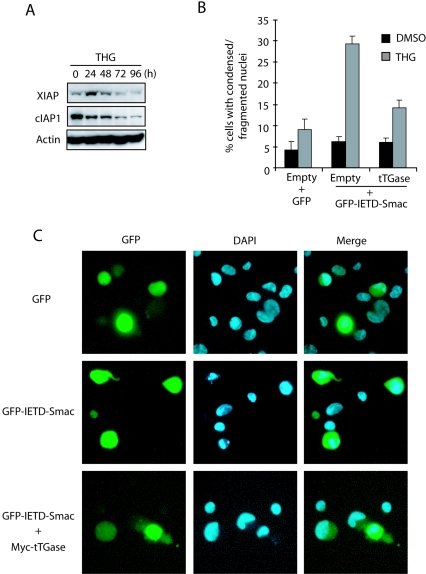
In a previous report (40), we showed that Smac and Omi are released from mitochondria in response to THG through a Bax-dependent pathway and that this is a critical step for HCT116 cells to achieve THG-induced apoptosis by removing the inhibitory effect of IAPs from caspase 3. However, we also observed that inhibition of tTGase restored caspase 3 activation in Bax-deficient HCT116 cells after prolonged exposure to THG. If the IAP family proteins are maintained at high levels throughout THG treatment, inhibition of tTGase should not be able to restore caspase 3 activation in Bax knockout cells. Therefore, we examined by immunoblot analysis the expression of IAP family proteins, including XIAP and c-IAP1, in HCT116 cells exposed to THG for various periods of time. As shown in Fig. 7A, both XIAP and cIAP-1 decreased after THG treatment. Notably, this decrease was followed by an increase in tTGase (Fig. 6A), suggesting that tTGase may take a role in inhibition of caspase 3 after XIAP and cIAP-1 are decreased. To test this possibility, we examined the ability of tTGase to suppress THG-induced apoptosis after the IAP antiapoptotic function is removed by overexpression of active cytosolic Smac. Bax-deficient HCT116 cells were transfected with GFP-IETD-Smac with or without tTGase expression plasmid, treated with THG, and subjected to DAPI staining. The GFP-IETD-Smac fusion protein becomes mature cytosolic Smac after cleavage by caspase 8 and sensitizes Bax-deficient HCT116 cells to THG-induced apoptosis (33, 40). Consistently, transfection of GFP-IETD-Smac sensitized Bax-deficient HCT116 cells to THG-induced apoptosis as demonstrated by nuclear condensation and fragmentation (Fig. 7B and C). In contrast, coexpression of tTGase suppressed the enhanced sensitivity of GFP-IETD-Smac-transfected cells to THG-induced apoptosis. These results support the notion that tTGase functions as a caspase 3 inhibitor compensating for the IAP family proteins at the late stage of THG treatment.
FIG. 7.
XIAP and cIAP-1 are decreased during THG treatment, and tTGase compensates for the lost IAP function for cell survival. (A) HCT116 Bax−/− cells were exposed to 1 μM THG for the indicated periods of time and subjected to immunoblot analysis with antibodies specific for β-actin, XIAP, or cIAP-1. (B and C) HCT116 Bax−/− cells were transfected with GFP, GFP-IETD-Smac, or GFP-IETD-Smac plus Myc-tTGase expression plasmids and treated with 1 μM THG for 48 h. The cells were stained with DAPI and analyzed under a fluorescence microscope (C), and GFP-positive cells with condensed or fragmented nuclei were counted as apoptotic cells (B). DMSO, dimethyl sulfoxide.
