Abstract
We have sequenced the entire genome of herpesvirus papio 2 (HVP-2; Cercopithecine herpesvirus 16) strain X313, a baboon herpesvirus with close homology to other primate alphaherpesviruses, such as SA8, monkey B virus, and herpes simplex virus (HSV) type 1 and type 2. The genome of HVP-2 is 156,487 bp in length, with an overall GC content of 76.5%. The genome organization is identical to that of the other members of the genus Simplexvirus, with a long and a short unique region, each bordered by inverted repeats which end with an “a” sequence. All of the open reading frames detected in this genome were homologous and colinear with those of SA8 and B virus. The HSV gene RL1 (γ134.5; neurovirulence factor) is not present in HVP-2, as is the case for SA8 and B virus. The HVP-2 genome is 85% homologous to its closest relative, SA8. However, segment-by-segment bootstrap analysis of the genome revealed at least two regions that display closer homology to the corresponding sequences of B virus. The first region comprises the UL41 to UL44 genes, and the second region is located within the UL36 gene. We hypothesize that this localized and defined shift in homology is due to recombination events between an SA8-like progenitor of HVP-2 and a herpesvirus species more closely related to the B virus. Since some of the genes involved in these putative recombination events are determinants of virulence, a comparative analysis of their function may provide insight into the pathogenic mechanism of simplexviruses.
Herpesvirus papio 2 (HVP-2; Cercopithecine herpesvirus 16) is a simian alphaherpesvirus (family Herpesviridae; subfamily Herpesvirinae). HVP-2 was first isolated in 1995 during an outbreak in a captive colony of African baboons (23), and it was initially misidentified as SA8 virus (Cercopithecine herpesvirus 2), an alphaherpesvirus that also infects primates of the genus Papio (21, 27, 28). Based on a difference in sequence and antigenicity of the glycoproteins B, D, and J, HVP-2 was subsequently recognized as a distinct virus species (13). HVP-2 and SA8 share close homology with the monkey B virus (Cercopithecine herpesvirus 1) and human herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2). These viruses have been grouped in the genus Simplexvirus, which also includes Bovine herpesvirus 2, Saimiriine herpesvirus 1, and Ateline herpesvirus 1 (51).
As with other members of the genus Simplexvirus, HVP-2 causes orogenital and neuronal infections that closely resemble those caused by HSV in humans (13, 14, 23, 29). The prevalence of HVP-2 in baboons is about 90%, but clinical disease is much less common (14). Neither SA8 nor HVP-2 is virulent in primates other than their natural host. However, B virus causes lethal encephalomyelitis when it is transmitted from its natural host, Asian macaques, to other primates, including humans (8, 54). In mice, SA8 is not known to produce disease (45), while both HVP-2 and B virus have the ability to cause encephalitis, but the severity of infection varies widely among different strains (16, 46, 47). This broad spectrum of pathogenicity is an interesting feature of the genus Simplexvirus, and comparison of different strains may lead to the identification of simplexvirus genes responsible for neurovirulence.
Comparison of the complete genome sequences of HSV-1 (11, 12, 31, 32, 35, 43), HSV-2 (1, 12, 15, 33, 34), B virus (42), and SA8 (50) has confirmed that these viruses are closely related and all of their genes are homologous and colinear. The only notable difference is that the simian viruses, SA8 and B virus, lack the RL1 (γ134.5) open reading frame. In HSV-1 and HSV-2, RL1 codes for a protein that has been shown to possess two genetically separable functions: neurovirulence in mice (5) and inhibition of the protein kinase R (PKR) system of the host cell (6). The interferon-induced PKR system shuts down the host cell protein synthesis in response to the presence of double-stranded viral RNA, and it is therefore an important innate mechanism of defense against viral infection from a variety of RNA and DNA viruses (for a review, see reference 7). Since inhibition of the PKR system seems to be essential for proper replication of HSV-1 in cell culture (6), the lack of RL1 may indicate a significant difference of the pathogenetic mechanism in the simian viruses.
