Abstract
The C-type lectins DC-SIGN and DC-SIGNR bind mannose-rich glycans with high affinity. In vitro, cells expressing these attachment factors efficiently capture, and are infected by, a diverse array of appropriately glycosylated pathogens, including dengue virus. In this study, we investigated whether these lectins could enhance cellular infection by West Nile virus (WNV), a mosquito-borne flavivirus related to dengue virus. We discovered that DC-SIGNR promoted WNV infection much more efficiently than did DC-SIGN, particularly when the virus was grown in human cell types. The presence of a single N-linked glycosylation site on either the prM or E glycoprotein of WNV was sufficient to allow DC-SIGNR-mediated infection, demonstrating that uncleaved prM protein present on a flavivirus virion can influence viral tropism under certain circumstances. Preferential utilization of DC-SIGNR was a specific property conferred by the WNV envelope glycoproteins. Chimeras between DC-SIGN and DC-SIGNR demonstrated that the ability of DC-SIGNR to promote WNV infection maps to its carbohydrate recognition domain. WNV virions and subviral particles bound to DC-SIGNR with much greater affinity than DC-SIGN. We believe this is the first report of a pathogen interacting more efficiently with DC-SIGNR than with DC-SIGN. Our results should lead to the discovery of new mechanisms by which these well-studied lectins discriminate among ligands.
The first step in viral entry is the stable attachment of the virion to the surface of a new target cell, a process that can be inefficient for many viruses (16, 34, 62, 73). Cellular proteins that facilitate productive infection by increasing the efficiency of virus binding, but whose presence is not absolutely required for viral entry, are often referred to as attachment factors (4). Two of the most extensively studied attachment factors are the lectins DC-SIGN (CD209) (18, 29) and DC-SIGNR (L-SIGN) (CD209L) (7, 67, 78). Both are tetrameric type II transmembrane proteins containing calcium-dependent (C-type) carbohydrate recognition domains (CRDs) (55). DC-SIGN is highly expressed in monocyte-derived dendritic cells (MDDCs) in vitro (29) and at lower levels (86) in vivo in subsets of macrophages (45, 53, 79) and dendritic cells (23, 29, 40, 80, 86). DC-SIGNR is expressed on microvascular endothelial cells, especially in the liver sinusoids and lymph nodes (7, 67, 81). Byfacilitating virion attachment, DC-SIGN and DC-SIGNR [henceforth referred to collectively as DC-SIGN(R)] can greatly increase the susceptibility of permissive cells to infection by a wide array of enveloped viruses or allow nonpermissive cells to capture and transmit these viruses to target cells in trans (3, 17, 35, 47, 52, 60, 76, 84).
Viruses that bind to DC-SIGN(R) appear to do so via high-mannose, N-linked glycans on their glycoproteins (44, 48, 51). This fact is readily explained by crystallographic studies demonstrating that mannose-rich oligosaccharides fit into elongated binding sites in the CRDs of DC-SIGN(R) (24). In addition to recognizing viral ligands based on their carbohydrate compositions, these lectins may bind preferentially to viruses displaying particular spatial arrangements of N-linked glycans that fit optimally onto the DC-SIGN(R) tetramers (55). Although the interaction of an individual DC-SIGN(R) CRD with a single high-mannose glycan is strong to begin with, certain viral ligands bind with much higher affinity to DC-SIGN(R) tetramers (11, 66, 77), demonstrating the importance of multivalent interactions in pathogen recognition.
Because their glycoproteins assume a regular arrangement on the viral membrane (59), flaviviruses represent attractive ligands for studying the role of multivalent interactions in binding to DC-SIGN(R). In addition, interactions between flavivirus virions and DC-SIGN(R) may impact human disease outcomes. Dengue virus has been shown to utilize DC-SIGN and DC-SIGNR for infection (60, 84), and a recent genetic study has indicated a strong link between a DC-SIGN promoter polymorphism and the risk of dengue fever (70).
To extend what is known about how flaviviruses interact with DC-SIGN(R), we selected West Nile virus (WNV) as a model viral ligand. WNV virions, like those of other flaviviruses, contain the two viral surface proteins E and prM/M, the capsid protein, and the positive-stranded genomic RNA (59). The envelope protein (E), which forms an icosahedral protein shell covering the surface of the virion (58), is a class II fusion protein responsible for mediating receptor engagement (69) and membrane fusion (38). Many WNV isolates, including virus strains responsible for the outbreak of WNV encephalitis in the Western Hemisphere, encode a single N-linked glycosylation site in the E protein (9, 12, 46). Interestingly, several studies have linked the presence of this site to increased neuroinvasion in mouse models of WNV infection (8, 9, 74). The premembrane glycoprotein (prM) of WNV and other flaviviruses facilitates the folding and trafficking of the E protein during virus particle biogenesis (2, 37). During particle egress, prM is cleaved by the cellular protease furin, releasing an N-terminal fragment (pr) containing the single N-linked glycan of prM. This cleavage event, which is required for infectivity in flaviviruses (22), leaves behind the small membrane protein (M) which is present in the mature virus particle (82). Although prM is not visualized in cryoelectron microscopic structural studies of mature flavivirus particles (25, 58, 91), biochemical studies have shown that significant quantities of uncleaved prM remain in infectious preparations of WNV and other flaviviruses (32, 42, 88).
In this study, we investigated whether DC-SIGN(R) can serve as attachment factors for WNV. We found that DC-SIGNR enhanced WNV infection to a much greater extent than did DC-SIGN, particularly for WNV grown in human cells. Enhanced infection was dependent on the N-linked glycosylation of WNV and mapped to preferential binding of WNV to the DC-SIGNR CRD. Unexpectedly, glycosylation of either prM or E was sufficient to allow WNV to interact with DC-SIGNR. Since DC-SIGNR-selective ligands have not been identified by previous studies, we anticipate future biochemical and structural dissection of this interaction will provide new insights into how DC-SIGN(R) interact with viral ligands containing a regular arrangement of glycans.
MATERIALS AND METHODS
Cells.
All cell lines were grown at 37°C in the presence of 5% CO2, except for Aedes albopictus C6/36 cells, which were maintained at 28°C in the presence of 5% CO2. Human embryonic kidney 293T cells (ATCC), HeLa cells, and baby hamster kidney clone 15 (BHK-21cl15) cells (provided by Michael Diamond, Washington University Medical School, St. Louis, Mo.) were grown in complete DMEM, consisting of 90% Dulbecco's modified Eagle medium high glucose (Invitrogen) plus 10% fetal calf serum (FCS; HyClone), 100 U/ml penicillin-streptomycin, and 2 mM added l-glutamine. BHK WNIIrep-REN cells (65a) were maintained in complete DMEM supplemented with 10 μg/ml blasticidin. CHO-K1, Vero, and C6/36 cells (ATCC) were grown as recommended (http://www.atcc.org). Human erythroleukemia K562 cells (ATCC), Raji B cells (provided by Vineet Kewalramani, NCI, Frederick, MD), and SupT1/CCR5 T cells (54) were cultured in complete RPMI growth medium consisting of 90% RPMI 1640 medium (Invitrogen) plus 10% FCS, 100 U/ml penicillin-streptomycin, 2 mM l-glutamine, and 10 mM HEPES (pH 7.5). K562 cell lines expressing the various DC-SIGN and DC-SIGNR constructs or an empty vector control were grown in this medium in the presence of 5 μg/ml blasticidin (Invitrogen). Elutriated blood monocytes (provided by the University of Pennsylvania Center for AIDS Research) were differentiated into MDDCs in complete RPMI supplemented with 10 ng/ml granulocyte-macrophage colony-stimulating factor and 20 ng/ml interleukin 4 (Peprotech). Monocytes were cultured at 106 cells/ml with medium changes at day 3 and day 5 of culturing and used in experiments on day 7. MDDCs were routinely CD1a+, CD11chi, DC-SIGNhigh, CD14low, CD83−, and CD86low. Pooled human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex and cultured according to the manufacturer's instructions in Endothelial Cell Medium-2 (EGM-2; Cambrex) supplemented with SingleQuots (Cambrex).
Generation of cell lines stably expressing DC-SIGN(R) constructs.
K562 cell lines were generated by electroporation with vector pcDNA6.2/V5-DEST (Invitrogen) to generate K562 control cells or with various DC-SIGN(R) constructs cloned into this vector. Cells stably expressing the lectins were selected by two to three rounds of magnetic sorting 7 days apart. Sorting was performed using antibody 120526 (see below) along with goat anti-mouse immunoglobulin G (IgG) microbeads and LS columns (Miltenyi Biotec), using a MidiMACs system (Miltenyi Biotec).
Antibodies, soluble glycoproteins, and reagents.
