Abstract
The gammaherpesvirus immediate-early genes are critical regulators of virus replication and reactivation from latency. Rta, encoded by gene 50, serves as the major transactivator of the lytic program and is highly conserved among all the gammaherpesviruses, including Epstein-Barr virus, Kaposi's sarcoma-associated herpesvirus, and murine gammaherpesvirus 68 (γHV68). Introduction of a translation stop codon in γHV68 gene 50 (gene 50.stop γHV68) demonstrated that Rta is essential for virus replication in vitro. To investigate the role that virus replication plays in the establishment and maintenance of latency, we infected mice with gene 50.stop γHV68. Notably, the gene 50.stop virus established a long-term infection in lung B cells following intranasal infection of mice but was unable to establish latency in the spleen. This complete block in the establishment of latency in the spleen was also seen when lytic virus production was inhibited by treating mice infected with wild-type virus with the antiviral drug cidofovir, implicating virus replication and not an independent function of Rta in the establishment of splenic latency. Furthermore, we showed that gene 50.stop γHV68 was unable to prime the immune system and was unable to protect against a challenge with wild-type γHV68, despite its ability to chronically infect lung B cells. These data indicate gammaherpesviruses that are unable to undergo lytic replication in vivo may not be viable vaccine candidates despite the detection of cells harboring viral genome at late times postinfection.
Background.
Gammaherpesviruses are characterized by their ability to establish latency in lymphocytes and can persist for the lifetime of the host. It is still undetermined whether reactivation and lytic virus replication are required for the maintenance of latency in the host. Gene 50 encodes Rta, a critical regulator of the viral lytic cascade of gammaherpesviruses which is highly conserved among all known gammaherpesviruses, including the human gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), and the rodent virus murine gammaherpesvirus 68 (γHV68) (24). Rta has been shown to transactivate a number of downstream viral genes, as well as having a role in DNA replication. Overexpression of Rta in cell lines latently infected with EBV, KSHV, or γHV68 leads to expression of early and late viral genes and the generation of viral particles (16, 19, 27, 30, 32). Rta has been shown to be essential for EBV replication, and deletion of gene 50 in KSHV inhibits replication and reactivation of the virus in vitro (31). All of these studies have investigated the role of gene 50 in tissue culture systems and have yet to determine the role of Rta and virus replication in the establishment and maintenance of gammaherpesvirus latency in vivo.
γHV68 is closely related to both EBV and KSHV, and infection of mice provides a tractable small animal model in which to study gammaherpesviruses in vivo. Generation of a gene 50 null virus demonstrated that expression of Rta is essential for virus replication in vitro (11, 15). Here we report the analysis of infection of mice with a gene 50 null virus.
Long-term carriage of G50.stop virus in lung B cells following intranasal (i.n.) infection, but failure to establish infection in spleen B cells.
To assess the role of virus replication in the establishment of a chronic infection, we used a γHV68 mutant that has a translation stop codon inserted into gene 50 at bp 67,975 to create G50.stop, as previously described (15). To use this virus in vivo, we reintroduced the gene 50 stop mutation onto a γHV68 backbone that contains both a Cre-recombinase expression cassette and a green fluorescent protein (GFP) expression cassette. Since γHV68 containing BAC sequences is attenuated in vivo compared to wild-type γHV68, the BAC sequences must be removed prior to conducting in vivo analyses (1). In γHV68-BAC, the BAC sequences are flanked by loxP sites and the expression of Cre-recombinase from the virus results in the efficient excision of the BAC sequences from the viral genome during growth of the G50.stop virus in the gene 50 complementary cell line, S27 (1, 15). The Cre-recombinase expression cassette was inserted into a previously identified neutral locus in the virus between open reading frames (ORFs) 27 and 29b (14). Enhanced GFP, driven by the human cytomegalovirus immediate-early promoter, was inserted into the XcmI site at bp 112,501 within ORF75b of γHV68. Due to difficulty plaquing the G50.stop virus on the S27 complementing cell line, GFP expression was utilized to determine viral titer using fluorescence foci-forming units (FFU) (3, 28). A wild-type control virus (γHV68-CreGFP) harboring the Cre-recombinase and enhanced GFP expression cassettes inserted at the same locations was also generated. The titers of both G50.stop and γHV68-CreGFP virus stocks were determined on S27 cell monolayers using the cell-to-cell spread of GFP expression from infectious virions to form fluorescent foci. Similar antibody-based fluorescence assays were originally developed for quantification of non-plaque-forming virus (3, 28). Notably, a direct comparison of wild-type virus (γHV68-CreGFP) titer determined by FFU versus PFU revealed that FFU was ca. 200-fold less sensitive than PFU (M. Farrell, J. Upton, and S. H. Speck, unpublished data). As such, we assume that expression of GFP from the gene 75b region of the viral genome is rapidly shut down during lytic virus growth in NIH 3T12 fibroblasts. Notwithstanding the observed difference between FFU- and PFU-determined titers, we standardized the G50.stop and γHV68-CreGFP virus stocks by determining FFU titers and used this as a basis for infecting mice.