Since HVP-2 appears to be most closely related to SA8 (4), its complete genomic sequence is useful in the study of the evolution of alphaherpesviruses, as well as for determining areas in the genome that may be important for pathogenesis, by comparison with related viruses. We present in this paper the complete sequencing of the genome of HVP-2 (strain X313) and a comparative analysis with the genomes of SA8 and B virus. Strain X313 was isolated during an outbreak in a captive baboon colony (13, 23) and has been shown to be neurovirulent in mice (47).
MATERIALS AND METHODS
Viruses and cells.
HVP-2 virus strain X313 was cultured in Vero cells (ATCC number CCL-81). HVP-2 DNA was prepared from confluent monolayers that were infected at a multiplicity of infection of 1 and incubated until the cytopathic effect was close to 100%, based on the technique previously described (50).
Sequencing and analysis.
The genome of HVP-2 was sequenced as described earlier for the sequencing of the SA8 genome (50). Briefly, a random shotgun library was used in combination with PCR for gap closure and sequence verification. The final sequencing redundancy was approximately 6.5-fold for the entire genome and 10- to 15-fold coverage for the inverted repeat regions. The genomic termini were identified by end-repairing the genomic DNA with T4 and Klenow DNA polymerases (New England Biolabs), followed by digestion with ClaI or AvrII (New England Biolabs) and cloning into a suitably prepared pBluescript vector (Stratagene). A minimum of six independent clones were sequenced for each terminus. Sequence analysis was performed as previously described (50), and the software package Mega3 (22) was used for phylogenetic analysis. SimPlot (version 2.5; distributed by the author, Stuart C. Ray, Division of Infectious Diseases, John Hopkins University School of Medicine) in conjunction with PHYLIP (Phylogeny Inference Package, version 3.6; distributed by the author, J. Felsenstein, Department of Genome Sciences, University of Washington, Seattle) was used for the identification of potential recombination events.
Southern blotting.
For the identification of terminal fragments, genomic DNA was digested with ClaI and/or AvrII (New England Biolabs) and the fragments were separated on a 1.5% agarose gel. Following transfer to a nylon membrane (Hybond N+; Amersham Biosciences), the blots were probed with PCR products specific for the terminal ends of either the short or long repeat units and developed using the ECL Direct Labeling and Detection System (Amersham Biosciences).
Nucleotide sequence accession number.
The genome sequence of HVP-2 strain X313 as described in this work was deposited in GenBank under accession number DQ149153.
RESULTS AND DISCUSSION
Genomic organization.
The genome of HVP-2 strain X313 was 156,487 bp in length, as sequenced by us. As expected, the HVP-2 genome illustrated the typical genetic arrangement of simplexviruses: a unique long (UL) and unique short (US) regions, each delimited by long (RL) and short inverted repeats. Also typical of the other herpesviruses was the presence of four isomers that differed in the relative orientation of the UL and US regions, the result of homologous recombination at the inverted repeats. The overall GC content of the HVP-2 genome was 76.5%, similar to the GC content of SA8. Like the other simplexviruses, the GC content was higher in the regions containing inverted repeats (80.2%).
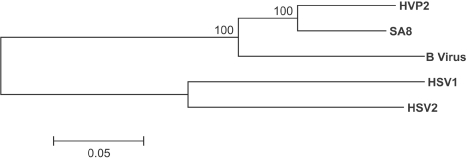
As expected, the overall identity at the DNA level was greatest (85.5%) with the SA8 virus (GenBank accession no. AY714813) (50) and was 79.1%, 60.1%, and 61.4% when compared with B virus (NC_004812) (42), HSV-1 (NC_001806) (11, 12, 31, 32, 35, 42), and HSV-2 (NC_001798) (1, 12, 15, 33, 34), respectively. Figure 1 illustrates the phylogenetic relationship of these five primate alphaherpeviruses based on their entire genomic sequences. Of note is the very close homology between HVP-2 and SA8, which is closer than the homology between HSV-1 and HSV-2, confirming a previous prediction based on partial sequencing of the HVP-2 open reading frames (4).
