Abstract
T-cell death-associated gene 8 (TDAG8) is a G-protein-coupled receptor transcriptionally upregulated by glucocorticoids (GCs) and implicated by overexpression studies in psychosine-mediated inhibition of cytokinesis and in GC-induced apoptosis. To examine the physiological function of TDAG8, we generated knockout (KO) mice by homologous recombination. An enhanced green fluorescent protein reporter was knocked into the disrupted tdag8 locus to allow the analysis of TDAG8 expression in living cells. Interestingly, we found that during thymocyte development, TDAG8 expression resembled the dynamic regulation described for known modulators of GC-induced apoptosis, including Bcl-2, Notch1, and GC receptor. TDAG8 was expressed in double-negative cells, was downregulated at the double-positive transition, and was upregulated in single-positive thymocytes. However, despite this striking expression pattern, maturation and selection of thymocytes, as well as major immune functions, were not affected in TDAG8 KO mice. In contrast to previous overexpression results, TDAG8 was dispensable for psychosine-induced formation of multinucleated cells. Furthermore, TDAG8 KO thymocytes showed normal apoptosis following in vivo and in vitro GC treatment. These results, while establishing a useful reporter strain to study T-lymphocyte maturation, argue against a critical role for TDAG8 in immune development, psychosine-mediated inhibition of cytokinesis, and GC-induced cell death.
Among the myriad factors that regulate the development and function of the immune system, the effects of glucocorticoid (GC) hormones on lymphoid cells and tissues were recognized as early as the 1940s (reviewed in reference 1). Given the efficacy of GCs in the treatment of leukemia, lymphoma, myeloma, and various inflammatory disorders, the molecular mechanisms of GC-induced apoptosis of normal and malignant lymphoid cells have been studied intensively (reviewed in references 1 and 6). The proapoptotic effects of GCs involve the activation of the glucocorticoid receptor (GR), a member of the nuclear receptor family of ligand-dependent transcription factors (reviewed in 2). Studies using mice expressing a dimerization-deficient mutant of the GR indicate that GC-induced thymocyte apoptosis requires the gene transactivation function of this receptor (28). This finding is supported by observations that lytic effects of GCs on thymocytes are ATP dependent (29) and are prevented by protein synthesis inhibitors (5). Therefore, it appears likely that, in thymocytes, GCs induce the expression of one or more genes that activate the “mitochondrial” apoptotic pathway in an Apaf-1-dependent (37) and caspase-9-dependent (8, 17) manner. The identities of the proapoptotic GC-induced genes have remained uncertain, despite several studies isolating potential candidates (9, 26; reviewed in reference 1).
T-cell death-associated gene 8 (TDAG8) is a G-protein-coupled receptor (GPCR) first identified by differential mRNA display during thymocyte apoptosis induced by T-cell receptor (TCR) engagement (4). Compared with TCR stimulation, GCs were found to be much more potent inducers of TDAG8 expression (4), suggesting a role for TDAG8 in the death and development of thymocytes. This hypothesis was further investigated using transgenic mice in which TDAG8 expression was driven by the strong Lck-proximal promoter (31). By examining the effects of different apoptotic stimuli, the authors found that TDAG8 overexpression resulted in a specific increase in the susceptibility of double-positive (DP) thymocytes to GC-induced cell death. They concluded that TDAG8 plays a critical role in GC-induced thymocyte apoptosis (31).
More recently, Malone et al. (21) explored the connection between TDAG8 and apoptosis of transformed lymphoid cells. Specifically, the authors examined the effects of psychosine (PSY), a glycosphingolipid shown by Im et al. (13) to induce a block in cytokinesis in TDAG8-overexpressing cells. Basing their study on the assumption that psychosine is a TDAG8-specific agonist, Malone et al. proposed that this glycosphingolipid synergizes with GCs to induce apoptosis in a TDAG8-dependent manner (21). However, several lines of evidence question the identification of psychosine as a physiological TDAG8 agonist, as well as its biological significance for GC-induced apoptosis. First, direct binding of psychosine to TDAG8 has never been demonstrated. Second, with the exception of globoid cell leukodystrophy (Krabbe's disease) (16), in vivo psychosine levels (35) are significantly lower than concentrations shown in vitro to block cytokinesis (13) or to induce apoptosis (21) in TDAG8-overexpressing cells. Third, several independent studies (14, 27, 33) have recently demonstrated that TDAG8 is specifically activated by protons and not by psychosine. In these studies—with one exception, where it was shown to play a nonspecific inhibitory role (33)—psychosine had no effect on the pH-sensing function of TDAG8 (14, 27).
The objectives of the current study were threefold: (i) to precisely delineate the compartmentalization and magnitude of TDAG8 expression during thymocyte development and to examine if TDAG8 is required for normal immune development, (ii) to determine if psychosine-mediated inhibition of cytokinesis requires TDAG8 expression, and (iii) to analyze if this GPCR is essential for GC-induced thymocyte apoptosis. For this, we generated mice in which TDAG8 expression was inactivated by homologous recombination. To analyze TDAG8 expression in living cells, an enhanced green fluorescent protein (EGFP) marker was knocked into the disrupted tdag8 locus, under the control of the endogenous TDAG8 promoter. Analysis of thymocytes from TDAG8-heterozygous mice revealed a highly regulated expression pattern, reminiscent of that previously described for genes involved in thymocyte development and GC-induced apoptosis, including interleukin-7 receptor (24), Bcl-2 (7), Notch1 (10), and GR (3). Despite this striking transcriptional regulation, TDAG8 deficiency did not detectably affect normal immune development, and examination of adult (8- to 16-week-old) TDAG8 knockout (KO) mice revealed no major immunological defects. In light of the study by Im et al. (13) suggesting that TDAG8 is required for psychosine-induced formation of giant, multinucleated cells, we examined this process using receptor-deficient mice. While we found that psychosine is a potent inhibitor of cytokinesis in activated T lymphocytes, this effect was independent of TDAG8 expression. Furthermore, in contrast to previous overexpression data (21, 31) suggesting a proapoptotic function for TDAG8, inactivation of this GPCR did not alter the sensitivity of thymocytes to GC or activation-induced cell death.
MATERIALS AND METHODS
Targeting strategy and generation of the TDAG8 KO mice.
