Abstract
Herpes simplex virus type 1 (HSV-1) ICP27 protein is an essential regulator of viral gene expression with roles at various levels of RNA metabolism in the nucleus. Using the tethered function assay, we showed a cytoplasmic activity for ICP27 in directly enhancing mRNA translation in vivo in the absence of other viral factors. The region of ICP27 required for translational stimulation maps to the C terminus. Furthermore, in infected cells, ICP27 is associated with polyribosomes, indicating a function in translation during the lytic cycle.
The multifunctional ICP27 phosphoprotein (40) is essential for herpes simplex virus type 1 (HSV-1) replication, has counterparts in all the herpesvirus families (3, 15), and is necessary for efficient expression of early (37) and late (17, 20, 26, 27, 31) viral genes. While ICP27 influences viral transcription (12, 17, 24), much evidence indicates that it acts posttranscriptionally. An RNA binding protein (23, 36), ICP27 inhibits pre-mRNA splicing (2, 11, 14, 34, 35), stimulates pre-mRNA 3′ processing (18, 19), affects mRNA stability (1), and shuttles between the nucleus and the cytoplasm (25), promoting viral RNA nuclear export (4, 13, 32, 35). An RGG box is required for RNA binding (23, 32), and C-terminal regions, including a zinc finger-like motif, have roles in transactivation and repression of reporter and viral genes (1, 27-28). Interestingly, two recent reports have provided indirect evidence for a role in the cytoplasm. First, ICP27 was shown to stimulate the polysomal association of HSV-1 VP16 RNA and the levels of VP16 protein (6); however, it remains to be determined whether this is due to a direct effect of ICP27 on translation. Second, association of ICP27 with translation factors eIF3, eIF4G, and cytoplasmic poly(A)-binding protein 1 (PABP) was reported (7), suggesting a potential role in viral and/or host mRNA translation or mRNA stability. Here we present data demonstrating that ICP27 directly stimulates mRNA translation.
ICP27 expressed from wild-type (wt) virus cosediments with polyribosomes.
To determine whether ICP27 protein is associated with the translation machinery in HSV-1-infected cells, sucrose gradient analysis was used to separate polyribosomes from monoribosomes and uncomplexed ribosomal subunits. BHK-21 cells were either mock infected or infected with wt virus or the ICP27 mutant M15, carrying missense mutations (P465L and G466E) within the C-terminal zinc finger region, which is deficient in expression of certain viral late genes (28). After 5 h, cycloheximide was added to arrest protein synthesis and to fix polyribosomes (30). Cell extracts were prepared and fractionated on sucrose gradients, and fractions were analyzed by Western blotting as described previously (33). The UV absorbance profile from mock-infected cells is shown in Fig. 1A; infected-cell extracts showed slightly reduced levels of polyribosomes (data not shown), presumably due to shutoff of host protein synthesis as a consequence of infection. The polyribosome distribution of ICP27 was compared with that of PABP, a general translation factor for poly(A)+ mRNA (8, 16). With all extracts, PABP was present across the gradient from polyribosomal to mRNP fractions (Fig. 1B) (33) and moves to the lighter fractions following EDTA treatment (33), which dissociates monosomes and polyribosomes into ribosomal subunits (data not shown). In extracts of wt-infected cells, ICP27 was also present in the heavier 80S ribosome and polyribosomal fractions (Fig. 1B). Furthermore, ICP27 that was associated with the polysomal and heavier fractions was released following EDTA treatment and moved to the lighter fractions, similar to PABP (data not shown), indicating an association with the cellular translational machinery. In contrast, the M15 mutant protein was absent from the polyribosome fractions (Fig. 1B). As M15 protein has been reported to have an exclusively nuclear distribution (22), we cannot rule out a degree of nuclear contamination during extract preparation contributing to the M15 protein cosedimenting with mRNP fractions. The association of ICP27 with polyribosomes is indicative of a function in mRNA stability or translation.
FIG. 1.
ICP27 is associated with polyribosomes in infected cells. Cytoplasmic extracts of BHK cells either mock infected or infected with wt or M15 mutant virus were fractionated on 10% to 50% sucrose gradients, and fractions were examined by Western blotting using a mixture of anti-ICP27 1119 and 1113 monoclonal antibodies (Goodwin Institute for Cancer Research, Plantation, Fla.) or anti-PABP 10E10 antibody (9), kindly supplied by G. Dreyfuss. (A) UV absorbance profile (254 nm) of gradient fractions from mock-infected cells; the positions of polysomes, 80S monosomes, 60S and 40S ribosomal subunits, and mRNPs are indicated. (B) Western blot analysis of gradient fractions following mock infection or infection with the indicated viruses.
