Abstract
Lrp1 knock-in mice carrying either a wild-type allele or three different mutated alleles encoding the multifunctional endocytic receptor LRP1 were generated by recombinase-mediated cassette exchange (RMCE). Reinsertion by RMCE of a wild-type allele led to a normal pattern and level of gene expression and a completely normal phenotype, indicating that the RMCE procedure itself is neutral with respect to the function of the gene locus. In contrast, reinsertion of mutated LRP1 alleles carrying either inactivating mutations in the proximal NPXY motif (NPTY→AATA) of the cytoplasmic domain or in the furin cleavage site (RHRR→AHAA) caused distinctive liver phenotypes: respectively, either a late fetal destruction of the organ causing perinatal death or a selective enlargement of von-Kupffer cell lysosomes reminiscent of a mild lysosomal storage without an apparent negative effect on animal survival. Notably, mutation of the distal NPXY motif overlapping with an YXXL motif (NPVYATL→AAVAATL) did not cause any obvious pathological effect. The mutations showed no effect on the LRP1 expression level; however, as expected, the proteolytic maturation of LRP1 into its two subunits was significantly impaired, although not completely abolished, in the furin cleavage mutant. These data demonstrate that RMCE is a reliable and efficient approach to generate multiple mutant knock-in alleles for in vivo functional analysis of individual domains or motifs of large multidomain proteins. Its application in Lrp1 reveals dramatically variant phenotypes, of which further characterization will definitively contribute to our understanding of the biology of this multifunctional receptor.
The multifunctional low-density lipoprotein receptor-related protein 1 (LRP1) is a large receptor that participates in endocytosis, phagocytosis, and signaling pathways (reviewed in references 12 and 31). LRP1 binds a variety of unrelated ligands including ApoE-containing lipoproteins, lipoprotein lipase, complexes of proteinases-proteinase inhibitors, and hormones and growth factors like insulin and platelet-derived growth factor (PDGF). Initially synthesized as a single-chain 600-kDa precursor protein, LRP1 is proteolytically cleaved by a furin-like endoprotease into two subunits of 515 kDa and 85 kDa (10, 11). The functional significance of this maturation step is not known; however, it does not seem to be essential for its endocytic functions (14). The very large, extracellular α-subunit contains the ligand-binding domains and remains attached to the membrane by a noncovalent association with the smaller β-subunit, containing an extracellular part, the membrane spanning domain and the cytoplasmic or intracellular domain (LRP1-ICD).
The complex LRP1-ICD contains many potential signaling motifs, especially for internalization: two NPXY motifs, the distal one overlapping with an YXXL motif, and two dileucine motifs. The YXXL might be the most dominant endocytosis signal (15). The two NPXY motifs are capable of interacting with cytoplasmic adaptor proteins and scaffold proteins, like DAB1, FE65, JIP1, PSD-95, SHC, and CED-6/GULP (2, 7, 17, 22, 32, 33). Furthermore, phosphorylation of LRP1-ICD is believed to regulate and modulate endocytosis and association with adaptor molecules. Binding of Shc, an adaptor protein involved in signaling pathways, requires phosphorylation of Tyr63 within the distal NPXY motif of LRP1-ICD (1, 17). In addition, Tyr63 phosphorylation of the coreceptor LRP1 modulates functional activity of the PDGF-BB receptor in vitro (4, 17) and in vivo in atherosclerotic mouse models (3). Also, serine and threonine phosphorylation by protein kinase A or C modulates endocytosis and association with adaptor molecules (16, 23). Thus, association with specific adaptor molecules in combination with LRP1-ICD phosphorylation is believed to be essential for differentiation of LRP1 function in cargo transport or in signaling pathways upon binding of different ligands to the receptor. LRP1 might also be directly involved in nuclear signaling by regulated intramembrane proteolysis that releases its intracellular domain (13, 18, 19). The precise mechanisms behind all this, however, remain largely unknown. Further molecular dissection of these signals in vitro, and surely also in vivo by mutagenesis of LRP1, will be instrumental to unraveling these mechanisms.
During the past 15 years, studies employing classical or conditional knockout mouse models have been a gold standard (albeit time and labor intensive) for functional analysis in vivo of a given gene or protein. For Lrp1 these approaches resulted in generation of an early embryonic lethal full knockout phenotype (8, 9) and successful tissue-specific inactivation in liver, brain, and vascular smooth muscle cells using the LoxP/Cre system (3, 20, 24, 25). However, this seemingly straightforward strategy approaches its limits when it is used for large multidomain proteins, especially when individual domains of the same protein are involved in very diverse functions, as in the case of LRP1. Such topics have been previously addressed by the use of either exon-specific knockouts or full knockouts “rescued” with different constructs in which individual domains have been inactivated. Alternatively, a knock-in procedure aiming at changing or inactivating a particular domain by mutagenesis of the endogenous gene could be used. However, combination of a knock-in strategy with a recombinase-mediated cassette exchange (RMCE) allowing repetitive use of the same “parental” embryonic stem (ES) cell line (into which different variants of the same endogenous gene can be inserted easily) would be very advantageous if multiple, different knock-ins are the aim. The laborious search for individual, correctly homologous, recombined variant knock-in ES cell lines would no longer be necessary.
In the present study, such an RMCE approach, based upon the method of the laboratory of Bode and colleagues (27-29), using heterospecific FRT (FLP-recombinase target) sites and FLP recombinase, was designed to introduce multiple knock-in mutations in endogenous LRP1. In order to molecularly dissect the function of different motifs in endogenous LRP1 in vivo, mutations were introduced into the furin proteolytic cleavage site and the NPXY motifs in the LRP1-ICD. Here we report the design and one of the first successful applications of an RMCE procedure to generate multiple Lrp1 knock-in mutants in mice for gene function analysis. Furthermore, we describe our initial characterization of the phenotypes corresponding to the different LRP1 knock-in mutations. These phenotypes varied from no phenotype as a result of inactivation of the distal NPXY motif, which overlaps with the YXXL motif, to perinatal death due to inactivation of the proximal NPXY motif.
MATERIALS AND METHODS
Plasmids and constructs.
A targeting construct for homologous recombination in the Lrp1 gene in ES cells, made in pBluescript-SK(−), contained centrally an exchangeable cassette encoding a HygTK selection marker gene flanked by heterospecific FRT sites, which are recognition sequences for the FLP recombinase (kindly provided by J. Bode). A modified FRT site (F3) is present at the 5′ end of the HygTK cassette, whereas a wild-type FRT site (F) is located downstream (29). This hygromycin B-herpes simplex virus thymidine kinase selection marker gene can be used for negative (ganciclovir) or positive (hygromycin B) selection. The upstream and downstream flanking sequences for homologous recombination were derived from murine Lrp1 genomic cosmid clones of the 129P3/J mouse strain (35). The upstream flanking sequences consisted of 2.1 kbp of Lrp1 sequences starting in intron 70 at an SmaI site and ending at an EcoRI site in intron 75. The downstream flanking sequences consisted of about 1.9 kbp starting at an HindIII site 1.1 kbp downstream of the last Lrp1 exon (exon 89). The HygTK cassette with flanking FRT sites, which replaces about 7.8 kbp of Lrp1 sequences, is in an opposite transcription orientation compared to the Lrp1 sequences.