Following i.n. infection with wild-type γHV68, viral latency can readily be detected in the spleen as early as day 16 postinfection by using a limiting dilution nested PCR assay (LDPCR) as previously described (20, 29) and continues to persist in the spleen at late times postinfection (e.g., 1 to 2 years postinfection). To determine whether a replication-defective virus can establish latency in vivo, the frequency of γHV68 genome-positive cells was analyzed for the replication-defective virus G50.stop at different times postinfection (Fig. 1). C57BL/6 mice were infected i.n. with 940 FFU of either G50.stop virus or the control γHV68-CreGFP virus. Viral stocks were simultaneously plated onto a mouse embryonic fibroblast (MEF) cell monolayer and monitored for cytopathic effect (CPE). No CPE was ever detected from the G50.stop viral stocks used for these experiments, indicating the absence of revertant replication-competent virus.
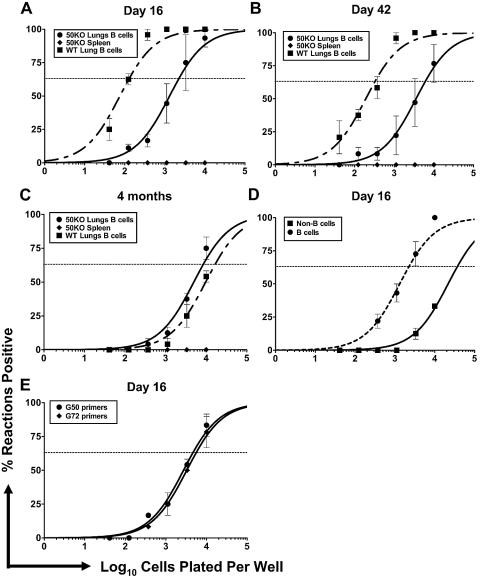
FIG. 1.
Analysis of γHV68 latency following infection with G50.stop. (A to C) Lungs from C57BL/6J mice infected i.n. with either γHV68-CreGFP (WT) or G50.stop (50KO) at day 16 (A), day 42 (B), and 4 months (C) postinfection were harvested, digested with collagenase (type IV; Worthington Biochemical Corporation, Lakewood, NJ), and sorted by magnetic bead cell sorting (MACS; Miltenyi Biotec, Auburn, CA) to enrich for B cells. Bulk spleen cells were also harvested at the same time points. Cells were analyzed by limiting dilution viral genome PCR as previously described (25). Serial dilutions of cells were plated into a background of 104 uninfected cells, lysed, and analyzed by nested PCR to detect viral genome. Curve fit lines were derived from nonlinear regression analysis, and symbols represent mean percentages of wells positive for virus ± the standard error of mean. The dashed line represents 63.2%, from which the frequency of viral genome-positive cells was calculated based on the Poisson distribution. (D) Lung cells from G50.stop-infected mice were harvested and digested with collagenase. Cells were stained with anti-CD19 conjugated to allophycocyanin and anti-B220 conjugated to fluorescein isothiocyanate (BD Pharmingen), sorted by flow cytometry on the MoFlo (Cytomation, Fort Collins, CO), and analyzed by LDPCR. Purity of sorted cells exceeded 94%. (E) Bulk lung cells from G50.stop-infected mice at day 16 postinfection were isolated as described above and assayed by LDPCR as previously described (14, 18). For all experiments, the data shown represent at least three independent experiments with cells pooled from five mice per experimental group.