The following previously described anti-DC-SIGN(R) antibodies were used in this study: anti-CRD antibodies (from R&D Systems) 120526 and 120612 [DC-SIGN(R) cross-reactive], 120507 (SIGN specific), and 120604 (SIGNR specific) (40); anti-repeat domain antibodies DC28 and DC11 [DC-SIGN(R) cross-reactive] (6); and phycoerythrin (PE)-labeled DC11 (DC11-PE) (5). Anti-WNV E protein antibody 4E1 was recently described (71). An Alexa-647 monoclonal antibody labeling kit (Molecular Probes) was used to make 4E1-647. Antibodies used for MDDC phenotyping (fluorescein isothiocyanate-conjugated anti-CD1a, -CD11c, -CD83, and -CD86; PE-conjugated anti-CD14; and matched isotype controls) were from Caltag. Control mouse serum IgG, 1-deoxymannojirimycin (DMJ), and mannan were purchased from Sigma. Six-His-tagged soluble WNV E protein (strain NY99-6480) was produced by recombinant vaccinia virus expression in HEK 293T cells and purified by affinity chromatography (71).
Plasmids.
Sequences of all plasmids described in this study are available upon request. Plasmids encoding an infectious lineage II WNV molecular clone (WNII-Not) (65) or a subgenomic replicon encoding a Renilla luciferase reporter gene (WNIIrep-REN) (65a) have been described elsewhere. Expression plasmids encoding the envelope (pWNIIprME) and capsid (pWNIIcap) genes of WNII-Not were made by TOPO cloning the appropriate PCR fragments into pENTR/D-TOPO (Invitrogen), followed by transfer of the genes into pcDNA6.2/V5-DEST by using the Gateway LR recombination reaction (Invitrogen). A plasmid (pCBWN) (19) encoding the prM and E genes of WNV strain NY99-6480 with an N-terminal signal sequence derived from Japanese encephalitis virus was generously provided by Gwong Jen J. Chang (CDC). Derivatives of pCBWN encoding asparagine-to-glutamine substitutions at amino acid positions 15 and 154 of prM and E, respectively, have been recently described (36). An expression vector encoding the prM-E genes of dengue virus serotype 1 (WestPac strain; pDV1 prM-E VAX) was obtained from Wellington Sun and Robert Putnak (WRAIR). An expression vector encoding the capsid protein of dengue virus 1 (pDEN1cap) was constructed using the same approach used to build pWNIIcap. Plasmid pcDNA3.1furin (13) encoding human furin was provided by John Moore, Weill Medical College of Cornell University, New York, NY.
The full-length non-epitope-tagged coding sequences of DC-SIGN(R) were amplified from previously described pcDNA3 expression vectors (67) and TOPO cloned into vector pENTR/D-TOPO. The various DC-SIGN(R) chimeras were generated in pENTR by standard techniques and transferred by LR recombination into pcDNA6.2/V5-DEST. Lentiviral expression constructs for DC-SIGN and DC-SIGNR were generated by LR recombination from pENTR into vector plenti6/V5-DEST (Invitrogen).
Viruses and pseudotypes.
Low-passage lineage I WNV strain 3000.0259 (21) was provided by Michael Diamond. A seed stock of this virus (referred to henceforth as NY2000) was produced by infection of C6/36 mosquito cells. To generate NY2000 viral stocks grown in mammalian cell lines, this C6/36 seed stock was used at low multiplicities of infection (MOIs) to infect the following cell types: 293T, HeLa, BHK, CHO-K1, K562, Raji, SupT1/CCR5, Vero, and MDDCs (from two separate donors). Input virus was removed by extensive washing, and supernatant containing progeny virus was harvested and filtered 3 days postinfection for the cell lines or 2 days postinfection for MDDCs. A seed stock of lineage II WNV strain WN 956 D117 3B (90) was produced by transfecting 293T cells with plasmid WNII-Not and harvesting 48 h later. This seed stock was used to infect 293T cells, and progeny virus was harvested after 3 days as described above. We refer to this virus stock in the text as WNII-Not. NY2000 and WNII-Not virus stocks were titered by infection of K562 control cells and intracellular fluorescence-activated cell sorter (FACS) staining for WNV E protein expression after 20 h. In this study, the MOI for infections is defined by the number of K562 infectious units added divided by the number of cells infected. For example, infection of K562s at an MOI of 0.01 would be expected to result in 1% infection. Some viral stocks were titered by plaque assay on BHK cells, and it was found that one K562 infectious unit is approximately equal to four BHK PFU.
To generate reporter virus particles (RVPs), BHK WNIIrep-REN cells were seeded into six-well plates and transfected with capsid and prM-E expression vectors as indicated in the text. Transfections were performed using Lipofectamine 2000 (Invitrogen) with 3 μg capsid plasmid (WNV or dengue virus) and 0.1 μg (WNV) or 1 μg (dengue virus) prM-E plasmid. No differences were observed in these studies when 1 μg WNV prM-E was used instead of 0.1 μg (data not shown). In some cases, 1 μg furin plasmid was included in the transfections to enhance prM cleavage. After overnight transfection, medium was replaced with 2 ml of a low-glucose formulation of complete DMEM. When indicated, 1 mM DMJ was added to producer cells after transfection to generate RVPs containing high-mannose glycans. RVPs were harvested 48 h posttransfection, stored at 4°C, and used within 1 week of harvesting.
Pseudotyped lentiviral stocks used for transduction of HUVECs with lacZ, DC-SIGN, or DC-SIGNR were generated using a Virapower lentiviral expression system (Invitrogen) and the plenti6 expression vectors described above.
Infections.
All infections of K562 cells were performed using cells growing in log phase, corresponding to cell densities of 3 × 105 to 6 × 105/ml, at the time of infection. Cells (100,000 per well) were seeded into 96-well plates and, when indicated, incubated for 30 min at 37°C with specific inhibitors before the addition of serial fourfold dilutions of virus in growth medium. Infection was allowed to proceed for 20 h without removal of the virus inoculum. Cells were transferred to cluster tube racks and fixed by the addition of paraformaldehyde to a final concentration of 1.3%. Cells were fixed for 1 hour at 4°C and then spun down and resuspended in 200 μl of iFACS buffer (Dulbecco's phosphate-buffered saline without calcium or magnesium, 2% FCS, 5 mM EDTA, 0.05% sodium azide, 0.1% saponin) containing 1 μg/ml 4E1-647 and, when indicated, 2.5 μg/ml DC11-PE. After staining for 30 min at 4°C, cells were washed in iFACS buffer and resuspended in 200 μl of iFACS buffer without saponin and containing 1.3% paraformaldehyde. Infection was assessed by flow cytometry using a Becton Dickinson FACSCalibur instrument with CellQuest software for data acquisition. Data were analyzed using FlowJo software (TreeStar). Infected cells were identified as the cell population expressing high levels of WNV E protein, as seen by 4E1-647 fluorescence (channel FL-4). Essentially identical levels of infection were calculated when an antibody to the WNV NS1 protein (provided by Michael Diamond) was used for intracellular staining, indicating that the anti-E protein intracellular staining protocol detects infected cells and not uninfected cells to which virus is bound.
Dendritic cell infections were performed as described above for K562 infections, except that after infection, both nonadherent and adherent cell populations were trypsinized and then pooled before fixing and processing for intracellular staining and flow cytometry. HUVECs were plated into six-well plates at 100,000 cells per well; infected with lentiviral pseudotypes expressing lacZ, DC-SIGN, or DC-SIGNR for 72 h; and then infected with 293T cell-derived NY2000 at an MOI of 0.05. After 20 h, cells were harvested by trypsinization, fixed, and processed for intracellular FACS as done for K562 cells. DC11-PE was included during intracellular staining, and percentages of infection in the lentivirus-transduced (DC11-PE positive) and nontransduced (DC11-PE negative) populations were determined. For RVP infections, 50,000 K562 cells per well were infected in 96-well plates with serial fourfold dilutions of RVPs in 200 μl medium. Cells were cultured for 48 h without removal of input RVPs. Cells in each well were then washed and processed to assess Renilla luciferase activity by use of commercially available luminescence substrates (Promega).
SVPs.
WNV subviral particles (SVPs) consisting of prM/M and E only were made by transfection of 106 293T cells with 4 μg plasmid pCBWN or pWNIIprME. After overnight incubation, medium was replaced with low-glucose complete DMEM containing 25 mM HEPES plus 1 mM DMJ when indicated. SVPs were harvested 48 h after transfection and filtered. The WNV E protein content of SVP preps was determined by a recently described capture enzyme-linked immunosorbent assay (36) using purified, soluble, monomeric WNV E protein as a standard.
Expression and binding assays.
Expression levels of DC-SIGN and DC-SIGNR were measured on K562, Raji, and SupT1 cell lines by staining with a saturating concentration (2.5 μg/ml) of DC11-PE in binding buffer (Dulbecco's phosphate-buffered saline with 1.2 mM CaCl2 total, 0.5 mM MgCl2 total, 2% FCS, 0.05% azide). To measure SVP binding, 105 cells growing at log phase were resuspended in 100 μl binding buffer, which was followed by the addition of 100 μl SVPs diluted in low-glucose DMEM. After incubation for 1 h at 4°C, cells were washed with binding buffer and resuspended in 200 μl binding buffer containing 1 μg/ml 4E1-647. After 30 min, cells were again washed and then spun down and resuspended in binding buffer containing 1.3% paraformaldehyde before analysis by flow cytometry. Live cells were identified based on their forward- and side-scatter properties, and SVP binding was assessed in this population.
RESULTS
Expression of DC-SIGNR greatly increases the susceptibility of cell lines to infection by WNV.
