Abstract
A major hurdle to the successful clinical use of some viral vectors relates to the innate, adaptive, and memory immune responses that limit the efficiency and duration of transgene expression. Some of these drawbacks may be circumvented by using vectors derived from nonhuman viruses such as canine adenovirus type 2 (CAV-2). Here, we evaluated the potential of CAV-2 vectors for gene transfer to the respiratory tract. We found that CAV-2 transduction was efficient in vivo in the mouse respiratory tract, and ex vivo in well-differentiated human pulmonary epithelia. Notably, the in vivo and ex vivo efficiency was poorly inhibited by sera from mice immunized with a human adenovirus type 5 (HAd5, a ubiquitous human pathogen) vector or by human sera containing HAd5 neutralizing antibodies. Following intranasal instillation in mice, CAV-2 vectors also led to a lower level of inflammatory cytokine secretion and cellular infiltration compared to HAd5 vectors. Moreover, CAV-2 transduction efficiency was increased in vitro in human pulmonary cells and in vivo in the mouse respiratory tract by FK228, a histone deacetylase inhibitor. Finally, by using a helper-dependent CAV-2 vector, we increased the in vivo duration of transgene expression to at least 3 months in immunocompetent mice without immunosuppression. Our data suggest that CAV-2 vectors may be efficient and safe tools for long-term clinical gene transfer to the respiratory tract.
Current treatments for lung diseases such as cystic fibrosis, α1-antitrypsin deficiency, lung cancer, and pulmonary fibrosis, as well as neonatal disorders such as respiratory distress syndrome and bronchopulmonary dysplasia, have partial to poor success rates (15, 16, 18, 41, 61). In search of alternative treatments, the straightforward access to respiratory epithelial cells via the trachea has initiated numerous gene transfer strategies (52). The often self-limiting respiratory tract infections caused by some human adenovirus (HAd) serotypes (e.g., 2 and 5) suggested ipso facto that respiratory epithelia are readily infected and could be genetically modified using vectors derived from some members of the Adenoviridae family. Although HAd2/5 (from species C) are the prototype vector backbones and have been widely used for gene transfer for more than 20 years, other human serotypes (either the entire capsid or parts thereof) are also being tested for gene transfer. The latest and most efficient adenovirus vectors for long-term gene transfer are referred to as “helper dependent” (HD) and are gutted of all viral coding regions. Their improved efficacy and duration of transgene expression (1, 29, 44, 59) is due primarily to the elimination of the adaptive cell-mediated immune response in immunologically naive animals. HD vectors have several other advantages, including variable cell tropism, relatively easy production to yield high titers (>1013 physical particles [p.p.]/ml) (43), and a high cloning capacity (>30 kb).
Although a few phase I trials have been encouraging, numerous obstacles dampened much of the early enthusiasm, especially concerning gene transfer to the respiratory tract. In spite of the improvements, a major hurdle to the successful use of Ad vectors in humans relates to memory immunity (humoral and cellular) that limits the efficiency and duration of transgene expression. Although many HAd are prevalent in most populations (10, 53), most infections lead to subclinical morbidity. Repeated exposure to multiple HAd serotypes leads to long-term protective memory cellular immunity (42, 45), which in turn may hinder Ad vectors' long-term efficacy. In addition, the progress in vector design has not eliminated the possibility of mobilization of HD Ad vector DNA following wild-type virus infection. Finally, HAd vectors are associated with a dose-dependent, transcription-independent acute innate inflammation (40).
To try to circumvent some of these drawbacks, we are continuing our analysis of the clinical potential of canine adenovirus type 2 (CAV-2) vectors (28, 30, 58, 59). CAV-2 vectors with E1 deleted (ΔE1) are, to the best of our knowledge, replication-defective in all cells (except the CAV-2 E1-transcomplementing cells), and are not significantly neutralized in vitro by most human sera containing anti-HAd5 neutralizing Ab (NAb) (30). Furthermore, no recombination or coreplication has been observed in human cells coinfected with HAd5 (27).
In this study, we tested CAV-2 vectors for their potential for gene transfer to the respiratory tract in humans. We found that CAV-2 vector transduction was efficient in vitro in human lung-derived cell lines, in vivo in the mouse respiratory tract, and ex vivo in primary cultures of well-differentiated human pulmonary epithelia. Notably, in vivo CAV-2 vector transduction efficiency was poorly inhibited in mice immunized with a HAd5 vector, despite the presence of relatively high levels of HAd5 NAb. CAV-2 vector intranasal instillation also led to a lower level of cytokine secretion and cellular infiltration compared to HAd5 vectors. While trying to optimize gene transfer, we found that we could increase transduction efficiency by pretreating mice with the histone deacetylase inhibitor FK228. Finally, we found that the duration of transgene expression in the murine respiratory tract could be increased to at least 3 months by using a helper-dependent CAV-2 (HDCAV) vector. Our data suggest that HDCAV vectors may be a clinically relevant option for gene therapy to the respiratory tract.
MATERIALS AND METHODS
Cell lines and vectors.
Canine DKCre (57) and human 911 (11) cells are E1-transcomplementing cell lines. A549 cells (human) present the same characteristics as type II alveolar epithelial cells (32). The bronchial epithelial cell line BEAS2B was generated from a healthy donor after autopsy and obtained after infection with the hybrid virus Ad12SV40 (50). All cell lines were grown as monolayer cultures in Dulbecco modified Eagle's minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) (GIBCO-BRL).
Adβgal, AdGFP, CAVβgal, and CAVGFP are HAd5- or CAV-2-derived vectors with deletions in the E1 region (ΔE1) and encode β-galactosidase or enhanced green fluorescent protein (EGFP) downstream of the immediate early cytomegalovirus (CMV) promoter (30). CAVDsRed (29) is a ΔE1 CAV-2 vector encoding DsRed2 (Clontech) from the CMV promoter (cloning details available upon request). Spike is a helper dependent (also known as high-capacity, or gutless) CAV-2 (HDCAV) vector encoding EGFP from the CMV promoter (59). Vectors were amplified in DKCre or 911 cells, purified by double CsCl density gradient centrifugation as previously described (30), and stored in 10% glycerol-phosphate-buffered saline (PBS) at −80°C.
All ΔE1 vectors (CAV-2 and HAd5) had titers of 5 × 1012 p.p./ml as determined by the optical density at 260 nm (OD260) as described previously (37). CAVGFP has a p.p./infectious particle ratio of 3:1. CAVDsRed had a p.p./infectious particle ratio of 20:1. AdGFP and Adβgal have p.p./infectious particle ratios of 10:1. Spike (HDCAV-GFP) had titer of 2 × 1011 p.p./ml and a p.p./infectious particle ratio of 10:1. Spike was amplified using the helper vector JBΔ5 (59), which contains a lacZ expression cassette. The helper vector contamination varied between 1 and 10% infectious particles for Spike preparations. This corresponds roughly to ∼1.0 to 0.1% contaminating PFU (59). For the titration of infectious particle of CAV-2 vectors, 105 DKCre cells were seeded in 12-well plates and incubated overnight with gentle rocking with serial dilutions starting from 10 p.p./cell. Twenty-four hours postinfection, the cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson) and the percentage of EGFP-positive cells was determined and used to calculate the infectious particles concentration as described previously (30). AdGFP was similarly titrated on 911 cells.
Viral transduction of human airway epithelia.