At day 16 postinfection, both γHV68-CreGFP and G50.stop could be detected by LDPCR analysis in lung cells that were B cell enriched by magnetic-activated cell sorter magnetic bead separation (Fig. 1A). At this time point the frequency of G50.stop latent virus-infected cells was a log lower than γHV68-CreGFP. At day 42 postinfection, G50.stop viral DNA was found in 1 in 6,000 cells of the B-cell-enriched population compared to 1 in 335 for γHV68-CreGFP (Fig. 1B). By 4 months postinfection, while the frequency of wild-type latently infected cells had dramatically decreased in the lung B-cell-enriched population (1 in 17,000), the level of G50.stop virus-infected B cells remained relatively steady (1 in 8,500) (Fig. 1C). Notably, at day 16 postinfection there was a low frequency of viral genome-positive cells detected in the non-B-cell population for both G50.stop (Fig. 1D) and γHV68-CreGFP (data not shown). Importantly, this substantiates the presence of virus in lung B cells and rules out any significant contribution of viral genome-positive cells from contaminating non-B cells in the previous analyses. By 4 months postinfection, viral genome-positive non-B cells were not detected in the lungs of mice infected with either G50.stop or γHV68-CreGFP (data not shown).
At no time postinfection could we detect latent virus in the spleens of G50.stop mice that were infected intranasally (Fig. 1A to C). We questioned whether the absence of splenic latency was related to the low dose of inoculating virus used in these experiments. Thus, using a higher-titer G50.stop stock that was available in a limited amount, mice were infected intranasally with 2 × 104 FFU. Following high-dose infection, we observed a higher frequency of viral genome-positive B cells in the lungs but still failed to detect viral latency in the spleens at any time point postinfection (data not shown). Limited studies infecting mice via intraperitoneal inoculation with 940 FFU of virus demonstrated that the G50.stop virus was able to establish low-level latency in peritoneal exudate cells compared to γHV68-CreGFP but again failed to establish latency in the spleens of the infected mice (data not shown). Thus, taken together, these data suggest that while lytic viral replication is not necessary to establish and maintain the presence of cells harboring viral genome at the primary site of infection, it appears to be required for the virus to traffic to the spleen and establish latency. One possibility is that establishing latency in the spleen involves trafficking of latently infected B cells which, upon arrival in the spleen, reactivate and seed secondary virus replication in the spleen, culminating in the establishment of splenic latency. Alternatively, virus replication may stimulate an immune response which the virus utilizes to traffic throughout the mouse (see Discussion, below).
It is possible that the viral genome-positive lung B cells detected by the LDPCR assay may reflect some form of abortive infection. To partially address this issue, we ran the LDPCR assay on bulk lung cells at day 16 post-G50.stop infection using primers to gene 72 (v-cyclin) as well as our standard primers to gene 50 (Rta). Using primer sets to these unlinked genes, we detected nearly identical frequencies of viral genome-positive cells in the lungs of mice infected with G50.stop (Fig. 1E), consistent with the presence of the entire genome in these cells. However, we cannot rule out the possibility that the viral genomes present are either defective or integrated. Notably, however, we have detected a low frequency of cells harboring gene 73 (LANA) transcripts at day 16 postinfection in lung cells of mice infected with G50.stop (S. M. Dickerson, R. D. Allen, and S. H. Speck, unpublished data), consistent with latency-associated viral gene expression. Thus, although it seems likely that the G50.stop virus establishes a latent infection in lung B cells, we currently cannot rule some form of nonfunctional/abortive infection resulting in stable carriage of the viral genome.
Treatment with the antiviral drug cidofovir prevents the establishment of γHV68 latency in the spleen.
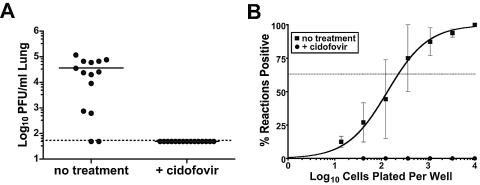
To assess whether the failure of the G50.stop virus to seed latency to the spleen reflects an absence of virus replication or a replication-independent function of the gene 50 product Rta, we blocked wild-type γHV68 replication with the antiviral drug cidofovir (2, 5). Mice were administered cidofovir the day before, the day of infection (1,000 PFU γHV68 i.n.), 2 days postinfection, and then every 3 days subcutaneously in the scruff of the neck at a dose of 25 mg/kg of body weight, as previously described (2, 5). At day 7 postinfection, no virus replication was detected in the lungs of cidofovir-treated mice as determined by plaque assay, while normal levels of lytic virus were detected in the untreated mice (Fig. 2A). At day 16 postinfection no latently infected cells could be detected in the spleens of cidofovir-treated mice, while normal levels of γHV68 latently infected cells were detected in untreated mice (Fig. 2B). Since cidofovir inhibits virus replication downstream of Rta expression, this analysis demonstrates that it is the absence of virus replication and not a replication-independent function of Rta that is responsible for the failure to establish splenic latency with the G50.stop virus.