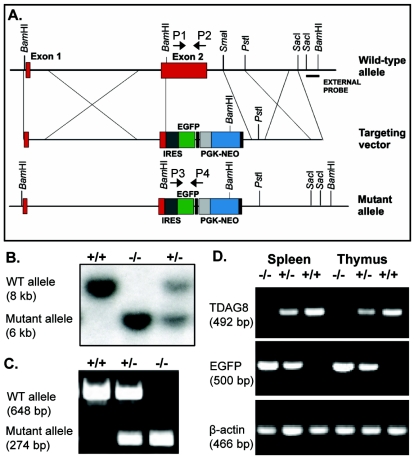
The TDAG8 targeting vector was constructed from two 7-kb fragments of genomic DNA isolated from a 129-Sv mouse embryonic stem (ES) cell genomic library, using TDAG8 cDNA as a probe. A 2.5-kb BamHI-SmaI fragment containing exon 2-derived TDAG8 coding sequences was replaced by a 3.4-kb fragment encoding promoterless internal ribosomal entry site-EGFP sequences and a 1.5-kb PGK1-Neo resistance cassette flanked by loxP sites. The vector contained 6.3 kb of 5′ flanking (the left arm) and 3.6 kb of 3′ flanking (the right arm) sequence homology and was linearized by NotI restriction. A 540-bp SacI-BamHI fragment 200 bp downstream of the right arm was used as the external probe for Southern blot hybridization. The targeting vector was electroporated into 129SvJ ES cells (Genomes System). Southern blot analysis of 150 neomycin-resistant colonies yielded two positive clones. ES cells from these individual clones were injected into blastocysts harvested from C57BL/6 mice (University of California—Los Angeles Transgenic Facility). The resulting chimeric males were crossed with C57BL/6 females. Germ line transmission of the TDAG8 mutant allele was detected by Southern blot analysis of tail DNA from F1 mice. The primers used for PCR analysis of genomic DNA were P1 (5′ACTGCCACTGTGAAGACCATG), P2 (5′CTCTTCTCGCTGTTTTCCGTG), P3 (5′GTAAACG-GCCACAAGTTCAGC), and P4 (5′CTTCAGCTCGATGCGGTTCAC). The phenotypic studies reported here used mice backcrossed for at least six generations onto the C57BL/6 (Ptprca; CD45.1) and BALB/c strains. TDAG8-deficient mice were also backcrossed for six generations on the DO11.10 TCR-transgenic strain (25). All mice were housed in the conventional animal facility at the University of California—Los Angeles, following current institutional regulations.
RNA isolation, reverse transcriptase-PCR (RT-PCR), and Northern blotting procedures.
DNA-free RNA was prepared using the Absolutely RNA Microprep Kit (no. 400800; Stratagene). The RNA was reverse transcribed using oligo(dT) primers and the SuperScript First-Strand Synthesis System (no. 11904-018; Invitrogen). The PCR conditions and primers were described previously (27). For Northern blot analysis, 10 μg of total RNA was electrophoresed on a formaldehyde-agarose gel, transferred to a nylon membrane, and hybridized with 32P-labeled probes (TDAG8, EGFP, and β-actin).
Humoral-immunity analysis.
A battery of tests (32) was used to analyze humoral immune responses of TDAG8-deficient mice. Preimmune serum was collected from male mice at 8 to 12 weeks of age. Baseline immunoglobulin titers were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (18). To analyze primary antibody responses, mice were immunized intraperitoneally (i.p.) with a mixture of 25 μg azobenzenearsonate hapten conjugated to chicken gamma globulin (ABA-CGG) (no. A-1210-10; Biosearch Technologies) and 5 × 108 heat-killed Bordetella pertussis cells (Lee Laboratories, Grayson, Georgia). Both antigens were precipitated in Imject Alum (no. 77161; Pierce) at a 1:1 ratio. For T-cell-independent responses, mice were injected with soluble 2,4,6-trinitrophenyl (TNP)-aminoethylcarboxymethyl-Ficoll (no. F-1300-10; Biosearch). Two weeks postimmunization, primary antibody responses against CGG (immunoglobulin G1 [IgG1]) and B. pertussis (IgG2a) were determined by ELISA using plates coated with 2,4-dinitrophenyl-CGG (no. D-05052; Biosearch) and with an extract of B. pertussis. IgM responses to TNP-Ficoll were determined by ELISA 7 days after immunization, using plates coated with TNP-bovine serum albumin (no. T-5050-10; Biosearch). Four weeks after the first immunization, the mice were rechallenged with ABA-CGG, and 7 days later, the titers of CGG-specific IgG1 antibodies were determined by ELISA.
Analysis of psychosine-induced inhibition of cytokinesis.
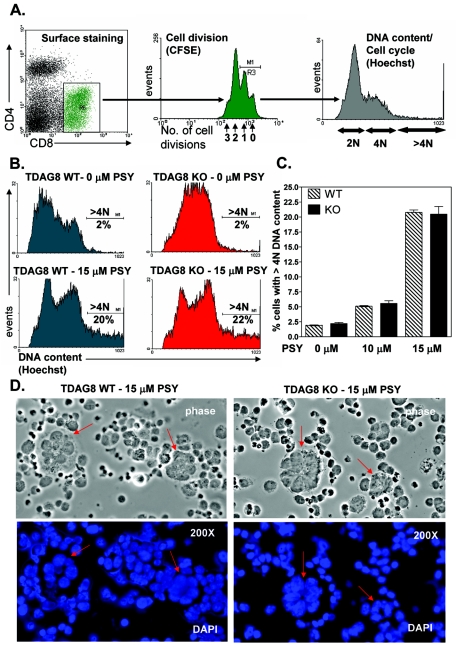
PSY (Avanti no. 860537 or Matreya, LLC, no. 1305) was dissolved in ethanol at 20 mM and stored as single-use aliquots in glass vials at −20°C. Single-cell suspensions of total splenocytes from 6- to 8-week-old wild-type (WT) TDAG8 and KO mice on the C57BL/6 background were labeled for 5 min at room temperature with 5 μM carboxyfluorescein succinimidyl ester (CFSE) (Vybrant CFDA Cell Tracker; no. V-12883; Molecular Probes) and then washed three times with Hanks' balance salt solution containing 5% fetal calf serum (FCS). Labeled splenocytes were cultured in X-VIVO 15 medium (no. 04-418Q; Cambrex) at 2 × 106 cells/ml in 24-well tissue culture plates (no. 3047; BD Labware). The cells were activated with various concentrations (10, 100, and 1,000 ng/ml) of the anti-CD3ɛ antibody (clone no. 145-2C11 from the American Type Culture Collection) in the presence of 1, 5, 10, or 15 μM psychosine (or ethanol, as a vehicle control). After 48 and 72 h, the cells were labeled with Hoechst 33342 dye (no. H21492; Molecular Probes), to determine the DNA content and with anti-CD4-phycoerythrin (PE) and anti-CD8-TC antibodies (BD Pharmingen) for phenotypic identification. The cells were analyzed by fluorescence-activated cell sorter (FACS) (the BD FACS Vantage SE system running BD Cell Quest software). The characteristic morphological changes induced by psychosine were examined on cytospins from duplicate cultures. Following fixation with 4% paraformaldehyde, the cytospins were mounted in mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories) and then analyzed by fluorescence microscopy (×200 magnification).