Airway epithelial cells were obtained from surgical polypectomies of non-cystic fibrosis patients or from trachea and bronchi of lungs removed for organ donation. Cells were isolated by enzyme digestion as previously described (74). Freshly isolated cells were seeded at 5 × 105 cells/cm2 on collagen-coated, 0.6-cm2 diameter Millicell polycarbonate filters (Millipore Corp., Bedford, MA). The cells were maintained at 37°C in a humidified atmosphere of 7% CO2/air. Twenty-four hours postplating, the mucosal medium was removed and the cells were allowed to grow at the air-liquid interface. The culture medium consisted of a 1:1 mix of DMEM-Ham's F-12, 5% Ultroser G (Biosepra SA, France), 100 U/ml penicillin, 100 μg/ml streptomycin, 1% nonessential amino acids, and 0.12 U/ml insulin.
To compare the transduction efficiency of AdGFP with CAVGFP, we incubated the epithelia with 2.5 × 103 p.p. in 5 μl. Following the indicated time, the vector suspension was removed and the epithelia were rinsed twice with PBS. The epithelia were incubated at 37°C for an additional 72 h before quantification of gene transfer by flow cytometry.
For the neutralizing assay, human serum containing anti-HAd5 Ab to all 3 major external capsid proteins was used (not shown). This serum was chosen because it had the highest neutralizing activity on 293 cells (1:10,000 dilution) of six sera tested. The human airway epithelia were incubated with the serum (only on the basolateral side) for 24 h. Epithelia transport both immunoglobulin G (IgG) and IgA to the thin layer of liquid that covers the apical surface of the epithelia and may prevent the epithelia from wild-type HAd infection (60).
In vivo gene transfer.
Six- to eight-week-old male C57BL/6J mice were purchased from the Centre d'Elevage R. Janvier (Le Genest Saint-Isle, France). All animal procedures were carried out according to the European Communities Council directive (86/609/EEC) and convention (ETS123) issued in 1986.
For intranasal (i.n.) instillation, mice were lightly anesthetized under methoxyflurane vapor (Isoflurane) and 1 × 1010 p.p. of either Adβgal or CAVβgal in 50 μl of 10% glycerol-PBS, pH 7.4, using a micropipette. Mice recovered within minutes and remained active after the procedure. For intravenous (i.v.) delivery, mice were injected via the tail vein in a total volume of 100 μl. In some cases, blood was collected by retro-orbital puncture and serum was obtained after 1 h of incubation at room temperature by centrifugation at 400 × g for 15 min and frozen at −20°C until further assay. At a predetermined time, mice were killed by cervical dislocation under methoxyflurane anesthesia.
The lungs destined for histological analysis (n = 3/group) were fixed with 4% paraformaldehyde-PBS cardiac perfusion and placed in 40% sucrose overnight. The fixed tissues were embedded in OCT (Tissue-Tek) and sliced using a cryostat (Jung CM3000; Leica). Sections (30 μm) were mounted on glass slides using Fluoprep (BioMerieux, Marcy l'Etoile, France) and analyzed by fluorescence microscopy (Zeiss Axiovert 200) or confocal microscopy (Zeiss Axioplan 2 with an LSM 510 25× oil immersion objective). Nuclei were counterstained with TO-PRO-3-iodide (Molecular Probes, Eugene, OR), and slides were mounted using Fluoprep.
Neutralization assays.
Anti-HAd5 neutralizing antibodies were quantified in the mouse serum collected on day −1 and day 30 postinstillation by evaluating the ability of the sera to inhibit transduction of 911 cells by AdGFP. Monolayers of 911 cells were seeded at 105/well in 12-well plates 24 h before infection with increasing ratios of p.p./cell of AdGFP. The 30% tissue culture infective dose (TCID30), which corresponds to 30% of EGFP-positive cells 24 h postinfection, was determined by flow cytometry. The TCID30 of AdGFP was 10 p.p./cell on 911 cells, and the TCID30 of CAVGFP was 10 p.p./cell on DKCre cells. We used a TCID30 because this corresponds to 1 infectious particle/GFP+ cell, and a twofold decrease in infectious particle/cell corresponds approximately to a twofold reduction in GFP+ cells.
For the neutralization test, we used 1 or 2 μl of decomplemented (56°C, 30 min) serum from mice injected i.v. or i.n. with HAd vector, respectively. AdGFP or CAVGFP (TCID30) was incubated with serum for 20 min at room temperature in serum-free DMEM in a final volume of 20 μl. The vector-serum mixture was added to 0.5 ml of complete medium and then incubated with a monolayer of 911 cells for 3 h at 37°C, and the medium was replaced with fresh 10% FCS-DMEM. Fluorescence levels were measured using flow cytometry 24 h postinfection. Controls using human anti-HAd5 serum and canine anti-CAV-2 serum were also included. Titration of NAb was performed using serial twofold dilutions of serum in FCS-free DMEM. The highest reciprocal serum dilution resulting in a reduction of at least 50% was considered the vector NAb titer. All serum were decomplemented by heating to 56°C for 15 min.
Quantification of β-Gal activity.
The lungs were harvested after intracardiac perfusion with PBS, frozen in liquid nitrogen, and homogenized with mortar and pestle in 1 ml of 100 mM H2KPO4, 100 mM HK2PO4, and 1 mM dithiothreitol. The homogenate was centrifuged for 10 min at 15,000 × g to remove particulate material, and the supernatant was frozen at −80°C. β-Galactosidase (β-Gal) activity in lung homogenate was assayed using a luminescent assay according to the manufacturer's instructions (Luminescent β-galactosidase detection kit II; BD Biosciences-Clontech) and a Microlumat LB96P luminometer (EG & G Berthold). Activity was corrected for tissue lysate protein concentration (Bradford reagent; Sigma-Aldrich Chemical Co.) and was expressed as relative light units/μg of protein.
Inflammatory cells and cytokines in BALF.
Bronchoalveolar lavage fluids (BALF) were obtained while the lung was in the thoracic cavity by making a thin incision in the trachea, cannulating it, rinsing three times with 1 ml of ice-cold 2 mM EDTA-PBS, and pooling. BALF cells were pelleted by centrifugation at 500 × g and resuspended in 1 ml of PBS, and 100 μl of cell suspension was mixed in 100 μl of 0.4% trypan blue and counted with a hemocytometer. The cell-free BALF supernatants were split into 300-μl aliquots and frozen at −80°C for cytokine analysis. The concentration of murine tumor necrosis factor alpha (TNF-α) in the BALF was determined using commercial enzyme-linked immunosorbent assay kits (Genzyme Immunologicals, Cambridge, MA).
Statistical Analysis.
All data are presented as means ± standard errors of the means (SEM). P values were calculated using one-way statistical analysis of variance with Newman-Keuls tests.
RESULTS
CAV-2 vectors in respiratory epithelia.
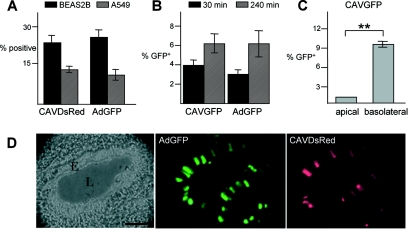
Initially, we assayed CAV-2-mediated gene transfer in two human pulmonary cell lines: BEAS2B (bronchial) and A549 (alveolar) cells. After incubation with CAVDsRed (a CAV-2 vector harboring a DsRed expression cassette) or AdGFP (a HAd5 vector harboring an EGFP expression cassette), cells were analyzed by flow cytometry. Each vector transduced human pulmonary cell lines with similar efficiency (Fig. 1A).
FIG. 1.