FIG. 1.
Phylogenetic relationship among simplexviruses. Whole genome alignments of HVP-2, SA8, B virus, HSV-1, and HSV-2 were compiled and compared using Mega v3.0 with ClustalW, with a gap-opening penalty of 20 and a gap extension penalty of 1 for both the pairwise and multiple parameters. Bootstrap analysis was performed using neighbor joining and the Kimura 2-parameter model with 500 replicates. Gaps or missing data were treated with complete deletion of the sites for the analysis. The resulting bootstrap values are indicated on the dendrogram.
As in the case of SA8 (50), the genome of HVP-2 was found to contain numerous sets of short (2- to 66-bp) tandem repeat sequences. In this strain, 32 sets of perfect repeats were present and an additional 73 sets of imperfect repeats were also identified in which one or more units diverged slightly from the consensus sequence (data not shown). As previously reported, a similar analysis of the published sequences for HSV-1, HSV-2, and B virus was performed and a total of 39, 49, and 76 sets were detected, respectively, including perfect and imperfect repeats (50).
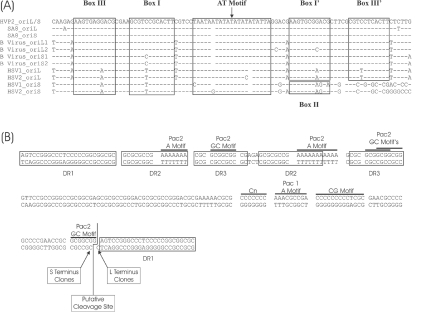
Three single-copy putative origins of replication were detected in the HVP-2 genome, one in the unique long region (oriL) and two in the short inverted repeat regions (oriS). oriL consisted of an almost perfect inverted repeat sequence of 179 bp in length. oriS had an identical sequence, but the inverted repeat extended to 199 bp. The HVP-2 putative origins of replication were highly homologous to the core sequence of 96 bp for the origin sequences of SA8, B virus, and the oriL of HSV-1 and HSV-2. A comparative alignment of the simplexvirus origin core regions and the putative binding sites for the origin binding protein (19) are shown in Fig. 2A.
Fig. 2.
Origins of replication and the “a” sequence of HVP-2. (A) Comparison of the origins of replication for HVP-2, SA8, B virus, HSV-1 and HSV-2. The boxes indicate the sites that bind to origin binding proteins in HSV-1, as previously defined (11, 32). (B) Sequence and features of the “a” region of HVP-2. The boxes indicate the various direct repeats (DR) identified, and the lines above the sequence highlight specific motifs of interest.
Copies of the “a” sequences carrying the DNA cleavage/packaging signals (10, 39, 52) were present at the genomic termini and at the internal junction between the inverted repeats. The “a” sequence of HVP-2 is shown in Fig. 2B, which also highlights the pac1 and pac2 motifs found in most herpesviruses (10, 39, 52) and the sets of direct repeats. Figure 2B also shows the putative site of cleavage of concatemers and the structure of the left and right genomic termini, with a GC 3′ overhang. This site was inferred from the sequences of the cloned genomic termini by using the method previously described for SA8 (50) and HSV-1 (40). Length and structure of “a” sequences and direct repeats are known to vary in different strains of HSV, and as such, the repeat arrangement reported for this HVP-2 genome may be a strain-specific feature.
In order to establish the presence of multiple copies of the “a” sequence at the genomic termini or in the internal L/S junction, we performed a Southern blot of intracellular HVP-2 DNA (containing both replicating and mature genomes), digested with the restriction enzyme AvrII and/or ClaI. The predicted length of the fragments produced in the presence of only one “a” sequence is 846 bp for the AvrII right terminus fragment, 1,769 bp for the ClaI left terminus fragment, and 2,615 bp for the internal junction digested with both ClaI and AvrII. The results of Southern blots hybridized with probes specific for the short or long termini show a ladder of at least three bands above the fragment corresponding to the left terminus (1,769, 1,995, and 2,221 bp) and the L/S junction (2,615, 2,841, and 3,067 bp), indicative of multiple copies of the “a” sequence (226 bp), but not above the fragment corresponding to the right terminus (data not shown). This result mirrors what was previously published for SA8 (50), and it confirms that in HVP-2, as in all simplexviruses studied to date (9, 40, 42, 50), multiple copies of the “a” sequence can be found, but not at the right terminus.
