Abstract
It is commonly accepted that infection of nondividing cells by gammaretroviruses such as the murine leukemia viruses is inefficient due to their inability to cross the nuclear envelope barrier. Challenging this notion, we now show that human nondividing macrophages display a specific window of susceptibility to transduction with a Friend murine leukemia virus (F-MLV)-derived vector during their differentiation from monocytes. This finding suggests that factors other than the nuclear membrane govern permissiveness to gammaretroviral infection and raises the possibility of using the macrophage tropism of F-MLV in gene therapy.
The nuclear envelope poses itself as a barrier between the initial and final phases of the early steps of retroviral infection: that is, between viral genome entry into the cytoplasm and its integration into the host genome in the nucleus. Two basic mechanisms have been proposed for the passage of viral nucleoprotein complexes (called reverse transcription or preintegration complexes [RTCs and PICs, respectively]) (3, 7, 8, 25) through the nuclear membrane: active import through the nuclear pores or passive passage in the nucleus following nuclear membrane disassembly during mitosis. Active nuclear import can occur either through hijacking of the normal cytonucleoplasmic transport machinery or through the induction of distortions that perturb the integrity of the nuclear envelope, as proposed for the human immunodeficiency virus type 1 (HIV-1) Vpr protein (5).
Among Retroviridae, lentiviruses such as HIV-1 have developed probably the most efficient way to traverse an intact nuclear membrane, and this results in the broad tropism of HIV-derived lentiviral vectors for most nondividing cells. Although the exact mechanism by which this is accomplished remains to be elucidated, lentiviral PICs appear to be gated through the nuclear pore and a number of viral proteins that compose it have nucleophilic properties, like Vpr, matrix (MA), and integrase (IN). An additional signal for nuclear transport may be provided by a particular single-gap structure determined by the central polypurine tract (cPPT) present on double-stranded proviral DNA (43). However, no consensus has yet been formed as to the importance of each of these elements in driving viral nuclear import. MA, Vpr, and cPPT can be deleted without completely abolishing either nuclear entry or viral infection (6, 9, 30), and the role played by IN nuclear localization signals is still being debated (6).
If nuclear localization of certain PIC components is taken as evidence suggesting—but not proving—they may concur in driving the entire viral nucleoprotein complex inside the nucleus, then the ability to infect nondividing cells should hardly be restricted to lentiviruses. Simple retroviruses previously referred to as “oncoretroviruses” should share a similar theoretical ability. For instance, the INs of certain alpharetroviruses are localized in the nucleus (18, 19, 39), and for gammaretroviruses such as the murine leukemia virus (MLV), clear evidence exists that cellular proteins with nuclear localization such as the barrier-to-autointegration factor (BAF) and the high-mobility protein HMG 1(Y) are recruited to PICs as efficiently as is the case with HIV-1 (21, 35). Moreover, nuclear localization of IN and NC following MLV infection of nondividing NIH 3T3 cells has been shown, suggesting that nuclear import of gammaretroviruses could occur (31).
Direct experimental evidence suggests that simple retroviruses can infect nondividing cells, albeit not to the same extent as lentiviruses. Among alpharetroviruses, nuclear accumulation of avian sarcoma virus (ASV) genomic DNA prior to mitosis has been demonstrated (14, 37) and both a Rous sarcoma-modified virus (RSV) and an ASV can infect growth-arrested cell lines, as well as differentiated primary murine neurons (12, 13, 15). These results strongly suggest that alpharetroviruses can infect nondividing cells.
For gammaretroviruses, although IN and NC can be found in the nucleus shortly after infection and despite the fact that proteins with nuclear localization are recruited by MLV PICs, infectivity seems mostly dependent upon passage through mitosis (20, 28, 32). However, this conclusion was mostly inferred from the use of chemically arrested cell lines, raising the possibility that chemical treatment affects steps other than nuclear entry per se. Alternatively, the passage through mitosis itself may be required for steps other than nuclear import, like, for example, proviral integration or expression.
Human macrophages and dendritic cells (DCs) are two populations of differentiated cells that can be derived from blood monocytes, whose properties include antigen presentation and immune system regulation (10). In addition to wide interest in them for gene therapy and vaccination, macrophages and DCs are a model of primary differentiated nondividing cells with which the relationship between cell cycle progression and retroviral infection can be tested.
In the course of our studies on lentiviral infection of primary human macrophages using retroviral vectors as a simplified model for viral infection, we realized that a Friend murine leukemia virus-derived retroviral vector (F-MLV), used as a negative control, yielded a significant proportion of cell transduction in granulocyte-macrophage colony-stimulating factor (GM-CSF)-derived macrophages. Given the importance of this finding, we decided to characterize this initial observation in detail.
MATERIALS AND METHODS
Retroviral vectors.
The HIV-1 and F-MLV packaging constructs expressed from a cytomegalovirus (CMV) promoter (8.2, coding for structural and accessory proteins of HIV-1, except for Env and Vpu; TG5349, coding for Gag-Pro-Pol of F-MLV) and their respective vector genomes (RRL.PPT.hPGK.GFPpre and TG13077, respectively) have been described elsewhere (22, 28, 42). Both genomes contain a CMV-driven enhanced green fluorescent protein reporter gene (eGFP) expression cassette, plus the relevant cis elements required for their mobilization. The F-MLV vector is based on the FB29 strain of Friend MLV (34), except that its packaging sequence (Ψ) was replaced with that of the rat VL30 retrotransposon (36). Retroviral particles were pseudotyped with the vesicular stomatitis virus glycoprotein (VSVg) envelope expressed from the plasmid MD.G to confer on them ample cellular tropism (28). The Moloney MLV (Mo-MLV)-based vector used here has been previously described (23, 24). The D1513A IN mutation was introduced in the pTG13077 Friend gag-pro-pol vector by standard molecular biology techniques. The resulting mutant (D1513A, with respect to the entire Gag-Pro-Pol coding sequence of strain FB29; accession no. Z1118) contains a single point mutation that changes the second aspartic acid of the IN catalytic site to alanine. Vectors were produced by transient DNA transfection of 293T cells, as described below.