Efficacy of CAV-2 vectors in lung tissue. (A) Transduction of human pulmonary cell lines with HAd5 and CAV-2 vectors. The transduction efficiency of CAV-2 and HAd5 vectors was assayed in BEAS2B (epithelial, bronchial) and A549 (epithelial, alveolar) cells. Cells were incubated with CAVDsRed or AdGFP (50 infectious particles/cell) and analyzed by flow cytometry 24 h later. (B) Transduction efficiency of well-differentiated human airway epithelia. Bronchial epithelium was reconstituted in vitro from human primary lung cells after culturing at the medium-airway interface. These cells reconstitute a well-differentiated epithelia, with tight junctions and a basolateral and apical surface. Epithelia were infected with 2.5 × 103 p.p. of either CAVGFP or AdGFP on the apical surface for 30 or 240 min, and cells were analyzed by FACS 72 h postinfection (n = 8). (C) Polarity of human airway epithelium infection with CAV-2. Epithelia were incubated with 2.5 × 103 p.p. of CAVGFP on either the apical or basolateral surface for 30 min. Flow cytometry analysis was performed 48 h later (n = 4). **, P < 0.01. (D) Coinstillation in the mouse respiratory tract. CAVDsRed and AdGFP (5 × 1010 p.p.) were codelivered to 6-week-old C57BL/6 mice by i.n. instillation (n = 4). The lungs were recovered 6 days postinfection. The figure shows an example of a bronchiole in cross-section in which epithelial cells were transduced by CAVDsRed and AdGFP. E, epithelium; L, lumen. Bar, 50 μm.
We then assayed CAV-2 vector transduction efficiency in well-differentiated human airway epithelia, a more clinically relevant model. Bronchial epithelium was reconstituted in vitro from human primary lung cells after culturing at the air-liquid interface. Airway epithelia were allowed to differentiate and to reach confluence by culturing for at least 14 days. Epithelia were tested for transepithelial electrical resistance (Rt) and for morphology by scanning electron microscopy (not shown). All epithelia had Rt values of >500 Ω cm2, indicating the development of tight junctions and an intact barrier (67). To compare the transduction efficiency of AdGFP with CAVGFP (a CAV-2 vector harboring the EGFP expression cassette), we incubated the epithelia with an equal number of vector particles on the apical surface. Two time points were assayed, and transduction was quantified by flow cytometry. Under these conditions in mature epithelia, CAVGFP and AdGFP showed an equal transduction efficiency that increased upon prolonged incubation time (Fig. 1B), in agreement with previous observations using HAd5 vectors (74).
Previous data suggested that the primary receptor for CAV-2 and species C HAd is CAR (coxsackie and adenovirus receptor) (4, 56), which in polarized epithelia traffics to the basolateral membrane (9, 46). When CAVGFP was applied at the basolateral surface of the epithelia, transduction efficiency significantly increased (Fig. 1C). These data support our previous results suggesting that CAR is the primary attachment molecule for CAV-2 (56) and demonstrating the polarity of the in vitro-reconstituted epithelia (67).
We then compared the efficiency of CAV-2 and HAd5 vectors in vivo, in mouse respiratory airways. Mice were coinfected with CAVDsRed and AdGFP by i.n. instillation and sacrificed, and the lungs were screened for fluorescence. In all mice, we observed equal DsRed and EGFP expression throughout both lobes of the lung, from the upper airway (Fig. 1D) to the alveoli (not shown). Due to the unsaturating conditions in this assay, we do not believe that there was a significant level of competition for the receptor (e.g., CAR) binding. Taken together, our data demonstrated that CAV-2 vectors efficiently transduced pulmonary cells in vitro, ex vivo, and in vivo.
Effect of memory anti-HAd5 humoral immunity on CAV-2 vector transduction efficiency.
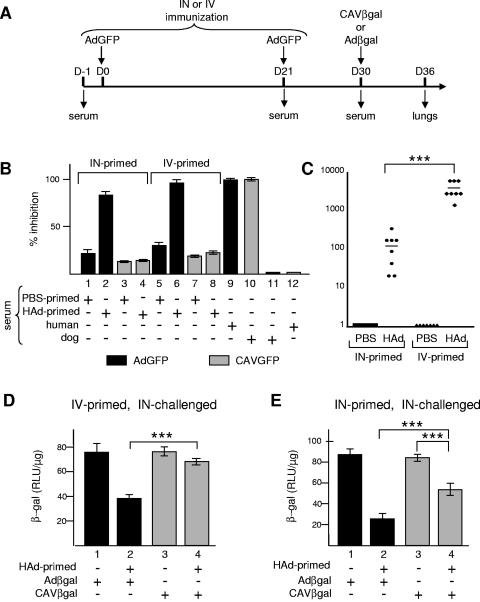
HAd-derived vectors (in particular serotypes 2 and 5) have had relatively disappointing transduction efficiencies in the human respiratory tract, possibly in part because of a widespread memory humoral immunity (neutralizing and opsonizing antibodies) (10, 73). In contrast, we previously found that only 2% of a random cohort had a detectable level of anti-CAV-2 NAb (30). To investigate whether the humoral immunity to HAd5 could be circumvented using CAV-2 vectors, we immunized mice either via i.n. instillation or via i.v. injection with AdGFP according to the experimental protocol described in the legend to Fig. 2A. After development of the HAd5-specific humoral response, animals were inoculated by i.n. instillation with CAVβgal, a ΔE1 CAV-2 vector encoding β-Gal, or Adβgal, a HAd5 vector with the same expression cassette. The lungs were recovered 6 days postinfection. To determine transduction efficiency, we used β-Gal activity as the readout.
FIG. 2.
Affect of anti-HAd5 humoral immunity on CAV-2 vector transduction efficiency in vivo. (A) C57BL/6 mice (n = 4/group) were primed i.v. or i.n. with AdGFP (5 × 1010 p.p. i.n. or 2 × 1011 p.p. i.v.) or PBS on days 0 and 21. Mice were then instilled i.n. with either Adβgal or CAVβgal (5 × 1010 p.p.) on day 30. The mice were sacrificed 6 days later. Lungs were recovered after intracardiac perfusion and bronchoalveolar lavage with PBS. Blood was also collected at days −1, 21, 30, and 36. On day 6, an i.n. primed mouse was sacrificed to confirm the efficacy of AdGFP transduction in the lung. We found EGFP-positive cells throughout the respiratory airways (not shown). Mock priming and i.n. instillations were done with an equivalent volume of 10% glycerol-PBS. (B) Neutralization assays. The level of HAd5-specific or cross-reacting NAb was assayed in the sera obtained from mice immunized with 1 μl i.v. or 2 μl i.n. at day 30. For HAd5-neutralization assay, 911 cells were infected with AdGFP previously incubated with a fixed volume of serum and analyzed by flow cytometry 24 h postinfection. Cells were infected with 10 p.p. of AdGFP/cell; a multiplicity of infection that results in approximately 30% of EGFP-positive cells 24 h postinfection. For cross-neutralization assays, DKCre cells were infected with CAVGFP previously incubated with sera. Human and dog sera containing NAb against HAd5 or CAV-2 were used as controls in each case. The results are shown as the percentages of transduction inhibition of each vector. Each bar represents the mean from the cohort (n = 8). (C) NAb titers from i.n. and i.v. primed mice. 911 cells were infected with AdGFP, incubated with serial dilutions of sera, and analyzed by flow cytometry 24 h postinfection. Relative NAb titers are expressed as the highest reciprocal serum dilution resulting in reduction of transduction by at least 50%. Titers are the mean values of the results from three independent experiments. The bar is the mean titer value for each cohort (n = 8). (D and E) Effect of anti-HAd5 humoral immunity against on CAV-2 transduction efficiency in mouse lung. The level of β-Gal activity in the lung of mice primed i.v (D) or i.n. (E) with AdGFP or PBS and i.n. instilled with Adβgal or CAVβgal. Each bar represents the mean value of the results for four animals ± standard error of the mean. RLU, relative light units; +, present; −, absent; ***, P < 0.001.