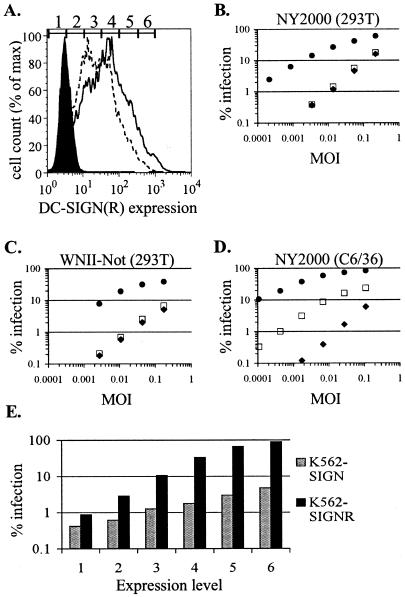
To determine if DC-SIGN or DC-SIGNR expression could influence the efficiency of WNV infection, we stably expressed each lectin or a control message in K562 erythroleukemia cells. K562 cells are substantially less infectible by WNV than several other cell lines we tested, such as Vero and BHK (data not shown). We reasoned that the relatively poor infectibility of K562 cells might be due in part to inefficient virus attachment, providing a context in which expression of an attachment factor would have the most dramatic effects. Cell surface expression of the lectins in these stable lines was assessed by flow cytometry (Fig. 1A). Each K562 line was infected with serial dilutions of a lineage I strain of WNV (NY2000 [strain 3000.0259]) (21) produced in human HEK 293T cells. Infection was monitored 20 hours later by intracellular FACS staining for E antigen expression (65, 75). K562-SIGNR cells showed levels of WNV infection greatly increased over those shown by control cells over the entire range of virus inputs tested (Fig. 1B). The magnitude of the increased infection was greatest (up to 40-fold enhancement) at the lowest MOIs. In contrast, K562-SIGN cells showed only slightly increased infectibility compared to control cells. When we infected cells with a lineage II WNV strain (WNII-Not [strain WN 956 D117 3B]) (65, 90) grown in human cells, we saw a pattern of DC-SIGNR-mediated enhancement similar to that seen for NY2000 (Fig. 1C). Notably, this lineage II strain lacks the N-linked glycosylation site found on the E protein of NY2000, although it still utilizes a glycosylation site present on prM (36). Thus, two different WNV strains, grown in human cells and differing by the presence or absence of an N-linked glycan on the viral E protein, infected cells expressing DC-SIGNR much more efficiently than they infected control cells or cells expressing DC-SIGN.
FIG. 1.
WNV preferentially infects K562 cell lines expressing DC-SIGNR. (A) Histogram analysis of DC-SIGN(R) expression in K562 cell lines. Cells were stained with DC11-PE and analyzed by flow cytometry. Filled area, control cells; dotted line, K562-SIGN cells; solid line, K562-SIGNR cells. Numbers and gates shown above histograms define the expression levels referred to in panel E. (B) K562-SIGN (open squares), K562-SIGNR (closed circles), or control K562 (closed diamonds) cells were infected with serial fourfold dilutions of lineage I WNV strain NY2000 grown in HEK 293T cells. Percentages of infection were assessed 20 h later by intracellular FACS staining for WNV E protein. Similar results were observed in more than five separate experiments. (C) Infection of K562 cells was performed as described for panel B, except cells were infected with lineage II WNV strain WNII-Not grown in HEK 293T cells. This strain lacks an N-linked glycosylation site on the viral E protein. A representative experiment of three performed is shown. (D) Infection was performed as described for panel B, except cells were infected with NY2000 grown in C6/36 mosquito cells. (E) K562-SIGN and K562-SIGNR cells were infected with 293T-derived virus at an MOI of 0.004. DC11-PE was included during staining for E antigen to assess DC-SIGN(R) expression. Cells were grouped by expression level according to the gates defined in panel A, and infection was assessed in each subset.
It was previously observed that, when grown in C6/36 mosquito cells, the alphavirus Sindbis virus can utilize DC-SIGN(R) for infection (44), an effect attributed to the predisposition of viruses produced in mosquito cells to contain mannose-rich N-linked glycans (39). Since WNV is also transmitted to humans through the bite of an infected mosquito, we grew WNV in the mosquito cell line C6/36 to see if this would result in the production of virus capable of utilizing DC-SIGN. Western blot analysis revealed the presence of high-mannose, endoglycosidase H-sensitive, N-linked glycans on the E protein of C6/36-derived but not 293T-derived virus (http://www.med.upenn.edu/micro/domslab/davisjvi.pdf and data not shown). C6/36-derived NY2000 infected K562-SIGN cells at levels 10-fold greater than those observed on control K562 cells (Fig. 1D), which was in contrast to the lack of enhancement seen for 293T-derived virus. C6/36-derived WNV infected K562-SIGNR cells with even greater efficiency than did 293T-derived virus, suggesting that the interaction of WNV with DC-SIGNR can be made more efficient by the incorporation of high-mannose glycans into the virus.
To rule out the possibility that the slight difference in expression levels between DC-SIGN and DC-SIGNR on K562 cells was responsible for the differing abilities of the two lectins to promote WNV infection, NY2000-infected cells were stained simultaneously for WNV antigen and DC-SIGN(R) expression. When cells were grouped according to their level of expression and infection was assessed in each subset, greatly enhanced infection of K562-SIGNR cells relative to that of K562-SIGN cells was seen at all levels of infection (Fig. 1E).
We also grew NY2000 in a wide variety of other cellular contexts, including human monocyte-derived dendritic cells and the cell lines K562, Raji, SupT1, HeLa, Vero, BHK, and CHO-K1. With the exception of virus grown in Vero cells, all virus stocks preferentially infected K562-SIGNR cells but showed little enhanced infection on K562-SIGN cells (data not shown). Similarly to C6/36-derived virus, Vero-derived NY2000 infected both K562-SIGN and K562-SIGNR cells relatively efficiently in comparison to control cells.
Previous studies (85, 89) have indicated that the cell type in which DC-SIGN(R) is expressed can have a significant impact on the efficiency with which these lectins promote viral infection. We therefore expressed DC-SIGN(R) in a variety of other contexts, including Raji, SupT1, BHK, 293T, and CHO-K1 cells. In all cases, DC-SIGNR expression made cells substantially more susceptible to infection by mammalian-derived NY2000 (data not shown). Except on Raji cells, DC-SIGN expression had only a modest effect on susceptibility to WNV infection. Raji-SIGN cells, which expressed DC-SIGN at the highest levels, were up to 10-fold more infectible than control Raji cells. However, in all contexts, including Raji cells, cells expressing DC-SIGNR were at least 10-fold more infectible than DC-SIGN-expressing cells. Thus, the superior ability of DC-SIGNR to promote infection by human cell-derived WNV was consistent regardless of cellular context.
Specific inhibitors block DC-SIGN(R)-mediated enhancement of WNV infection.
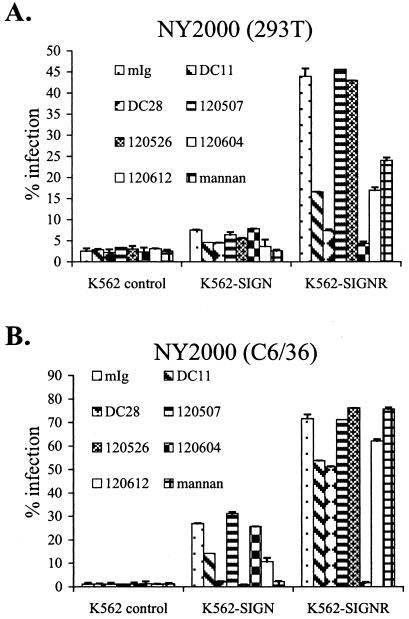
We screened a panel of monoclonal antibodies (MAbs) against DC-SIGN(R) for the ability to block WNV infection on K562-SIGN and K562-SIGNR cells (Fig. 2). The most effective inhibitor of DC-SIGNR-mediated infection was the anti-CRD, DC-SIGNR-specific MAb 120604, which reduced infection of K562-SIGNR cells almost to the levels seen in control cells. DC28, which binds to the repeat domain of both DC-SIGN and DC-SIGNR, was also an effective inhibitor. Generally, these antibodies blocked infection by 293T-derived virus (Fig. 2A) more efficiently than they blocked infection by C6/36-derived virus (Fig. 2B), consistent with a higher-affinity interaction between DC-SIGNR and the mannose-rich mosquito-derived virus. The most effective inhibitor of DC-SIGN-mediated infection by C6/36-derived WNV was the anti-CRD MAb 120526, followed by DC28. Notably, while 120526 completely blocked utilization of DC-SIGN, reducing infection of K562-SIGN cells to the level observed in control cells, it did not substantially affect WNV utilization of DC-SIGNR, even though this MAb does bind to DC-SIGNR (40). The yeast cell wall component mannan, a competitive inhibitor that binds to the carbohydrate binding site of mannose-specific lectins, also blocked DC-SIGN utilization by NY2000 but demonstrated only a modest effect on DC-SIGNR usage, suggesting either higher-affinity binding of WNV to DC-SIGNR or a lower affinity of mannan binding. A similarly inefficient blockade of DC-SIGNR utilization by mannan has been reported previously for Sindbis and dengue viruses (44, 84).