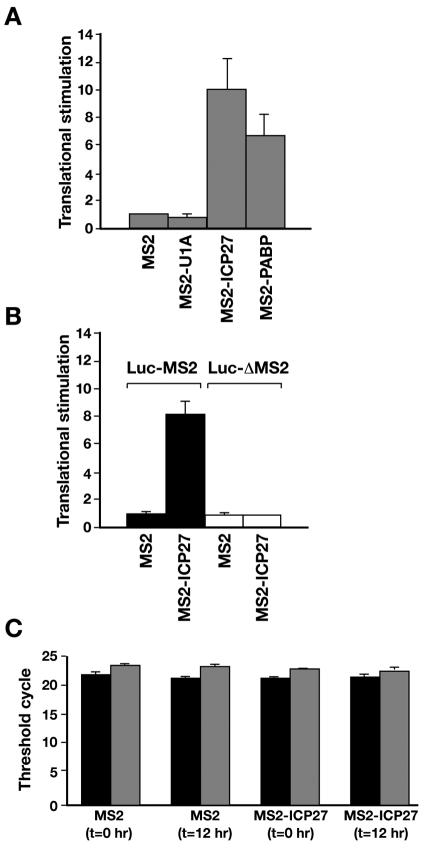
ICP27 enhances translation in vivo.
The widely used tethered function assay of Xenopus oocytes (5, 10, 33, 41) was employed to test the effect of ICP27 on translation in vivo. This allows putative translational regulators to be studied independently of any action on transcription, polyadenylation, or mRNA export and without knowledge of any natural RNA binding target. The assay has two components: a luciferase reporter mRNA containing binding sites for the bacteriophage MS2 coat protein within its 3′ untranslated region and a fusion of MS2 coat protein with ICP27. Interaction of the MS2 coat protein with its target mRNA binding sites brings ICP27 to the mRNA. Luciferase activity is normalized against an internal standard by coinjection of a β-galactosidase reporter mRNA lacking MS2 sites. Reporter mRNAs were introduced directly into the cytoplasm of stage VI oocytes containing the following proteins expressed from previously injected RNAs (10): MS2 coat protein alone, MS2-tethered splicing factor U1A (MS2-U1A), PABP (MS2-PABP), or ICP27 (MS2-ICP27). Importantly, MS2-ICP27 stimulated reporter gene expression more than 10-fold compared with MS2 coat protein (Fig. 2A). By contrast, MS2-U1A, an unrelated RNA binding protein, showed no stimulation, while MS2-PABP, a known activator of translation in this assay (10, 41), also enhanced expression. Furthermore, the stimulatory effect of ICP27 required MS2 binding, as no effect was observed in the absence of MS2 binding sites in the reporter mRNA (Fig. 2B). Quantitative reverse transcription-PCR showed that the levels of reporter mRNA remained unchanged at 12 h postinjection in oocytes expressing either MS2 coat protein or MS2-ICP27 (Fig. 2C), and thus stimulation of luciferase activity is not a consequence of altered mRNA stability. These data show that ICP27 can directly stimulate the translation of mRNAs to which it is bound and establish that ICP27 is sufficient to stimulate translation in the absence of any other viral factors.
FIG. 2.
ICP27 stimulates translation in the tethered function assay. (A) Xenopus oocytes expressing MS2, MS2-U1A, MS2-PABP, or MS2-ICP27 were injected with a luciferase reporter mRNA containing MS2 coat protein binding sites (Luc-MS2) within its 3′ untranslated region and a β-galactosidase internal control mRNA (10). Shown are normalized luciferase/β-galactosidase activity ratios, with that of MS2 coat protein alone set as 1. The data represent the average stimulations from at least three independent experiments with standard errors. (B) mRNAs encoding MS2 coat protein or an MS2-ICP27 coat protein fusion were injected with β-galactosidase mRNA and either the reporter mRNA used for panel A (Luc-MS2; black bars) or a reporter lacking MS2 coat protein binding sites (Luc-ΔMS2; white bars [10]). The data represent the averages from three independent experiments with standard errors. (C) Oocytes expressing MS2 coat protein alone or the MS2-ICP27 fusion were injected with the Luc-MS2 and β-galactosidase reporter mRNAs (black and gray bars, respectively). Total RNA was extracted either immediately or after 12 h of incubation. Quantitative reverse transcription-PCR was performed with primers directed against the reporter mRNAs, using a TaqMan PCR machine (Applied Biosystems) linked to ABI Prism software. The threshold cycle shown is the number of amplification cycles required to obtain a detectable product in the linear range of the assay. The data represent the averages from three independent experiments with standard errors.
The ICP27 C terminus is required for stimulation of translation.