We first checked whether AdGFP-inoculated mice developed anti-HAd5 NAb using an in vitro assay based on transduction of 911 cells. Vector neutralization was assessed by the reduction in the percent of EGFP-positive cells following preincubation of the vector with a fixed volume of sera. Mouse sera did not contain HAd5-NAb before immunization (not shown). All mice immunized i.v. or i.n. with AdGFP developed NAb (Fig. 2B). When serum from i.v. primed mice was tested, we observed a 97% inhibition of AdGFP transduction (Fig. 2B, lane 6) in contrast to the control (Fig. 2B, lane 5). Serum from i.n. primed mice also contained HAd5 NAbs, although the level of AdGFP inhibition was lower (83%) (lane 2), but still highly significant (P < 0.001), than that of the control (lane 1). To assay for the cross-neutralization of anti-HAd5 antibodies, we repeated the above experiment but substituted CAVGFP for AdGFP and DKCre for 911 cells. Serum from immunized mice did not block CAVGFP transduction more than control serum (Fig. 2B, lanes 4 versus 3 and 8 versus 7), demonstrating no significant cross-neutralization in this assay. Cross-neutralization was also tested using human and dog sera: complete inhibition of AdGFP and CAVGFP transduction was achieved using serum from the respective host (lanes 9 and 10, respectively). Notably, dog serum did not neutralize AdGFP and, as previously shown, human serum did not neutralize CAVGFP (lanes 11 and 12, respectively). We also found that serum from a nonhuman primate (Microcebus murinus) did not neutralize CAVGFP or AdGFP (not shown).
We then quantified the NAb titer using twofold serial dilutions of each serum in our in vitro assay. The highest reciprocal serum dilution resulting in reduction of vector transduction inhibition by at least 50% was considered the NAb titer. For animals i.v. primed with AdGFP, the mean HAd5 NAb titer was 3,360. Following i.n. priming, the mean HAd5 NAb titer was 120 (Fig. 2C). These data are consistent with those from Kanesaki et al. and Meitin et al. (24, 36), who showed that the efficacy of the humoral response depends on the route of inoculation: i.n. instillation led to a local production of Ab, whereas i.v. injection resulted in a stronger systemic response. In serum from control mice in our study, none reached 50% inhibition of AdGFP transduction, and therefore, their titer was defined as 1 (Fig. 2C).
Finally, after i.n. instillation of CAVβgal or Adβgal, we quantified gene transfer to the respiratory tract using β-Gal activity as the readout. When mice were i.n. instilled with Adβgal, β-Gal activity was up to threefold lower (versus controls) in AdGFP i.v. or i.n. primed mice (Fig. 2D and E, lanes 1 and 2) (P < 0.001). In contrast, β-Gal activity following CAVβgal instillation in AdGFP i.v. primed mice was similar to that of control mice (Fig. 2D, lane 4), demonstrating that i.v. immunization with AdGFP had little effect on CAV-2 transduction efficiency in the mouse respiratory tract. However, CAVβgal transduction efficiency was slightly affected (1.5-fold reduction) (P < 0.001) by i.n. immunization (Fig. 2E, lane 4), although less than for Adβgal. This was possibly due to local production of IgA, which is less specific and involved in virus cross-reactivity (66).
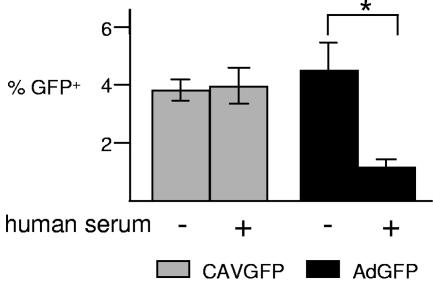
To simulate a potential clinical scenario, we incubated CAVGFP or AdGFP with well-differentiated human airway epithelia at the apical surface, after preincubation or not with a human serum containing a high titer of anti-HAd5 NAb, and compared the transduction efficiency by flow cytometry. Preincubation with human serum inhibited AdGFP transduction of human airway epithelia by 3.6-fold (P < 0.05), whereas CAVGFP transduction was unaffected (Fig. 3).
FIG. 3.
Effect of NAb on transduction of human airway epithelia with HAd5 and CAV-2. CAVGFP or AdGFP (2.5 × 103 p.p.) was added to the apical surface of well-differentiated human airway epithelia in normal medium or to epithelia in medium preincubated for 24 h with a 1:100 dilution of human serum. Flow cytometry analysis was performed 72 h postinfection. +, present; −, absent.
Together, these data demonstrated that in most cases anti-HAd5 NAb do not significantly alter CAV-2 transduction in vivo or ex vivo and suggest that the widespread memory humoral immunity against HAd5 could be circumvented by CAV-2 vectors. Our results suggest that the potential vector neutralization caused by virus-specific NAb could be avoided using CAV-2-derived vectors.
Helper-dependent CAV-2 vectors allow long-term transgene expression in the respiratory tract.
Although CAV-2 vectors could circumvent the memory humoral immunity, this does not address the problem posed by the adaptive cellular immune response directed against the transduced cells expressing the residual viral genes present in ΔE1 vectors (69, 70). This CD8+ cytotoxic T cell response, which eliminates transduced cells, begins 5 to 7 days postinfection and partially explains why ΔE1 CAV-2 vectors did not lead to significant transgene expression for greater than 3 weeks postinstillation (30). HD Ad vectors, void of viral coding sequences, retain the advantages of Ad vectors (including high-efficiency in vivo transduction and high-level transgene expression). Furthermore, HD vector transduction led to a reduced cell-mediated adaptive immune response in immunologically naive animals and led to a significant increase in the duration of transgene expression in many tissues and in many animal models (1, 29, 44, 59).
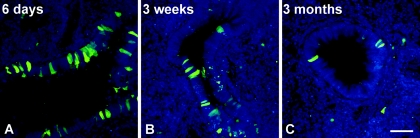
We therefore assayed whether an HDCAV vector could lead to long-term transgene expression in the mouse respiratory tract. C57BL/6 mice were i.n. instilled with Spike, a helper-dependent CAV-2 vector encoding EGFP (59). Mice were sacrificed 6 days, 3 weeks, or 3 months postinstillation, and EGFP expression was assayed in lung sections by fluorescence microscopy. We found similar EGFP expression (in terms of intensity and number of cells) in bronchial epithelial cells 6 days and 3 weeks postinstillation (Fig. 4A and B). More notably, we detected EGFP expression in the bronchial epithelia 3 months postinstillation (Fig. 4C). As expected, we found fewer EGFP-positive cells at 3 months than after 3 weeks, reflecting the natural turnover of pulmonary epithelial cells in vivo. In alveolar epithelial cells, we detected EGFP expression at 6 days and 3 weeks postinstillation (although there were fewer cells at 3 weeks than at 6 days). No EGFP-positive alveolar cells were found at 3 months (not shown). In our previous experiments (30), we used 8-week-old BALB/c mice to test the efficacy of CAV-2 vectors in the mouse lung. To determine if the prolonged duration of transgene expression from Spike was due to different mouse strains and/or age, we instilled CAVGFP and/or CAVβgal in age-matched C57BL/6 mice. Mice (n = 3/time point) were sacrificed at 1, 3, or 5 weeks postinstillation. Similar to our previous results, essentially no transgene expression was detected at 3 (or 5) weeks (see Fig. S1 in the supplemental material). The advantage of GFP as a transgene is that it is easily detected; however, quantification of transgene delivery is difficult. Therefore, in mice instilled with CAVβgal, we quantified β-Gal activity as described above. Similar to our results with EGFP as a transgene, we found significant β-Gal activity at 1 week postinstillation but no significant difference between control mice (PBS instilled) and CAVβgal-instilled mice sacrificed at 3 weeks (see Fig. S2 in the supplemental material). Altogether, these data demonstrated that the HDCAV vector could generate long-term transgene expression in the mouse upper respiratory tract.