Virion particle production and purification.
Vectors were produced by calcium phosphate DNA transfection of 293T cells. Forty-eight hours after transfection, the supernatant was purified by ultracentrifugation as described before through a double-step sucrose cushion (45 to 25% [wt/vol]) followed by an additional centrifugation step through a 25% sucrose cushion (11). Pelleted virions were resuspended and frozen in RPMI 1640 devoid of serum and supplemented with 10 mM MgCl2 and 100 μM deoxynucleoside triphosphates (dNTPs); their infectious titers were determined on dividing HeLa cells.
Cells.
Human primary lymphocytes and monocytes were obtained from peripheral blood mononuclear cells (PBMCs) of healthy donors at the Etablissement Français du Sang de Lyon (EFS-Lyon), as described previously (11). Briefly, after isolation of PBMCs by centrifugation in lymphocyte separation medium (Eurobio, France), cells were layered onto a two-step discontinuous density gradient (50 to 40% Percoll at a density of 1.130 g/ml, Pharmacia, Sweden), and monocytes and peripheral blood lymphocytes (PBLs) were recovered after centrifugation as low- and high-density fractions, respectively. Prior to freezing, monocytes were further purified by negative selection using a cocktail of hapten CD3, CD7, CD19, CD45RA, and CD56 anti-immunoglobulin E antibodies coupled to MACS microbeads (Miltenyi Biotec, France), yielding a 92 to 95% pure monocyte population. DCs were differentiated from monocytes upon culture for 4 to 6 days in GM-CSF (100 ng/ml; Schering Plough) and interleukin 4 (IL-4; 100 ng/ml; R&D systems), as described previously (11), and were of immature phenotype in the absence of further stimuli. Macrophages were obtained from monocytes plated at a density of 2 × 105/ml in 48-well plates (105/well) in complete RPMI 1640 in the absence of serum and cytokine for 3 h at 37°C. By this time, monocytes had adhered to the plate, and fetal calf serum (FCS) and GM-CSF were added at a final concentration of 10% and 100 ng/ml, respectively. When indicated, GM-CSF was substituted for macrophage colony-stimulating factor (M-CSF) in FCS or human sera obtained from donors of the AB group (hAB) in the absence of cytokines. The human Jurkat T-cell line and PBLs were cultured in RPMI 1640 medium and 10% FCS, while 293T and HeLa fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) plus 10% FCS. When indicated, PBLs were treated for 24 h with 1 μg/ml phytohemagglutinin (PHA; Sigma catalog no. L8902) and 150 U/ml of human recombinant IL-2 (from the NIH AIDS Reagent and Reference Program) or with 25 ng/ml of IL-7 (R&D Systems), while HeLa cells were either gamma irradiated (6,000 rads) or treated with aphidicolin (10 μg/ml) to induce G2/M or G1/S arrest, respectively (as determined by propidium iodide [PI] incorporation; data not shown) for 24 h prior to viral transduction. Flow cytometry antibodies were from DAKO and Becton Dickinson. Phycoerythrin (PE)-labeled latex beads were purchased from Sigma.
Cell transduction with retroviral vectors.
A total of 105 cells were used for viral transduction, and generally macrophages and DCs were used between days 4 and 6 of differentiation, unless otherwise specified. Transductions were carried out for 2 h at 37°C, and cell transduction efficiency was examined 3 to 4 days later by flow cytometry. For GFP and surface marker labeling, analysis was performed 2 days posttransduction to minimize problems of compensation due to the strong intensity of GFP. When indicated, control infections were performed in presence of nucleoside analogs zidovudine (AZT) and 2′,3′-dideoxycitidine (ddC) (10 and 20 μg/ml, respectively; no. 3485 and 220, respectively, from the AIDS Reagent and Reference Program, NIH). Each of the data presented is representative of three to seven independent experiments obtained with cells of different donors.
PCR analysis.
Seven days after transduction, cells were lysed and the presence of proviral DNA was analyzed by PCR with primers specific for the full length (from 5′ to 3′, upstream, CTCAGCAGTTTCTTAAGACCC; downstream, GATCTGAGCCTATTGATCGATC) and two long terminal repeats (2LTRs) (from 5′ to 3′, upstream, GCTGTTGCATCCGACTCGTG; downstream, CACCGCAGATATCCTGTTTG). Cell lysis and PCR were conducted as described previously (11). PCRs were carried out for 30 cycles of 96°C for 30 s, 55°C for 30 s, and 72°C for 60 s, and amplified products migrated on an agarose gel; their identity was confirmed by sequencing.
[3H]thymidine, CFSE, and BrdU incorporation.