DISCUSSION
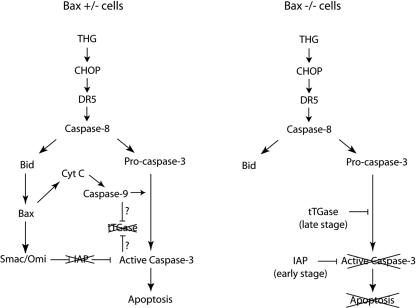
THG triggers caspase 3 activation and apoptosis in HCT116 cells through a Bax-dependent mechanism (40). In Bax-deficient cells, the THG-induced release of Smac and Omi is blocked, allowing the IAP family proteins to suppress caspase 3 activation (Fig. 8). However, the cells became rounded and detached from the plates after prolonged exposure to THG (data not shown). Nonetheless, a significant number of Bax-deficient cells could survive even after 5 days of treatment with THG, as demonstrated by clonogenic assay (Fig. 1B). These cells ceased cell cycle progression but were not in a senescent state because no senescence-associated β-galactosidase activity was observed (data not shown). Interestingly, these cellular characteristics were accompanied by the formation of high-molecular-weight species (p40 and p64) of caspase 3. In contrast, BFA, an inhibitor of the ER-Golgi transport that also causes ER stress, could induce caspase 3 activation and cell death independently of Bax (Fig. 1 and 2). These results suggest that THG specifically produces the high-molecular-weight forms of caspase 3 that may contribute to the resistance of Bax-deficient cells to THG-induced apoptosis.
FIG. 8.
A model of THG-induced apoptosis. In Bax+/− cells, Smac is released from mitochondria to inhibit IAPs in order to achieve caspase 3 activation and apoptosis. The tTGase-mediated cross-linking of caspase 3 is suppressed by unknown mechanisms, which may be controlled by caspase 9 and/or caspase 3. In Bax−/− cells, the full processing and activation of caspase 3 are blocked by IAPs at the early stage of THG treatment. At the late stage, IAPs are decreased and subsequently induced, and activated tTGase takes the role in inhibition of apoptosis by cross-linking caspase 3. Cyt C, cytochrome c.
Since caspase 3 is ubiquitinated by XIAP and undergoes proteasome-dependent degradation (34), we first speculated that the high-molecular-weight species of caspase 3 are caused by ubiquitination. If this is the case, inhibition of proteasome-mediated degradation should accumulate the high-molecular-weight forms of caspase 3. However, when we treated Bax-deficient HCT116 cells with THG in the presence of the proteasome inhibitor MG132, the high-molecular-weight species of caspase 3 disappeared rather than accumulated (data not shown), suggesting that they are not ubiquitinated forms of caspase 3. It is worth noting that the combinational treatment of THG with MG132 induced cell death in Bax knockout HCT116 cells (data not shown). Therefore, we next focused on tTGase, because tTGase is activated by Ca2+ and catalyzes a highly stable cross-linking of proteins during apoptosis (12, 25, 36).
It has been reported that tTGase has both pro- and antiapoptotic activities that depend on cell type and stimulation (1, 2, 4, 22, 25). However, the exact mechanism by which this enzyme regulates cell death is far from clear. One potential antiapoptotic mechanism is that tTGase prevents the degradation of retinoblastoma (Rb) protein and maintains its antiapoptotic function during apoptosis (4, 22). On the other hand, it has been reported that tTGase has a BH3-like domain and serves as a BH3-only protein to promote cytochrome c release and Bax conformational change (30). However, HCT116 cells transfected with tTGase expression plasmid did not alter THG-induced Bax conformational change (data not shown). Therefore, it is likely that tTGase plays no significant role in upstream Bax activation and mitochondrial outer membrane permeabilization induced by THG treatment. In contrast, overexpression of tTGase increased the formation of high-molecular-weight species of caspase 3 in THG-treated, Bax-deficient cells, and purified tTGase catalyzed caspase 3 cross-linking in vitro (Fig. 5). Conversely, inhibition of tTGase by MDC or shRNA suppressed caspase 3 cross-linking and promoted cell death in Bax-deficient HCT116 cells (Fig. 4 and 6). However, knockdown of tTGase by shRNA only partially suppressed THG-induced p40 and p64 species of caspase 3, and the protein levels of tTGase did not correlate absolutely with the sensitivity to THG-induced caspase 3 activation (Fig. 6). In contrast, MDC treatment almost completely abrogated caspase 3 cross-linking and restored its full processing and enzymatic activities (Fig. 4). Since the human transglutaminase family consists of eight different members (20), it is possible that other types of transglutaminase are involved in caspase 3 cross-linking as well.
Unlike the findings for Bax-deficient cells, we could not detect the high-molecular-weight species of caspase 3 induced by THG in Bax-positive cells under conditions favorable for caspase 3 proteolytic cleavage (Fig. 2, 3, and 5). But, these results do not exclude the possibility that tTGase can also cross-link the fully processed subunits of caspase 3 and inactivate its enzymatic activity. It is also possible that the in vivo process of caspase 3 cross-linking is blocked by some unidentified factor(s) in Bax-positive cells. Caspase 9 and caspase 3 are activated by THG treatment in Bax-positive cells but not in Bax knockout cells. Since z-VAD-fmk restored the cross-linking of caspase 3, active caspase 9 and/or caspase 3 may affect the accessibility of tTGase to caspase 3 through unknown mechanisms.