FIG. 4.
Helper-dependent CAV-2 vector allows long-term expression of the transgene in the mouse respiratory tract. C57BL/6 mice (n = 3/group) received 5 × 1010 p.p. of Spike by i.n. instillation. Mice were sacrificed 6 days (A), 3 weeks (B), or 3 months (C) postinfection, and the lungs were recovered after fixation by intracardiac perfusion using 4% paraformaldehyde-PBS. Nuclei are in blue. Bar, 20 μm.
Equal doses of CAV-2 vectors are less inflammatory than HAd5 vectors.
In addition to inducing adaptive anti-capsid and anti-transgene immunity, HAd vectors activate the innate arm of the immune system (38). The acute local inflammation triggered by high doses of HAd vectors can limit gene transfer efficiency and can result in adverse side effects. In mice and cotton rats, inflammation due to HAd transduction mimics that seen in humans (12, 48). In the lung, alveolar macrophages efficiently take up vectors and release proinflammatory cytokines such as TNF-α, interleukin 6 (IL-6), IL-1, and gamma interferon within the first 6 hours postinfection (72, 76). This leads to the rapid recruitment of neutrophils, natural killer cells, and macrophages to the site of transduction (39, 75). In contrast to the adaptive response, the Ad-induced innate response is transcription independent (33, 44). Although adenoviruses induce the innate response in many ways, it may be initiated by the capsid endocytosis that follows the low-affinity interaction between integrins (e.g., αv, αL, αM and β1, β2, β3, β5 dimers) and an arginine-glycine-aspartic acid (RGD) motif present in the penton base protein of most HAd (14). Another point of induction is probably endosomal escape, but little is currently known concerning the differences between CAV-2 and HAd5 intracellular trafficking (7). The three major external capsid proteins (fiber, penton base, and hexon) of CAV-2 do not contain known integrin-interacting motifs (7, 56), and therefore, CAV-2's mode of internalization may differ from that of species C HAd, suggesting that CAV-2 vectors may not activate the innate immune response like that of HAd. To test the effect of CAV-2, we compared the inflammatory reaction induced after CAVβgal or Adβgal i.n. instillation. We measured the TNF-α concentration and quantified the infiltrating cells in the BALF.
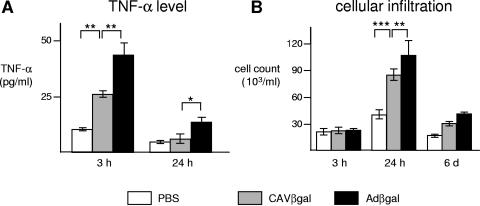
Both CAVβgal and Adβgal i.n. instillations induced TNF-α secretion within 3 h, which was still detectable 24 h postinfection (Fig. 5A). Both vectors also induced cell migration into mice lung airways 24 h postinstillation compared to mock-treated mice. Cellular infiltration was still present 6 days postinstillation (Fig. 5B). However, HAd induced 1.7-fold more TNF-α secretion than CAV-2 (P < 0.05) and 1.3-more more cellular infiltration (P < 0.05) at 3 and 24 h postinstillation, respectively. We observed a local inflammation characterized by peribronchiolar cuffs, mainly composed of lymphocyte infiltration, 6 days postinstillation of AdGFP but not with Spike (see Fig. S3 in the supplemental material). In addition, we saw no obvious signs of inflammation at 3 weeks or at 3 months. These data are consistent with long-term expression from the HDCAV vector and similar to the results of Toietta et al. using HD Ad vectors (64). Our data thus showed that CAV-2 activation of the innate immune response in the mouse lung is detectable but less than that of HAd5 vectors.
FIG. 5.
CAV-2 vectors are less inflammatory than HAd5 in the mouse respiratory tract. C57BL/6 mice (n = 4/group) received 1011 p.p. of either Adβgal or CAVβgal by i.n. instillation. Mice were sacrificed 3 h, 24 h, or 6 days postinfection, and the BALF were recovered. The inflammation induced by i.n. inoculation was monitored by measuring the TNF-α levels (A) or counting the total cells (B) in mouse BALF. Data are expressed as means ± standard errors of the means. **, P < 0.01; ***, P < 0.001.
FK228 increases CAV-2 transduction efficiency in mouse lung.
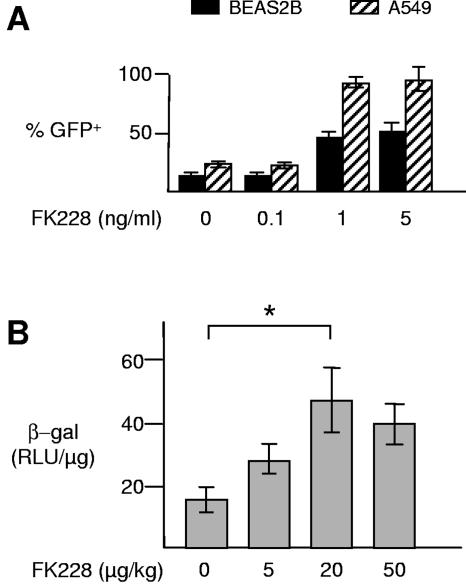
If one could obtain equal or greater transgene expression using fewer input vector particles, one may be able to reduce the dose-dependent, acute inflammatory reaction seen in most tissues and animal models. Thus, in addition to using CAV-2 vectors, we tried to optimize the transduction efficiency in the respiratory tract. One possible approach is to transiently increase the expression level of CAR (51). We therefore evaluated the ability of FK228 (also known as FR901228 or depsipeptide), a naturally occurring polypeptide isolated from Chromobacterium violaceum (65), to increase transduction efficiency in the respiratory tract. FK228 is currently in phase II clinical trials for cutaneous T-cell lymphoma (34, 47, 54). FK228 treatment leads to increased cell surface levels of CAR and αv integrins in human lymphocytes and, as a consequence, improves the transduction efficiency of HAd5 vectors (25, 26). Initially, we preincubated BEAS2B or A549 cells with three concentrations of FK228 prior to infection with CAVGFP. The percentage of EGFP-positive cells (as well as the mean fluorescence, not shown) reached a plateau between 1 and 5 ng FK228/ml with an increase of 3.4-fold in BEAS2B cells and 4-fold in A549 (Fig. 6A). One nanogram of FK228 per milliliter was also less cytotoxic (not shown). It is likely that the increase in mean fluorescence was due to a greater number of particles being internalized per transduced cells as well as an increase in transgene expression (13).
FIG. 6.
Effect of FK228 pretreatment on CAV-2 vector transduction efficiency in lungs. (A) BEAS2B and A549 human pulmonary cells were treated with FK228 (0 to 5 ng/ml) for 24 h before infection with CAVGFP (50 or 100 infectious particles/cell, respectively). Cells were analyzed by flow cytometry 24 h postinfection. (B) C57BL/6 mice were treated with FK228 (0 to 50 μg/kg) by i.n. instillation 24 h before i.n. inoculation with CAVβgal (5 × 1010 p.p.). Lungs were then recovered after intracardiac perfusion with PBS, and β-Gal activity was measured using a luminescent assay and normalized with the concentration of proteins in each homogenate. β-Gal activity in lungs from PBS- and FK228-treated mice was not significantly different (not shown). Each bar represents the mean ± standard error of the mean (n = 4/group). RLU, relative light units; *, P < 0.05.