In vivo analysis of thymocyte apoptosis.
Female mice (6 to 8 weeks old; C57BL/6 and BALB/c backgrounds) were treated with 100 μg dexamethasone (DEX) (no. D8893; Sigma) in 500 μl phosphate-buffered saline (PBS) or with vehicle by i.p. injection. Thymi were harvested 20 h postinjection, and cells were analyzed by flow cytometry (the BD FACSCanto system running BD FACSDiva software), using annexin V-PE, 7-AAD, anti-CD3-APC, anti-CD4-APC-Cy7, and anti-CD8-PE-Cy5 (all from BD Pharmingen). A Xenogen-IVIS cooled charge-coupled device (CCD) optical system (Xenogen, Alameda, CA) was used for ex vivo detection of EGFP signals from explanted thymi. The captured images were analyzed with Living Image software 2.20 (Xenogen), and values were expressed in photons/s/cm2/steridian.
In vitro analysis of thymocyte apoptosis.
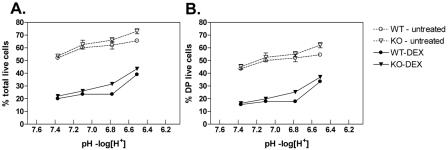
Thymocytes from 4- to 6-week-old WT and TDAG8 KO mice (C57BL/6 background) were cultured overnight (106 cells/well) in Opti-MEM (no. 22600-050; Invitrogen), supplemented with 2% FCS, in the presence or absence of DEX. For anti-TCR-induced apoptosis, 96-well flat-bottom tissue culture plates were coated with 5 μg/ml of anti-CD3ɛ antibody prior to the addition of 106 thymocytes in 200 μl of X-VIVO 15. To examine the role of pH in apoptosis, thymocytes were cultured in Opti-MEM supplemented with 2% FCS, 10 mM N,N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (no. B4554; Sigma), and 1 mM MES (morpholineethanesulfonic acid) (no. BP300; Fisher Biotech). The pH of the medium was adjusted using serial dilutions of 1 M NaOH or HCl. The cells were incubated overnight in a humidified 37°C incubator at atmospheric levels of CO2. The pH values (see Fig. 8) were measured in duplicate cultures at 37°C during the course of the assay using a microelectrode (no. 9802BN; Thermo Electron Corporation).
FIG. 8.
Exposure to acidic extracellular pH does not differentially modulate the susceptibility of TDAG8 WT and KO thymocytes to GC-induced apoptosis. To evaluate whether pH had an effect on DEX-induced apoptosis, thymocytes were exposed to different pH conditions in the presence and absence of 2.5 nM DEX. Viable cells were identified by FACS (annexin V, 7-AAD, CD4, and CD8 staining), as described in Materials and Methods. The percentages of live (7-AAD-negative and annexin V-negative) total thymocytes (A) and live DP thymocytes (B) are displayed. All P values were greater than 0.05. These results are representative of three independent experiments. The error bars indicate SEM.
Data presentation and statistical analysis.
Graphs were constructed using GraphPad Prism software, version 4.02. P values were calculated using Student's t test. P values of <0.05 were considered significant. Data are presented as means ± standard errors of the mean (SEM).
RESULTS
Generation of TDAG8 KO mice and validation of EGFP knockin expression.
The mouse tdag8 gene consists of two exons with the coding region located in exon 2 (Fig. 1A). In addition to the deletion of tdag8 by homologous recombination, our targeting strategy introduced a knockin fluorescent reporter of TDAG8 expression. Thus, we generated a targeting construct in which 90% of exon 2, including the entire TDAG8 open reading frame, was replaced by an internal ribosomal entry site element, followed by the EGFP gene and a neomycin resistance cassette (Fig. 1A). Homologous integration of this construct into the tdag8 locus was expected to position the EGFP knockin under the control of the TDAG8 endogenous promoter. Correctly targeted ES cell clones were injected into C57BL/6 blastocysts, producing chimeric mice. Germ line transmission of the tdag8-inactivating mutation was confirmed in the progeny by Southern blotting (Fig. 1B) and by PCR (Fig. 1C). Loss of TDAG8 expression and the presence of the EGFP transcripts in heterozygous and KO mice were examined by RT-PCR analysis of RNA extracts from the thymus and spleen (Fig. 1D). We also considered the possibility that TDAG8 function could be affected by the strain genetic background. Therefore, we crossed these mice for six consecutive generations onto the C57BL/6 and BALB/c strains. On both strains, TDAG8 KO mice were normal in appearance, size, and mating. Genotype analysis indicated that these mice were born at the expected Mendelian frequency without sexual bias, and a detailed histological examination of major internal organs did not reveal any morphological abnormalities (data not shown).
FIG. 1.
Generation of TDAG8 KO mice. (A) Strategy for homologous recombination in the tdag8 locus. (B) Genotypic determination by Southern blot analysis of BamHI-digested tail genomic DNA hybridized with an external probe. (C) PCR analysis of tail genomic DNA using primers specific for the WT (+/+) allele (P1 and P2) and for the recombinant mutant allele (P3 and P4). (D) RT-PCR to demonstrate the absence of TDAG8 transcripts in KO mice and the expression of EGFP in TDAG8 heterozygous (+/−) and KO (−/−) mice.
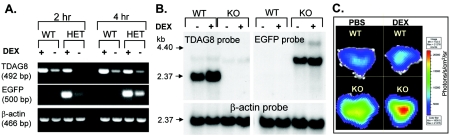
Next, we determined if the expression of the EGFP reporter reproduced the expression of the WT TDAG8 allele. We reasoned that, similar to TDAG8 (4), EGFP expression in thymocytes would be sensitive to the synthetic GC DEX. As shown in Fig. 2A (RT-PCR), Fig. 2B (Northern blot), and Fig. 2C (ex vivo imaging with a Xenogen CCD system of thymi explanted from DEX-treated mice), levels of EFGP mRNA and fluorescence in TDAG8+/− or TDAG8−/− (KO) thymocytes were indeed upregulated by DEX. These results demonstrate that we successfully generated a TDAG8-deficient mouse strain with an integrated fluorescent reporter.