A second construct was made in pGEM-7Zf(+) as a replacement plasmid for application of RMCE. From the 5′ end to 3′ end, it contained the following sequences: (i) a wild-type FRT site (F) (including linker sequences of 90 bp in total), (ii) Lrp1 genomic sequences starting at an EcoRI site in intron 75 up to an SmaI site in exon 76 (about 0.5 kbp), (iii) Lrp1 cDNA sequences starting at an SmaI site in exon 76 up to an EcoRI site in exon 89 (about 2.7 kbp), (iv) Lrp1 genomic sequences starting at an EcoRI site in exon 89 up to an HindIII site in the downstream sequences (about 1.1 kbp), (v) a neomycin selection marker gene (Neo) flanked by LoxP sites (in same orientation as the Lrp1 gene) (about 1.7 kbp), and (vi) a modified FRT site (F3) (including linker sequences of 90 bp in total). The 2.7-kbp SmaI-EcoRI Lrp1 cDNA sequences consisted of complete wild-type sequences (30) or mutated sequences. These mutated Lrp1 cDNA sequences were generated by use of a QuickChange site-directed mutagenesis kit (Stratagene). The different primers for mutagenesis (only the sense primer is shown) are given in Table 1. The mutated Lrp1 cDNA sequences were always entirely checked by sequence analysis.
TABLE 1.
Primers used to introduce Lrp1 knock-in mutations
| Motif | Primera | Mutation |
|---|---|---|
| Furin cleavage site | CCT ACC ACT TCC AAC GCC CAC GCG GCG CAG ATC GAC CGG | RHRR → AHAA |
| NPXY1 | AAT GTG GAA ATT GGA GCC GCT ACC GCC AAG ATG TAT G | NPTY → AATA |
| NPYX2 | CCT ACC AAC TTC ACC GCC GCA GTG GCT GCC ACG CTC T | NPVYATL → AAVAATL |
Only the sense sequences are shown. Residues in bold represent mutated residues compared to wild type.
For transient expression of the FLP recombinase, the expression plasmid pCAGGS-FLPe-IRES-puro was used (26) (kindly provided by F. Stewart).
RMCE in Lrp1 in ES cells.
Lrp1 mutant ES cells were generated by a novel knock-in strategy in ES cells, which was based on an RMCE method initially worked out by the laboratory of Jürgen Bode and colleagues (27-29). First, an exchangeable cassette encoding a selection marker gene was introduced into the Lrp1 gene by homologous recombination in ES cells replacing the 3′ end Lrp1 exons. A linear 7-kbp fragment of the targeting construct was electroporated into E14 ES cells (strain 129 derived) (6) according to standard procedures. Hygromycin B-resistant colonies were analyzed by Southern blot analysis using several external and internal probes to identify correctly recombined clones. One correct clone was obtained, which was subsequently used for application of RMCE to restore the Lrp1 gene with the initially removed Lrp1 coding sequences together with a neomycin selection marker gene. The ES cell line with the initial exchangeable cassette was used repeatedly to restore the Lrp1 gene, either wild type or mutant. In the RMCE procedure itself, a mixture of 100 μg of FLPe expression plasmid and 30 μg of replacement plasmid DNA (both supercoiled circular DNA) in a volume of 30 μl of Tris-EDTA buffer was electroporated into 5 × 106 ES cells. This very high concentration of plasmid DNA is identical to that used by the laboratory of Bode and colleagues (29). The final molecular ratio between the FLPe expression plasmid and the replacement plasmid was about 5 to 1. Cells were plated on feeders in a 250-ml culture flask for about 30 to 48 h (depending on the density and growth of the ES cells) without selection pressure to let the recombination events happen. Subsequently, ES cells were trypsinized and replated at a density of 2.5 × 105 ES cells per 75-ml culture flask. The selection for G418-resistant clones was started immediately or after 12 h. G418-resistant clones (about 5 to 25 surviving clones per flask) were picked for further growth and analysis after 8 to 10 days. Correct exchange by the RMCE method was checked by PCR and Southern blot analysis. The PCR primer pairs used (AB to GH) are given in Table 2, and the relative map positions of these pairs are also indicated in Fig. 1B.
TABLE 2.
Primer pairs used to genotype Lrp1 knock-in ES cell lines and mice
| Primer pair | Primersa | Amplimer size (bp) | PCR result
|
|
|---|---|---|---|---|
| Before RMCE | After RMCE | |||
| AB | CACAGTGTCCCCTCCCCTCTC | 453 | + | − |
| GCCCTGCCATAGCCACTG | ||||
| CD | TTAATATGCGAAGTGGACCTC | 449 | + | − |
| GAGTCATAAGGAGTCTGGAG | ||||
| EF | GTTCCCTCCATGCCCTGACA | 487 (wt) | + | + |
| GGAGCCTGCCGGAGTGAGA | 577 | − | + | |
| GH | CTGAAGGCTCTTTACTATTGC | 405 | − | + |
| GAGTCATAAGGAGTCTGGAG | ||||
| IJb | ACTTCCAACGCCCACGCGGC | 776 | ||
| GGGGCATCTGGAGGGGGTGTTG | ||||
| KLc | CCGCTGATGGCTCCCGACAAT | 466 | ||
| TACATCTTGGCGGTAGCGGC | ||||
| MNd | GCCTACCAACTTCACCGCCG | 442 | ||
| GCCGCCAGCTTCCAGGGGTATG | ||||
| OPe | AATCCTGCTCCTCTCCCTCTC | 398 | ||
| TATCTCCGCAGTCGTTGTCGT | ||||
Primer pairs IJ to MN were used in mutation-specific PCRs. Nucleotides in bold represent mutated residues not present in wild-type Lrp1. wt, wild type.
PCR result was positive only in the case of a furin site mutation.
PCR result was positive only in the case of an NPXY1 mutation.
PCR result was positive only in the case of an NPXY2 mutation.
Genomic probe A for Southern blot analysis.
FIG.1.