To analyze cell proliferation, monocytes were seeded at day 0 with 1 μCi of [3H]thymidine and GM-CSF and, when indicated, irradiated (6,000 rads). An equal number of cycling Jurkat T cells was included as a positive control. Plates were analyzed at day 15, but similar results were obtained after 4, 6, or 8 days. For carboxyfluorescein diacetate succinimidyl ester (CFSE) analysis, freshly thawed monocytes and PBLs were incubated for 10 min at 37°C with 0.05 μM CFSE in phosphate-buffered saline before washing and replating in complete macrophage differentiation medium or with anti-CD3, anti-CD28 (Becton Dickinson), and IL-2 (at 1 μg/ml and 150 U/ml, respectively) for 8 days prior to analysis. Bromodeoxyuridine (BrdU) labeling was performed on day 4 macrophages (i.e., at the day at which maximal transduction occurred) by incubating them with 100 μM BrdU for 24 h prior to fixation, staining with a fluorescein-5-isothiocyanate (FITC)-conjugated anti-BrdU antibody, and flow cytometry analysis. Jurkat cells were added as a positive control.
RESULTS
Transduction of primary GM-CSF-derived human macrophages and of growth-arrested HeLa cells with an F-MLV-derived retroviral vector.
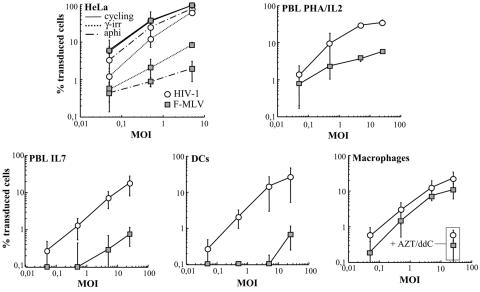
The ability of F-MLV to transduce nondividing cells was compared directly to that of an HIV-1-derived vector whose transduction abilities are independent from the cell's cycling status. The two retroviral vectors have been described previously and are presented schematically in Fig. 1. To minimize the risks of GFP carryover and thus pseudotransduction, VSVg-pseudotyped vectors produced by transfection of 293T cells were purified onto a double-sucrose cushion. As a first step toward the analysis of the requirement for nuclear membrane removal in gammaretroviral infection, equal HeLa infectious units of F-MLV and HIV-1 viral preparations were used to transduce cycling and growth-arrested HeLa cells in parallel. Cell cycle arrest was induced 24 h prior to transduction at G2/M by gamma irradiation (6,000 rads) and at G1/S by aphidicolin treatment (as determined by propidium iodide incorporation; not shown). As these cells do not progress into the cell cycle, their nuclear membrane is present throughout the course of viral infection and thus should pose itself as a barrier to gammaretroviral infection under both circumstances. As expected, HIV-1 infection was largely independent from the cell cycling status and high transduction rates were obtained in cycling and arrested cells (Fig. 2). On the contrary, F-MLV transduction was dependent on the phase at which the cell cycle arrest was induced. Compared to cycling cells, F-MLV transduction efficiency dropped 100-fold in G1/S-arrested cells but only 10-fold in G2/M-arrested cells. Although it is true that HIV-1 has a marked advantage over F-MLV in the infection of noncycling cells, it also seems true that F-MLV is able in certain instances to transduce growth-arrested cells, in line with previous observations obtained with other so-called “simple” retroviruses (13, 15).
FIG. 1.
Schematic representation of the retroviral vectors used here. The HIV-1 (22, 28) and F-MLV (42) vectors are shown by open and gray boxes, respectively. For simplicity, only the viral accessory proteins coded for by the HIV-1 packaging construct (all except Vpu) are drawn in the figure; the main viral elements of each transfer vector are shown as follows: ψ, packaging sequence; RRE, Rev-responsive element; cPPT, central polypurine tract; U3*, partially deleted and self-inactivating LTR; SD and SA, slice donor and acceptor sites, respectively. Both vectors express GFP driven from a CMV promoter.
FIG. 2.
Transduction of growth-arrested HeLa cells and of primary human blood cells with HIV-1 and F-MLV vectors. VSVg-pseudotyped vectors were purified on a double-sucrose cushion after production from 293T cells, and normalized amounts of HeLa infectious particles were used on target cells at different MOIs (as indicated). The percentage of GFP-positive cells was scored 3 to 4 days later by flow cytometry. The different primary cells shown here were derived from the same donor, and average results from three to seven different donors are presented. HeLa cells were arrested in either G2/M by gamma irradiation (γ-irr; 6,000 rads) or in G1/S by aphidicolin (aphi) treatment (10 μg/ml), and PBLs were treated with PHA plus IL-2 (to induce cell proliferation) or IL-7 (to induce G0 to G1b transition) 24 h prior to transduction. Human blood monocytes were differentiated in macrophages and DCs in presence of GM-CSF and GM-CSF plus IL-4, respectively, and transduced between days 4 and 6. When indicated, AZT and ddI were added to cells prior to viral transduction.
Transduction of PBLs was examined next (Fig. 2). Lymphocytes were treated with either PHA plus IL-2 or with IL-7, which induce cell proliferation or G0/G1b transition but induced only marginal cell proliferation under the conditions used here, respectively (4, 11). Dividing PHA-IL-2-stimulated lymphocytes were susceptible to transduction with both retroviral vectors, although the percentage of F-MLV-transduced cells was lower than that of HIV-1-transduced cells, as reported by others (26). On the contrary, although IL-7-treated lymphocytes were susceptible to HIV-1 transduction, albeit with variations depending on the donor, they were largely resistant to F-MLV and were transduced at rates below 1% for a multiplicity of infection (MOI) of 25. In our experience, a score of transduced cells below 1% for high viral inputs (equal to or higher than 5) is not to be considered the result of a true infection event but rather as a background signal (which is maintained if infection is performed in presence of reverse transcriptase [RT] inhibitors and which does not increase proportionally to the viral input, not shown). This is certainly not the case for aphidicolin-treated HeLa cells in which the low percentage of transduced cells is attained at lower MOIs (0.5 and lower) and is sensitive to the presence of RT inhibitors during infection (not shown).