Procaspase 3 is represented as p32 (32 kDa), and active caspase 3 consists of p20/p19/p17 (large subunit with or without prodomain) and p12 (small subunit) (8). Procaspase 3 exists as a stable dimer, p32/p32, in vivo, and then partial cleavage by caspase 8 forms a dimer of p32/p20/p12 or p20/p12/p12/p20. The cross-linking reactions by tTGase probably occur in the preformed caspase 3 complexes but not in monomers in vivo. Therefore, based on its estimated molecular weight, the p64 species is likely cross-linked p32/p32 or p20/p12/p12/20. However, Fig. 5D and E show that only procaspase 3 could form the p64 species in vitro. In addition, Fig. 3 shows that z-VAD-fmk treatment enhanced the formation of this species. Therefore, we speculate that the p64 band is most likely the cross-linked product of p32/p32. The results from Fig. 2 and 3 indicate that the p40 species appears only when procaspase 3 is cleaved. Because p20 cannot form a homodimer, the p40 form of caspase 3 may correspond to the cross-linked p32/p12 or p20/p12/p12. However, we could not detect a stable cross-linked product by in vitro cross-linking assay with either a p32/p12 or p20/p12 mixture. This result is not surprising since we did not produce p20 and p12 by cleavage of a stable homodimer of p32 by caspase 8, and it is probable that p20 and p12 may not be folded correctly to form the p20/p12/p12 heterodimer in vitro. Moreover, these high-molecular-weight species of caspase 3 could also be a part of tTGase-mediated cross-links of caspase 3 with other peptides. Therefore, a more precise analysis of the cross-linked species will provide further insight into the tTGase-mediated inactivation of caspase 3 during THG treatment.
Taken together, the present study demonstrates a novel role for tTGase as a new type of caspase 3 inhibitor during THG-induced apoptosis. Previously, we showed that inhibition of IAPs by Smac or Omi sensitizes Bax-deficient cells to THG (40). Here, we demonstrated that both XIAP and cIAP-1 expression were downregulated during long-term exposure to THG. Concomitant with this downregulation, both tTGase expression and activity were upregulated. Therefore, we propose a model (Fig. 8) that at early time points of THG treatment, IAPs bind to and prevent caspase 3 activation in Bax-deficient cells. However, prolonged exposure to THG leads to the downregulation of IAPs, which is compensated for by subsequent upregulation and activation of tTGase that cross-links caspase 3 to continue keeping this effector caspase inactive. The Bax gene is often mutated in many types of human cancer, which contributes to chemoresistance (45). The finding that tTGase acts as a new type of apoptosis inhibitor, therefore, may provide a target for future attempts at modulating caspase 3 activity in Bax-deficient cancer cells for therapeutic benefit.
Acknowledgments
We thank Bert Vogelstein (Johns Hopkins University) for HCT116 Bax+/− and Bax−/− cells, Emad Alnemri (Kimmel Cancer Institute) for GFP-IETD-Smac plasmid, and Nicholas Woods for reading the manuscript. We acknowledge the services provided by the Moffitt Molecular Biology Core and Molecular Imaging Core.
This work was supported by NIH grant CA82197.