RMCE in Lrp1. (A) Principle of RMCE. Due to recombination (indicated by X) between identical FRT sites by a transiently expressed FLP recombinase, an HygTK exchangeable cassette, present in a particular locus and flanked by heterospecific FRT sites (filled and open triangles), is exchanged by a Neo replacement cassette, present in a circular plasmid and also flanked by the same two heterospecific FRT sites. By application of positive (G418) and/or negative (ganciclovir) selection, clones resulting from exchange between the HygTK cassette and the Neo cassette can be selected for. (B) Homologous recombination (HR) replacing the 3′ end part of the Lrp1 gene (3′ part of intron 75 to sequences downstream of exon 89) by an exchangeable cassette (1), which can subsequently be replaced via RMCE (2) with initially removed Lrp1 coding sequences (genomic and cDNA sequences) restoring the Lrp1 gene, either wild type or mutant (3). For selection purposes, a Neo expression cassette flanked by LoxP sites (black triangles) was included in the replacement cassette, downstream of the Lrp1 sequences. The relative positions of the probes A and B and the PCR primer pairs (AB→GH) for monitoring the recombination events are indicated. Restriction enzyme sites are as follows: E, EcoRI; H, HindIII; K, KpnI; N, NotI; S, SacI. (C) Southern blot analyses of ES cell DNA proving correct homologous recombination in the Lrp1 gene in E14 ES cells (HindIII digest and probe A). (D) Southern blot analyses of ES cell DNA showing successful application of RMCE restoring the Lrp1 gene (KpnI digest and probe B). (E) PCR analyses of tail DNA with primer pair EF, proving the presence of the 5′ end FRT site in the Lrp1 gene restored by RMCE.
Generation of mutant Lrp1 knock-in mice.
Four ES cell lines with a modified Lrp1 gene obtained by application of RMCE, one encoding wild-type LRP1 and three encoding a different mutant LRP1, were injected into C57BL6/J blastocysts using standard procedures. The resulting chimeras were mated with C57BL6/J mice. Southern blotting and PCR analysis of tail DNA samples were used to confirm transmission of the modified Lrp1 gene to the offspring. Heterozygous mice were intercrossed to obtain homozygous mice.
For subsequent generations, the genotype of the four obtained mouse lines was checked by use of mutation-specific PCR primer pairs (Table 2, pairs IJ to MN) and by sequencing PCR amplimers covering the modified sequences internally.
Derivation of mutant Lrp1 knock-in MEFs and PEA sensitivity assay.
Mouse embryonic fibroblasts (MEFs) were derived from embryonic day 18.5 (E18.5) homozygous wild-type and modified Lrp1 knock-in embryos by culturing small pieces of thorax tissue in Dulbecco's modified Eagle's medium-F12 medium supplemented with 50% fetal calf serum. Outgrowing fibroblasts were transferred two to three times to new culture flasks for further propagation, and the percentage of fetal calf serum was gradually reduced to 10%. To immortalize the fibroblasts, a plasmid encoding the simian virus 40 large T antigen was transfected to the cells using FuGene 6 (Roche). After an additional 8 to 10 passages, stable MEF cell cultures were established. Together with the Lrp1 knockout MEF cell line, PEA-13 (36), these MEFs were analyzed by Western blot analysis for LRP1 expression and for sensitivity for the Pseudomonas aeruginosa exotoxin A (PEA), for which LRP1 is the unique endocytic receptor (36). Cell survival of initially 0.3 × 106 cells plated in 25-cm2 flasks was monitored upon incubation with 20 or 40 ng/ml PEA in the time period 24 to 48 h after plating.
Western blotting.
Expression of LRP1 protein was analyzed in adult brain and liver tissue. Postnuclear supernatant of homogenized tissue was centrifuged for 1 h at 100,000 × g to obtain soluble and membrane fractions. The membrane fraction (40 μg of protein) was analyzed for LRP1 expression by Western blot analysis. For embryonic liver and brain (40 μg of protein) and MEF cell lines (20 μg of protein), total cell lysates were analyzed. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), 8% Tris-glycine and 4 to 12% NuPage (Invitrogen) gels were used. The two rabbit antibodies recognizing the LRP1 protein were raised against the C terminus of the LRP1 β-subunit (ELLGRGPEDEIGDPLA, amino acids [aa] 4530 to 4545) and against a mixture of two LRP1 α-subunit-specific peptides (DWEEDPKDSRRGRLE, aa 667 to 681, and ISRAKRDQTWREDVVT, aa 1947 to 1962).
Histology.
For mice of all genotypes, all major organs (brain, liver, kidney, spleen heart, lung, and intestine) were investigated by paraffin histology. Further analysis at higher resolution using epoxy resin-embedded material for semithin sectioning and electron microscopy was performed to further characterize the liver phenotypes of NPXY1 and the furin cleavage site mutants. Additionally, complete late prenatal embryos (E16.5) were serially sectioned and screened for further abnormalities of body and tissue architecture.
Mice of both embryonic and postnatal stages were perfused in deep anesthesia via the left ventricle with either modified Bouin's solution (original Bouin's formula, diluted to 25% of normal concentration in Dulbecco’s phosphate-buffered saline [PBS]) or 6% glutaraldehyde in Soerensen phosphate buffer. After dissection, tissues were postfixed in the same solution overnight on a shaker.
For paraffin embedding, Bouin-fixed specimens were rinsed in PBS, dehydrated, and passed through Clear-Rite as an exchange solution before vacuum infiltration with Paraplast Plus. Blocks were cut with a motorized Slee rotary microtome at 7 to 10 μm.
For semi- and ultrathin sectioning and subsequent transmission electron microscopy, glutaraldehyde-fixed tissues were postfixed in 2% OsO4, dehydrated, and embedded in Araldite or Epon 812. Semithin sections were cut on a Leica Ultracut UCT, and alternating slides were stained with toluidine blue and p-phenylene-diamine. Selected sections were remounted on acrylic stubs and resectioned at 70 nm, contrasted with lead citrate, and photographed with a Philips CM10 transmission electron microscope equipped with an SIS Megaview III digital camera or a JEOL JEM 2100 transmission electron microscope with a GATAN Tridiem EELS and camera system.
For scanning electron microscopy, glutaraldehyde-fixed specimens were postfixed in 1% OsO4, dehydrated in graded concentrations of ethanol, which was then stepwise exchanged with 100% acetone. After critical point drying in a Polaron CPD 7501 critical point dryer, samples were mounted on aluminum stubs and gold coated in an automated AGAR sputter coater. Samples were then examined in a JEOL JSM 7401 F scanning electron microscope.
For immunohistology, Paraplast-embedded sections were deparaffinized in Clear-Rite, rehydrated in descending ethanol concentrations, washed in PBS, and after antigen retrieval by either limited proteolysis or heating in citrate buffer, blocked in 2% serum-2% bovine serum albumin in TNB (0.1 M Tris-HCl, pH 7.5, 0.15 M NaCl, 0.5% blocking reagent) buffer, and reacted for 1 h at room temperature with the primary antibody diluted in the same solution. Bound primary antibodies were detected by the use of horseradish peroxidase-tagged secondary antibodies, followed by tyramide-fluorescein isothiocyanate and a counterstain with bisbenzimide.
Light microscopy specimens were photographed with a ZEISS Axioplan 2e microscope equipped with a Zeiss AxioCam Hr.
RESULTS
Efficient generation of mutant Lrp1 knock-in ES cell lines by application of RMCE.