FIG. 2.
Specific inhibitors prevent DC-SIGN(R)-mediated enhancement of WNV infection. (A) K562 cell lines were incubated with the indicated inhibitors for 30 min at 37°C and infected with 293T-derived NY2000 at an MOI of 0.02. Inhibitors were included in the virus input to keep the concentration of inhibitor constant. Infections were performed without removal of viral input or inhibitors, and percentages of infection were assessed by intracellular FACS after 20 h. Antibodies were used at a concentration of 5 μg/ml and mannan was used at 500 μg/ml. mIg refers to control mouse serum IgG. MAbs DC11 and DC28 are DC-SIGN(R) cross-reactive and bind to the repeat domain, 120526 and 120612 are DC-SIGN(R) cross-reactive and bind to the CRD, 120507 binds to the CRD of DC-SIGN only, and 120604 binds to the CRD of DC-SIGNR only. Values shown are the averages from duplicate wells with standard deviations indicated. A representative experiment of two performed is shown. (B) Infections were performed as described for panel A, except that C6/36-derived NY2000 was used.
DC-SIGN(R) expressed in primary cell types can contribute to more-efficient WNV infection.
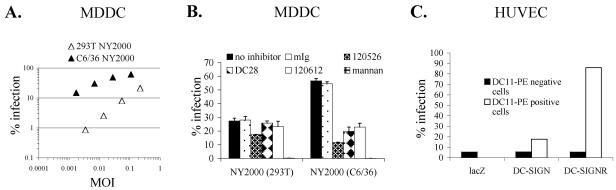
We examined whether the C6/36- and 293T-derived WNV stocks would have differing abilities to infect primary immature MDDCs, which express DC-SIGN from its native promoter. Virus stocks were normalized by their relative abilities to infect control K562 cells and used to infect MDDCs. We found that the mannose-rich C6/36-derived virus infected MDDCs approximately 10-fold better than 293T-derived virus (Fig. 3A), consistent with a role for DC-SIGN or other mannose-specific lectins in promoting infection. We next investigated the specific role of DC-SIGN in MDDC infection through the use of the inhibitors described above. The majority of MDDC infection by C6/36-derived virus could be blocked with antibodies directed against DC-SIGN, particularly the anti-CRD MAb 120526 (80% inhibition) (Fig. 3B). In contrast, MAb 120526 blocked only 35% of the total infection by 293T-derived NY2000, indicating a reduced role for DC-SIGN in infections with human cell-derived WNV. Preincubation of MDDCs with mannan (500 μg/ml) almost completely abolished MDDC infection for NY2000 produced both in C6/36 cells (99.9% inhibition) and in 293T cells (99.3% inhibition). This result may indicate the participation of other mannan-binding lectins besides DC-SIGN in WNV infection of MDDCs, though other mechanisms for this efficient inhibition of infection might exist.
FIG. 3.
DC-SIGN(R)-mediated enhancement of infection in primary cell cultures. (A) Titration of 293T-derived (open triangles) and C6/36-derived (closed triangles) WNV strain NY2000 on immature MDDCs. Percentages of infection were assessed after 20 h by intracellular FACS. Similar results were obtained using MDDCs from two other donors. (B) Blockade of MDDC infection by inhibitors of DC-SIGN. MDDCs were preincubated with the indicated inhibitors and infected with 293T- or C6/36-derived NY2000. Percentages of infection were assessed after 20 h by intracellular FACS. MAbs were used at 10 μg/ml, and mannan was used at 500 μg/ml. Infections were performed at an MOI of 0.09 for NY2000 grown in 293T cells and at an MOI of 0.04 for C6/36-derived virus. One representative experiment out of two performed is shown. (C) HUVECs were infected with lentiviruses encoding a control gene (lacZ), DC-SIGN, or DC-SIGNR at an MOI of 0.1. Seventy-two h posttransduction, cells were infected with 293T-derived NY2000 at an MOI of 0.05. After 20 h, intracellular staining for WNV E protein and DC-SIGN(R) expression was performed. For HUVECs transduced with DC-SIGN or DC-SIGNR lentiviruses, percentages of infection were assessed in both DC-SIGN(R)-expressing (DC11-PE-positive) and nonexpressing (DC11-PE-negative) cell populations.
In addition to studying DC-SIGN function on a relevant cell type, we studied DC-SIGNR in a more native context. DC-SIGNR is expressed naturally on liver sinusoidal endothelial cells and on a subset of endothelial cells in lymph nodes. To model this, we used HUVECs transduced at low MOI with pseudotyped lentiviruses encoding lacZ, DC-SIGN, or DC-SIGNR and infected 3 days later with 293T-derived NY2000 at an MOI of 0.05. Approximately 5% of HUVECs transduced with the lacZ control virus were infected by WNV (Fig. 3C). DC-SIGN-transduced HUVECs showed about 3-fold enhancement of infection compared to controls, and DC-SIGNR-transduced cells were infected at 18-fold-higher levels. In each experiment, nontransduced HUVECs (DC11-PE-negative cells) showed levels of infection similar to those of the HUVECs transduced with the lacZ control. Together, these results demonstrate that DC-SIGNR enhances infection similarly on endothelial cells and on the cell lines we examined above.
WNV subviral particles produced in human cells bind preferentially to DC-SIGNR.
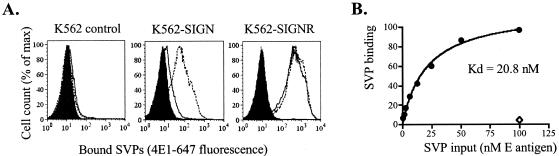
We hypothesized that the ability of DC-SIGNR to promote WNV infection, superior in comparison to that of DC-SIGN, was due to more-efficient attachment of WNV to DC-SIGNR. To test this, we utilized a FACS-based assay to evaluate the binding of noninfectious 293T-derived WNV SVPs to K562-SIGN(R) cells (Fig. 4A). SVPs bound much more efficiently to K562-SIGNR cells than to K562-SIGN cells or controls. When infectious 293T-derived NY2000 virus was used as a ligand instead of SVPs, binding was likewise seen only to K562-SIGNR cells (data not shown). To obtain SVPs containing high-mannose glycans on their envelope glycoproteins, particles were produced in cells treated with DMJ (26). DMJ, an inhibitor of Golgi mannosidase I, arrests glycan maturation primarily at the Man8GlcNAc2 stage. Production of SVPs in the presence of DMJ largely rescued their ability to bind to K562-SIGN cells, consistent with the increased infection of these cells by mannose-rich, C6/36-derived virus. The dissociation constant for the binding of SVPs to DC-SIGNR was an E protein concentration of approximately 20 nM (Fig. 4B), indicating a high-affinity interaction. Binding of SVPs could be blocked by treatment of K562-SIGNR cells with the calcium-chelating agent EGTA or MAb 120604 (data not shown). To confirm that the failure of WNV to bind DC-SIGN was not due to competition by endogenous, DC-SIGN-selective ligands present on K562 cells, we produced soluble tetrameric DC-SIGN(R) ectodomain proteins in bacteria. WNV SVPs were bound by purified DC-SIGNR but not by DC-SIGN, confirming the results seen with our FACS-based binding assay (data not shown).
FIG. 4.
WNV SVPs produced in human cells and containing native glycosylation patterns bind selectively to cells expressing DC-SIGNR. (A) Binding of WNV SVPs to K562 cells. The indicated cell lines were incubated with medium only (filled area) or with NY99-6480 SVPs (6 nM E protein). SVPs were produced in 293T cells under standard conditions (solid lines) to produce particles with native glycosylation patterns or in the presence of the Golgi mannosidase inhibitor 1-deoxymannojirimycin (dashed lines) to increase the incorporation of high-mannose N-linked glycans. Bound SVPs were detected by staining with anti-WNV MAb 4E1-647. (B) K562-SIGNR cells (circles) were incubated with serial twofold dilutions of SVPs produced under standard conditions and processed for FACS as described for panel A. The geometric mean 4E1-647 fluorescence was calculated at each concentration of SVPs, and the background fluorescence of cells incubated in the absence of SVPs was subtracted from each value. A one-site binding curve was fit to these data and is shown as a solid line, with the dissociation constant (Kd) indicated. For reference, the background-subtracted binding to K562 control cells (diamond) is shown at the highest SVP input.
Specific utilization of DC-SIGNR is a property of the WNV envelope glycoproteins.