Finally, we measured the effect of the pretreatment of the mouse respiratory tract with FK228 on the transduction efficiency. C57BL/6 mice were pretreated by i.n. instillation of FK228 24 h before i.n. instillation with CAVβgal. Mice were sacrificed 48 h postinstillation, and the lungs were recovered and assayed for β-Gal activity. We found that 20 μg/kg of FK228 increased CAVβgal transduction efficiency by threefold (P < 0.05) (Fig. 6B). We thus showed that FK228 increased CAV-2 transduction efficiency in the respiratory epithelia both in vitro and in vivo. These preliminary experimental results strongly suggest that a detailed analysis varying FK228 doses, time of instillation, and the number of physical particles instilled could lead to improved and long-term gene transfer efficacy.
DISCUSSION
One of the obstacles to successful clinical gene transfer using vectors derived from ubiquitous human pathogens (e.g., adenovirus, adeno-associated virus, or herpes simplex virus) relates to memory immunity that limits effective therapy. Furthermore, an acute inflammatory reaction in response to high doses of vectors can result in serious adverse side effects or death (49). As a potential solution to some of these obstacles, we continued the evaluation of the clinical relevance of vectors derived from the canine adenovirus serotype 2 for gene transfer to the respiratory tract. We showed that, in most cases, CAV-2 vectors efficiently transduced mice and human respiratory epithelia and were poorly inhibited by sera containing HAd5 neutralizing antibodies. We also found that CAV-2 vectors were less inflammatory than HAd5 vectors and that the transduction efficiency in the mouse lung can be increased using FK228. Finally, the use of a helper-dependent CAV-2 vector led to long-term transgene expression in the mouse lung.
One aim of this study was to determine whether the ubiquitous humoral immunity against human Adenoviridae could be circumvented using CAV-2 vectors. To mimic this immunity, we immunized mice with a vector derived from the HAd5, which is one of several serotypes that is prevalent in most populations (10, 53). In vitro CAV-2 vector transduction was not neutralized by the NAb developed in i.v. immunized mice, and only weakly transduced by the NAb developed in i.n. immunized mice. Moreover, the in vivo CAVβgal transduction efficiency was not affected by the humoral response of i.v. primed mice and only partially affected in the case of i.n. priming. Our observations are consistent with the type of response observed according to the route of inoculation: i.v. immunization results in a systemic immune response, whereas i.n. immunization leads to a higher IgA response, which is less specific and involved in NAb cross-reactivity (24, 36, 66). Our data suggest that CAV-2 vectors may avoid the memory humoral immunity against HAd.
However, because of the continuous renewal of airway epithelial cells (life span is ∼120 days in humans) (3), it is likely that multiple instillations will be required to maintain a sufficient number of transduced cells, (e.g., 5 to 10% to overcome the electrophysiological defect in cystic fibrosis) (23). In this context, successful readministration of most vectors could be prevented by neutralizing or opsonizing Abs induced after their first application. Thus, strategies involving immunomodulation, induction of tolerance, or modification of viral capsids need to be incorporated into vector delivery (55). Although most of these approaches are not unique to CAV-2 vectors, immunotolerance to CAV-2 capsid proteins would be much safer than a similar approach for HAds. HAd infections can be lethal in severely immunocompromised individuals and newborns (19). There are encouraging reports regarding the successful readministration of HAd vectors in the mouse muscle (17), liver (22, 71), pancreas (35), and respiratory tract (8, 71). These studies also underscore an important criterion: suppression of the adaptive immune response is more efficient when the host is immunologically naive. This will rarely be the case when using most HAd or adeno-associated virus vectors. We also predict that an anti-Ad memory T cell response that is found in most humans (45), which is poorly blunted by common (e.g., cyclosporine A or FK506) immunosuppression regimes (21), in combination with a vector-induced innate immune response, will lead to acute deleterious side effects in some patients.
We showed that CAV-2 vectors induced a lower innate response than HAd5 vectors, based on TNF-α secretion and acute cellular infiltration in the mouse lung. It would be advantageous to limit further the dose-dependent inflammation by using lower titers without reducing the global transgene expression level. This may be achieved by a pretreatment with the histone deacetylase inhibitor FK228, which increased CAV-2 vector transduction efficiency in vivo. There is, however, an important caveat: our encouraging preliminary data do not address the potential modification(s) (either an increase or decrease) of the innate and inflammatory response when FK228 and vector-mediated gene transfer are combined. Although it is beyond the scope of this study to address how FK228 increased transduction efficiency in the respiratory tract (e.g., activation of CAR transcription, improved CAR trafficking, activation of transgene transcription, disruption of epithelial junctions), future studies must also concentrate on the characterization of the immune response following the combination of FK228 and gene transfer.
Zsengeller et al. showed that, in the respiratory tract, resident alveolar macrophages internalize HAd5 vectors and upregulate the inflammatory cytokines IL-6 and TNF-α within 10 min postinstillation (76). HAd trafficking through the cell activates signaling pathways and induces the expression of various cytokines and proinflammatory genes in cells of the innate immune system (such as macrophages and monocytes) and in nonimmune targets (such as epithelial cells) (14, 38). Studies using UV/psoralen-inactivated HAd particles highlighted the role of the viral capsid proteins in the induction of the signaling cascade (20). Some, but not all, of the intracellular signaling is triggered by the interaction of the viral particle with integrins (14, 38). In many HAd5-transducible cells, a low-affinity interaction between the penton base RGD motif and integrins can mediate virus internalization by endocytosis through clathrin-coated vesicles (31, 68). In epithelial cells, HAd5 internalization induces upregulation of gamma interferon, inducible protein 10 (IP-10), RANTES, and IL-8 (2, 6, 63). Because of the inherent differences of the CAV-2 capsid (10-fold less negatively charged than HAd5 [56] and no known integrin-interacting motif) we hypothesized that CAV-2 vectors could trigger different intracellular signaling cascades. These differences could play a role in internalization and/or endosome acidification and escape (5, 62, 63). Notably, it is unlikely that the capsid-integrin interaction is exclusively responsible for cytokine induction: studies using competing RGD peptides or RGD deletion vectors demonstrated that RGD-dependent interactions play a role in the activation of inflammatory pathways in epithelial cells. In addition, although the mouse is an important and useful model, it does not ipso facto correspond to the response that will occur in human cells/tissues. For example, we have found notable differences between CAV-2 and HAd5 on the maturation of human monocyte-derived dendritic cells but little difference when incubated with murine bone marrow-derived dendritic cells (E. J. Kremer, unpublished data).
In summary, HDCAV vectors have the capacity to overcome the humoral immunity of viral vectors, allow long-term expression in the lung, and are less inflammatory than HAd5 vectors. CAV-2 vectors may be a clinically viable alternative, in combination with other therapies, for some inherited or congenital lung diseases.
Supplementary Material
Acknowledgments
We thank the association Vaincre la Mucoviscidose (VLM), Fondation pour la Recherche Medicale, Vaincre les Maladies Lysosomales, Association Française contre les Myopathies and the Association pour la Recherche sur le Cancer for financial support. A.K. was a VLM postdoctoral fellow, and E.J.K. is an Inserm Director of Research.