Mutant Lrp1 ES cells were generated by a novel knock-in strategy in ES cells, which was based on a RMCE method, initially worked out by the laboratory of Jürgen Bode and colleagues (27-29). In this RMCE method (Fig. 1A), the investigators first randomly introduced in the genome of ES cells an exchangeable cassette encoding a selection marker gene flanked by two different, heterospecific FRT sites. In the next step, this exchangeable cassette was efficiently replaced by a cassette encoding a different selection marker gene flanked by the same heterospecific FRT sites mediated by transiently expressed FLP recombinase. This replacement occurs, in fact, via a two-step mechanism as a result of recombination events between identical FRT sites, as explained in detail by Seibler and Bode (27). Correctly recombined clones were selected by medium containing G418 and/or ganciclovir.
A novel knock-in strategy that is described and applied here combined a homologous recombination event in mouse ES cells, replacing part of the 3′ end exons of the Lrp1 gene by the aforementioned exchangeable cassette encoding a selection marker gene, with subsequent application of RMCE to restore the Lrp1 gene with the initially removed Lrp1 coding sequences together with a different selection marker gene (Fig. 1B).
Instead of genomic sequences, predominantly cDNA sequences were used in the replacement vector to reduce the complexity of the construct and to facilitate the introduction of many different or multiple mutations in sequences corresponding to different exons of the endogenous gene. The ES cell line with the initial exchangeable cassette resulted from a homologous recombination event with a low efficiency: only one out of 300 hygromycin B-resistant clones proved to be correctly recombined, as was proven by Southern blot analysis using several internal and external probes (Fig. 1C and data not shown).
This ES cell line was used repeatedly to restore the Lrp1 gene, either wild-type or mutant, by application of RMCE. The following three mutations were introduced: (i) mutation of the furin cleavage site (RHRR→AHAA) encoded by exon 76 sequences, (ii) mutation of the proximal NPXY motif (NPXY1; NPTY→AATA) encoded by exon 88 sequences, and (iii) mutation of the distal NPXY motif (NPXY2; NPVY→AAVA) encoded by exon 89 sequences. This distal NPXY motif overlaps with an YXXL motif, which is concomitantly inactivated.
Application of this RMCE method in the Lrp1 gene using positive selection by G418 proved to be very efficient. The relative number of ES cell clones that underwent RMCE correctly, based upon initial PCR analysis using primer pairs AB, CD, EF, and GH (Fig. 1B), was 57% (wild-type cDNA construct), 38% (furin cleavage site mutation), 61% (NPXY1 mutation), and 46% (NPXY2 mutation). For each construct six clones were selected for further analysis by Southern blotting, and all proved to be correctly recombined (Fig. 1D and data not shown).
Generation of mutant Lrp1 knock-in mice.
Clones of the four different modified Lrp1 ES cell lines, one encoding wild-type LRP1 and three encoding a different mutant LRP1, were successfully used to generate chimeric mice by blastocyst injection. For all four lines, chimeras gave rise to germ line transmission after mating with C57BL6/J mice. Heterozygous mice carrying one of the Lrp1 knock-in modifications (C57BL6/J × 129 background) were intercrossed to obtain homozygous offspring. The viable offspring were genotyped by PCR (Fig. 1E). Southern blot analysis of a limited number of mice was used for further confirmation of the presence of modified Lrp1 alleles (data not shown). All modified Lrp1 mouse lines, except the NPXY1 mutant, resulted in viable and fertile homozygous modified offspring. The ratio of the three genotypes was according to a Mendelian distribution. The NPXY1 mutant showed only viable wild-type and heterozygous offspring at a ratio of 1 to 2, indicative for embryonic lethality for homozygous offspring.
Reinsertion of the wild-type Lrp1 gene by the RMCE method itself does not, as expected, show any phenotype.
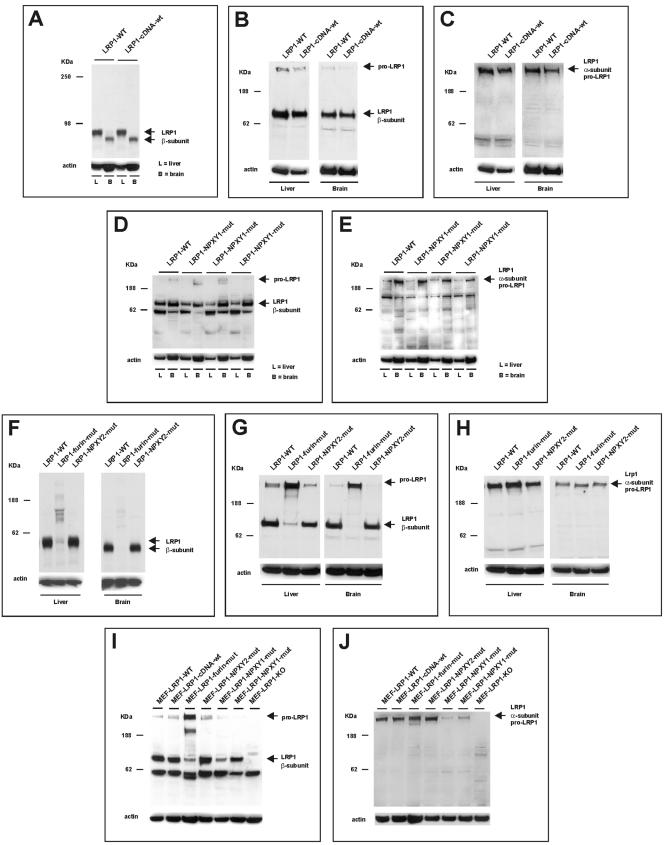
The modified Lrp1 allele encoding wild-type LRP1 contains some additional sequences outside the LRP1 coding sequences (internally, an FRT site; downstream, a floxed Neo cassette and an FRT site), whereas some 3′ end introns are no longer present. These modifications could theoretically have a functional impact on the Lrp1 locus and could give a phenotype, but this is not the case, as expected. The homozygous mice are viable and fertile and show neither biochemical nor histological differences compared to normal wild-type mice. Figure 2A, B, and C show a typical example of biochemical analysis and comparison to wild-type mice. They revealed similar expression levels both in liver and brain and normal maturation into the α- and β-subunits. Also the difference in mobility in SDS-PAGE between the β-subunits in liver and brain due to differences in glycosylation (18) is the same. Nor were histological differences noticed in the major organs that were analyzed (data not shown). So, the modified Lrp1 allele encoding wild-type LRP1 can be considered as a functional wild-type Lrp1 allele. This implies that the other three modifications, which introduce actual mutations in the LRP1 protein by the same RMCE method, can indeed be considered as genuine mutations in LRP1.
FIG.2.