REFERENCES
- 1.Antonyak, M. A., J. E. Boehm, and R. A. Cerione. 2002. Phosphoinositide 3-kinase activity is required for retinoic acid-induced expression and activation of the tissue transglutaminase. J. Biol. Chem. 277:14712-14716. [DOI] [PubMed] [Google Scholar]
- 2.Antonyak, M. A., U. S. Singh, D. A. Lee, J. E. Boehm, C. Combs, M. M. Zgola, R. L. Page, and R. A. Cerione. 2001. Effects of tissue transglutaminase on retinoic acid-induced cellular differentiation and protection against apoptosis. J. Biol. Chem. 276:33582-33587. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 4.Boehm, J. E., U. Singh, C. Combs, M. A. Antonyak, and R. A. Cerione. 2002. Tissue transglutaminase protects against apoptosis by modifying the tumor suppressor protein p110 Rb. J. Biol. Chem. 277:20127-20130. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J. S., and K. Mehta. 1999. Tissue transglutaminase: an enzyme with a split personality. Int. J. Biochem. Cell Biol. 31:817-836. [DOI] [PubMed] [Google Scholar]
- 6.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551-1570. [DOI] [PubMed] [Google Scholar]
- 7.Earnshaw, W. C., L. M. Martins, and S. H. Kaufmann. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68:383-424. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes-Alnemri, T., R. C. Armstrong, J. Krebs, S. M. Srinivasula, L. Wang, F. Bullrich, L. C. Fritz, J. A. Trapani, C. M. Croce, K. J. Tomaselli, G. Litwack, and E. S. Alnemri. 1996. Activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc. Natl. Acad. Sci. USA 93:7464-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fesus, L., and M. Piacentini. 2002. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem. Sci. 27:534-539. [DOI] [PubMed] [Google Scholar]
- 10.Furuya, Y., P. Lundmo, A. Short, D. Gill, and J. Isaacs. 1994. The role of calcium, pH, and cell proliferation in the programmed (apoptotic) death of androgen-independent prostatic cancer cells induced by thapsigargin. Cancer Res. 54:6167-6175. [PubMed] [Google Scholar]
- 11.Green, D. R., and G. I. Evan. 2002. A matter of life and death. Cancer Cell 1:19-30. [DOI] [PubMed] [Google Scholar]
- 12.Hebert, S. S., A. Daviau, G. Grondin, M. Latreille, R. A. Aubin, and R. Blouin. 2000. The mixed lineage kinase DLK is oligomerized by tissue transglutaminase during apoptosis. J. Biol. Chem. 275:32482-32490. [DOI] [PubMed] [Google Scholar]
- 13.Hegde, R., S. M. Srinivasula, Z. Zhang, R. Wassell, R. Mukattash, L. Cilenti, G. DuBois, Y. Lazebnik, A. S. Zervos, T. Fernandes-Alnemri, and E. S. Alnemri. 2002. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 277:432-438. [DOI] [PubMed] [Google Scholar]
- 14.Hirai, I., and H. G. Wang. 2002. A role of the C-terminal region of human Rad9 (hRad9) in nuclear transport of the hRad9 checkpoint complex. J. Biol. Chem. 277:25722-25727. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, P. H., E. S. Sorensen, T. E. Petersen, J. Gliemann, and L. K. Rasmussen. 1995. Residues in the synuclein consensus motif of the alpha-synuclein fragment, NAC, participate in transglutaminase-catalysed cross-linking to Alzheimer-disease amyloid beta A4 peptide. Biochem. J. 310:91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junn, E., R. D. Ronchetti, M. M. Quezado, S. Y. Kim, and M. M. Mouradian. 2003. Tissue transglutaminase-induced aggregation of alpha-synuclein: implications for Lewy body formation in Parkinson's disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 100:2047-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krajewska, M., H.-G. Wang, S. Krajewski, J. M. Zapata, A. Shabaik, R. Gascoyne, and J. C. Reed. 1997. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 57:1605-1613. [PubMed] [Google Scholar]
- 18.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 19.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 20.Lorand, L., and R. M. Graham. 2003. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4:140-156. [DOI] [PubMed] [Google Scholar]
- 21.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 22.Milakovic, T., J. Tucholski, E. McCoy, and G. V. Johnson. 2004. Intracellular localization and activity state of tissue transglutaminase differentially impacts cell death. J. Biol. Chem. 279:8715-8722. [DOI] [PubMed] [Google Scholar]
- 23.Miller, M. L., and G. V. Johnson. 1995. Transglutaminase cross-linking of the tau protein. J. Neurochem. 65:1760-1770. [DOI] [PubMed] [Google Scholar]