Lastly, transduction of monocyte-derived macrophages and DCs was evaluated. Human blood monocytes as well as macrophages and DCs are considered nondividing cells. Macrophages and DCs were differentiated from monocytes in the presence of GM-CSF or GM-CSF-IL-4, respectively and transduced between days 4 and 6. While both DCs and macrophages were susceptible to HIV-1 transduction, the two cell types differed with respect to F-MLV. Specifically, while DCs were resistant to F-MLV transduction (below 1% transduction for an MOI of 25), macrophages were unexpectedly susceptible to F-MLV transduction. Transduction rates varied with the donor, yet the relative differences between HIV-1 and F-MLV were generally contained within two- to fourfold and F-MLV transduction efficiency ranged from 10 to 25%. To exclude the possibility of pseudotransduction (i.e., the generation of a GFP-positive signal due to phagocytosis of GFP contaminating viral preparations), cells were incubated with a cocktail of reverse transcriptase inhibitors prior to transduction (Fig. 2, as indicated). Since only baseline levels of GFP-positive cells were observed under these conditions, these results indicate the presence of bona fide viral transduction in human macrophages.
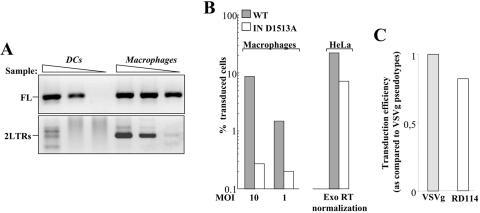
To demonstrate the presence of F-MLV proviral DNA in transduced macrophages, cells were infected at an MOI of 10 and lysed 24 h afterwards for analysis of reverse transcription intermediates by PCR. Despite the presence of comparable amounts of full-length proviral DNA in both DCs and macrophages, specific 2LTR forms could be observed only in the case of macrophages, suggesting that nuclear import of proviral DNA had occurred in these cells (Fig. 3A). To determine if integration was necessary for the productive transduction of macrophages, a single point mutation was introduced into one aspartic acid of the catalytic site of the MLV-IN (D1513A) in the context of the packaging vector F-MLV. This mutant transduced growing HeLa cells at a somewhat lower efficiency than its wild-type counterpart (two- to threefold) but was nonetheless infectious in this cell type (Fig. 3B), similarly to certain HIV-1 vectors whose IN catalytic site had been mutated (27, 40; data not shown). Despite its infectivity on HeLa cells, however, transduction with the IN mutant F-MLV vector yielded only background levels of transduction on macrophages, well below 1% even if MOIs higher than 10 were used (Fig. 3B). These results suggest that F-MLV proviral DNA is able to enter the nuclei of macrophages and that integration is required for expression of the GFP transgene. To determine if MLV transduction of macrophages was due to the entry pathway specified by VSVg, MLV vectors were pseudotyped with the feline endogenous virus RD114 envelope, which contrary to VSVg is a pH-independent envelope. Given the close infectivity of RD114- and VSVg-MLV vectors, the ability of MLV vectors to transduce macrophages does not seem to depend on the entry pathway of virion particles (Fig. 3C).
FIG. 3.
Proviral DNA analysis and requirement for IN activity in the transduction of human macrophages. (A) Day 4 DCs and macrophages were transduced with the same amount of F-MLV vectors at an MOI 10 and lysed 24 h postinfection for the analysis of late reverse transcription products that were full-length (FL) and 2LTR circles. Analysis was performed on threefold dilution of the sample with primers that allow the specific amplification of each form followed by agarose gel migration and detection. (B) A single point mutation was introduced in the catalytic aspartic acid of the F-MLV IN (D1513A) by site-directed mutagenesis. Mutant and wild-type (WT) viruses were produced in parallel, and their infectivity was tested on growing HeLa cells after normalization by exogenous (Exo) RT activity (right panel). Upon normalization of their infectious titer, wild-type and mutant viral preparations were used to infect macrophages (left panel). (C) VSVg- and RD114-pseudotyped MLV vectors were similarly produced and tested on macrophages at equal MOIs. The infectivity of RD114-pseudotyped MLV vectors is shown with respect to that of VSVg-MLV, set arbitrarily at 1.
By the time of transduction, DCs were largely immature, while macrophages were firmly adherent on the plate throughout the culture time, when cells underwent a 3-h serum starvation period after thaw. GFP expression in transduced macrophages remained stable for at least 2 months (Fig. 4A) (data not shown). Characterization of day 4 transduced GFP-positive cells 2 days postinfection (i.e., at day 6) indicated they expressed CD14 and CD40, two surface molecules present on human macrophages (Fig. 4B) and the expression of these markers remained stable at later times. One of the distinctive features of differentiated macrophages is an extensive phagocytic ability. Thus, the presence of viral transduction in phagocytosis-competent macrophages can be taken as evidence that transduction occurs in terminally differentiated cells, which are by definition nondividing. To prove that transduction occurred in functionally differentiated macrophages, PE-latex beads were added to macrophage cultures for 2 h and cells were extensively washed prior to viral transduction (the culture was homogenous with respect to its ability to internalize latex beads; not shown). Following viral transduction and fluorescence microscopy analysis 3 days later, all of the GFP-positive macrophages displayed internalized PE-labeled latex beads, indicating that transduction occurred in terminally differentiated and functional macrophages (Fig. 4C).