LRP1 protein expression and maturation in mutant Lrp1 knock-in mice and MEFs. Equal amounts of protein samples of adult (A to C and F to H) and E18.5 fetal (D and E) liver and brain tissue of mice and derived MEFs (I and J) were analyzed by Western blotting using 8% Tris-glycine (A and F) and 4 to 12% NuPage (B to E and G to J) gels. LRP1 protein expression was analyzed using an antibody recognizing the carboxy terminus of pro-LRP1 and the β-subunit of LRP1 (AB, D, F, G, and I) or an antibody recognizing epitopes shared by pro-LRP1 and the α-subunit of LRP1 (C, E, H, and J). Analysis with an antibody against actin was used to monitor the amount of protein loaded. The genotypes of the mice and derived MEFs are indicated above the lanes: LRP1-WT, wild-type; LRP1-cDNA-wt, wild-type Lrp1 cDNA knock-in; LRP1-furin-mut, mutant Lrp1 furin cleavage site knock-in; LRP1-NPXY1-mut, mutant Lrp1 NPXY1 knock-in; LRP1-NPXY2-mut, mutant Lrp1 NPXY2 knock-in; MEF-LRP1-KO, MEF cell line PEA-13 derived from Lrp1 knockout.
Mutation of the NPXY1 motif in LRP1-ICD results in embryonic lethality.
Several motifs in the LRP1-ICD are believed to play an essential role in the regulation of the function of the LRP1 receptor. The embryonic lethal phenotype of the homozygous NPXY1 mutant illustrates this dramatically. The NPXY1 motif appears to be of decisive importance for hepatic LRP1 function during late fetal development. Homozygous embryos appear to be phenotypically normal up to about E15. From this stage onwards, a rapidly progressing destruction of liver tissue occurs, whereby the loss of cells is paralleled by an edematous swelling and enlargement of the organ, strikingly evident at E18.5 (Fig. 3). The majority of homozygous mice die perinatally, and occasionally dead homozygous pups could be found in the cages. However, a more detailed analysis of the survival of the different genotypes during intrauterine development indicates that already beyond E14.5 homozygous embryos may be underrepresented (Table 3).
FIG. 3.
Mutation of the NPXY1 motif causes fetal hepatomegaly and perinatal death. The majority of fetuses with inactivated NPXY1 motifs survive through pregnancy up to term but then die shortly before or immediately after birth. At E18.5 (shown here) fetuses with mutated NPXY1 motifs (B and D) are mildly disproportionate, with a foreshortened neck and caudal body region and an enlarged abdomen, compared to wild-type littermates (A and C). Upon dissection, the fetuses feature a drastically enlarged liver (compare panels C and D) but no further macroscopic abnormalities. Sagittal sections indicate that already at E16.5 the liver is markedly enlarged (arrows), partially displacing the gut in the caudal abdominal cavity. (E) Wild type. (F) NPXY1 mutation. (Note that positions of stomach in panels E and F [arrowheads] indicate comparable paramedian planes of sectioning.) No major abnormalities of other organs are evident.
TABLE 3.
Survival table for Lrp1 NPXY1 knock-in mutants
| Age | No. of litters | No. of embryos | No. of living embryos by genotype
|
Additional implantations
|
||||
|---|---|---|---|---|---|---|---|---|
| Wild type (+/+) | Heterozygous (m/+) | Homozygous (m/m) | No. of resorptionsa | Dead embryos
|
||||
| No. | Genotype | |||||||
| E10.5 | 7 | 51 | 12 | 28 | 11 | 4 | 2 | +/+ |
| 2 | m/m | |||||||
| E14.5 | 16 | 86 | 17 | 48 | 21 | 6 | 2 | m/+ |
| 4 | m/m | |||||||
| E16.5 | 18 | 140 | 44 | 74 | 22 | 4 | 2 | m/+ |
| 5 | m/m | |||||||
| E18.5 | 20 | 124 | 27 | 79 | 18 | 2 | m/m | |
| Newborn | 13 | 72 | 50 | 22 | 0 | |||
No genotypes.
Histologically, hepatic lesions begin with a conspicuous enlargement of the space of Dissé between hepatocytes and sinusoid endothelia (Fig. 4), whereby scattered larger gaps occur that could be indicative of lost hematopoietic stem cells.
FIG. 4.
Hepatomegaly in NPXY1 embryos is accompanied by a dramatic cell loss and edematous swelling of the tissue. (A and B) During late pregnancy (shown here for E16.5 to birth) the murine liver is characterized by numerous hematopoietic stem cells (white arrow in panel B; black arrow indicates a megakaryocyte) interposed between hepatocytes (hep) and sinusoids (S) leading to the central veins (CV). While the liver of an NPXY1 mouse at E16.5 still looks relatively normal (C), from E17.5 onwards to E19.5 (D and E, respectively), the subendothelial space along the sinusoid lining develops a network of fluid-filled spaces (asterisks), while the density of cells drops. At E19.5 (E), the solid tissue between sinusoids has largely disappeared. Ultrastructurally, surviving hepatocytes often have large vacuoles and show a conspicuous load with glycogen (F and G). Scale bars: 150 μm (A), 75 μm (B to E), and 10 μm (F and G).
Between E16.5 and E18.5, the majority of hepatocytes degenerate, so that at E19.5 liver lobules mainly consist of endothelial tubes of the sinusoids, between which the hepatocyte strands are transformed into mainly fluid-filled spaces with scattered hematopoietic stem cells. Surviving hepatocytes often feature large accumulations of glycogen in their cytoplasm.
Biochemical analysis of liver and brain tissue from E18.5 mutant embryos revealed a slightly lower expression of LRP1 in liver compared to wild-type embryos, potentially reflecting only differences in relative composition of variant liver cell types, whereas in brain the expression levels appeared to be similar (Fig. 2D and E). Proteolytic maturation of LRP1 into its subunits seemed not to be affected by the mutation. Contrary to adult tissue, the relative expression in the brain seems to be higher than in the liver, both in wild-type and mutant embryos.
Mutation of the NPXY2 motif in LRP1-ICD does not show any phenotype.
In contrast to the NPXY1 motif, the NPXY2 motif, which overlaps with an YXXL motif, seems to be dispensable, since the NPXY2 mutation did not show any phenotype so far. The homozygous mice are viable and fertile, and biochemical analyses (Fig. 2F, G, and H) as well as histological analyses of the major organs (data not shown) revealed no differences compared to wild-type mice.
Mutation of the furin cleavage site in LRP1 impairs proteolytic maturation of LRP1 but causes only a very mild phenotype.
The functional significance of the proteolytic maturation of pro-LRP1 into α- and β-subunits by cleavage at a furin cleavage site in vivo is unknown but could have a crucial impact on LRP1 function. Biochemical analysis of tissues derived from mice homozygous for a cleavage site mutation revealed, however, a clear impairment of the maturation of pro-LRP1 into its subunits. Figure 2F, G, and H show a typical example of biochemical analysis of this mutation and comparison to tissues from wild-type mice. However, Western blot analysis showed some residual maturation clearly visible in liver (estimated to be less than 5%) and after prolonged exposure also in brain (data not shown). The consistent difference in mobility in SDS-PAGE between the β-subunits in liver and brain argues against a nonspecific antibody binding as an explanation. So this quite unexpected result points toward presence of a minor, alternative cleavage site close to the furin cleavage site.