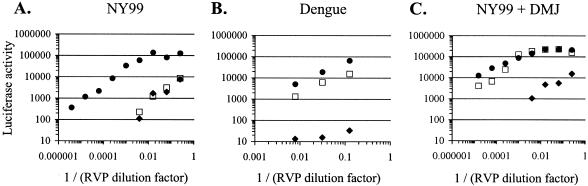
Having established that mammalian cell-derived WNV binds to and infects DC-SIGNR-expressing cells much more readily than it binds to and infects cells expressing DC-SIGN, we sought to identify features of the virus that contribute to this unusual selectivity. To do this, we employed a complementation approach for the production of RVPs that encapsidate a WNV subgenomic replicon (65a) (28,41, 43, 72). The RVPs were prepared by transfection of a BHKcell stably propagating a luciferase-expressing, bicistronic WNV replicon with plasmids encoding the flavivirus structural genes for capsid, prM, and E. Luciferase activity in cells infected with these RVPs is in direct proportion to the percent infection achieved (data not shown). As seen with infectious virus, RVPs made with WNV capsid and NY99-6480 prM/E proteins preferentially infected K562-SIGNR cells compared to K562 control or K562-SIGN cells (Fig. 5A). At high RVP inputs, luciferase activity reached a plateau in DC-SIGNR-expressing cells, due to saturating levels of infection. In contrast, RVPs incorporating the structural proteins of dengue virus demonstrated enhanced infection of both K562-SIGN and -SIGNR cells (Fig. 5B), in agreement with previously reported findings with live dengue virus (84). RVPs made using WNV capsid and NY99-6480 and derived from DMJ-treated producer cells infected both K562-SIGN and -SIGNR cells with high efficiency. Together, these results demonstrate that DC-SIGNR, compared to DC-SIGN, was preferentially utilized for infection and that this preferential utilization maps to the prM/E glycoproteins of WNV and requires native mammalian cell glycosylation patterns.
FIG. 5.
The selective usage of DC-SIGNR for infection is specific to WNV glycoproteins containing native glycosylation patterns. A Renilla luciferase-expressing WNV replicon was packaged into RVPs by transfection of BHK cells containing this replicon with expression plasmids encoding flavivirus capsid proteins and prM-E polyproteins. Serial fourfold dilutions of RVPs were added to K562 cell lines, and infection was assessed after 48 h by measuring luciferase activity. Open squares, K562-SIGN cells; closed circles, K562-SIGNR cells; closed diamonds, K562 control cells. (A) RVPs were made by use of pWNIIcap, encoding the WNV capsid protein, and pCBWN, encoding the prM-E polyprotein of WNV strain NY99-6480 (NY99). The E protein of NY99-6480 is identical to the E protein of NY2000. (B) RVPs were made using pDEN1cap, encoding the dengue virus capsid protein, and pDV1 prM-E VAX, encoding a serotype 1 dengue virus prM-E polyprotein. (C) RVPs were made as described for panel A, but DMJ was added during production to yield particles containing predominantly high-mannose N-linked glycans. Similar results were seen with more than four separate RVP preparations. Note the difference between the x axis scale in panels A and C and that in panel B.
Mutations in prM or E N-linked glycosylation sites modify DC-SIGNR usage by WNV.
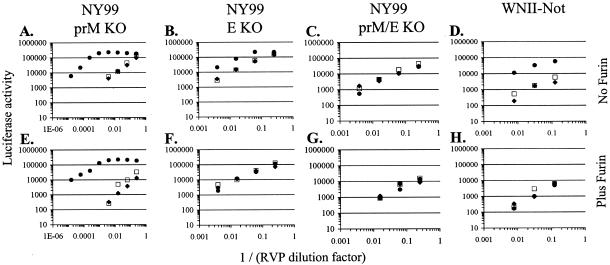
The NY99-6480 strain of WNV contains single N-linked glycosylation sites on both the E protein and the pr portion of prM. Since furin-dependent proteolysis of prM and subsequent removal of pr from the particle do not go to completion for WNV (88), the N-linked glycan on pr could play a role in attachment to DC-SIGNR. To investigate this, we produced RVPs that lacked either of the two glycosylation sites individually or in combination (36). We found that deletion of the N-linked site in NY99-6480 prM had minimal effect on DC-SIGNR utilization (compare Fig. 6A and Fig. 5A), whereas deletion of the site in E greatly reduced but did not fully eliminate the DC-SIGNR-mediated enhancement (compare Fig. 6B with Fig. 5A). Deletion of both sites abolished DC-SIGNR utilization (Fig. 6C). RVPs made using the prM/E genes of a lineage II strain (WN 956 D117 3B [WNII-Not]) (90) which naturally lacks the E protein glycosylation site found in NY99-6480 were capable of using DC-SIGNR (Fig. 6D), albeit less efficiently than wild-type NY99-6480 particles. Production of RVPs in cells transiently overexpressing human furin, which should drive the conversion of prM to M to completion and thus remove any N-linked glycans normally contributed by pr, eliminated the utilization of DC-SIGNR by the NY99-6480 E glycosylation mutant (Fig. 6F) and the lineage II RVPs (Fig. 6H). Importantly, the expression of furin had no effect on DC-SIGNR utilization by RVPs produced with the NY99-6480 prM glycosylation knockout (Fig. 6E). Thus, in the absence of glycosylation, pr does not contribute to the interaction with DC-SIGNR. Overall, our results demonstrate that a single N-linked glycosylation site on either prM or E is sufficient to allow WNV to interact with DC-SIGNR, although the presence of E glycosylation is necessary for optimal DC-SIGNR utilization.
FIG. 6.
The use of DC-SIGNR as an attachment factor by WNV requires at least one N-linked glycan on either prM or E. RVPs were produced by transfection of replicon-containing BHK cells with pWNIICap and different WNV prM-E expression plasmids (as indicated above the graphs) and used to infect K562 cell lines. Infection was assessed after 48 h by measuring luciferase activity. Open squares, K562-SIGN cells; closed circles, K562-SIGNR cells; closed diamonds, K562 control cells. prM-E and capsid expression plasmids were transfected alone (A through D) or cotransfected with an expression plasmid encoding human furin (E through H). “NY99 prM KO” refers to pCBWN containing an asparagine-to-glutamine substitution at prM residue 15 which resulted in the removal of the N-linked glycosylation site. “NY99 E KO” contains an asparagine-to-glutamine substitution removing the N-linked site at E protein residue 154. “NY99 prM/E KO” contains both prM N15Q and E N154Q substitutions. “WNII-Not” here refers to plasmid pWNIIprM-E, containing the prM-E polyprotein from lineage II WNV strain WNII-Not. This strain lacks an N-linked glycosylation site on the E protein but still possesses the site on prM. A representative experiment out of two performed for each RVP preparation is shown. Similar results were seen with two separate RVP preparations. Note the difference between the x axis scale in panels A and E and that in the other panels.
Mapping the regions of DC-SIGN and DC-SIGNR that account for their differing abilities to promote WNV infection.
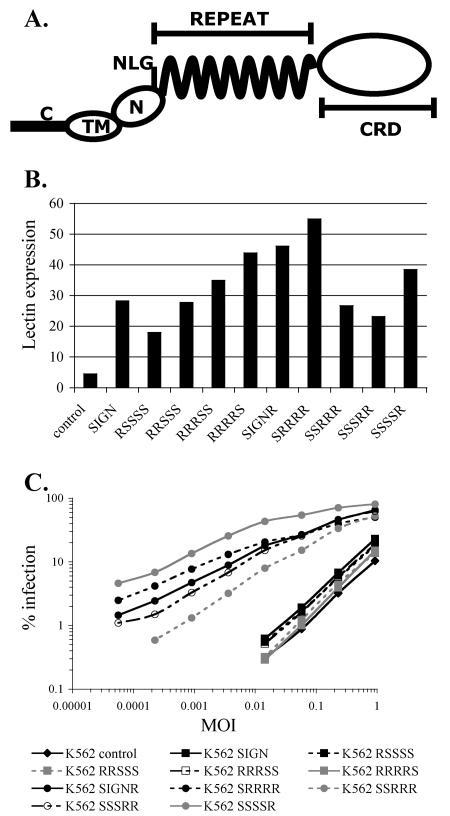
As a first step toward understanding why DC-SIGNR binds WNV and promotes infection more efficiently than DC-SIGN, we sought to identify the regions of DC-SIGNR responsible for this effect. The lectins were divided into five domains (Fig. 7A) by following a convention we used in a previous study (66), and chimeras between the two proteins were produced by making reciprocal exchanges at the boundaries between domains. Each chimera was expressed stably in K562 cells, and its expression was assessed by flow cytometry (Fig. 7B). When infected with 293T-derived NY2000, all cell lines expressing chimeras containing the DC-SIGNR CRD were much more infectible than control K562 cells, whereas none of the chimeras containing the DC-SIGN CRD increased infection more than approximately twofold (Fig. 7C). 293T-derived NY2000 bound with similar efficiency to all cell lines expressing chimeras containing the DC-SIGNR CRD and failed to bind to cells expressing chimeras containing the DC-SIGN CRD (data not shown).
FIG. 7.
Specific utilization of DC-SIGNR by WNV requires the DC-SIGNR CRD. (A) Schematic of DC-SIGN(R) domain structure. C, cytoplasmic domain (amino acids 1 to 41 of DC-SIGN/amino acids 1 to 49 of DC-SIGNR); TM, transmembrane domain (42 to 60/50 to 72); N, N-terminal domain (61 to 80/73 to 92); NLG, N-linked glycosylation site at asparagine (80/92); Repeat domain (81 to 252/93 to 264); CRD (253 to 404/265 to 399). (B) Expression of DC-SIGN(R) chimeras in stable K562 cell lines. Chimeras are named according to their five-domain compositions by the domain classifications given above. For example, chimera RRRSS consists of the DC-SIGNR cytoplasmic, transmembrane, and N-terminal domains fused to the repeat domain and the CRD of DC-SIGN. Cells were stained with DC11-PE and analyzed by flow cytometry. The geometric mean DC11-PE fluorescence is shown. (C) K562 cell lines expressing the indicated chimeras were infected with serial fourfold dilutions of 293T-derived NY2000. Percentages of infection were assessed by intracellular FACS after 20 h.