FIG. 4.
Features of F-MLV-transduced macrophages. (A) Transmission and fluorescence microscopy pictures of plated F-MLV-transduced macrophage cultures. (B) Cell surface marker analysis 2 days posttransduction of day 4 macrophages. Macrophages were incubated with the indicated fluorescently coupled antibody prior to flow cytometry analysis. (C) To demonstrate that viral transduction occurred in differentiated macrophages, day 4 macrophages were incubated with PE-labeled latex beads for 2 h followed by extensive cell washing to remove unbound beads. Two hours afterwards, the cell culture was infected. Cells were analyzed by fluorescence microscopy 3 days postinfection.
Overall, these results indicate that an F-MLV vector transduces efficiently primary GM-CSF-derived human macrophages under the conditions used here. Since macrophages and not DCs can be transduced despite their common origin from monocytes, these results suggest that either the Friend strain is an exception among gammaretroviruses in its ability to transduce macrophages, incubation with GM-CSF induces cell proliferation in macrophages as shown in at least one report (33), or gammaretroviral infection of nondividing cells is regulated by a complex set of factors rather than by the mere interposition of a nuclear membrane barrier.
F- and Mo-MLV-derived vectors display similar transduction efficiency on primary macrophages.
To determine if the Friend strain was an exception among gammaretroviruses, F-MLV vectors were compared to Moloney MLV-derived vectors produced and purified in parallel. The infectious titers obtained with the two vectors on HeLa cells were almost identical. When Mo- and F-MLV vectors were compared side by side on macrophages, the transduction efficiencies obtained with both were also identical (Fig. 5), suggesting that transduction of human macrophages is a more general property of gammaretroviruses, rather than the exception of the Friend strain used here.
FIG. 5.
Comparison between Moloney MLV and Friend MLV-derived retroviral vectors. The Friend MLV-derived vector was compared to a Moloney MLV-derived vector produced in parallel. This vector has been previously described elsewhere (23, 24), and it is comparable to the Friend MLV-derived vector used here. After normalization of their infectious titers on HeLa cells, equal MOIs were used in the transduction of day 4 macrophages, and infection was scored 3 days later by flow cytometry analysis.
Cell proliferation analysis of monocyte-derived macrophages.
To determine if macrophages underwent cell division, proliferation was examined by three distinct methods: [3H]thymidine, CFSE, and BrdU incorporation. Monocytes were seeded with [3H]thymidine and GM-CSF. As controls, seeded monocytes were gamma irradiated at day 0 and an equal number of cycling Jurkat T cells was included. Even after 15 days of incubation, no evidence of [3H]thymidine incorporation could be gathered in macrophages and similar results were obtained if the analysis was performed earlier (Fig. 6A) (data not shown). The CFSE dye incorporates into the cell cytoplasm and is divided equally between daughter and mother cells, resulting in a fluorescence decrease in cells that have divided. PBLs and monocytes were labeled with CFSE after cell thaw and cultured with CD3 or CD28 plus IL-2 or GM-CSF, respectively, for 8 days prior to analysis. While stimulation of PBLs resulted in the clear appearance of cells with decreased CFSE fluorescence, no such decrease was observed during monocyte-to-macrophage differentiation (Fig. 6B). Lastly, BrdU incorporation was evaluated. Like thymidine, BrdU is incorporated in the genome of cells undergoing active S phase, which can thus be demonstrated after incubation with an appropriate anti-BrdU fluorescent antibody by flow cytometry. After 24 h of incubation with BrdU of day 4 macrophages (the time at which macrophages were susceptible to F-MLV transduction), no BrdU-positive macrophages were present, contrary to control Jurkat cells (Fig. 6C). Taken together, these results indicate that under the conditions used here, monocytes differentiate into macrophages in the absence of detectable cell proliferation.
FIG. 6.
Absence of cell proliferation in human GM-CSF-derived macrophages. (A) Monocytes (gamma irradiated [γ-irr.] as a control or not, both with GM-CSF) and cycling Jurkat T cells were seeded in equal numbers in the presence of [3H]thymidine for 15 days prior to analysis. (B) Monocytes and PBLs were labeled with CFSE at day 0 and then differentiated with GM-CSF or stimulated with CD3 or CD28 plus IL-2 for 8 days, respectively, prior to flow cytometry analysis. The graph shows a comparison between CFSE fluorescence at day 0 and that at day 8, as indicated. (C) Day 4 GM-CSF-derived macrophages and Jurkat T cells were incubated for 24 h in BrdU, prior to staining with a fluorescently labeled anti-BrdU antibody and flow cytometry analysis. In each histogram plot, BrdU-negative and BrdU-positive samples (continuous and dotted lines, respectively) are superimposed.
Susceptibility to retroviral infection varies according to culture conditions and timing of macrophage differentiation.