FIG. 2.
Effects of DEX treatment on TDAG8 and EGFP expression. (A and B) In vitro and in vivo DEX treatment induced the upregulation of both the TDAG8 WT allele and the EGFP knockin mutant allele. Thymocytes from TDAG8+/+ (WT) and TDAG8+/− (HET) mice were treated in vitro with 10 nM DEX for 2 or 4 h, and the expression levels of TDAG8 and EGFP were analyzed at the RNA level by RT-PCR. (B) Northern blot analysis of TDAG8 and EGFP expression following in vivo DEX treatment. Mice were injected i.p. with 100 μg of DEX diluted in PBS and euthanized 20 h later. Predicted sizes for the WT and KO transcripts were 2.036 kb and 3.091 kb, respectively. (C) Upregulation of the EGFP signal in thymi from TDAG8 KO mice following in vivo DEX treatment. Images of thymi explanted 20 h after DEX injections (100 μg/mouse) were acquired using a Xenogen-IVIS cooled CCD optical system, as described in Materials and Methods.
TDAG8 displays a restricted distribution during development and differentiation of the immune system.
Previous studies at the mRNA level have demonstrated that TDAG8 expression is largely restricted to primary and secondary lymphoid organs (reference 4 and L. V. Yang, C. G. Radu, and O. N. Witte, unpublished data). However, a more precise delineation of TDAG8 expression at different stages of immune development and differentiation would be required, in light of the potential role of this receptor during T-cell death and maturation (4, 21, 31). We first examined the expression of the EGFP knockin reporter in thymocyte subsets identified by CD4 and CD8 expression (Fig. 3A). As shown in Fig. 3B, thymocyte maturation from the double-negative (DN) stage to the single-positive stage (CD4+ or CD8+) was accompanied by striking changes in TDAG8 levels. In particular, we noted the significant downregulation of TDAG8 expression coinciding with the transition from the DN to the DP stage. Further fractionation of the DN subset based on CD25 and CD44 expression indicated that TDAG8 levels peaked at the DN2 stage and then decreased progressively in DN3 and DN4 cells (Fig. 3C). Importantly, a similar pattern of dynamic modulation of TDAG8 expression during thymocyte development has emerged from recent microarray studies (12, 22), further validating our fluorescent-reporter-based expression analysis.
FIG. 3.
Differential regulation of TDAG8 expression during T-cell development and in various immune lineages. (A, B, and C) FACS analysis of CD4, CD8, and EGFP expression in thymocytes from TDAG8 WT, heterozygous (HET), and KO mice. EGFP expression is displayed in panel C as mean fluorescence intensity (MFI). The DN stage was subfractionated in four stages (DN1 to -4) based on CD44 and CD25 expression. (D) Expression of the EGFP “knockin” in splenic CD8+ cells from TDAG8 heterozygous mice. (E) Expression of the EGFP “knockin” in Gr1+ (granulocytes), CD11b+ (myeloid), and Ter119+ (erythroid) cells from the bone marrow and splenic B (B220+) and T (CD4+ and CD8+) lymphocytes, as well as dendritic cells (DCs) (CD11b+/CD11c+) and natural killer (NK) (CD3−/DX5+) cells. These results are representative of four independent experiments. The error bars indicate SEM.
Next, we analyzed the expression of the EGFP reporter in hematopoietic cells from bone marrow and spleen. Figure 3D exemplifies the levels of EGFP expression that were detected by flow cytometry in mature splenic CD8+ T cells from TDAG8-heterozygous mice. The results of a comprehensive analysis of different hematopoietic lineages from bone marrow and spleen are presented in Fig. 3E. TDAG8 was expressed at low levels in the erythroid (Ter119+) and B-lymphocyte lineages. Bone marrow Gr1+ cells, as well as splenic CD4+ and CD8+ T cells, expressed intermediate levels of TDAG8, while high levels of receptor expression were detected in CD11b+ myeloid cells, in CD11c+ dendritic cells, and also in NK1.1+ natural killer cells. Overall, these results were concordant with the expression pattern of TDAG8 determined at the RNA level (SymAtlas v0.8.0; Genomics Institute of the Novartis Research Foundation [http://symatlas.gnf.org/SymAtlas/]) and indicate that TDAG8 could potentially regulate the development and function of multiple immune lineages.
TDAG8 deficiency does not perturb normal immune development.
To determine if TDAG8 inactivation affects the development of lymphoid organs, we analyzed the cellular compositions of thymus, spleen, and peripheral lymph nodes. No significant differences were detected between 4- to 8-week-old WT and KO mice in thymic composition (Fig. 4A) and cellularity (Fig. 4B). Moreover, the composition and cellularity of spleens and lymph nodes (Fig. 4C and D and data not shown) from 8- to 16-week-old KO mice on the C57BL/6 background were also normal. Similar results were obtained using TDAG8 KO mice on the BALB/c background. Therefore, we conclude that TDAG8 is not critically required for the normal development of major lymphoid organs. Next, we examined the hypothesis suggested by previous studies (4, 31, 33), that TDAG8 could participate in thymic positive and negative T-cell selection. For this, we used DO11.10 mice, which are transgenic for a class II-restricted TCR specific for the ovalbumin-derived peptide, OVA323-339 (25). DO11 TCR-transgenic CD4+ cells are positively selected in mice expressing the major histocompatibility complex (MHC) class II I-Ad (25) and negatively selected in the presence of the I-Ab MHC class II (20). We therefore crossed the TDAG8 KO mice on positively selecting (BALB/c I-Ad) and negatively selecting (C57BL/6 I-Ab × BALB/c IAd; F1) strains (Fig. 4E). As anticipated, the numbers and percentages of thymic and splenic DO11 TCR-transgenic cells were significantly decreased on the negatively selecting background (Fig. 4F and data not shown). However, these reductions were independent of TDAG8 expression (Fig. 4F), suggesting that in this model, TDAG8 is not critically required for normal positive and negative T-cell selection.
FIG. 4.