Despite this significant defect of LRP1 maturation, the resultant mice developed normally and did not show any macroscopically visible alteration. Likewise, the histological analysis of both fetal and adult furin cleavage mutant mice revealed a normal phenotype for most tissues investigated. Only in the adult liver (Fig. 5) was a conspicuous vacuolization within the local macrophages, the von-Kupffer star cells, noted. Vacuoles were immunopositive for lamp1 (data not shown), identifying them as enlarged lysosomes. Ultrastructurally, these lysosomes appeared “empty,” with some strands of granular material, pointing toward a soluble content extracted during embedding.
FIG. 5.
Mutation of the furin cleavage site causes vacuolization of von-Kupffer cells. In contrast to the NPXY1 motif, mutation of the furin cleavage site is compatible with survival into adulthood. Microscopic analysis reveals an increased vacuolization (white arrows) only in adult liver due to enlarged lysosomes of hepatic von-Kupffer macrophages (white arrows in panel B; v-KC in panel C) compared to controls (A). Optically “empty” lysosomes fill a major part of the macrophage cell body (asterisk in panel C). Scale bars, 20 μm (A and B) and 5 μm (C).
All different mutant knock-in Lrp1 MEFs can still function as endocytic receptors.
All the homozygous wild-type and modified Lrp1 knock-in MEFs derived during this study expressed LRP1. Only the two independently isolated MEFs expressing the NPXY1 mutant revealed relatively lower expressions compared to wild-type MEFs, one moderately lower and one even much lower in expression (Fig. 2I and J). In the full LRP1 knockout MEF cell line (PEA-13), of course no LRP1 expression could be detected. All MEFs showed proteolytic maturation of LRP1 into its subunits. However, as in the parental homozygous mice, the furin cleavage site mutant MEFs showed only some weak residual maturation.
All MEFs, except the LRP1 knockout and the NPXY1 mutants, showed similar high sensitivity for exposure to PEA. Incubation with 20 or 40 ng/ml PEA resulted in death of all cells after 24 h. The LRP1 knockout and the NPXY1 mutant with the much lower LRP1 expression showed no cell death or reduced growth compared to nontreated controls. The NPXY1 mutant with the moderately lower LRP1 expression showed moderate cell death at a low PEA concentration but high cell mortality at a high PEA concentration. Despite a potential impact because of lower LRP1 expression levels, the NPXY1 mutant seemed to be sensitive for PEA, although maybe to a lesser extent compared to wild-type and the two other knock-in mutations. This indicates that the NPXY1 mutant is still capable of functioning as an endocytic receptor for PEA.
DISCUSSION
RMCE is an economic and reliable approach to characterize domain-specific functions in complex proteins.
The conventional techniques to study the role of a protein in a loss-of-function approach like knockout animals or RNA interference or overexpression of dominant negative protein variants in vitro typically affect the entire protein. While normally desired, this can be a significant disadvantage when complex multidomain proteins are addressed, since different domains might subserve entirely different functions. Exon- or domain-specific knockout and knock-in animals have been successfully created in numerous instances, but this technique is impractical for a systematic functional screen of large proteins, as for each modification a complete new knockout or knock-in project will have to be started.
Here, RMCE offers a significantly more economic approach of inserting any desired modified or mutated allele of a gene of interest into the respective locus of a parental ES cell line. In particular, the efficiency of the exchange was surprisingly high (38 to 61%). Similar high efficiencies were obtained in another locus, for which also only positive G418 selection was used (data not shown). In all experiments, the same large amounts of expression and replacement plasmids as used by the laboratory of Bode and colleagues (29) were applied. These high efficiencies observed made the further optimizing of plasmid amounts and ratios unnecessary. Possibly the presence of the exchange cassette in high-quality, supercoiled, circular plasmid DNA together with its recognition and binding by the highly expressed FLP recombinase (due to the even larger amounts of expression plasmid DNA used) provoked the occurrence of cassette exchange, which is finally almost as likely as random integration in this RMCE procedure. However, Cesari and colleagues (5) also showed previously the successful application of a similar RMCE procedure to generate Elk-1 knockout mice. They used the same heterospecific FRT sites, the same HygTK gene, the same FLPe plasmid, and similarly large amounts of plasmid for their RMCE application. The lower efficiency (6%) in their experiment might point toward locus-dependent effects having an impact on the ratio of cassette exchange versus random integration.
Furthermore, it should be noted that in an initial RMCE pilot experiment in the Lrp1 gene, a combination of positive selection by G418 and negative selection by ganciclovir resulted in 100% correctly recombined clones (8 out of 8), whereas only positive selection by G418 yielded 63% (15 out of 24). Because of the high efficiency of G418 selection alone and some adverse effects of ganciclovir on ES cell growth and ES cell morphology in our hands, it was decided to use only the positive selection procedure in further experiments. Use of only negative selection by ganciclovir was therefore not tested but should in principle be feasible, enabling exchange with a replacement cassette without a selection marker gene. In the application of RMCE by Cesari and colleagues (5), a combination of positive G418 and negative ganciclovir selection was used. So actual experimental proof that a RMCE method can indeed be used to generate mice carrying marker-free replacement constructs is still not available, but this is likely only a matter of time.
As application of RMCE requires the modification of the original locus by the addition of, e.g., FRT sites and, in our case, a floxed Neo marker gene, additional controls have to be performed to ensure that the RMCE procedure itself is neutral with respect to gene function. Most importantly, as a “positive” control, the reintroduction of a wild-type allele into the prepared locus should restore gene expression and protein function to physiological levels both in vitro and especially in derived knock-in mice. Here, our data show that, indeed, the reinsertion of a wild-type allele leads to the generation of fully normal “wild-type” mice. Both in tissues and derived cell lines, LRP1 expression is qualitatively and quantitatively normal, up to the level of a faithful reproduction of tissue-specific glycosylation and normal posttranslational processing, which confirms that the RMCE-specific modification of the locus itself is neutral toward gene function and that any effects caused by the insertion of modified alleles are, indeed, caused by these alleles themselves.
Mutation of the furin cleavage site introduced by RMCE causes the predicted molecular effect.
As a further control of fidelity of RMCE-induced mutations, we used an LRP1 allele with a mutated furin cleavage site, first to find out whether the theoretically predictable effect on LRP1 processing would indeed occur in tissues and derived cell lines and, second, to evaluate the significance of furin cleavage for LRP1 function.
The subtotal loss of furin-type processing of the mutated LRP1 allele first shows that reinserted mutated alleles indeed behave as predicted, i.e., that the RMCE procedure is also neutral with respect to the fate of the encoded protein beyond the mere regulation of expression levels, thereby adding to the respective data on other processing events like cleavage and glycosylation of the respective wild-type allele (see above).
In parallel, our data also reveal the existence of another, albeit minor, LRP1 processing activity that can cleave the protein close to the furin site. As no typical furin consensus sequence could be identified in that region, this activity probably belongs to a different protease family, to be further identified by inhibitor profiling and other means.