The different results described in the literature on the transduction of human macrophages with simple retroviruses, as well as the distinct outcome of F-MLV transduction following transduction of macrophages and DCs both derived from the same population, raised the possibility that the cell differentiation status modulates susceptibility to retroviral infection. To determine if this was the case, three different conditions normally used to differentiate macrophages from monocytes were used: GM-CSF, M-CSF, or hAB. Survival rates of differentiated macrophages varied in the following order: GM-CSF > M-CSF > hAB (from 80 to 50%, respectively). Cell surface marker analysis on day 4 macrophages showed a decrease in CD14 surface labeling in macrophages differentiated in GM-CSF, as compared to the starting monocyte population and to macrophages differentiated under the remaining conditions, as reported previously (Table 1) (16, 17). Macrophages differentiated under the three conditions, however, had similar functional properties (as determined by phagocytosis and mixed lymphocyte reaction; data not shown). At day 4, macrophages were transduced with HIV-1 and F-MLV retroviral vectors at an MOI of 25 and GFP expression was analyzed by flow cytometry (Fig. 7A). GM-CSF-derived macrophages were the most susceptible to retroviral infection, followed by M-CSF and hAB serum. HIV-1 and F-MLV transduction rates mirrored under these three conditions, but with a finer analysis, these differences were more stringent for F-MLV than for HIV-1 (3 versus 6 and 9 versus 15 times lower in M-CSF and in hAB serum, respectively, when compared to GM-CSF for HIV-1 versus F-MLV). Contrary to GM-CSF, F-MLV transduction of M-CSF- and hAB serum-derived macrophages was in general very low and generally approximated background levels (around 1%). These relative differences were maintained also if transduction was performed at different times during monocyte-to-macrophage differentiation (not shown).
TABLE 1.
Cell surface analysis of macrophages derived under different conditions
| Surface marker | Result fora:
|
|||
|---|---|---|---|---|
| Monocytes | Day 4 macrophages
|
|||
| GM-CSF | M-CSF | Human serum | ||
| HLA-DR | ++ | ++ | +++ | ++ |
| CD40 | +++ | +++ | +++ | +++ |
| CD14 | +++ | + | +++ | ++ |
| CD86 | ++ | ++ | ++ | ++ |
| CD80 | + | + | + | + |
Results are averages between cells derived from three different donors. +++, median fluorescence intensity (MFI) of 1,000 or higher; ++, MFI between 100 and 999; +, MFI between 10 and 99. Negative control, MFI = 3.
FIG. 7.
Susceptibility of macrophages to retroviral transduction is time and culture condition dependent. (A) Monocytes were differentiated into macrophages in GM-CSF, M-CSF, or human serum (hAB) prior to viral infection at an MOI of 25 and flow cytometry analysis. (B) Time curve susceptibility to retroviral transduction of GM-CSF-derived macrophages. Time zero indicates cells immediately after the 3-h serum deprivation step and addition of GM-CSF and FCS. The results presented here represent the average of three independent experiments with cells of different donors.
Given that the differentiation conditions modulated the macrophages' susceptibility to retroviral infection, we hypothesized that the same could be true during monocyte-to-macrophage differentiation. To test this hypothesis, transductions were performed along the monocyte-to-macrophage differentiation period at an MOI of 5. Monocytes transduced at that time were refractory to retroviral infection (not shown); however, a single 3-h serum deprivation step (by the end of which cells adhered firmly to the plate) was sufficient for appreciable transduction to occur. Again, HIV-1 and F-MLV transduction mirrored and reached a maximum generally between days 4 and 6, depending on the donor, and then decreased until day 15 (Fig. 7B). At day 15, F-MLV transduction efficiency decreased to background levels. In the absence of the initial 3-h serum deprivation step, cells adhered by days 6 to 7 and the peak of retroviral susceptibility was delayed 3 to 4 days (not shown). DCs were consistently resistant to F-MLV transduction throughout their differentiation period (not shown). These results suggest that the intracellular milieu of macrophages strongly influences the cell's susceptibility to retroviral infection and that the choice of the right moment for viral infection will determine both success and efficiency of transduction at least in this specific cell type.
DISCUSSION
The results described here show for the first time that gammaretrovirus-derived vectors possess the ability to transduce nondividing GM-CSF-differentiated human macrophages. Transduction occurs efficiently in these cells (between 10 and 25% at the highest MOI used, depending on the donor), in the clear absence of cell division, and with only a minor defect with respect to HIV-1. Our results with macrophages may at first appear to contrast with previous studies indicating that MLV-derived vectors are unable to infect primary macrophages (28, 41). Yet, we believe they also provide a rational explanation for this apparent contradiction. Indeed, we have shown that F-MLV transduction of macrophages is exquisitely sensitive to both timing and differentiation conditions, so that it is the exact choice of what is a relatively narrow optimal window that determines whether F-MLV transduction will succeed or not. As for the infection of growth-arrested cells, our results do agree with most previous observations indicating that G1/S-arrested aphidicolin-treated cells are mostly resistant to MLV (1, 13, 29).
Our study does not question the obvious advantage of HIV-1 in the infection of most nondividing cells, underscoring again the fact that lentiviruses have evolved probably the most efficacious way to traverse the nuclear envelope. However, the results presented here suggest that a deep revision of the dogma based on which lentiviruses can infect nondividing cells while “oncoretroviruses” cannot is warranted. This has already been strongly suggested for alpharetroviruses (12-15, 37), and we believe our results suggest it for gammaretroviruses as well.
The conception of the nuclear membrane as an impermeable barrier that stands between gammaretroviral PICs and the host genome must be revised, because this rule fails to explain why macrophages, that are clearly nondividing under the conditions used here and thus possess a normal intact nuclear membrane, are susceptible to MLV infection. The fact that human macrophages are the exception rather than the norm among the nondividing cells tested here suggests that gammaretroviral infection is normally diminished in the presence of a nuclear membrane, but it also indicates that gammaretroviral PICs do possess an intrinsic ability to cross such a barrier. This raises the possibility that factors other than or in addition to the nuclear envelope concur in determining the outcome of infection in nondividing cells. This is suggested by the different susceptibilities to F-MLV transduction between G1/S- and G2/M-arrested HeLa cells and between DCs and macrophages despite their common origin from a unique cell type. Furthermore, macrophages themselves show a narrow window of susceptibility to F-MLV outside which transduction either does not occur or occurs at very low frequency. For all of their differences, the cells mentioned above are nondividing and viable, and thus we must suppose they share the presence of an intact nuclear membrane. If the nuclear membrane were the only restrictive factor for MLV passage to the nucleus (since similar amounts of FL proviral DNA were found), all cells should have been equally resistant to MLV transduction. Since this is not the case, the hypothesis can be put forward that cellular factors present at the moment of MLV infection somehow influence its outcome. Accordingly, macrophages may express at a specific point of their differentiation a positive factor that gates incoming MLV PICs across the nuclear membrane or else lack a negative factor expressed in most nondividing cells.