To define the ICP27 regions that contribute to this novel function, we examined the ability of MS2 fused with various ICP27 mutants to stimulate translation in the tethered function assay. A diagram of ICP27 and the mutants used is shown in Fig. 3A. Two well-studied ICP27 N-terminal deletions, d1-2 (29) and d4-5 (21), stimulated translation to an extent similar to that for the wt protein (Fig. 3B), suggesting that these regions do not contribute to the translational activity. In contrast, deletion of the C-terminal 105 amino acids (Δ407) led to a complete loss of translational stimulation (Fig. 3C); this deletion mutant lacks the putative zinc finger that is conserved in ICP27 counterparts of various herpesviruses (1, 38). Interestingly, the ICP27 missense mutations in M15 (P465L and G466E) and M16 (C488L) (28), which map to this region, also fail to stimulate translation (Fig. 3B), although they are efficiently expressed in oocytes (Fig. 3D). Importantly, ICP27 expressed from the M15 mutant is absent from polyribosomes (Fig. 1B). Thus, the activity of ICP27 as a translational activator correlates with its ability to associate with polyribosomes in HSV-1-infected cells, and both assays implicate the C-terminal region as essential for the ability of ICP27 to stimulate mRNA translation.
FIG. 3.

The ICP27 C terminus is required to stimulate translation. (A) Top, schematic diagram of ICP27 showing the locations of the RGG box (amino acids 138 to 152) and putative zinc finger region (amino acids 468 to 508). Bottom, horizontal bars represent sequences of the various ICP27 truncation mutants used. Gaps represent regions absent in the mutants, and locations of point mutations in the M15 (P465L, G466E) and M16 (C488L) mutants are shown by vertical bars. Deletions in the mutants are as follows: d1-2, amino acids 12 to 63; d4-5, amino acids 139 to 153; Δ407, amino acids 408 to 512. (B) and (C) MS2 coat protein or the indicated MS2-ICP27 fusion proteins were examined using the tethered function assay. The results are averages from at least three independent experiments with standard errors. (D) Xenopus oocytes were injected with RNA encoding MS2 fused to wild-type or mutants of ICP27 as indicated. Fusion protein expression was analyzed by [35S]methionine labeling in vivo and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of protein extracts (lanes 1 to 5) or by translation in vitro (lanes 6 and 7). The upper and lower arrows mark the positions of full-length and truncated proteins, respectively.
The ICP27 zinc binding region is involved in protein self-interaction (39, 42) and in interactions with cellular partners such as splicing factor SRp20 (34) and the nuclear export factor TAP (3), but whether any of these properties relate to translational stimulation remains to be determined. It is likely, however, that this region mediates an interaction between ICP27 and a component of the translation machinery. Currently we are elucidating the viral RNA targets, protein cofactors, and mechanism of ICP27-mediated translational stimulation and evaluating the contribution of this novel cytoplasmic effect to the viral life cycle.
Acknowledgments
This work was supported by MRC grant G9826324 to J.B.C. and MRC Ph.D. studentship award G78/7826 to O.L. N.K.G. is funded by an MRC Career Development Award and an MRC Senior Fellowship; G.S.W. is funded by a Beit Memorial Fellowship.
We thank William Richardson, Ross Anderson, Barbara Gorgoni, and Brian Collier for technical assistance.
Footnotes
This work is dedicated to the memory of J. Barklie Clements, who was co-corresponding author and died during revision of the manuscript. We dedicate this article to Barklie for his long-standing contribution to the herpesvirus field and for being a wonderful person and a great scientist. He will be sadly missed by all of us in his group, his friends, and his colleagues for his enthusiasm, encouragement, and support.