DISCUSSION
DC-SIGN and DC-SIGNR have been shown to promote infection by a wide variety of pathogens in vitro, making it of general importance to understand fully how these lectins recognize their ligands. To our surprise, we found that human cell-derived WNV binds strongly to DC-SIGNR and only weakly to DC-SIGN. Preferential binding to DC-SIGNR by an infectious agent has not previously been observed, indicating that there are unappreciated differences in the ligand-binding profiles of these two lectins. WNV can serve as a probe for mapping the determinants responsible for this binding difference. Furthermore, the finding that these lectins can increase WNV infection may have relevance to the pathogenesis of WNV and other flaviviruses.
Much is already known about the ligand specificities of DC-SIGN(R). Monosaccharide competition experiments have indicated that both lectins preferentially bind pyranose sugars containing equatorial 3′ and 4′ OH groups, particularly mannose (55). However, DC-SIGN(R) differ from other mannose-specific lectins in their abilities to bind internal sugars within glycans rather than terminal sugars. Structural studies have shown that DC-SIGN(R) bind preferentially to the trisaccharide Man∝1-3(Man∝1-6)Man∝1 found at the outer branch point of high-mannose glycans but are unable to bind to the same trisaccharide found at the inner branch point of complex glycans due to a steric clash between a conserved phenylalanine and the β1-4-linked chitobiose core (24). In addition to binding high-mannose glycans, DC-SIGN, but not DC-SIGNR, has the additional ability to bind fucose-containing structures, such as the Lewisx trisaccharide (33, 87). Thus, while DC-SIGN is known to recognize a set of ligands not bound by DC-SIGNR, the reverse has not previously been shown to be true.
Thus far, only certain bacteria (10) and parasites (87) have been shown to interact with DC-SIGN via fucosylated glycan structures, while viruses known to bind to DC-SIGN appear to do so via high-mannose glycans attached to viral glycoproteins (44, 49, 50, 68). When WNV was grown in C6/36 mosquito cells, which promote the incorporation of mannose-rich glycans into virions (39), DC-SIGN utilization increased greatly. When WNV RVPs were generated in cells treated with DMJ, which blocks processing of N-linked glycans, primarily at the Man8GlcNAc2 stage (26), the resulting RVPs utilized DC-SIGN for infection even more efficiently than C6/36-derived virus, similar to findings reported previously for Sindbis virus (44). The increased infectivity of DMJ particles likely reflects both the increased number of high-mannose glycans per particle and the high affinity with which Man8 structures bind to DC-SIGN compared to the shorter high-mannose glycans and Man3 core structures known to be added to viruses grown in mosquito cells (33).
DC-SIGNR-mediated infection also increased when WNV virions or SVPs were produced under conditions that promoted the incorporation of high-mannose glycans. However, even when WNV was grown in 293T cells, infection was still facilitated by DC-SIGNR, despite the apparent absence of endoglycosidase H-sensitive, high-mannose structures on this virus stock. We cannot exclude the possibility that low levels of high-mannose structures are incorporated into WNV in 293T cells and are responsible for binding of the virus to DC-SIGNR. However, if this were true, it would not explain the relatively weak binding of this virus to DC-SIGN, since DC-SIGN has an ability to bind high-mannose structures that is similar tothat of DC-SIGNR (33). Further studies to understand the mechanism of the selective binding of WNV to DC-SIGNR are under way.
Based on the ability of DC-SIGN(R) to promote more-efficient infection of cells by mosquito-derived Sindbis and dengue viruses in vitro, Klimstra et al. have proposed that these lectins may represent common receptors for cellular attachment by all mosquito-borne viruses (44). Our results with C6/36-derived stocks of WNV are consistent with this hypothesis and confirm the importance of DC-SIGN in the capturing of mosquito-derived viruses by MDDCs in vitro. However, our results with WNV differ from those of Klimstra et al. in that the growth of WNV in mosquito cells is not required for its interaction with DC-SIGNR, whereas mammalian cell-derived Sindbis virus was unable to use either DC-SIGN or DC-SIGNR for infection. Thus, DC-SIGNR could promote cellular attachment not only of the initial, mosquito-derived WNV inoculum but also of virus released during subsequent rounds of replication.
Interestingly, mammalian cell-derived RVPs containing the prM/E genes of dengue virus preferentially infected cells expressing either DC-SIGN or DC-SIGNR, while WNV RVPs utilized only DC-SIGNR for infection. This suggests that dengue virus structural proteins possess some mechanism for promoting DC-SIGN engagement that is lacking in WNV. This may reflect the adaptation of dengue virus to humans as a natural host. Our RVP data support a model in which different flaviviruses use DC-SIGN and/or DC-SIGNR for continuing infection of the human host.
A surprising finding in this study was that DC-SIGNR could promote infection by WNV strains or RVPs that contain N-linked glycans solely on prM. Although it has been recognized for some time that flavivirus preparations contain some level of uncleaved prM, it was difficult to exclude the possibility that infection was mediated solely by a subset of particles in which prM was fully cleaved to M. Our results prove that retention of levels of prM in a WNV particle sufficient to mediate binding to DC-SIGNR does not abrogate its infectivity. Binding of WNV to DC-SIGNR via the prM glycan also establishes that under certain circumstances, the E protein of flaviviruses may not be the only viral determinant responsible for interacting with cellular receptors or attachment factors. Importantly, previous studies of the interaction between dengue virus and DC-SIGN have assumed that E is the only glycoprotein on the mature virus and therefore the only potential site for DC-SIGN binding (50, 56). Our WNV results suggest that this assumption merits direct testing using genetic knockouts of E and prM glycosylation sites.
The data presented in this report demonstrate that DC-SIGNR can function as an attachment factor for WNV. Importantly, these experiments do not establish or suggest that this lectin is a receptor for WNV in vitro or in vivo. For pH-independent enveloped viruses, the term “receptor” is generally reserved for those cell surface molecules whose binding triggers the conformational changes in the viral envelope protein that lead to membrane fusion (4). For some pH-triggered viruses, such as avian sarcoma and leukosis viruses, receptors are needed to prime the viral envelope protein for conformational changes induced by an acidic pH (57). For others, such as WNV, conformational rearrangements and fusion with target membranes occur at a low pH without a need for target proteins or carbohydrates (30).
For the latter class of viruses, the defining characteristics of a cellular receptor have not been established. We envision at least two requirements. First, the expression of a true receptor can be shown to convert a cell line which is refractory to virus entry into a permissive line. Second, the engagement of a true receptor is sufficient for inducing the endocytosis of the virus and trafficking to the intracellular compartment where fusion occurs. We have been unable to test the first criterion, as all metazoan cell lines we have tested support West Nile virus infection at some level. Thus, while DC-SIGNR can facilitate WNV infection, it is clearly not required. Whether DC-SIGNR plays a role in WNV endocytosis is not yet known, though a recent study examined whether endocytosis motifs in the DC-SIGN cytoplasmic tail are required for promotion of dengue virus infection (50). This study found that removal of the dileucine motif in the DC-SIGN cytoplasmic tail prevented antibody-induced internalization of the lectin but had no effect on its ability to promote dengue virus infection in the cell lines examined. This suggests that dengue virus internalization may involve interactions with other cell surface molecules after DC-SIGN binding. Another possibility is suggested by a recent theoretical study showing that recruitment of diffusible cell surface receptors by virus-sized particles may lead to alternative internalization pathways (27). It remains to be seen whether WNV must bind to additional surface molecules for internalization after binding to DC-SIGNR.
It was reported recently (15) that αvβ3 integrin is the receptor for West Nile virus on vertebrate cells and is required for infection. Although we have not directly addressed the role of this integrin in our studies, we note that several of the cell lines in which we successfully grew WNV in this study, including the K562, Raji, and CHO-K1 cell lines, previously have been shown to lack αvβ3 integrin expression (14, 61). Thus, αvβ3 is not absolutely required for WNV infection. Furthermore, HUVECs are known to express αvβ3 integrin (1), yet we found them to have a susceptibility to WNV infection similar to that of αvβ3-negative K562 cells. HUVECs were made greatly more infectible when transduced with DC-SIGNR, suggesting that the attachment of WNV to the cell surface can still be a limiting factor on cells expressing αvβ3 integrin.
It remains to be seen whether DC-SIGN and DC-SIGNR modulate WNV tropism or pathogenesis in vivo. In humans, the great majority of WNV infections are asymptomatic (80%) or characterized by a mild febrile illness (20%), but in approximately 1 out of every 150 cases, the virus invades the central nervous system (CNS), leading to meningitis or encephalitis often accompanied by muscle weakness or paralysis (64). Although the tropism of WNV within the CNS has been examined in fatal human cases (31), little is known about viral tropism in the periphery, where DC-SIGN(R) are expressed. Infection of some inbred mouse strains leads to an encephalitis that models many aspects of severe human CNS disease (20). However, interpreting the role of DC-SIGN(R) in human infections based on these studies will be difficult, because the multiple murine homologues of DC-SIGN(R) differ from their human counterparts in their structures, ligand specificities, and patterns of expression (63, 83).