Normal immune development in TDAG8 KO mice. (A) Equivalent distributions of DN, DP, and single-positive CD4+ and CD8+ populations in thymi from WT and KO mice. (B) Comparison of total cell numbers from thymi explanted from 4- to 8-week-old WT (n = 31) and KO (n = 24) mice. (C) Compositions of B220+ and CD3+ lymphoid populations from WT and KO spleens. (D) Comparison of total cell numbers from spleens explanted from 8- to 16-week-old WT (n = 36) and KO (n = 31) mice. (E) Strategy to examine the positive and negative selection of WT and KO DO11.10 TCR-transgenic splenocytes. These cells are positively selected on the BALB/c strain expressing the I-Ad MHC class II haplotype. In contrast, the DO11.10 cells are negatively selected on the C57BL6 × BALB/c F1 strain expressing both I-Ad and I-Ab MHC class II haplotypes. (F) Percentages of selected CD4+ DO11.10 TCR-transgenic T cells present in the spleens of mice on the BALB/c (n = 4 for each genotype) and C57BL6 × BALB/c F1 genetic backgrounds (n = 4 for each genotype). DO11.10 TCR-transgenic CD4+ T cells were identified by FACS based on their reactivity with the anti-DO11 idiotypic antibody KJ1-26 (BD Pharmingen). The error bars indicate SEM.
Major immune functions are intact in TDAG8 KO mice.
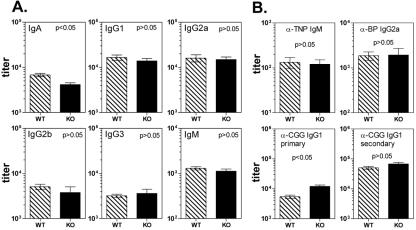
Expression of TDAG8 in peripheral lymphoid organs (4) and in multiple immune lineages (Fig. 3D and E) could indicate that this receptor has immunoregulatory functions. We therefore performed a preliminary characterization of major immune functions in 8- to 16-week-old KO mice on the BL/6 genetic background. With the exception of an approximately twofold decrease in the levels of serum IgA, the titers of other major immunoglobulin isotypes were comparable in WT and KO mice (Fig. 5A). While the significance of the reduction in IgA levels is currently unclear, these results suggest that TDAG8 expression is not critically required for immunoglobulin class switching. Furthermore, immunization of KO mice with T-cell-independent (TNP-Ficoll) and -dependent (CGG and B. pertussis) antigens led to antibody titers that matched or, in the case of primary IgG1 responses to CGG, slightly exceeded those from WT controls (Fig. 5B). Secondary immune responses to CGG were indistinguishable between WT and KO mice (Fig. 5B, lower right). Moreover, ovalbumin immunization of TDAG8 KO mice on the BALB/c background resulted in normal IgG1 and IgG2a antibody titers (data not shown). These results indicate that both T helper 1-dependent (IgG1) and T helper 2-dependent (IgG2) antibody responses (32) were not affected by TDAG8 inactivation. The overall integrity of the immune system of TDAG8 KO mice was further confirmed by the ability of these mice to mediate the immune rejection of Moloney murine sarcoma/leukemia virus complex-induced rhabdomyosarcomas (23), with an efficiency similar to that observed for WT animals (data not shown).
FIG. 5.
Humoral-immunity analyses of TDAG8 KO mice. Preimmune sera were collected from male WT and KO mice on the C57BL/6 background at 8 to 12 weeks of age. (A) Baseline immunoglobulin titers were determined by ELISA as previously described (18). Numbers of mice: 18 per genotype for IgA, IgG1, and IgG2a determinations; 4 WT mice and 3 KO mice for IgG2b, IgG3, and IgM determinations. The error bars indicate SEM. (B) Mice were immunized i.p. with a mixture of 25 μg ABA-CGG and 5 × 108 heat-killed Bordetella pertussis (BP) cells, as described in Materials and Methods. Seven days later, the mice were also immunized with TNP-Ficoll. Two weeks after the first immunization, primary antibody responses against TNP-bovine serum albumin (IgM; n = 4 mice/genotype), CGG (IgG1; n = 20 WT mice and n = 22 KO mice), and B. pertussis (IgG2a; n = 20 WT mice and n = 22 KO mice) were determined by ELISA. Four weeks postimmunization, the mice were rechallenged with ABA-CGG to measure secondary immune responses. Seven days later, titers of IgG1 antibodies against CGG were determined by ELISA (n = 8 mice/genotype).
TDAG8 is dispensable for psychosine-induced inhibition of cytokinesis.
In 2001, Im et al. (13) showed that treatment of TDAG8-overexpressing cells with psychosine induced a block in cytokinesis by uncoupling mitosis from cell division. The net result of this inhibition was the formation of giant, multinucleated cells. To test this potential connection between TDAG8 and formation of multinucleated cells in a more relevant biological setting, we examined the effects of psychosine on cytokinesis using immune cells explanted from TDAG8 WT and KO mice. The CD4+ and CD8+ T-cell fractions from total splenocyte cultures were activated and induced to proliferate using an antibody against the CD3ɛ chain of the TCR. The number of cell divisions, as well as the DNA contents of CD4+ and CD8+ subsets, was monitored by flow cytometry using the dilution of the vital dye CFSE, in conjunction with cell cycle analysis using the Hoechst 33342 dye (Fig. 6A). As shown in Fig. 6B for the CD8+ subset, treatment of activated T cells with 15 μM psychosine induced a significant accumulation of cells with greater than 4 N DNA content in both the WT and KO samples. Similar results were observed in the CD4+ subset (data not shown). The psychosine-mediated formation of these cells was strictly dependent on the anti-TCR stimulation, and the abnormal increase in DNA content was maximal among antibody-activated cells that had not divided or had completed only one round of cell division (data not shown). Importantly, the percentages of multinucleated cells following psychosine treatment at two concentrations (10 or 15 μM) were indistinguishable between TDAG8 WT and KO mice (Fig. 6C). The characteristic morphology of giant multinucleated cells was observed on cytospins from psychosine-treated cultures (Fig. 6D). Taken together, these results demonstrate that, in activated T lymphocytes, psychosine is a potent inhibitor of cytokinesis, but that TDAG8 is not essential for this effect.
FIG. 6.