Open reading frames.
All open reading frames in the HVP-2 genome show considerable homology to, and are colinear with, the corresponding open reading frames of SA8. The map of the SA8 genome that we previously published (50) also correctly represents the feature map of HVP-2. The exact coordinates of the HVP-2 genes and of other features are reported in Table 1 in the supplemental material. Gene prediction algorithms (GeneMark) (3, 25), trained with the codon usage of HVP-2, SA8, and B virus open reading frames, did not detect any additional open reading frames in the genome, including the putative UL53A open reading frame described in the B virus genome (42).
Analysis of the B virus (42) and SA8 (50) genomes failed to identify a probable open reading frame corresponding to the neurovirulence gene RL1 (γ134.5) found in HSV-1 and HSV-2, and this was also the case for HVP-2. The lack of an RL1 homologue in these simian herpesviruses may be significant, as the gene product of RL1 has been shown to function as both a neurovirulence factor in mice (5) and an essential inhibitor of the interferon-induced protein kinase R system (6).
Taking advantage of the fact that the DNA sequences of HVP-2 and SA8 are so closely related to each other, we conducted a more thorough analysis of all the possible translations of the region of the missing RL1 gene on the assumption that if a functional gene were present in this area, it would likely be conserved between these two viruses. With the exception of regions corresponding to the location of RL2, no similarities were found that would indicate the presence of a conserved open reading frame or exons (data not shown). These results add evidence for the hypothesis that the region 5′ to the RL2 gene was noncoding in the progenitor of primate simplexviruses and that the RL1 open reading frame was acquired from a host or another virus by the common progenitor of human herpes simplex viruses: RL1, in fact, shows homology with mammalian GADD proteins (30).
The codon usage of the HVP-2 open reading frames is similar to that previously published for SA8 (50), B virus (42), and HSV (17, 31, 49). For each amino acid, the codons with the highest possible GC content are used in over 90% of occurrences and this maximizes the GC content of the genome The GC frequency was found to be 75.2%, 56.6%, and 93.7% for the first, second, and third codon positions, respectively, and this mirrors the frequencies of 74.5%, 55.9%, and 91.4% previously published for SA8 (50).
The location of the polyadenylation signals (AATAAA) in HVP-2 correspond almost exactly to those of SA8 (50). The only difference found was the presence of a single polyadenylation signal for the putative latency-associated transcript (LAT) large transcript in the short repeat sequence, as opposed to three in SA8. Interestingly, two polyadenylation signals in the LAT region and one in the untranscribed strand complementary to UL53 were conserved between SA8 and HVP-2, although they do not appear to be associated with any open reading frame. One of the sites in the LAT region (position 5373) is followed by a GT-rich region, characteristic of functional polyadenylation signals in both HVP-2 and SA8. The locations of all putative polyadenylation signals are listed in Table 1 in the supplemental material.
Both strands of the HVP-2 genome were also scanned for the presence of variants of the polyadenylation signal, as described by Beaudoing et al. (2), and 92 occurrences were identified (data not shown). However, only two instances of the sequence ATTAAA at the end of UL56 (positions 115,888 and 115,892) and one instance of the sequence CAUAAA downstream of US8A (position 146,404) may be of functional significance because they are located at the end of an opening reading frame and they are followed by GT-rich regions.
Evidence of previous recombination events in the HVP-2 genome.