FIG. 2.
Analysis of γHV68 lytic replication and latency in mice treated with the antiviral drug cidofovir. Mice were given subcutaneous injections of cidofovir (25 mg/kg; Vistide; Gilead Sciences, Foster City, CA) starting at day −1, day 0, and day 2 followed by injections every third day. On day zero, mice were infected with 1,000 PFU γHV68 i.n. (A) Lung tissue was harvested at day 7 postinfection and the titer was determined on NIH 3T12 cells (ATCC CCC 164) as previously described (8). Each data point represents a mouse. (B) Bulk spleen cells were harvested at day 16 postinfection and analyzed for the frequency of cells harboring viral genome as determined by LDPCR as previously described.
Infection with G50.stop does not stimulate an immune response.
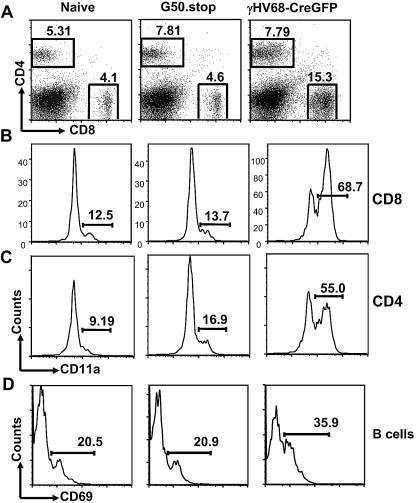
We next determined whether, in the absence of virus replication, the virus would initiate an immune response. At 14 days postinfection, we isolated lung cells from either naïve (mock infected; received a dose of medium i.n.), G50.stop-infected, or γHV68-CreGFP-infected mice by collagenase digestion and analyzed the levels of T-cell activation. In the control γHV68-CreGFP-infected mice, there was an increase in the percentage of CD8+ T cells found in the lungs, and the majority of these cells were CD11a (LFA-1) high (Fig. 3A and B). There was no change identified in the percentage of CD8+ T cells in the lungs of G50.stop-infected mice compared to naïve mice, and there was no increase in the percentage of CD8+ T cells that were activated (Fig. 3A and B). We only looked at the gross activation of the immune response, since there are only two known CD8 T-cell epitopes in C57BL/6 mice and these are directly against lytic cycle-associated proteins (ORF6 and ORF61). Notably, gene chip analyses have demonstrated that these antigens are not expressed upon infection with G50.stop virus (R. D. Allen and S. H. Speck, unpublished data). There was also no change in the percentage of CD4+ T cells that were activated in the G50.stop-infected mice compared to the naïve mice, while the majority of CD4+ T cells in the mice that were infected with γHV68-CreGFP were activated, as shown by CD11a staining (Fig. 3A and C). We also could not detect any increase in B-cell activation in the lungs of G50.stop-infected mice compared to naïve mice, whereas approximately 40% of the B cells isolated from the lungs of wild-type virus-infected mice were activated (Fig. 3D).
FIG. 3. .