TDAG8 is dispensable for psychosine-induced inhibition of cytokinesis and formation of multinucleated cells. (A) Flow cytometry-based approach used to examine the effects of PSY on cell division and DNA content. Total splenocytes from TDAG8 WT and KO were stimulated with a soluble anti-CD3 antibody, as described in Materials and Methods. CD4+ and CD8+ subsets were identified by surface staining. The gate for cell cycle analysis (Hoechst 33342) included cells that had undergone no more than one division, as indicated by the CFSE profile. (B) Treatment with 15 μM PSY of splenocytes activated for 48 h with 100 ng/ml soluble anti-CD3 antibody resulted in the accumulation of cells with greater than 4 N DNA content. (C) Percentages of TDAG8 WT and KO CD8+ cells with greater than 4 N DNA content following treatment with 10 or 15 μM PSY. The error bars indicate SEM. (D) Morphological examination of psychosine-induced accumulation of multinucleated cells using DAPI-stained cytospins. These results are representative of three independent experiments.
TDAG8-deficient thymocytes undergo normal GC-induced apoptosis.
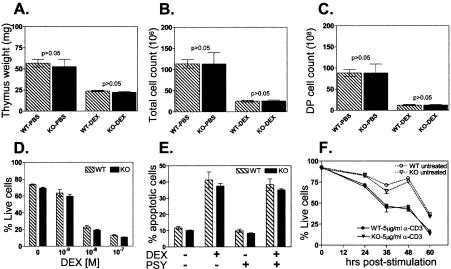
Previous overexpression studies (21, 31) have suggested that TDAG8 plays a critical role in regulating GC-induced apoptosis. To examine this possibility, we first evaluated the susceptibility of thymocytes from WT and TDAG8 KO mice (on the BALB/c strain) to apoptosis induced by systemic administration of the synthetic GC DEX. In WT mice, treatment with DEX induced an approximately 50% reduction in thymic mass (Fig. 7A). Surprisingly, a similar reduction was observed with KO thymi (Fig. 7A). Furthermore, identical numbers of viable total thymocytes (Fig. 7B) or DP cells (Fig. 7C) were recovered from DEX-treated WT and KO mice. Similar results were obtained with TDAG8 KO mice on the C57BL/6 genetic strain (data not shown). These findings are inconsistent with a critical role for TDAG8 as a promoter of GC-induced apoptosis. Alternatively, the proapoptotic effect of TDAG8 could be dependent on the dose of DEX used to induce apoptosis. However, equal numbers of viable thymocytes were recovered from overnight cultures of WT and KO thymocytes exposed to a wide range of DEX concentrations (Fig. 7D). We were also unable to confirm the hypothesis of Malone et al. (21) regarding a synergistic effect of psychosine and DEX on TDAG8-expressing cells. Thus, the addition of PSY during DEX treatment failed to differentially regulate the rates of apoptosis of WT and KO thymocytes (Fig. 7E). Similar results were obtained when we examined the susceptibility of WT and KO thymocytes to apoptosis induced by anti-CD3 (Fig. 7F) or phorbol ester-phorbol myristate acetate and calcium ionophore-ionomycin stimulation (data not shown), processes shown to upregulate TDAG8 expression (4). Taken together, these results dispute the identification of TDAG8 as a critical regulator of thymocyte apoptosis induced by GC treatment or by TCR engagement.
FIG. 7.
TDAG8 KO thymocytes show normal apoptotic responses to DEX and anti-TCR stimulation. Apoptosis was examined by flow cytometry using annexin V, 7-AAD, CD4, and CD8, as described in Materials and Methods. (A, B, and C) Six- to 8-week-old mice (n = 4/group) were treated with 100 μg/500 μl of DEX or vehicle (PBS) by i.p. injection. The mice were sacrificed 20 h later to analyze the DEX-induced reduction in thymic size, cellularity, and composition. The error bars indicate SEM. (A) Weights of the explanted thymi. (B) Numbers of viable (annexin V- and 7-AAD-negative) total thymocytes. (C) Numbers of viable CD4+/CD8+ (DP) thymocytes. The results shown are representative of three independent experiments on two genetic backgrounds (C57BL/6 and BALB/c). (D) Thymocytes from TDAG8 WT and KO mice (n = 3/group) were treated in vitro for 16 h with different doses of DEX. All P values were greater than 0.05. These results are representative of four independent experiments. (E) Psychosine treatment does not augment DEX-induced apoptosis of thymocytes. Thymocytes were treated in vitro for 6 h with 1 μM DEX ± 10 μM PSY. All P values were greater than 0.05. These results are representative of two independent experiments. (F) Normal thymocyte apoptosis following anti-TCR stimulation. Thymocytes were stimulated for the indicated periods with plate-bound anti-CD3ɛ antibody. All P values were greater than 0.05. These results are representative of three independent experiments.
Finally, we considered the possibility that the recently identified function of TDAG8 as a proton sensor (14, 27, 33) could be functionally related to GC-induced thymocyte apoptosis. However, exposure of thymocytes to DEX under extracellular pH values ranging from 7.4 to 6.6 did not reveal significant differences in the survival of WT and KO thymocytes (Fig. 8A and B). Interestingly, as previously reported (19), the acidification of the extracellular environment increased the viability of both WT and TDAG8 KO thymocytes, irrespective of DEX treatment (Fig. 8A and B).
DISCUSSION
Key findings emerging from the analysis of TDAG8 KO mice.
The physiological role of TDAG8 is currently unknown despite previous studies that have implicated this GPCR in diverse biological processes, such as T-cell development and apoptosis (4, 14, 21, 31), cellular responses to glycosphingolipids involved in hereditary metabolic disorders (13, 21, 33), and, more recently, detection of acidic extracellular environments (14, 27, 33). The generation of receptor-deficient mice described in the current study provided an important genetic tool to help examine the hypotheses formulated during the last decade in connection with TDAG8. Several conclusions have emerged from our characterization of TDAG8 KO mice. First, despite a broad expression pattern of TDAG8 across multiple hematopoietic lineages, lymphoid organs from TDAG8 KO mice have a normal structure and cellular composition. Our results also argue against a critical role for TDAG8 during the stringent positive and negative selection processes that govern T-cell development. Moreover, major immune functions, such as immunoglobulin class switching, production of T-cell-independent and -dependent antibodies, and rejection of strongly immunogenic tumors, are not impaired by TDAG8 inactivation. Second, we found that TDAG8 was dispensable for psychosine-induced inhibition of cytokinesis and formation of multinucleated cells. Third, thymocyte apoptosis was not affected by TDAG8 inactivation, as shown in several in vivo and in vitro experimental models.
TDAG8 is not essential for psychosine-induced inhibition of cytokinesis.