However, it remains to be determined whether such a factor could target one or several viral proteins. Chimeric swapping between Gag portions of HIV-1 and Mo-MLV suggested that the capsid protein (CA) of gammaretroviruses may doom incoming PICs to failure in the presence of a nuclear membrane (41). It is thus possible that gammaretroviral CA is targeted by negative cellular factors present during growth arrest or, as suggested by our study, absent during a short time window during monocyte-to-macrophage differentiation. Differences in CA sequences among gamma- and alpharetroviruses and thus their relative affinity for such a factor or factors may explain the observed advantage of RSV over Mo-MLV in the transduction of G1/S-arrested cells (13). It remains possible that viral determinants other than or together with CA concur in specifying the passage of gammaretroviral PICs through the nuclear envelope.
Macrophages have not been described as natural targets for MLV replication in vivo (34, 38). Yet this failure may be due to blocks at stages other than nuclear import, such as cell entry, viral expression, or virion assembly. As retroviral vectors recapitulate the early phases of viral infection, we may expect these phases to proceed successfully even in the case of wild-type virus. These results open up the possibility to use F-MLV transduction of monocyte-derived macrophages for gene therapy purposes, since macrophages are considered as long-term storage cells with potential usage in the treatment of diverse pathologies such as cancer (2). Although the efficacy of the macrophage-based gene therapy approaches awaits confirmation, gammaretroviral transduction now provides a possible new tool toward their modification.
Acknowledgments
We are grateful to Transgene for MLV-VL30, to Aymeric Rivollier for providing blood material, to the AIDS Reagent and Reference Program of the NIH for providing some of the material used here, to Schering Plough for GM-CSF, to Eran Bacharach for the Moloney MLV-based vector, and to Christelle Daude for technical help.
A.C. is supported by Sidaction and ANRS, and J.-L.D. is supported by the TRIoH Consortium of the EC.
REFERENCES
- 1.Bieniasz, P. D., R. A. Weiss, and M. O. McClure. 1995. Cell cycle dependence of foamy retrovirus infection. J. Virol. 69:7295-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke, B. 2003. Macrophages as novel cellular vehicles for gene therapy. Expert Opin. Biol. Ther. 3:919-924. [DOI] [PubMed] [Google Scholar]
- 3.Chen, H., and A. Engelman. 1998. The barrier-to-autointegration protein is a host factor for HIV type 1 integration. Proc. Natl. Acad. Sci. USA 95:15270-15274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dardalhon, V., S. Jaleco, S. Kinet, B. Herpers, M. Steinberg, C. Ferrand, D. Froger, C. Leveau, P. Tiberghien, P. Charneau, N. Noraz, and N. Taylor. 2001. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc. Natl. Acad. Sci. USA 98:9277-9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 6.Dvorin, J. D., P. Bell, G. G. Maul, M. Yamashita, M. Emerman, and M. H. Malim. 2002. Reassessment of the roles of integrase and the central DNA flap in human immunodeficiency virus type 1 nuclear import. J. Virol. 76:12087-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fassati, A., and S. P. Goff. 2001. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J. Virol. 75:3626-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fassati, A., and S. P. Goff. 1999. Characterization of intracellular reverse transcription complexes of Moloney murine leukemia virus. J. Virol. 73:8919-8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freed, E. O., G. Englund, and M. A. Martin. 1995. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J. Virol. 69:3949-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon, S. 1998. The role of the macrophage in immune regulation. Res. Immunol. 149:685-688. [DOI] [PubMed] [Google Scholar]
- 11.Goujon, C., L. Jarrosson-Wuilleme, J. Bernaud, D. Rigal, J.-L. Darlix, and A. Cimarelli. 2003. Heterologous human immunodeficiency virus type 1 lentiviral vectors packaging a simian immunodeficiency virus-derived genome display a specific postentry transduction defect in dendritic cells. J. Virol. 77:9295-9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greger, J. G., R. A. Katz, K. Taganov, G. F. Rall, and A. M. Skalka. 2004. Transduction of terminally differentiated neurons by avian sarcoma virus. J. Virol. 78:4902-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatziioannou, T., and S. P. Goff. 2001. Infection of nondividing cells by Rous sarcoma virus. J. Virol. 75:9526-9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries, E. H., C. Glover, and M. E. Reichmann. 1981. Rous sarcoma virus infection of synchronized cells establishes provirus integration during S-phase DNA synthesis prior to cellular division. Proc. Natl. Acad. Sci. USA 78:2601-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz, R. A., J. G. Greger, K. Darby, P. Boimel, G. F. Rall, and A. M. Skalka. 2002. Transduction of interphase cells by avian sarcoma virus. J. Virol. 76:5422-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreutz, M., B. Hennemann, U. Ackermann, E. Grage-Griebenow, S. W. Krause, and R. Andreesen. 1999. Granulocyte-macrophage colony-stimulating factor modulates lipopolysaccharide (LPS)-binding and LPS-response of human macrophages: inverse regulation of tumour necrosis factor-alpha and interleukin-10. Immunology 98:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruger, M., J. G. Van de Winkel, T. P. De Wit, L. Coorevits, and J. L. Ceuppens. 1996. Granulocyte-macrophage colony-stimulating factor down-regulates CD14 expression on monocytes. Immunology 89:89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kukolj, G., K. S. Jones, and A. M. Skalka. 1997. Subcellular localization of avian sarcoma virus and human immunodeficiency virus type 1 integrases. J. Virol. 71:843-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kukolj, G., R. A. Katz, and A. M. Skalka. 1998. Characterization of the nuclear localization signal in the avian sarcoma virus integrase. Gene 223:157-163. [DOI] [PubMed] [Google Scholar]