- 24.Newmeyer, D. D., and S. Ferguson-Miller. 2003. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112:481-490. [DOI] [PubMed] [Google Scholar]
- 25.Oliverio, S., A. Amendola, C. Rodolfo, A. Spinedi, and M. Piacentini. 1999. Inhibition of “tissue” transglutaminase increases cell survival by preventing apoptosis. J. Biol. Chem. 274:34123-34128. [DOI] [PubMed] [Google Scholar]
- 26.Ozoren, N., and W. S. El-Deiry. 2003. Cell surface death receptor signaling in normal and cancer cells. Semin. Cancer Biol. 13:135-147. [DOI] [PubMed] [Google Scholar]
- 27.Piacentini, M., L. Fesus, M. G. Farrace, L. Ghibelli, L. Piredda, and G. Melino. 1991. The expression of “tissue” transglutaminase in two human cancer cell lines is related with the programmed cell death (apoptosis). Eur. J. Cell Biol. 54:246-254. [PubMed] [Google Scholar]
- 28.Purhonen, P., K. Pursiainen, and H. Reunanen. 1997. Effects of brefeldin A on autophagy in cultured rat fibroblasts. Eur. J. Cell Biol. 74:63-67. [PubMed] [Google Scholar]
- 29.Reed, J. C. 2001. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol. Med. 7:314-319. [DOI] [PubMed] [Google Scholar]
- 30.Rodolfo, C., E. Mormone, P. Matarrese, F. Ciccosanti, M. G. Farrace, E. Garofano, L. Piredda, G. M. Fimia, W. Malorni, and M. Piacentini. 2004. Tissue transglutaminase is a multifunctional BH3-only protein. J. Biol. Chem. 279:54783-54792. [DOI] [PubMed] [Google Scholar]
- 31.Shin, D. M., J. H. Jeon, C. W. Kim, S. Y. Cho, J. C. Kwon, H. J. Lee, K. H. Choi, S. C. Park, and I. G. Kim. 2004. Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J. Biol. Chem. 279:15032-15039. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasula, S. M., M. Ahmad, T. Fernandes-Alnemri, G. Litwack, and E. S. Alnemri. 1996. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc. Natl. Acad. Sci. USA 93:14486-14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasula, S. M., P. Datta, X. J. Fan, T. Fernandes-Alnemri, Z. Huang, and E. S. Alnemri. 2000. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J. Biol. Chem. 275:36152-36157. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, Y., Y. Nakabayashi, and R. Takahashi. 2001. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. USA 98:8662-8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szondy, Z., Z. Sarang, P. Molnar, T. Nemeth, M. Piacentini, P. G. Mastroberardino, L. Falasca, D. Aeschlimann, J. Kovacs, I. Kiss, E. Szegezdi, G. Lakos, E. Rajnavolgyi, P. J. Birckbichler, G. Melino, and L. Fesus. 2003. Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc. Natl. Acad. Sci. USA 100:7812-7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucholski, J., and G. V. Johnson. 2002. Tissue transglutaminase differentially modulates apoptosis in a stimuli-dependent manner. J. Neurochem. 81:780-791. [DOI] [PubMed] [Google Scholar]
- 37.van Loo, G., X. Saelens, M. van Gurp, M. MacFarlane, S. J. Martin, and P. Vandenabeele. 2002. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 9:1031-1042. [DOI] [PubMed] [Google Scholar]
- 38.Verderio, E. A., T. Johnson, and M. Griffin. 2004. Tissue transglutaminase in normal and abnormal wound healing: review article. Amino Acids 26:387-404. [DOI] [PubMed] [Google Scholar]
- 39.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933.11711427 [Google Scholar]
- 40.Yamaguchi, H., K. Bhalla, and H. G. Wang. 2003. Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer Res. 63:1483-1489. [PubMed] [Google Scholar]
- 41.Yamaguchi, H., J. Chen, K. Bhalla, and H. G. Wang. 2004. Regulation of Bax activation and apoptotic response to microtubule-damaging agents by p53 transcription-dependent and -independent pathways. J. Biol. Chem. 279:39431-39437. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi, H., and H. G. Wang. 2002. Bcl-XL protects BimEL-induced Bax conformational change and cytochrome c release independent of interacting with Bax or BimEL. J. Biol. Chem. 277:41604-41612. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi, H., and H. G. Wang. 2004. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 279:45495-45502. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, J., M. Lesort, R. P. Guttmann, and G. V. Johnson. 1998. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J. Biol. Chem. 273:2288-2295. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, L., J. Yu, B. H. Park, K. W. Kinzler, and B. Vogelstein. 2000. Role of BAX in the apoptotic response to anticancer agents. Science 290:989-992. [DOI] [PubMed] [Google Scholar]