Finally, it is striking that furin-dependent maturation seems to play such a small role for overall LRP1 function, as evidenced both by the largely normal phenotype of the mice—as opposed to the very early embryonic lethality of the classical full knockout (8, 9)—and the preserved uptake of PEA. Nonetheless, it cannot be excluded that the presumed residual processing by a different protease may contribute to the rescue of the phenotype. On the other hand, the results are in agreement with analysis in vitro, which showed no effect of mutation of the furin cleavage site on endocytic functions, although ER exit was somewhat retarded (14). Thus, the furin-dependent processing of LRP1 to form the characteristic heterodimer of the mature receptor (an event not unlike the Notch receptor) is apparently of minor importance for its function. However, the enlargement of von-Kupffer cell lysosomes may be related to some kind of storage-like phenomenon, pointing toward a possible role of the heterodimer structure in the further processing of internalized material and/or receptor downstream of actual phagocytosis.
Mutation of individual domains of LRP1-ICD by RMCE reveals involvement of NPXY1 in organ- and stage-specific functions.
LRP1 plays a role in several putatively independent functional pathways, ranging from the internalization of bound ligands, via protein kinase- and phosphorylation-mediated signaling involving its intracellular domain, to regulated intramembrane proteolysis releasing its intracellular domain for a possible nuclear signaling (reviewed in references 12 and 31). Therefore, it is at present largely unclear which of the many functions ascribed to LRP1 uses which signaling mechanism or combinations of mechanisms. Here we have started to address this question by individually mutating the two NPXY motifs in LRP1-ICD, which are thought to serve as important internalization signals and binding motifs. Previous in vitro studies using LRP1 mini-receptor constructs revealed that the YXXL motif, which overlaps with the NPXY2 motif and is concomitantly inactivated in the NPXY2 knock-in mutation, serves as the dominant endocytosis signal for LRP1 (15). However, the complete lack of phenotype for the NPXY2 knock-in mutation suggests that the distal NPXY2 and the YXXL motifs are dispensable. The PEA assay of NPXY2 knock-in MEFs, which revealed no differences compared to wild-type LRP1, confirms this. The lack of a pathological phenotype of this mutant is very unexpected, especially because this motif occurs via interaction with many different cytosolic adaptor and scaffold proteins and modulation thereof by phosphorylation of Tyr63 in this motif involved several different signal pathways and processes, like PDGF-BB signaling, phagocytosis, and modulation of postsynaptic responses (2, 7, 17, 22, 32, 33).
On the other hand, direct proof of the interaction of proteins with the NPXY1 motif is limited to FE65, which was shown to be capable of binding to both NPXY motifs (22).
So, a phenotype for one of the two NPXY mutations was expected for the NPXY2 mutation rather than for the NPXY1 mutation. However, in striking contrast to an inactivated NPXY2 motif, mutation of the NPXY1 motif caused a novel lethal liver phenotype characterized by the late dramatic destruction of liver tissue between E16.5 and E18.5. Since the NPXY1 motif does not seem to fulfill an important role as an endocytosis signal in vitro (15) and the NPXY1 mutant MEFs are still capable of functioning as endocytic receptors for PEA, our data would argue for an endocytosis-independent signaling function of LRP1 to be of critical importance for late prenatal liver development and possibly also for the transient hematopoietic microenvironment in this tissue. Here, a potential role of LRP1 in cytokine signaling could depend on the NPXY1 motif, the mutation of which would then cause the degradation of this hematopoietic environment.
In summary, our data on the effects of the targeted mutation of different LRP1 domains show as a proof-of-principle that RMCE can be used to insert different variants of a gene into its original locus without side effects from the procedure itself. This opens up numerous possibilities to study protein function from the inactivation of functional domains like internalization signals, the removal of glycosylation sites, the expression of humanized proteins, etc. In the case of LRP1, additional knock-in mice are presently being generated and will be analyzed. Derived knock-in MEFs, which can be reused for application of RMCE, will be used to generate additional knock-in MEF mutants with even more subtle mutations in the endogenous Lrp1 gene. These additional MEFs together with the ones already described here will be used to study in vitro the impact of the mutations in the endogenous Lrp1 gene on ligand internalization, cell proliferation, migration, etc. Furthermore, it would be of major clinical interest to establish which of the subdomains of LRP1-ICD are of relevance for the internalization or metabolism of amyloid precursor protein and Aβ peptides or PDGF-BB receptor. The presumed role of LRP1 in the development of Alzheimer's disease (13, 21, 22, 34) and atherosclerosis (3, 4, 17), in which Tyr63 of the NPXY2 motif potentially plays a central role, will also be studied further in vivo by crossing of the viable LRP1 NPXY2 mutant with mouse models for Alzheimer's disease and atherosclerosis. These analyses will likely reveal a phenotype for the NPXY2 mutation relevant for our understanding of the etiology of these diseases.
Acknowledgments
We thank Jürgen Bode and Francis Stewart for the plasmid carrying the HygTK cassette flanked by heterospecific FRT sites and the plasmid pCAGGS-FLPe-IRES-puro, respectively. We also thank An Snellinx and Elke Maes for technical assistance.
We thank the Fonds voor Wetenschappelijk Onderzoek Vlaanderen for financial support granted to A.R. and D.H. This work was also financially supported by the IUAP program and the Alzheimer's Association.