Determination of the immune response to G50.stop. Lungs were harvested from naïve mice and mice infected with G50.stop and γHV68-CreGFP at day 14 postinfection, collagenase digested, and stained for flow cytometry analysis. Lung cells were stained with anti-mouse CD8, anti-mouse CD4, and anti-CD11a (all from BD Pharmingen) and analyzed on a FACSCalibur (BD Biosciences Immunocytometry Systems, San Jose, CA). Data from representative mice are shown (a total of six mice were individually analyzed for each condition in two independent experiments). (A) Collagenase-digested lung cells were gated on CD8+ and CD4+ T cells. Numbers represent percentages of lung cells. (B) Plots gated on CD8+ T cells. Numbers represent percentages of CD11ahi CD8+ T cells. Among the mice analyzed, there was a statistically significant difference between the percentage of CD11ahi CD8+ T cells between naïve and γHV68-CreGFP-infected mice (P < 0.0001), but not between naïve and G50.stop virus-infected mice (P = 0.58). (C) Plots gated on CD4+ T cells. Numbers represent percentages of CD11ahi CD4+ T cells. Among the mice analyzed, there was a statistically significant difference between the percentage of CD11ahi CD4+ T cells between naïve and γHV68-CreGFP-infected mice (P < 0.0001), but not between naïve and G50.stop virus-infected mice (P = 0.20). (D) Plots gated on B cells. The numbers represent percentages of CD69hi B cells. Among the mice analyzed, there was a statistically significant difference between the percentage of CD69hi B cells between naïve and γHV68-CreGFP-infected mice (P = 0.0013), but not between naïve and G50.stop virus-infected mice (P = 0.50).
Vaccination with G50.stop is unable to protect against wild-type γHV68 challenge.
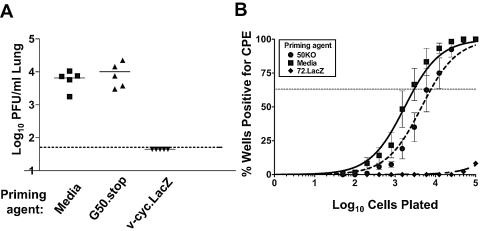
γHV68 ORF 72 encodes v-cyclin which, when interrupted by the insertion of a β-galactosidase expression cassette (or a translation stop codon), is severely compromised in its ability to reactivate from latency (23). Previously it has been shown that priming with the reactivation-defective virus v-cyclin.LacZ protected against both lytic virus replication and the establishment of latency upon a low-dose challenge with wild-type γHV68 (21). We wanted to determine whether G50.stop was able to protect against lytic virus replication and establishment of latency following an intranasal challenge with wild-type virus, despite our not being able to detect any activation of the immune system following infection with G50.stop. Mice were initially primed with either medium, G50.stop, or v-cyclin.LacZ. At 28 days postinfection, the mice were challenged with 100 PFU of wild-type γHV68 via intranasal inoculation and analyzed for their ability to control both lytic virus replication and the establishment of latency. At day 7 postchallenge, the mice primed with v-cyclin.LacZ had no detectable acute virus replication in the lungs, while mice primed with either medium alone or G50.stop virus had similar levels of acute virus replication in the lungs (Fig. 4A). At day 16 postchallenge, we analyzed the level of latency established by wild-type γHV68 using a limiting dilution reactivation assay (25). The reactivation assay consists of limiting dilution plating of spleen cells on a monolayer of MEFs, which are permissive for virus growth, and monitoring the wells for CPE on the MEF monolayer. Also plated in parallel are samples of cells that have been hypotonically lysed and mechanically disrupted, to distinguish between preformed infectious virus and virus reactivating from latency. In the analysis presented here, there were no significant levels of preformed infectious virus detected in any of the samples (data not shown). Notably, as previously reported (13), the v-cyclin.LacZ virus was able to protect against the establishment of latency by wild-type γHV68. However, priming with G50.stop virus did not protect against establishment of latency upon challenge with wild-type virus (Fig. 4B). The latter is completely consistent with the failure to detect any activation of the immune system following infection with G50.stop virus (Fig. 3).
FIG. 4.
Comparison of vaccination with G50.stop and v-cyclin.LacZ. Mice were primed with either medium, 940 PFU v-cyclin.LacZ, or 940 PFU G50.stop i.n. 28 days prior to challenge. Mice were challenged with 100 PFU wild-type γHV68 i.n. (A) Lung tissue was harvested at day 7 postinfection, and the titer was determined on NIH 3T12 cells. Each data point represents a mouse. (B) Bulk spleen cells were harvested at day 16 postinfection and analyzed for cells reactivating virus by an ex vivo limiting dilution reactivation assay as previously described (18, 19). Data are representative of at least two independent experiments. A statistically significant difference was observed between the frequency of viral genome-positive cells in control mice (media) and those immunized with the v-cyclin.LacZ virus (P = 0.0003), but not mice immunized with the G50.stop virus (P = 0.50).
Conclusions.