Overall, HVP-2 is most closely related to SA8 at both the DNA (Fig. 1) and amino acid levels (see Table 1 in the supplemental material). However, the protein homology data indicate that the amino acid sequences of UL42, UL43, UL44, and UL36 of HVP-2 have a higher homology to the corresponding genes of the B virus than to those of SA8. In order to investigate the reason for this anomaly, we compared the DNA sequences of HVP-2, SA8, and B virus and a consensus sequence of HSV-1 and HSV-2 using the Bootscan function of SimPlot. SimPlot was originally developed to examine recombination in various human immunodeficiency virus isolates (24, 48) and has subsequently been applied to the study of recombination in other viruses (18, 53, 55) as well as to the analysis of clinical isolates of HSV-1 (70). The program works in conjunction with the phylogenetic analysis suite PHYLIP to plot the results of successive bootstrap analysis on a group of sequences by using a sliding window. This window is moved across the alignment in incremental steps until the entire alignment has been assessed.
The SimPlot Bootscan analysis of the full sequence alignment (not shown) showed that HVP-2 clustered with SA8 over most of the genome, with probability approaching 100%. However, close examination of the graph revealed that there were at least 2 areas of the alignment in which the HVP-2 sequence significantly deviates from this pattern.
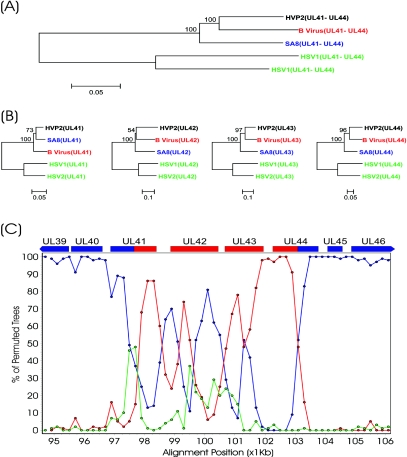
The first area comprises the UL41 to UL44 open reading frames that showed greater homology for B virus than for SA8. Figure 3C illustrates that in this region, the percentage of clustering of the HVP-2 sequence with SA8 dropped from the 100% levels, while the percentage of clustering with B virus increased. To confirm these results, we performed an independent bootstrap analysis of the segment of DNA corresponding to the area of inversion of homology, and the resulting dendrogram is presented in Fig. 3A. This region clearly clusters HVP-2 with B virus, which is in contrast to the clustering with SA8 when the entire genome is considered (Fig. 1). A similar analysis was performed at the amino acid level for the genes encompassed by this region (Fig. 3B). UL42, UL43, and UL44 were all found to more closely resemble their counterparts from the B virus than those from SA8.
Fig. 3.
Putative recombination event in the UL41/UL44 region of the HVP-2 genome. DNA-based bootstrap analysis of the UL41/UL44 region (A) and comparison of the individual proteins from this region (B) are shown. Alignments were compiled and compared using Mega v3.0 with ClustalW. Bootstrap analysis was performed using neighbor joining and the Kimura 2-parameter (DNA) or Poisson correction (protein) models with 500 replicates. The resulting bootstrap values are indicated on the dendrograms. (C) SimPlot analysis of the UL41/UL44 region. Bootscan analysis was conducted on the whole genome alignment illustrated in Fig. 1 using a window setting of 1,000 bp and a step value of 200 bp. The program did not perform bootstrap analysis in a given window if the percentage of gaps in the alignment exceeded 50% (gap stripping set to 50%). Using HVP-2 as the query sequence, the comparison with SA8 is plotted in blue, B virus in red, and the HSV-1/HSV-2 consensus in green.
The anomaly shown in Fig. 3 could be due to a region of high sequence variability that generates a chaotic bootstrap clustering. Visual inspection of the alignment did not reveal repeats or poorly conserved regions, with the exception of the last 550 nucleotides of UL42, which are very poorly conserved among these five herpesviruses. The identities at the DNA level of HVP-2 in the putative recombination area were 81.2% and 85.1% with SA8 and B virus, respectively, similar to the level of identity for the whole genome.