REFERENCES
- 1.Brown, C. R., M. S. Nakamura, J. D. Mosca, G. S. Hayward, S. E. Straus, and L. P. Perera. 1995. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J. Virol. 69:7187-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryant, H. E., S. E. Wadd, A. I. Lamond, S. J. Silverstein, and J. B. Clements. 2001. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J. Virol. 75:4376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, I. H., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 79:3949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, I. H., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 76:12877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coller, J. M., N. K. Gray, and M. P. Wickens. 1998. mRNA stabilization by poly(A)-binding protein is independent of poly(A) and requires translation. Genes Dev. 12:3226-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellison, K. S., R. A. Maranchuk, K. L. Mottet, and J. R. Smiley. 2005. Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J. Virol. 79:4120-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontaine-Rodriguez, E. C., T. J. Taylor, M. Olesky, and D. M. Knipe. 2004. Proteomics of herpes simplex virus infected cell protein 27: association with translation initiation factors. Virology 330:487-492. [DOI] [PubMed] [Google Scholar]
- 8.Gorgoni, B., and N. K. Gray. 2004. The roles of cytoplasmic poly(A)-binding proteins in regulating gene expression: a developmental perspective. Brief Funct. Genomic Proteomic 3:125-141. [DOI] [PubMed] [Google Scholar]
- 9.Gorlach, M., C. G. Burd, and G. Dreyfuss. 1994. The mRNA poly(A)-binding protein: localization, abundance, and RNA-binding specificity. Exp. Cell Res. 211:400-407. [DOI] [PubMed] [Google Scholar]
- 10.Gray, N. K., J. M. Coller, K. S. Dickson, and M. Wickens. 2000. Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J. 19:4723-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jean, S., K. M. LeVan, B. Song, M. Levine, and D. M. Knipe. 2001. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (gamma 2) genes in infected cells. Virology 283:273-284. [DOI] [PubMed] [Google Scholar]
- 13.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindberg, A., and J. P. Kreivi. 2002. Splicing inhibition at the level of spliceosome assembly in the presence of herpes simplex virus protein ICP27. Virology 294:189-198. [DOI] [PubMed] [Google Scholar]
- 15.Malik, P., D. J. Blackbourn, and J. B. Clements. 2004. The evolutionarily conserved Kaposi's sarcoma-associated herpesvirus ORF57 protein interacts with REF protein and acts as an RNA export factor. J. Biol. Chem. 279:33001-33011. [DOI] [PubMed] [Google Scholar]
- 16.Mangus, D. A., M. C. Evans, and A. Jacobson. 2003. Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 4:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy, A. M., L. McMahan, and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J. Virol. 63:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGregor, F., A. Phelan, J. Dunlop, and J. B. Clements. 1996. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J. Virol. 70:1931-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLauchlan, J., A. Phelan, C. Loney, R. M. Sandri-Goldin, and J. B. Clements. 1992. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J. Virol. 66:6939-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahan, L., and P. A. Schaffer. 1990. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J. Virol. 64:3471-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mears, W. E., V. Lam, and S. A. Rice. 1995. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 69:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242:128-137. [DOI] [PubMed] [Google Scholar]
- 23.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 70:7445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olesky, M., E. E. McNamee, C. Zhou, T. J. Taylor, and D. M. Knipe. 2005. Evidence for a direct interaction between HSV-1 ICP27 and ICP8 proteins. Virology 331:94-105. [DOI] [PubMed] [Google Scholar]
- 25.Phelan, A., and J. B. Clements. 1997. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J. Gen. Virol. 78:3327-3331. [DOI] [PubMed] [Google Scholar]
- 26.Rice, S. A., and D. M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 62:3814-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice, S. A., V. Lam, and D. M. Knipe. 1993. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J. Virol. 67:1778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers, J., and H. Munro. 1987. Translation of ferritin light and heavy subunit mRNAs is regulated by intracellular chelatable iron levels in rat hepatoma cells. Proc. Natl. Acad. Sci. USA 84:2277-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandri-Goldin, R. M. 1998. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanford, J. R., N. K. Gray, K. Beckmann, and J. F. Caceres. 2004. A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 18:755-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sciabica, K. S., Q. J. Dai, and R. M. Sandri-Goldin. 2003. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 22:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, R. W., P. Malik, and J. B. Clements. 2005. The herpes simplex virus ICP27 protein: a multifunctional post-transcriptional regulator of gene expression. Biochem. Soc. Trans. 33:499-501. [DOI] [PubMed] [Google Scholar]
- 36.Sokolowski, M., J. E. Scott, R. P. Heaney, A. H. Patel, and J. B. Clements. 2003. Identification of herpes simplex virus RNAs that interact specifically with regulatory protein ICP27 in vivo. J. Biol. Chem. 278:33540-33549. [DOI] [PubMed] [Google Scholar]
- 37.Uprichard, S. L., and D. M. Knipe. 1996. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J. Virol. 70:1969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaughan, P. J., K. J. Thibault, M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. The herpes simplex virus immediate early protein ICP27 encodes a potential metal binding domain and binds zinc in vitro. Virology 189:377-384. [DOI] [PubMed] [Google Scholar]
- 39.Wadd, S., H. Bryant, O. Filhol, J. E. Scott, T. Y. Hsieh, R. D. Everett, and J. B. Clements. 1999. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J. Biol. Chem. 274:28991-28998. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox, K. W., A. Kohn, E. Sklyanskaya, and B. Roizman. 1980. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J. Virol. 33:167-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkie, G. S., P. Gautier, D. Lawson, and N. K. Gray. 2005. Embryonic poly(A)-binding protein stimulates translation in germ cells. Mol. Cell. Biol. 25:2060-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhi, Y., K. S. Sciabica, and R. M. Sandri-Goldin. 1999. Self-interaction of the herpes simplex virus type 1 regulatory protein ICP27. Virology 257:341-351. [DOI] [PubMed] [Google Scholar]