The experiments presented here represent a necessary first step toward assessing the in vivo role of DC-SIGNR in WNV infection, using transgenic animal models or genetic studies of WNV-infected humans. We speculate that DC-SIGN might be important for the infection of macrophages or dendritic cells in the skin following a bite by a WNV-infected mosquito, while DC-SIGNR might facilitate the capture of progeny virus by endothelial cells in the liver and lymph nodes. Once infection is established in these tissues, cell-to-cell spread of WNV to DC-SIGNR-negative cells would likely occur, as attachment factors play a small role in affecting viral tropism when the local virus concentration is high and attachment to the cell surface is no longer a limiting factor. It is difficult to predict whether capture of WNV by DC-SIGNR-expressing cells would be detrimental to the host, by facilitating more efficient viral replication, or beneficial, by promoting more-rapid immune activation. Regardless of whether DC-SIGN and DC-SIGNR are ultimately found to play significant roles in WNV infection in vivo, we believe that further exploration of the selective interaction between WNV and DC-SIGNR will lead to novel insights into multivalent ligand recognition by these lectins, which have been suggested to play important roles in the pathogenesis of a wide variety of human diseases.
Acknowledgments
This work was supported by grant NIH AI 50469 and U54 AI 57168. C.W.D. received support from grant T32 AI 07632, S.L.H. was supported by grants T32-GM-007229 and T32-AI-07324-13, and M.D.S. was supported by grant NIH F31 RR05074. This research was also supported in part by the Intramural Research Program of the NIH, National Institutes of Allergy and Infectious Diseases (NIAID).
We thank Michael Diamond, Vladimir Yamshchikov, Wellington Sun, Robert Putnak, Gwong Jen Chang, and Fang-Hua Lee for providing reagents. We thank members of the Doms lab for valuable discussions and Chris Buck, Bertrand Saunier, and Ed Berger for discussions and critical reading of the manuscript.
REFERENCES
- 1.Albelda, S. M., M. Daise, E. M. Levine, and C. A. Buck. 1989. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J. Clin. Investig. 83:1992-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, S. L., K. Stadler, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J. Virol. 69:5816-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muniz, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baribaud, F., R. W. Doms, and S. Pohlmann. 2002. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets 6:423-431. [DOI] [PubMed] [Google Scholar]
- 5.Baribaud, F., S. Pohlmann, G. Leslie, F. Mortari, and R. W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135-9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baribaud, F., S. Pohlmann, T. Sparwasser, M. T. Kimata, Y. K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, S. J. van Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beasley, D. W., C. T. Davis, M. Whiteman, B. Granwehr, R. M. Kinney, and A. D. Barrett. 2004. Molecular determinants of virulence of West Nile virus in North America. Arch. Virol. Suppl. 18:35-41. [DOI] [PubMed] [Google Scholar]
- 9.Beasley, D. W., M. C. Whiteman, S. Zhang, C. Y. Huang, B. S. Schneider, D. R. Smith, G. D. Gromowski, S. Higgs, R. M. Kinney, and A. D. Barrett. 2005. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 79:8339-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman, M. P., A. Engering, H. H. Smits, S. J. van Vliet, A. A. van Bodegraven, H. P. Wirth, M. L. Kapsenberg, C. M. Vandenbroucke-Grauls, Y. van Kooyk, and B. J. Appelmelk. 2004. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200:979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernhard, O. K., J. Lai, J. Wilkinson, M. M. Sheil, and A. L. Cunningham. 2004. Proteomic analysis of DC-SIGN on dendritic cells detects tetramers required for ligand binding but no association with CD4. J. Biol. Chem. 279:51828-51835. [DOI] [PubMed] [Google Scholar]
- 12.Berthet, F. X., H. G. Zeller, M. T. Drouet, J. Rauzier, J. P. Digoutte, and V. Deubel. 1997. Extensive nucleotide changes and deletions within the envelope glycoprotein gene of Euro-African West Nile viruses. J. Gen. Virol. 78:2293-2297. [DOI] [PubMed] [Google Scholar]
- 13.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buttgereit, P., S. Weineck, G. Ropke, A. Marten, K. Brand, T. Heinicke, W. H. Caselmann, D. Huhn, and I. G. Schmidt-Wolf. 2000. Efficient gene transfer into lymphoma cells using adenoviral vectors combined with lipofection. Cancer Gene Ther. 7:1145-1155. [DOI] [PubMed] [Google Scholar]
- 15.Chu, J. J., and M. L. Ng. 2004. Interaction of West Nile virus with alpha v beta 3 integrin mediates virus entry into cells. J. Biol. Chem. 279:54533-54541. [DOI] [PubMed] [Google Scholar]
- 16.Chuck, A. S., M. F. Clarke, and B. O. Palsson. 1996. Retroviral infection is limited by Brownian motion. Hum. Gene Ther. 7:1527-1534. [DOI] [PubMed] [Google Scholar]
- 17.Cormier, E. G., R. J. Durso, F. Tsamis, L. Boussemart, C. Manix, W. C. Olson, J. P. Gardner, and T. Dragic. 2004. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc. Natl. Acad. Sci. USA 101:14067-14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis, B. S., G. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebel, G. D., A. P. Dupuis II, K. Ngo, D. Nicholas, E. Kauffman, S. A. Jones, D. Young, J. Maffei, P. Y. Shi, K. Bernard, and L. D. Kramer. 2001. Partial genetic characterization of West Nile virus strains, New York state, 2000. Emerg. Infect. Dis. 7:650-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elshuber, S., S. L. Allison, F. X. Heinz, and C. W. Mandl. 2003. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J. Gen. Virol. 84:183-191. [DOI] [PubMed] [Google Scholar]
- 23.Engering, A., S. J. Van Vliet, T. B. Geijtenbeek, and Y. Van Kooyk. 2002. Subset of DC-SIGN(+) dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 100:1780-1786. [DOI] [PubMed] [Google Scholar]
- 24.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 25.Ferlenghi, I., M. Clarke, T. Ruttan, S. L. Allison, J. Schalich, F. X. Heinz, S. C. Harrison, F. A. Rey, and S. D. Fuller. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593-602. [DOI] [PubMed] [Google Scholar]
- 26.Fuhrmann, U., E. Bause, G. Legler, and H. Ploegh. 1984. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature 307:755-758. [DOI] [PubMed] [Google Scholar]
- 27.Gao, H., W. Shi, and L. B. Freund. 2005. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 102:9469-9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gehrke, R., M. Ecker, S. W. Aberle, S. L. Allison, F. X. Heinz, and C. W. Mandl. 2003. Incorporation of tick-borne encephalitis virus replicons into virus-like particles by a packaging cell line. J. Virol. 77:8924-8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 30.Gollins, S. W., and J. S. Porterfield. 1986. pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J. Gen. Virol. 67:157-166. [DOI] [PubMed] [Google Scholar]
- 31.Guarner, J., W. J. Shieh, S. Hunter, C. D. Paddock, T. Morken, G. L. Campbell, A. A. Marfin, and S. R. Zaki. 2004. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum. Pathol. 35:983-990. [DOI] [PubMed] [Google Scholar]
- 32.Guirakhoo, F., F. X. Heinz, C. W. Mandl, H. Holzmann, and C. Kunz. 1991. Fusion activity of flaviviruses: comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J. Gen. Virol. 72:1323-1329. [DOI] [PubMed] [Google Scholar]
- 33.Guo, Y., H. Feinberg, E. Conroy, D. A. Mitchell, R. Alvarez, O. Blixt, M. E. Taylor, W. I. Weis, and K. Drickamer. 2004. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 11:591-598. [DOI] [PubMed] [Google Scholar]
- 34.Haim, H., I. Steiner, and A. Panet. 2005. Synchronized infection of cell cultures by magnetically controlled virus. J. Virol. 79:622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 36.Hanna, S. L., T. C. Pierson, M. D. Sanchez, A. A. Ahmed, M. M. Murtadha, and R. W. Doms. 2005. N-linked glycosylation of West Nile virus envelope proteins influences particle assembly and infectivity. J. Virol. 79:13262-13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinz, F. X., G. Auer, K. Stiasny, H. Holzmann, C. Mandl, F. Guirakhoo, and C. Kunz. 1994. The interactions of the flavivirus envelope proteins: implications for virus entry and release. Arch. Virol. Suppl. 9:339-348. [DOI] [PubMed] [Google Scholar]
- 38.Heinz, F. X., K. Stiasny, and S. L. Allison. 2004. The entry machinery of flaviviruses. Arch. Virol. Suppl. 18:133-137. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh, P., and P. W. Robbins. 1984. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J. Biol. Chem. 259:2375-2382. [PubMed] [Google Scholar]
- 40.Jameson, B., F. Baribaud, S. Pohlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones, C. T., C. G. Patkar, and R. J. Kuhn. 2005. Construction and applications of yellow fever virus replicons. Virology 331:247-259. [DOI] [PubMed] [Google Scholar]