Here we have shown that γHV68 with a stop mutation in the gene encoding the immediate-early activator Rta (G50.stop) appears to establish a long-term latent infection in the lungs of mice following intranasal infection. As expected, this virus was completely defective for virus replication in vivo, consistent with the previous demonstration that it is essential for virus replication in tissue culture (9). The lack of virus replication in vivo prevented the establishment of latency in the spleen, even at late times postinfection. It should be noted that we cannot rule out the possibility that the presence of viral genome-positive cells might reflect abortive infection of lung B cells. If the latter is true, it is clearly a stable association of the viral genome with these cells. Notably, in independent analyses we have detected a low frequency of ORF73 (LANA) transcripts in lung cells of mice infected with G50.stop at day 16 postinfection (unpublished data), consistent with latency-associated viral gene expression.
To determine whether the block in the establishment of latency in the spleen was specific to the deletion of Rta or reflected the lack of virus replication, mice were treated with the antiviral drug cidofovir. Treatment of mice prior to, during, and after intranasal infection with wild-type virus blocked both lytic virus replication in the lung and the establishment of latency in the spleen. There were also reduced levels of latently infected cells detected in the lungs (data not shown). These data provide strong evidence that virus replication is necessary for the trafficking of virus from the lungs to other tissues in the mouse but that Rta and virus replication are not required for the establishment of latency at the site of infection. It is unclear whether virus trafficking to secondary sites requires virus replication at the primary site of infection (e.g., lung or peritoneum) with the subsequent induction of a viremia and seeding of virus to peripheral tissues, or if trafficking of latently infected cells which are then reactivated leads to virus replication in secondary tissues. Notably, establishment of splenic latency in B-cell-deficient mice is severely impaired following intranasal inoculation (22, 26) and can be rescued by adoptively transferring B cells (18). However, acute virus replication in the lungs is not impaired by the absence of B cells (22), directly implicating infected B cells as the major mechanism in trafficking virus from the lung to the spleen.
A previous study has shown that γHV68 with a stop mutation in ORF31 (ORF31STOP) is replication defective in vitro (9). In a recently published study (4), when mice were infected with ORF31STOP via intranasal inoculation, no latent virus was detected in the spleen. Differing from our analysis with G50.stop, ORF31STOP was able to establish low-level latency in the spleen following intraperitoneal infection. While both mutant viruses are replication defective, ORF31STOP is likely to express immediate-early and early genes whose expression may lead to a limited immune response, and this may facilitate trafficking of latently infected cells to the spleen following infection by the intraperitoneal route, but not following intranasal inoculation. The data on replication-defective γHV68 in vivo are somewhat distinct from those observed with replication-impaired alpha- and betaherpesviruses (6, 7, 10, 12). A possible difference is that both alpha- and betaherpesviruses encode several critical immediate-early regulators of viral gene expression (13, 17), while γHV68 (and KSHV) appears to encode a single major lytic transactivator, Rta (15, 31).
With respect to utilizing replication-defective gammaherpesviruses as vaccines, we have shown that G50.stop fails to stimulate an acquired immune response in the lungs as demonstrated by a lack of activation of CD4+ and CD8+ T cells as well as B cells. This lack of activation of the immune response correlated with an inability of G50.stop to successfully vaccinate mice against a challenge with wild-type γHV68. Thus, this raises the question of whether replication-defective gammaherpesviruses, despite their ability to establish a long-term latent infection at the site of inoculation, are viable vaccine candidates. It is possible that they may be effective when administered at high inoculating doses. In addition, inactivation of an essential late gene, rather than an immediate-early gene, would allow expression of a wide array of gene products associated with virus replication that may limit virus replication during the early stages of infection.
Acknowledgments
This research was supported by NIH grants CA52004 and CA95318 to S.H.S. and the Yerkes National Primate Research Center base grant P51 RR00165. J.M.M. is supported by a postdoctoral fellowship from the American Cancer Society, and M.L.F. was supported by a postdoctoral fellowship from the Leukemia and Lymphoma Society.
We thank Michael Hulsey for his assistance and expertise with fluorescence-activated cell sorting and members of the Speck lab for helpful comments on this research.