The continuous and localized nature of this inversion of homology in a region of high identity suggests that a recombination event may have occurred between a common progenitor of SA8 and HVP-2 with a simplexvirus related to the B virus. Given the close homology between SA8 and HVP-2 outside this recombination area, we can presume that the putative recombination event occurred relatively recently in the evolutionary history of these viruses. Comparing the phylogenetic distance between HVP-2 and SA8, as shown in Fig. 1, the event occurred well after the divergence of HSV-1 and HSV-2, which was placed in one study at about 8.5 million years before the present time (36). The virus that supposedly donated the recombination segment is clearly more related to B virus than it is to the other simplexviruses; however, it is apparent from the graph in Fig. 3C that the recombination segment is more divergent from the sequences of the B virus than HVP-2 is from SA8. We therefore hypothesize that the donor virus diverged from the B virus much earlier than the divergences of SA8 from HVP-2, and therefore, the donor virus may not be a recent precursor of the B virus but rather a simplexvirus species that is yet undiscovered or extinct.
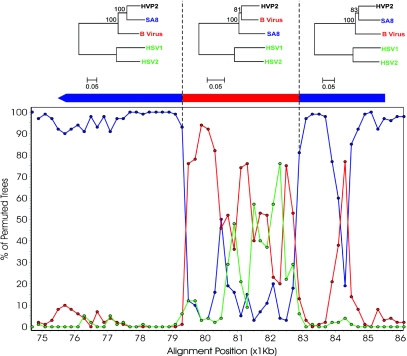
A second area that shows evidence of recombination corresponds to the central part of the large UL36 open reading frame, is shown in Fig. 4. As with the UL41/UL44 region, this region is characterized by a drop in homology of the HVP-2 sequence to SA8 and a corresponding rise in homology to the B virus. The phylogenetic trees based on segments of the amino acid sequence of UL36 show a clear inversion of the relationship for the central segment of UL36, and as in the case of the UL42/UL44 region, this is strongly suggestive of a recombination event that occurred during the evolution of HVP-2. This region is also highly conserved at the DNA level (91.2% and 92.1% identities with SA8 and B virus, respectively). The red peak in the right panel of Fig. 4, outside the putative recombination area, corresponds to a variable region with a deletion in the SA8 genome.
Fig. 4.
Putative recombination event in the UL36 gene. SimPlot analysis is based on the DNA alignment of UL36, and the dendrograms above are based on protein comparisons of the regions indicated. Alignments where compiled and compared using Mega v3.0 with ClustalW. The dendrograms were generated using neighbor joining and the Poisson correction model with 500 replicates. The resulting bootstrap values are indicated on the dendrograms. Bootscan analysis was conducted using a window setting of 1,000 bp and a step value of 200 bp with gap stripping set to 50%. Using HVP-2 as the query sequence, the comparison with SA8 is plotted in blue, B virus in red, and the HSV-1/HSV-2 composite in green.
Reductions in the degree of homology of the HVP-2 sequence to that of SA8 were also observed in other locations in the genome. The regions identified corresponded to the UL14/UL15, UL23/UL24, and UL31/UL32 junctions as well as a segment of UL30. However, these spots consist of short spans of DNA similar to that seen at the left and right ends of UL36 (Fig. 4) and, as such, may not truly indicate regions of recombination. In addition, a reduction in the similarity of HVP-2 with SA8 was observed in the region of the inverted repeats. However, these regions are not conserved among the simplexviruses and the lack of homology with SA8 is not likely due to recombination.
Of the genes involved in the recombination events described above, UL42 codes for the viral polymerase accessory protein (41), a conserved gene that has the same function in all herpesviruses. UL43 codes for a transmembrane protein of unclear function, which is not required for viral infection and replication in cell culture, nor for neuroinvasiveness in mice (26). The UL41 gene product is the viral host shutoff protein (vhs), a tegument protein that degrades the host mRNA upon infection (reviewed in reference 44). The UL44 gene produces glycoprotein C, an envelope component that inhibits complement-mediated lysis of infected cells (31, 38) and is necessary, together with glycoprotein B, for binding of HSV to its principal receptor, the cell surface heparan sulfate (20). The UL36 gene product is found in the virion tegument, and its function is still uncertain (37). Like other tegument proteins, it may be involved in the early pathogenesis events.