Using ectopic overexpression, Im et al. (13) established a correlation between high levels of TDAG8 and the propensity of transfected RH7777 or HEK293 cells to undergo psychosine-mediated formation of giant, multinucleated cells. Our studies, done in a more relevant biological context, do not support an essential role for TDAG8 in the inhibition of cytokinesis. It remains possible that TDAG8 overexpression could sensitize certain cell types to the effects of psychosine on cell division. Given the lack of binding studies, it is currently uncertain if the effects of psychosine on TDAG8-overexpressing cells are the result of a “true” ligand-receptor relationship or the reflection of an indirect mechanism.
Normal glucocorticoid-induced apoptosis in TDAG8-deficient thymocytes.
Our finding that TDAG8 is not essential for GC-induced thymocyte apoptosis directly conflicts with previous studies claiming a critical role for TDAG8 in GC-induced cell death (21, 31). We believe our study to be more reliable, primarily because the TDAG8−/− model avoids inherent problems associated with very high levels of receptor expression from transgenic inserts relative to those of the endogenous wild-type TDAG8. Nevertheless, our results do not exclude the possibility that GCs and TDAG8 are functionally linked during intrathymic T-cell development in promoting a process other than apoptosis. In this context, it did not escape our attention that regulation of TDAG8 expression during thymic development closely resembles that observed for GR (3). The most likely interpretation for this finding is that TDAG8 expression is controlled via GR by basal GCs released from the adrenal gland or from ectopic production in thymic epithelial cells. Moreover, the precise functions of GCs in intrathymic T-cell development, with the exception of GC-induced apoptosis, are not fully understood (reviewed in reference 15). It is tempting to speculate that future clarifications in this controversial field will also shed light on the real physiological significance of GC-dependent transcriptional regulation of TDAG8.
TDAG8 as a proton sensor and the functional-redundancy hypothesis.
The recent identification of TDAG8 as a pH sensor represents another intriguing feature of this GPCR. In contrast to the presumed connection to psychosine, data in support of the proton-sensing abilities of TDAG8 have been reported by three independent groups, including ours (14, 27, 33). Importantly, we have recently demonstrated that TDAG8 is critically required for the production of cyclic AMP by primary thymocytes and splenocytes exposed to acidic extracellular environments (27). However, since major immune functions investigated by us to date appear unaffected by TDAG8 inactivation, the biological significance of TDAG8-mediated pH sensing remains uncertain. The lack of obvious immune defects in TDAG8 KO mice could be explained by functional redundancy with the sequence-related GPCRs, G2A (34), OGR1 (36), and GPR4 (11). Based on the similarities in expression pattern (27), the most likely functional-redundancy mechanism would involve TDAG8 and G2A. Although the role of G2A as a pH sensor is less clear (27), we are currently investigating the redundancy hypothesis using mice deficient for both receptors. Alternatively, the biological functions of TDAG8 could be restricted to immune processes associated with pathophysiological conditions characterized by local perturbations in acid-base homeostasis (reviewed in reference 30). Nonetheless, the availability of TDAG8 KO mice will greatly facilitate further studies of the molecular mechanisms used by immune cells to detect and respond to microenvironmental pH variations.
Acknowledgments
We thank Shirley Quan for outstanding technical assistance; LaKeisha Perkins, Mathew Au, and Rosalyn Taijeron for maintaining the mouse colony; and Renee L. H. Lim for designing the TDAG8 targeting construct. We also thank Barbara Anderson for excellent preparation of the manuscript and Chengyi J. Shu for help with CCD imaging. We are indebted to Yongwon Choi (The Rockefeller University) for advice and support during the initial phases of this project.
O.N.W. is an Investigator of the Howard Hughes Medical Institute. C.G.R. was supported by a Cancer Research Institute Fellowship during a portion of these studies. A.N. was supported by the Howard Hughes Medical Institute Research Training Fellowship for Medical Students.
REFERENCES
- 1.Ashwell, J. D., F. W. Lu, and M. S. Vacchio. 2000. Glucocorticoids in T cell development and function. Annu. Rev. Immunol. 18:309-345. [DOI] [PubMed] [Google Scholar]
- 2.Beato, M., P. Herrlich, and G. Schutz. 1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851-857. [DOI] [PubMed] [Google Scholar]
- 3.Brewer, J. A., B. P. Sleckman, W. Swat, and L. J. Muglia. 2002. Green fluorescent protein-glucocorticoid receptor knockin mice reveal dynamic receptor modulation during thymocyte development. J. Immunol. 169:1309-1318. [DOI] [PubMed] [Google Scholar]
- 4.Choi, J. W., S. Y. Lee, and Y. Choi. 1996. Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol. 168:78-84. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, J. J., and R. C. Duke. 1984. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J. Immunol. 132:38-42. [PubMed] [Google Scholar]
- 6.Frankfurt, O., and S. T. Rosen. 2004. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr. Opin. Oncol. 16:553-563. [DOI] [PubMed] [Google Scholar]
- 7.Gratiot-Deans, J., R. Merino, G. Nunez, and L. A. Turka. 1994. Bcl-2 expression during T-cell development: early loss and late return occur at specific stages of commitment to differentiation and survival. Proc. Natl. Acad. Sci. USA 91:10685-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakem, R., A. Hakem, G. S. Duncan, J. T. Henderson, M. Woo, M. S. Soengas, A. Elia, J. L. de la Pompa, D. Kagi, W. Khoo, J. Potter, R. Yoshida, S. A. Kaufman, S. W. Lowe, J. M. Penninger, and T. W. Mak. 1998. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94:339-352. [DOI] [PubMed] [Google Scholar]
- 9.Harrigan, M. T., G. Baughman, N. F. Campbell, and S. Bourgeois. 1989. Isolation and characterization of glucocorticoid- and cyclic AMP-induced genes in T lymphocytes. Mol. Cell. Biol. 9:3438-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasserjian, R. P., J. C. Aster, F. Davi, D. S. Weinberg, and J. Sklar. 1996. Modulated expression of notch1 during thymocyte development. Blood 88:970-976. [PubMed] [Google Scholar]