REFERENCES
- 1.Adler, H., M. Messerle, and U. H. Koszinowski. 2001. Virus reconstituted from infectious bacterial artificial chromosome (BAC)-cloned murine gammaherpesvirus 68 acquires wild-type properties in vivo only after excision of BAC vector sequences. J. Virol. 75:5692-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dal Canto, A. J., H. W. t. Virgin, and S. H. Speck. 2000. Ongoing viral replication is required for gammaherpesvirus 68-induced vascular damage. J. Virol. 74:11304-11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinoza, J. C., and J. Kuznar. 2002. Rapid simultaneous detection and quantitation of infectious pancreatic necrosis virus (IPNV). J. Virol. Methods 105:81-85. [DOI] [PubMed] [Google Scholar]
- 4.Flano, E., Q. Jia, J. Moore, D. L. Woodland, R. Sun, and M. A. Blackman. 2005. Early establishment of gamma-herpesvirus latency: implications for immune control. J. Immunol. 174:4972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangappa, S., S. B. Kapadia, S. H. Speck, and H. W. t. Virgin. 2002. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J. Virol. 76:11460-11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghazal, P., A. E. Visser, M. Gustems, R. Garcia, E. M. Borst, K. Sullivan, M. Messerle, and A. Angulo. 2005. Elimination of ie1 significantly attenuates murine cytomegalovirus virulence but does not alter replicative capacity in cell culture. J. Virol. 79:7182-7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino, Y., S. K. Dalai, K. Wang, L. Pesnicak, T. Y. Lau, D. M. Knipe, J. I. Cohen, and S. E. Straus. 2005. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 79:410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacoby, M. A., H. W. t. Virgin, and S. H. Speck. 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J. Virol. 76:1790-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia, Q., T. T. Wu, H. I. Liao, V. Chernishof, and R. Sun. 2004. Murine gammaherpesvirus 68 open reading frame 31 is required for viral replication. J. Virol. 78:6610-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavail, J. H., A. N. Tauscher, J. W. Hicks, O. Harrabi, G. T. Melroe, and D. M. Knipe. 2005. Genetic and molecular in vivo analysis of herpes simplex virus assembly in murine visual system neurons. J. Virol. 79:11142-11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, S., I. V. Pavlova, H. W. t. Virgin, and S. H. Speck. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J. Virol. 74:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 13.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 14.Moser, J. M., J. W. Upton, R. D. Allen III, C. B. Wilson, and S. H. Speck. 2005. Role of B-cell proliferation in the establishment of gammaherpesvirus latency. J. Virol. 79:9480-9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlova, I. V., H. W. t. Virgin, and S. H. Speck. 2003. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J. Virol. 77:5731-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffen, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 18.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibbetts, S. A., J. Loh, V. Van Berkel, J. S. McClellan, M. A. Jacoby, S. B. Kapadia, S. H. Speck, and H. W. t. Virgin. 2003. Establishment and maintenance of gammaherpesvirus latency are independent of infective dose and route of infection. J. Virol. 77:7696-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tibbetts, S. A., J. S. McClellan, S. Gangappa, S. H. Speck, and H. W. t. Virgin. 2003. Effective vaccination against long-term gammaherpesvirus latency. J. Virol. 77:2522-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usherwood, E. J., J. P. Stewart, K. Robertson, D. J. Allen, and A. A. Nash. 1996. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J Gen. Virol. 77:2819-2825. [DOI] [PubMed] [Google Scholar]
- 23.van Dyk, L. F., H. W. t. Virgin, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virgin, H. W. t., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. I. Virgin. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weck, K. E., S. S. Kim, H. I. Virgin, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West, J. T., and C. Wood. 2003. The role of Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 regulator of transcription activation (RTA) in control of gene expression. Oncogene 22:5150-5163. [DOI] [PubMed] [Google Scholar]
- 28.Wheelock, E. F., and I. Tamm. 1961. Enumeration of cell-infecting particles of Newcastle disease virus by the fluorescent antibody technique. J Exp. Med. 113:301-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willer, D. O., and S. H. Speck. 2003. Long-term latent murine gammaherpesvirus 68 infection is preferentially found within the surface immunoglobulin D-negative subset of splenic B cells in vivo. J. Virol. 77:8310-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu, T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu, Y., D. P. AuCoin, A. R. Huete, S. A. Cei, L. J. Hanson, and G. S. Pari. 2005. A Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50 deletion mutant is defective for reactivation of latent virus and DNA replication. J. Virol. 79:3479-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]