- 20.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, L., C. M. Farnet, W. F. Anderson, and F. D. Bushman. 1998. Modulation of activity of Moloney murine leukemia virus preintegration complexes by host factors in vitro. J. Virol. 72:2125-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manganini, M., M. Serafini, F. Bambacioni, C. Casati, E. Erba, A. Follenzi, L. Naldini, S. Bernasconi, G. Gaipa, A. Rambaldi, A. Biondi, J. Golay, and M. Introna. 2002. A human immunodeficiency virus type 1 pol gene-derived sequence (cPPT/CTS) increases the efficiency of transduction of human nondividing monocytes and T lymphocytes by lentiviral vectors. Hum. Gene Ther. 13:1793-1807. [DOI] [PubMed] [Google Scholar]
- 23.Mark-Danieli, M., N. Laham, M. Kenan-Eichler, A. Castiel, D. Melamed, M. Landau, N. M. Bouvier, M. J. Evans, and E. Bacharach. 2005. Single point mutations in the zinc finger motifs of the human immunodeficiency virus type 1 nucleocapsid alter RNA binding specificities of the Gag protein and enhance packaging and infectivity. J. Virol. 79:7756-7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markowitz, D., S. Goff, and A. Bank. 1988. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J. Virol. 62:1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, M. D., C. M. Farnet, and F. D. Bushman. 1997. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J. Virol. 71:5382-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhlebach, M. D., I. Schmitt, S. Steidl, J. Stitz, M. Schweizer, T. Blankenstein, K. Cichutek, and W. Uckert. 2003. Transduction efficiency of MLV but not of HIV-1 vectors is pseudotype dependent on human primary T lymphocytes. J. Mol. Med. 81:801-810. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima, N., R. Lu, and A. Engelman. 2001. Human immunodeficiency virus type 1 replication in the absence of integrase-mediated DNA recombination: definition of permissive and nonpermissive T-cell lines. J. Virol. 75:7944-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 29.Patton, G. S., O. Erlwein, and M. O. McClure. 2004. Cell-cycle dependence of foamy virus vectors. J. Gen. Virol. 85:2925-2930. [DOI] [PubMed] [Google Scholar]
- 30.Reil, H., A. A. Bukovsky, H. R. Gelderblom, and H. G. Gottlinger. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risco, C., L. Menendez-Arias, T. D. Copeland, P. Pinto da Silva, and S. Oroszlan. 1995. Intracellular transport of the murine leukemia virus during acute infection of NIH 3T3 cells: nuclear import of nucleocapsid protein and integrase. J. Cell Sci. 108:3039-3050. [DOI] [PubMed] [Google Scholar]
- 32.Roe, T., T. C. Reynolds, G. Yu, and P. O. Brown. 1993. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 12:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuitemaker, H., N. A. Kootstra, R. A. Fouchier, B. Hooibrink, and F. Miedema. 1994. Productive HIV-1 infection of macrophages restricted to the cell fraction with proliferative capacity. EMBO J. 13:5929-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitbon, M., B. Sola, L. Evans, J. Nishio, S. F. Hayes, K. Nathanson, C. F. Garon, and B. Chesebro. 1986. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell 47:851-859. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki, Y., and R. Craigie. 2002. Regulatory mechanisms by which barrier-to-autointegration factor blocks autointegration and stimulates intermolecular integration of Moloney murine leukemia virus preintegration complexes. J. Virol. 76:12376-12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torrent, C., C. Gabus, and J.-L. Darlix. 1994. A small and efficient dimerization/packaging signal of rat VL30 RNA and its use in murine leukemia virus-VL30-derived vectors for gene transfer. J. Virol. 68:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varmus, H. E., T. Padgett, S. Heasley, G. Simon, and J. M. Bishop. 1977. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell 11:307-319. [DOI] [PubMed] [Google Scholar]
- 38.Vogt, M., C. Haggblom, S. Swift, and M. Haas. 1985. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J. Virol. 55:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner, S., P. Hindmarsh, M. Napirei, K. Vogel-Bachmayr, and B. M. Wohrl. 2002. Subcellular localization and integration activities of Rous sarcoma virus reverse transcriptase. J. Virol. 76:6205-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiskerchen, M., and M. A. Muesing. 1995. Human immunodeficiency virus type 1 integrase: effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates, and sustain viral propagation in primary cells. J. Virol. 69:376-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita, M., and M. Emerman. 2004. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J. Virol. 78:5670-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu, Q., and J.-L. Darlix. 1996. The zinc finger of nucleocapsid protein of Friend murine leukemia virus is critical for proviral DNA synthesis in vivo. J. Virol. 70:5791-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]