- 11.Heiber, M., J. M. Docherty, G. Shah, T. Nguyen, R. Cheng, H. H. Heng, A. Marchese, L. C. Tsui, X. Shi, S. R. George, et al. 1995. Isolation of three novel human genes encoding G protein-coupled receptors. DNA Cell Biol. 14:25-35. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann, R., L. Bruno, T. Seidl, A. Rolink, and F. Melchers. 2003. Rules for gene usage inferred from a comparison of large-scale gene expression profiles of T and B lymphocyte development. J. Immunol. 170:1339-1353. [DOI] [PubMed] [Google Scholar]
- 13.Im, D. S., C. E. Heise, T. Nguyen, B. F. O'Dowd, and K. R. Lynch. 2001. Identification of a molecular target of psychosine and its role in globoid cell formation. J. Cell Biol. 153:429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii, S., Y. Kihara, and T. Shimizu. 2005. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J. Biol. Chem. 280:9083-9087. [DOI] [PubMed] [Google Scholar]
- 15.Jondal, M., A. Pazirandeh, and S. Okret. 2004. Different roles for glucocorticoids in thymocyte homeostasis? Trends Immunol. 25:595-600. [DOI] [PubMed] [Google Scholar]
- 16.Kanazawa, T., and Y. Kozutsumi. 2002. Biological function of sphingolipids involved in cytokinesis. Tanpakushitsu Kakusan Koso 47:394-399. [PubMed] [Google Scholar]
- 17.Kuida, K., T. F. Haydar, C. Y. Kuan, Y. Gu, C. Taya, H. Karasuyama, M. S. Su, P. Rakic, and R. A. Flavell. 1998. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325-337. [DOI] [PubMed] [Google Scholar]
- 18.Le, L. Q., J. H. Kabarowski, Z. Weng, A. B. Satterthwaite, E. T. Harvill, E. R. Jensen, J. F. Miller, and O. N. Witte. 2001. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity 14:561-571. [DOI] [PubMed] [Google Scholar]
- 19.Lei, H. Y., M. J. Tang, and N. Tsao. 1997. Intracellular alkalinization in dexamethasone-induced thymocyte apoptosis. Apoptosis 2:304-312. [DOI] [PubMed] [Google Scholar]
- 20.Liu, C. P., J. W. Kappler, and P. Marrack. 1996. Thymocytes can become mature T cells without passing through the CD4+ CD8+, double-positive stage. J. Exp. Med. 184:1619-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malone, M. H., Z. Wang, and C. W. Distelhorst. 2004. The glucocorticoid-induced gene tdag8 encodes a pro-apoptotic G protein-coupled receptor whose activation promotes glucocorticoid-induced apoptosis. J. Biol. Chem. 279:52850-52859. [DOI] [PubMed] [Google Scholar]
- 22.Mick, V. E., T. K. Starr, T. M. McCaughtry, L. K. McNeil, and K. A. Hogquist. 2004. The regulated expression of a diverse set of genes during thymocyte positive selection in vivo. J. Immunol. 173:5434-5444. [DOI] [PubMed] [Google Scholar]
- 23.Milan, G., A. Zambon, M. Cavinato, P. Zanovello, A. Rosato, and D. Collavo. 1999. Dissecting the immune response to Moloney murine sarcoma/leukemia virus-induced tumors by means of a DNA vaccination approach. J. Virol. 73:2280-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munitic, I., J. A. Williams, Y. Yang, B. Dong, P. J. Lucas, N. El Kassar, R. E. Gress, and J. D. Ashwell. 2004. Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. Blood 104:4165-4172. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, K. M., A. B. Heimberger, and D. Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250:1720-1723. [DOI] [PubMed] [Google Scholar]
- 26.Owens, G. P., W. E. Hahn, and J. J. Cohen. 1991. Identification of mRNAs associated with programmed cell death in immature thymocytes. Mol. Cell. Biol. 11:4177-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radu, C. G., A. Nijagal, J. McLaughlin, L. Wang, and O. N. Witte. 2005. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. USA 102:1632-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichardt, H. M., K. H. Kaestner, J. Tuckermann, O. Kretz, O. Wessely, R. Bock, P. Gass, W. Schmid, P. Herrlich, P. Angel, and G. Schutz. 1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93:531-541. [DOI] [PubMed] [Google Scholar]
- 29.Stefanelli, C., F. Bonavita, I. Stanic, G. Farruggia, E. Falcieri, I. Robuffo, C. Pignatti, C. Muscari, C. Rossoni, C. Guarnieri, and C. M. Caldarera. 1997. ATP depletion inhibits glucocorticoid-induced thymocyte apoptosis. Biochem. J. 322:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomura, H., C. Mogi, K. Sato, and F. Okajima. 2005. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal 17:1466-1476. [DOI] [PubMed] [Google Scholar]
- 31.Tosa, N., M. Murakami, W. Y. Jia, M. Yokoyama, T. Masunaga, C. Iwabuchi, M. Inobe, K. Iwabuchi, T. Miyazaki, K. Onoe, M. Iwata, and T. Uede. 2003. Critical function of T cell death-associated gene 8 in glucocorticoid-induced thymocyte apoptosis. Int. Immunol. 15:741-749. [DOI] [PubMed] [Google Scholar]
- 32.Vinuesa, C. G., and C. C. Goodnow. 2004. Illuminating autoimmune regulators through controlled variation of the mouse genome sequence. Immunity 20:669-679. [DOI] [PubMed] [Google Scholar]
- 33.Wang, J. Q., J. Kon, C. Mogi, M. Tobo, A. Damirin, K. Sato, M. Komachi, E. Malchinkhuu, N. Murata, T. Kimura, A. Kuwabara, K. Wakamatsu, H. Koizumi, T. Uede, G. Tsujimoto, H. Kurose, T. Sato, A. Harada, N. Misawa, H. Tomura, and F. Okajima. 2004. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 279:45626-45633. [DOI] [PubMed] [Google Scholar]
- 34.Weng, Z., A. C. Fluckiger, S. Nisitani, M. I. Wahl, L. Q. Le, C. A. Hunter, A. A. Fernal, M. M. Le Beau, and O. N. Witte. 1998. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc. Natl. Acad. Sci. USA 95:12334-12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitfield, P. D., P. C. Sharp, R. Taylor, and P. Meikle. 2001. Quantification of galactosylsphingosine in the twitcher mouse using electrospray ionization-tandem mass spectrometry. J. Lipid Res. 42:2092-2095. [PubMed] [Google Scholar]
- 36.Xu, Y., and G. Casey. 1996. Identification of human OGR1, a novel G protein-coupled receptor that maps to chromosome 14. Genomics 35:397-402. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida, H., Y. Y. Kong, R. Yoshida, A. J. Elia, A. Hakem, R. Hakem, J. M. Penninger, and T. W. Mak. 1998. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94:739-750. [DOI] [PubMed] [Google Scholar]