We are grateful to Isabelle Serre (Lapeyronie Hospital, Montpellier) for histopathological analysis, Chantal Jacquet and Yannick Fraisse for help with the in vivo assays, and Marie-Catherine Romey for BEAS2B and A549 cell lines. FK228 was a generous gift from the Fujisawa company (Tokyo, Japan). We thank our colleagues in the Institut de Génétique Moléculaire de Montpellier for their help, constructive comments during these studies, and critical reading of the manuscript. We have no conflicting financial interests.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Amalfitano, A., and R. J. Parks. 2002. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr. Gene Ther. 2:111-133. [DOI] [PubMed] [Google Scholar]
- 2.Amin, R., R. Wilmott, Y. Schwarz, B. Trapnell, and J. Stark. 1995. Replication-deficient adenovirus induces expression of interleukin-8 by airway epithelial cells in vitro. Hum. Gene Ther. 6:145-153. [DOI] [PubMed] [Google Scholar]
- 3.Ayers, M. M., and P. K. Jeffery. 1988. Proliferation and differentiation in mammalian airway epithelium. Eur. Respir. J. 1:58-80. [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Bowen, G. P., S. L. Borgland, M. Lam, T. A. Libermann, N. C. Wong, and D. A. Muruve. 2002. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-kappa B. Hum. Gene Ther. 13:367-379. [DOI] [PubMed] [Google Scholar]
- 6.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chillon, M., and E. J. Kremer. 2001. Trafficking and propagation of canine adenovirus vectors lacking a known integrin-interacting motif. Hum. Gene Ther. 12:1815-1823. [DOI] [PubMed] [Google Scholar]
- 8.Chirmule, N., A. Truneh, S. E. Haecker, J. Tazelaar, G. Gao, S. E. Raper, J. V. Hughes, and J. M. Wilson. 1999. Repeated administration of adenoviral vectors in lungs of human CD4 transgenic mice treated with a nondepleting CD4 antibody. J. Immunol. 163:448-455. [PubMed] [Google Scholar]
- 9.Cohen, C. J., J. Gaetz, T. Ohman, and J. M. Bergelson. 2001. Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J. Biol. Chem. 276:25392-25398. [DOI] [PubMed] [Google Scholar]
- 10.D'Ambrosio, E., N. Del Grosso, A. Chicca, and M. Midulla. 1982. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J. Hyg. (London) 89:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallaux, F. J., O. Kranenburg, S. J. Cramer, A. Houweling, H. Van Ormondt, R. C. Hoeben, and A. J. Van Der Eb. 1996. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum. Gene Ther. 7:215-222. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg, H., L. Moldawer, P. Sehgal, M. Redington, P. Kilian, R. Chanock, and G. Prince. 1991. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc. Natl. Acad. Sci. USA 88:1651-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldsmith, M., M. Kitazono, P. Fok, T. Aikou, S. Bates, and T. Fojo. 2003. The histone deacetylase inhibitor FK228 preferentially enhances adenovirus transgene expression in malignant cells. Clin. Cancer Res. 9:5394-5401. [PubMed] [Google Scholar]
- 14.Greber, U. F. 2002. Signalling in viral entry. Cell. Mol. Life Sci. 59:608-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griesenbach, U., S. Ferrari, D. M. Geddes, and E. W. Alton. 2002. Gene therapy progress and prospects: cystic fibrosis. Gene Ther. 9:1344-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griesenbach, U., D. M. Geddes, and E. W. Alton. 2004. Gene therapy for cystic fibrosis: an example for lung gene therapy. Gene Ther. 11(Suppl. 1):S43-S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guibinga, G. H., H. Lochmuller, B. Massie, J. Nalbantoglu, G. Karpati, and B. J. Petrof. 1998. Combinatorial blockade of calcineurin and CD28 signaling facilitates primary and secondary therapeutic gene transfer by adenovirus vectors in dystrophic (mdx) mouse muscles. J. Virol. 72:4601-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hege, K. M., and D. P. Carbone. 2003. Lung cancer vaccines and gene therapy. Lung Cancer 41(Suppl. 1):S103-S113. [DOI] [PubMed] [Google Scholar]
- 19.Hierholzer, J. C. 1992. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 5:262-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higginbotham, J. N., P. Seth, R. M. Blaese, and W. J. Ramsey. 2002. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum Gene Ther. 13:129-141. [DOI] [PubMed] [Google Scholar]
- 21.Ho, S., N. Clipstone, L. Timmermann, J. Northrop, I. Graef, D. Fiorentino, J. Nourse, and G. R. Crabtree. 1996. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 80:S40-S45. [DOI] [PubMed] [Google Scholar]
- 22.Ilan, Y., B. Sauter, N. R. Chowdhury, B. V. Reddy, N. R. Thummala, G. Droguett, A. Davidson, M. Ott, M. S. Horwitz, and J. R. Chowdhury. 1998. Oral tolerization to adenoviral proteins permits repeated adenovirus-mediated gene therapy in rats with pre-existing immunity to adenoviruses. Hepatology 27:1368-1376. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, L. G., J. C. Olsen, B. Sarkadi, K. L. Moore, R. Swanstrom, and R. C. Boucher. 1992. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 2:21-25. [DOI] [PubMed] [Google Scholar]
- 24.Kanesaki, T., B. R. Murphy, P. L. Collins, and P. L. Ogra. 1991. Effectiveness of enteric immunization in the development of secretory immunoglobulin A response and the outcome of infection with respiratory syncytial virus. J. Virol. 65:657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitazono, M., M. E. Goldsmith, T. Aikou, S. Bates, and T. Fojo. 2001. Enhanced adenovirus transgene expression in malignant cells treated with the histone deacetylase inhibitor FR901228. Cancer Res. 61:6328-6330. [PubMed] [Google Scholar]
- 26.Kitazono, M., V. K. Rao, R. Robey, T. Aikou, S. Bates, T. Fojo, and M. E. Goldsmith. 2002. Histone deacetylase inhibitor FR901228 enhances adenovirus infection of hematopoietic cells. Blood 99:2248-2251. [DOI] [PubMed] [Google Scholar]
- 27.Klonjkowski, B., P. Gilardi-Hebenstreit, J. Hadchouel, V. Randrianarison, S. Boutin, P. Yeh, M. Perricaudet, and E. J. Kremer. 1997. A recombinant E1-deleted canine adenoviral vector capable of transduction and expression of a transgene in human-derived cells and in vivo. Hum. Gene Ther. 8:2103-2115. [DOI] [PubMed] [Google Scholar]
- 28.Kremer, E. J. 2004. CAR chasing: canine adenovirus vectors-all bite and no bark? J. Gene Med. 69(Suppl. 1):S139-S151. [DOI] [PubMed] [Google Scholar]
- 29.Kremer, E. J. 2005. Gene transfer to the central nervous system: current state of the art of the viral vectors. Curr. Genomics 6:13-39. [Google Scholar]
- 30.Kremer, E. J., S. Boutin, M. Chillon, and O. Danos. 2000. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J. Virol. 74:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieber, M., B. Smith, A. Szakal, W. Nelson-Rees, and G. Todaro. 1976. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 17:62-70. [DOI] [PubMed] [Google Scholar]
- 33.Liu, Q., A. Zaiss, P. Colarusso, K. Patel, G. Haljan, T. Wickham, and D. Muruve. 2003. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 14:627-643. [DOI] [PubMed] [Google Scholar]
- 34.Marshall, J., N. Rizvi, J. Kauh, W. Dahut, M. Figuera, M. Kang, W. Figg, I. Wainer, C. Chaissang, M. Li, and M. Hawkins. 2002. A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J. Exp. Ther. Oncol. 2:325-332. [DOI] [PubMed] [Google Scholar]
- 35.McClane, S. J., N. Chirmule, C. V. Burke, and S. E. Raper. 1997. Characterization of the immune response after local delivery of recombinant adenovirus in murine pancreas and successful strategies for readministration. Hum. Gene Ther. 8:2207-2216. [DOI] [PubMed] [Google Scholar]