REFERENCES
- 1.Barnes, H., E. J. Ackermann, and P. van der Geer. 2003. v-Src induces Shc binding to tyrosine 63 in the cytoplasmic domain of the LDL receptor-related protein 1. Oncogene 22:3589-3597. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, H., B. Larsen, M. Tyers, and P. van Der Geer. 2001. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J. Biol. Chem. 276:19119-19125. [DOI] [PubMed] [Google Scholar]
- 3.Boucher, P., M. Gotthardt, W. P. Li, R. G. Anderson, and J. Herz. 2003. LRP: role in vascular wall integrity and protection from atherosclerosis. Science 300:329-332. [DOI] [PubMed] [Google Scholar]
- 4.Boucher, P., P. Liu, M. Gotthardt, T. Hiesberger, R. G. Anderson, and J. Herz. 2002. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J. Biol. Chem. 277:15507-15513. [DOI] [PubMed] [Google Scholar]
- 5.Cesari, F., V. Rennekampff, K. Vintersten, L. G. Vuong, J. Seibler, J. Bode, F. F. Wiebel, and A. Nordheim. 2004. Elk-1 knock-out mice engineered by Flp recombinase-mediated cassette exchange. Genesis 38:87-92. [DOI] [PubMed] [Google Scholar]
- 6.Doetschman, T., R. G. Gregg, N. Maeda, M. L. Hooper, D. W. Melton, S. Thompson, and O. Smithies. 1987. Targeted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature 330:576-578. [DOI] [PubMed] [Google Scholar]
- 7.Gotthardt, M., M. Trommsdorff, M. F. Nevitt, J. Shelton, J. A. Richardson, W. Stockinger, J. Nimpf, and J. Herz. 2000. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 275:25616-25624. [DOI] [PubMed] [Google Scholar]
- 8.Herz, J., D. E. Clouthier, and R. E. Hammer. 1992. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71:411-421. [DOI] [PubMed] [Google Scholar]
- 9.Herz, J., D. E. Couthier, and R. E. Hammer. 1993. Correction: LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 73:428. [DOI] [PubMed] [Google Scholar]
- 10.Herz, J., U. Hamann, S. Rogne, O. Myklebost, H. Gausepohl, and K. K. Stanley. 1988. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 7:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herz, J., R. C. Kowal, J. L. Goldstein, and M. S. Brown. 1990. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 9:1769-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herz, J., and D. K. Strickland. 2001. LRP: a multifunctional scavenger and signaling receptor. J. Clin. Investig. 108:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinoshita, A., T. Shah, M. M. Tangredi, D. K. Strickland, and B. T. Hyman. 2003. The intracellular domain of the low-density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J. Biol. Chem. 278:41182-41188. [DOI] [PubMed] [Google Scholar]
- 14.Ko, K. W., R. S. McLeod, R. K. Avramoglu, J. Nimpf, D. J. FitzGerald, J. Vukmirica, and Z. Yao. 1998. Mutation at the processing site of chicken low-density lipoprotein receptor-related protein impairs efficient endoplasmic reticulum exit, but proteolytic cleavage is not essential for its endocytic functions. J. Biol. Chem. 273:27779-27785. [DOI] [PubMed] [Google Scholar]
- 15.Li, Y., M. P. Marzolo, P. van Kerkhof, G. J. Strous, and G. Bu. 2000. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low-density lipoprotein receptor-related protein. J. Biol. Chem. 275:17187-17194. [DOI] [PubMed] [Google Scholar]
- 16.Li, Y., P. van Kerkhof, M. P. Marzolo, G. J. Strous, and G. Bu. 2001. Identification of a major cyclic AMP-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: implication for receptor-mediated endocytosis. Mol. Cell. Biol. 21:1185-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loukinova, E., S. Ranganathan, S. Kuznetsov, N. Gorlatova, M. M. Migliorini, D. Loukinov, P. G. Ulery, I. Mikhailenko, D. A. Lawrence, and D. K. Strickland. 2002. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low-density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function between LRP and the PDGF. J. Biol. Chem. 277:15499-15506. [DOI] [PubMed] [Google Scholar]
- 18.May, P., H. H. Bock, J. Nimpf, and J. Herz. 2003. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J. Biol. Chem. 278:37386-37392. [DOI] [PubMed] [Google Scholar]
- 19.May, P., Y. K. Reddy, and J. Herz. 2002. Proteolytic processing of low-density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J. Biol. Chem. 277:18736-18743. [DOI] [PubMed] [Google Scholar]
- 20.May, P., A. Rohlmann, H. H. Bock, K. Zurhove, J. D. Marth, E. D. Schomburg, J. L. Noebels, U. Beffert, J. D. Sweatt, E. J. Weeber, and J. Herz. 2004. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol. Cell. Biol. 24:8872-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrzik, C. U., T. Busse, D. E. Merriam, S. Weggen, and E. H. Koo. 2002. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 21:5691-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrzik, C. U., I. S. Yoon, S. Jaeger, T. Busse, S. Weggen, and E. H. Koo. 2004. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J. Neurosci. 24:4259-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranganathan, S., C. X. Liu, M. M. Migliorini, C. A. Von Arnim, I. D. Peltan, I. Mikhailenko, B. T. Hyman, and D. K. Strickland. 2004. Serine and threonine phosphorylation of the low-density lipoprotein receptor-related protein by protein kinase Calpha regulates endocytosis and association with adaptor molecules. J. Biol. Chem. 279:40536-40544. [DOI] [PubMed] [Google Scholar]
- 24.Rohlmann, A., M. Gotthardt, R. E. Hammer, and J. Herz. 1998. Inducible inactivation of hepatic LRP gene by Cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Investig. 101:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohlmann, A., M. Gotthardt, T. E. Willnow, R. E. Hammer, and J. Herz. 1996. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat. Biotechnol. 14:1562-1565. [DOI] [PubMed] [Google Scholar]
- 26.Schaft, J., R. Ashery-Padan, F. van der Hoeven, P. Gruss, and A. F. Stewart. 2001. Efficient FLP recombination in mouse ES cells and oocytes. Genesis 31:6-10. [DOI] [PubMed] [Google Scholar]
- 27.Schlake, T., and J. Bode. 1994. Use of mutated FLP recognition target (FRT) sites for the exchange of expression cassettes at defined chromosomal loci. Biochemistry 33:12746-12751. [DOI] [PubMed] [Google Scholar]
- 28.Seibler, J., and J. Bode. 1997. Double-reciprocal crossover mediated by FLP-recombinase: a concept and an assay. Biochemistry 36:1740-1747. [DOI] [PubMed] [Google Scholar]
- 29.Seibler, J., D. Schubeler, S. Fiering, M. Groudine, and J. Bode. 1998. DNA cassette exchange in ES cells mediated by Flp recombinase: an efficient strategy for repeated modification of tagged loci by marker-free constructs. Biochemistry 37:6229-6234. [DOI] [PubMed] [Google Scholar]
- 30.Smeijers, L., S. Willems, A. Lauwers, E. Thiry, F. van Leuven, and A. J. Roebroek. 2002. Functional expression of murine LRP1 requires correction of Lrp1 cDNA sequences. Biochim. Biophys. Acta 1577:155-158. [DOI] [PubMed] [Google Scholar]
- 31.Strickland, D. K., and S. Ranganathan. 2003. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J. Thromb. Haemost. 1:1663-1670. [DOI] [PubMed] [Google Scholar]
- 32.Su, H. P., K. Nakada-Tsukui, A. C. Tosello-Trampont, Y. Li, G. Bu, P. M. Henson, and K. S. Ravichandran. 2002. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP). J. Biol. Chem. 277:11772-11779. [DOI] [PubMed] [Google Scholar]
- 33.Trommsdorff, M., J. P. Borg, B. Margolis, and J. Herz. 1998. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem. 273:33556-33560. [DOI] [PubMed] [Google Scholar]
- 34.Ulery, P. G., J. Beers, I. Mikhailenko, R. E. Tanzi, G. W. Rebeck, B. T. Hyman, and D. K. Strickland. 2000. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J. Biol. Chem. 275:7410-7415. [DOI] [PubMed] [Google Scholar]
- 35.van der Zee, A., L. Stas, C. Hilleker, F. van Leuven, K. W. van Dijk, L. Havekes, R. Frants, and M. Hofker. 1994. Genomic cloning of the mouse LDL receptor related protein/alpha 2-macroglobulin receptor gene. Genomics 23:256-259. [DOI] [PubMed] [Google Scholar]
- 36.Willnow, T. E., and J. Herz. 1994. Genetic deficiency in low density lipoprotein receptor-related protein confers cellular resistance to Pseudomonas exotoxin A. Evidence that this protein is required for uptake and degradation of multiple ligands. J. Cell Sci. 107:719-726. [PubMed] [Google Scholar]