- 42.Keelapang, P., R. Sriburi, S. Supasa, N. Panyadee, A. Songjaeng, A. Jairungsri, C. Puttikhunt, W. Kasinrerk, P. Malasit, and N. Sittisombut. 2004. Alterations of pr-M cleavage and virus export in pr-M junction chimeric dengue viruses. J. Virol. 78:2367-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khromykh, A. A., M. T. Kenney, and E. G. Westaway. 1998. trans-complementation of flavivirus RNA polymerase gene NS5 by using Kunjin virus replicon-expressing BHK cells. J. Virol. 72:7270-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klimstra, W. B., E. M. Nangle, M. S. Smith, A. D. Yurochko, and K. D. Ryman. 2003. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 77:12022-12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krutzik, S. R., B. Tan, H. Li, M. T. Ochoa, P. T. Liu, S. E. Sharfstein, T. G. Graeber, P. A. Sieling, Y. J. Liu, T. H. Rea, B. R. Bloom, and R. L. Modlin. 2005. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 11:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 47.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin, G., G. Simmons, S. Pohlmann, F. Baribaud, H. Ni, G. J. Leslie, B. S. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and R. W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lozach, P. Y., A. Amara, B. Bartosch, J. L. Virelizier, F. Arenzana-Seisdedos, F. L. Cosset, and R. Altmeyer. 2004. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J. Biol. Chem. 279:32035-32045. [DOI] [PubMed] [Google Scholar]
- 50.Lozach, P. Y., L. Burleigh, I. Staropoli, E. Navarro-Sanchez, J. Harriague, J. L. Virelizier, F. A. Rey, P. Despres, F. Arenzana-Seisdedos, and A. Amara. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 280:23698-23708. [DOI] [PubMed] [Google Scholar]
- 51.Lozach, P. Y., H. Lortat-Jacob, A. de Lacroix de Lavalette, I. Staropoli, S. Foung, A. Amara, C. Houles, F. Fieschi, O. Schwartz, J. L. Virelizier, F. Arenzana-Seisdedos, and R. Altmeyer. 2003. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 278:20358-20366. [DOI] [PubMed] [Google Scholar]
- 52.Marzi, A., T. Gramberg, G. Simmons, P. Moller, A. J. Rennekamp, M. Krumbiegel, M. Geier, J. Eisemann, N. Turza, B. Saunier, A. Steinkasserer, S. Becker, P. Bates, H. Hofmann, and S. Pohlmann. 2004. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 78:12090-12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCully, M. L., T. A. Chau, P. Luke, P. G. Blake, and J. Madrenas. 2005. Characterization of human peritoneal dendritic cell precursors and their involvement in peritonitis. Clin. Exp. Immunol. 139:513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Means, R. E., T. Matthews, J. A. Hoxie, M. H. Malim, T. Kodama, and R. C. Desrosiers. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J. Virol. 75:3903-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell, D. A., A. J. Fadden, and K. Drickamer. 2001. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 276:28939-28945. [DOI] [PubMed] [Google Scholar]
- 56.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2005. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J. Virol. 79:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 58.Mukhopadhyay, S., B. S. Kim, P. R. Chipman, M. G. Rossmann, and R. J. Kuhn. 2003. Structure of West Nile virus. Science 302:248. [DOI] [PubMed] [Google Scholar]
- 59.Mukhopadhyay, S., R. J. Kuhn, and M. G. Rossmann. 2005. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 3:13-22. [DOI] [PubMed] [Google Scholar]
- 60.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neff, S., D. Sá-Carvalho, E. Rieder, P. W. Mason, S. D. Blystone, E. J. Brown, and B. Baxt. 1998. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αvβ3 as its receptor. J. Virol. 72:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park, C. G., K. Takahara, E. Umemoto, Y. Yashima, K. Matsubara, Y. Matsuda, B. E. Clausen, K. Inaba, and R. M. Steinman. 2001. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 13:1283-1290. [DOI] [PubMed] [Google Scholar]
- 64.Petersen, L. R., and A. A. Marfin. 2002. West Nile virus: a primer for the clinician. Ann. Intern. Med. 137:173-179. [DOI] [PubMed] [Google Scholar]
- 65.Pierson, T. C., M. S. Diamond, A. A. Ahmed, L. E. Valentine, C. W. Davis, M. A. Samuel, S. L. Hanna, B. A. Puffer, and R. W. Doms. 2005. An infectious West Nile virus that expresses a GFP reporter gene. Virology 334:28-40. [DOI] [PubMed] [Google Scholar]
- 65a.Pierson, T. C., M. D. Sanchez, B. A. Puffer, A. A. Ahmed, B. J. Geiss, L. E. Valentine, L. A. Altamura, M. S. Diamond, and R. W. Doms. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology, in press. [DOI] [PubMed]
- 66.Pohlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Munch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pohlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pohlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 70.Sakuntabhai, A., C. Turbpaiboon, I. Casademont, A. Chuansumrit, T. Lowhnoo, A. Kajaste-Rudnitski, S. M. Kalayanarooj, K. Tangnararatchakit, N. Tangthawornchaikul, S. Vasanawathana, W. Chaiyaratana, P. T. Yenchitsomanus, P. Suriyaphol, P. Avirutnan, K. Chokephaibulkit, F. Matsuda, S. Yoksan, Y. Jacob, G. M. Lathrop, P. Malasit, P. Despres, and C. Julier. 2005. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 37:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanchez, M. D., T. C. Pierson, D. McAllister, S. L. Hanna, B. A. Puffer, L. E. Valentine, M. M. Murtadha, J. A. Hoxie, and R. W. Doms. 2005. Characterization of neutralizing antibodies to West Nile virus. Virology 336:70-82. [DOI] [PubMed] [Google Scholar]
- 72.Scholle, F., Y. A. Girard, Q. Zhao, S. Higgs, and P. W. Mason. 2004. trans-packaged West Nile virus-like particles: infectious properties in vitro and in infected mosquito vectors. J. Virol. 78:11605-11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seisenberger, G., M. U. Ried, T. Endress, H. Buning, M. Hallek, and C. Brauchle. 2001. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science 294:1929-1932. [DOI] [PubMed] [Google Scholar]
- 74.Shirato, K., H. Miyoshi, A. Goto, Y. Ako, T. Ueki, H. Kariwa, and I. Takashima. 2004. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J. Gen. Virol. 85:3637-3645. [DOI] [PubMed] [Google Scholar]
- 75.Shrestha, B., D. Gottlieb, and M. S. Diamond. 2003. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 77:13203-13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleux, J. L. Riley, R. W. Doms, P. Bates, and S. Pohlmann. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123. [DOI] [PubMed] [Google Scholar]
- 77.Snyder, G. A., M. Colonna, and P. D. Sun. 2005. The structure of DC-SIGNR with a portion of its repeat domain lends insights to modeling of the receptor tetramer. J. Mol. Biol. 347:979-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soilleux, E. J., R. Barten, and J. Trowsdale. 2000. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J. Immunol. 165:2937-2942. [DOI] [PubMed] [Google Scholar]
- 79.Soilleux, E. J., L. S. Morris, B. Lee, S. Pohlmann, J. Trowsdale, R. W. Doms, and N. Coleman. 2001. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 195:586-592. [DOI] [PubMed] [Google Scholar]
- 80.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, Q. Luo, E. Levroney, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. 2002. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71:445-457. [PubMed] [Google Scholar]
- 81.Soilleux, E. J., L. S. Morris, S. Rushbrook, B. Lee, and N. Coleman. 2002. Expression of human immunodeficiency virus (HIV)-binding lectin DC-SIGNR: consequences for HIV infection and immunity. Hum. Pathol. 33:652-659. [DOI] [PubMed] [Google Scholar]
- 82.Stadler, K., S. L. Allison, J. Schalich, and F. X. Heinz. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 71:8475-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahara, K., Y. Yashima, Y. Omatsu, H. Yoshida, Y. Kimura, Y. S. Kang, R. M. Steinman, C. G. Park, and K. Inaba. 2004. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int. Immunol. 16:819-829. [DOI] [PubMed] [Google Scholar]
- 84.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trumpfheller, C., C. G. Park, J. Finke, R. M. Steinman, and A. Granelli-Piperno. 2003. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int. Immunol. 15:289-298. [DOI] [PubMed] [Google Scholar]
- 86.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 87.van Die, I., S. J. van Vliet, A. K. Nyame, R. D. Cummings, C. M. Bank, B. Appelmelk, T. B. Geijtenbeek, and Y. van Kooyk. 2003. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology 13:471-478. [DOI] [PubMed] [Google Scholar]
- 88.Wengler, G. 1989. Cell-associated West Nile flavivirus is covered with E+pre-M protein heterodimers which are destroyed and reorganized by proteolytic cleavage during virus release. J. Virol. 63:2521-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]
- 90.Yamshchikov, V. F., G. Wengler, A. A. Perelygin, M. A. Brinton, and R. W. Compans. 2001. An infectious clone of the West Nile flavivirus. Virology 281:294-304. [DOI] [PubMed] [Google Scholar]
- 91.Zhang, W., P. R. Chipman, J. Corver, P. R. Johnson, Y. Zhang, S. Mukhopadhyay, T. S. Baker, J. H. Strauss, M. G. Rossmann, and R. J. Kuhn. 2003. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat. Struct. Biol. 10:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]