- 36.Meitin, C., B. Bender, and P. J. Small. 1994. Enteric immunization of mice against influenza with recombinant vaccinia. Proc. Natl. Acad. Sci. USA 91:11187-11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muruve, D. A. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15:1157-1166. [DOI] [PubMed] [Google Scholar]
- 39.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 40.Muruve, D. A., M. J. Cotter, A. K. Zaiss, L. R. White, Q. Liu, T. Chan, S. A. Clark, P. J. Ross, R. A. Meulenbroek, G. M. Maelandsmo, and R. J. Parks. 2004. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 78:5966-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutlu, G. M., A. T. Ahkmedov, H. Lum, and P. Factor. 2004. Potential genetic therapies for acute lung injury. Curr. Gene Ther. 4:487-495. [DOI] [PubMed] [Google Scholar]
- 42.Olive, M., L. Eisenlohr, N. Flomenberg, S. Hsu, and P. Flomenberg. 2002. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum. Gene Ther. 13:1167-1178. [DOI] [PubMed] [Google Scholar]
- 43.Palmer, D., and P. Ng. 2003. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 8:846-852. [DOI] [PubMed] [Google Scholar]
- 44.Palmer, D. J., and P. Ng. 2005. Helper-dependent adenoviral vectors for gene therapy. Hum. Gene Ther. 16:1-16. [DOI] [PubMed] [Google Scholar]
- 45.Perreau, M., and E. J. Kremer. 2005. Frequency, proliferation and activation of human memory T cells induced by a nonhuman adenovirus. J. Virol. 79:14595-14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickles, R. J., J. A. Fahrner, J. M. Petrella, R. C. Boucher, and J. M. Bergelson. 2000. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J. Virol. 74:6050-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piekarz, R., and S. Bates. 2004. A review of depsipeptide and other histone deacetylase inhibitors in clinical trials. Curr. Pharm. Des. 10:2289-2298. [DOI] [PubMed] [Google Scholar]
- 48.Prince, G., D. Porter, A. Jenson, R. Horswood, R. Chanock, and H. Ginsberg. 1993. Pathogenesis of adenovirus type 5 pneumonia in cotton rats (Sigmodon hispidus). J. Virol. 67:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raper, S. E., N. Chirmule, F. S. Lee, N. A. Wivel, A. Bagg, G. P. Gao, J. M. Wilson, and M. L. Batshaw. 2003. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80:148-158. [DOI] [PubMed] [Google Scholar]
- 50.Reddel, R. R., Y. Ke, B. I. Gerwin, M. G. McMenamin, J. F. Lechner, R. T. Su, D. E. Brash, J. B. Park, J. S. Rhim, and C. C. Harris. 1988. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 48:1904-1909. [PubMed] [Google Scholar]
- 51.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin, B. K. 1999. Emerging therapies for cystic fibrosis lung disease. Chest 115:1120-1126. [DOI] [PubMed] [Google Scholar]
- 53.Russell, W. C. 2000. Update on adenovirus and its vectors. J Gen. Virol. 81:2573-2604. [DOI] [PubMed] [Google Scholar]
- 54.Sandor, V., S. Bakke, R. Robey, M. Kang, M. Blagosklonny, J. Bender, R. Brooks, R. Piekarz, E. Tucker, W. Figg, K. Chan, B. Goldspiel, A. Fojo, S. Balcerzak, and S. Bates. 2002. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin. Cancer Res. 8:718-728. [PubMed] [Google Scholar]
- 55.Schagen, F. H., M. Ossevoort, R. E. Toes, and R. C. Hoeben. 2004. Immune responses against adenoviral vectors and their transgene products: a review of strategies for evasion. Crit. Rev. Oncol. Hematol. 50:51-70. [DOI] [PubMed] [Google Scholar]
- 56.Soudais, C., S. Boutin, S. S. Hong, M. Chillon, O. Danos, J. M. Bergelson, P. Boulanger, and E. J. Kremer. 2000. Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J. Virol. 74:10639-10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soudais, C., S. Boutin, and E. J. Kremer. 2001. Characterisation of cis-acting sequences involved in the canine adenovirus packaging domain. Mol. Ther. 3:631-640. [DOI] [PubMed] [Google Scholar]
- 58.Soudais, C., C. Laplace-Builhe, K. Kissa, and E. J. Kremer. 2001. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 15:2283-2285. [DOI] [PubMed] [Google Scholar]
- 59.Soudais, C., N. Skander, and E. J. Kremer. 2004. Long-term in vivo transduction of neurons throughout the rat central nervous system using novel helper-dependent CAV-2 vectors. FASEB J. 18:391-393. [DOI] [PubMed] [Google Scholar]
- 60.Spiekermann, G. M., P. W. Finn, E. S. Ward, J. Dumont, B. L. Dickinson, R. S. Blumberg, and W. I. Lencer. 2002. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 196:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stecenko, A. A., and K. L. Brigham. 2003. Gene therapy progress and prospects: alpha-1 antitrypsin. Gene Ther. 10:95-99. [DOI] [PubMed] [Google Scholar]
- 62.Suomalainen, M., M. Y. Nakano, K. Boucke, S. Keller, and U. F. Greber. 2001. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 20:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tibbles, L. A., J. C. Spurrell, G. P. Bowen, Q. Liu, M. Lam, A. K. Zaiss, S. M. Robbins, M. D. Hollenberg, T. J. Wickham, and D. A. Muruve. 2002. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 76:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toietta, G., D. R. Koehler, M. J. Finegold, B. Lee, J. Hu, and A. L. Beaudet. 2003. Reduced inflammation and improved airway expression using helper-dependent adenoviral vectors with a K18 promoter. Mol. Ther. 7:649-658. [DOI] [PubMed] [Google Scholar]
- 65.Ueda, H., H. Nakajima, Y. Hori, T. Fujita, M. Nishimura, T. Goto, and M. Okuhara. 1994. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J. Antibiot. (Tokyo) 47:301-310. [DOI] [PubMed] [Google Scholar]
- 66.Van Ginkel, F. W., C. Liu, J. W. Simecka, J. Y. Dong, T. Greenway, R. A. Frizzell, H. Kiyono, J. R. McGhee, and D. W. Pascual. 1995. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and beta-galactosidase. Hum. Gene Ther. 6:895-903. [DOI] [PubMed] [Google Scholar]
- 67.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 68.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 69.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433-442. [DOI] [PubMed] [Google Scholar]
- 70.Yang, Y., F. A. Nunes, K. Berencsi, E. E. Furth, E. Gonczol, and J. M. Wilson. 1994. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 91:4407-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang, Y., Q. Su, I. S. Grewal, R. Schilz, R. A. Flavell, and J. M. Wilson. 1996. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J. Virol. 70:6370-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yei, S., N. Mittereder, S. Wert, J. A. Whitsett, R. W. Wilmott, and B. C. Trapnell. 1994. In vivo evaluation of the safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lung. Hum. Gene Ther. 5:731-744. [DOI] [PubMed] [Google Scholar]
- 73.Zabner, J., B. W. Ramsey, D. P. Meeker, M. L. Aitken, R. P. Balfour, R. L. Gibson, J. Launspach, R. A. Moscicki, S. M. Richards, T. A. Standaert, et al. 1996. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J. Clin. Investig. 97:1504-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zabner, J., B. G. Zeiher, E. Friedman, and M. J. Welsh. 1996. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J. Virol. 70:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, Y., N. Chirmule, G. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]
- 76.Zsengeller, Z., K. Otake, S. A. Hossain, P. Y. Berclaz, and B. C. Trapnell. 2000. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J. Virol. 74:9655-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.