While the recombination involving UL42 and UL43 is unlikely to affect the biological properties of HVP-2, it is tempting to speculate that acquisition by HVP2 through recombination of non-SA8 pathogenesis genes, such as UL41, UL44, and perhaps UL36, may confer the characteristic HVP-2 neurovirulence in mice (47).
Conclusions.
The complete sequencing of HVP-2 confirms that this virus is very closely related to SA8 and that all of its genes are homologous and colinear with those of the other simian simplexviruses. Like SA8 and B virus, HVP-2 lacks the RL1 gene, a key virulence and pathogenesis gene of HSV-1 and HSV-2. At least two regions of the HVP-2 genome were identified which appear to be the result of relatively recent recombination with a simplexvirus related to, but distinct from, the B virus. Some of the genes involved in these recombination events have an identifiable role in the virulence and pathogenesis of simplexviruses. This suggests that the evolutionary history of HVP-2 is more complex than a simple divergence from a recent progenitor of SA8. The complete sequencing of two genomes, like SA8 and HVP-2, which are so closely related and yet display focused genetic and biological differences, may greatly help in defining genes of interest for further study of their potential role in simplexvirus pathogenesis.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the Health Canada Genomic Research and Development Program. The support of the DNA Core Facility, National Microbiology Laboratory, was invaluable for the completion of this project.
We thank Michael Coulthart, Morag Graham, and Sherrie Kelly at the National Microbiology Laboratory for helpful discussions on the analysis of the data for this article, Claire Sevenhuysen for a critical review of the manuscript, and Johnny Lee for technical assistance during his co-op term at the University of Victoria, Victoria, British Columbia, Canada.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Barnett, B. C., A. Dolan, E. A. Telford, A. J. Davison, and D. J. McGeoch. 1992. A novel herpes simplex virus gene (UL49A) encodes a putative membrane protein with counterparts in other herpesviruses. J. Gen. Virol. 73:2167-2171. [DOI] [PubMed] [Google Scholar]
- 2.Beaudoing, E., S. Freier, J. R. Wyatt, J. M. Claverie, and D. Gautheret. 2000. Patterns of variant polyadenylation signal usage in human genes. Genome Res. 10:1001-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besemer, J., A. Lomsadze, and M. Borodovsky. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 29:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigger, J. E., and D. W. Martin. 2003. The genome of herpesvirus papio 2 is closely related to the genomes of human herpes simplex viruses. J. Gen. Virol. 84:1411-1414. [DOI] [PubMed] [Google Scholar]
- 5.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to γ134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 6.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with γ134.5- mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens, M. J., and A. Elia. 1997. The double-stranded RNA-dependent protein kinase PKR: structure and function. J. Interferon Cytokine Res. 17:503-524. [DOI] [PubMed] [Google Scholar]
- 8.Davidson, W. L., and K. Hummeler. 1960. B virus infection in man. Ann. N. Y. Acad. Sci. 85:970-979. [DOI] [PubMed] [Google Scholar]
- 9.Davison, A. J., and N. M. Wilkie. 1981. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J. Gen. Virol. 55:315-331. [DOI] [PubMed] [Google Scholar]
- 10.Deiss, L. P., J. Chou, and N. Frenkel. 1986. Functional domains within the a sequence involved in the cleavage-packaging of herpes simplex virus DNA. J. Virol. 59:605-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolan, A., E. McKie, A. R. MacLean, and D. J. McGeoch. 1992. Status of the ICP34.5 gene in herpes simplex virus type 1 strain 17. J. Gen. Virol. 73:971-973. [DOI] [PubMed] [Google Scholar]
- 12.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberle, R., D. H. Black, S. Lipper, and J. K. Hilliard. 1995. Herpesvirus papio 2, an SA8-like alpha-herpesvirus of baboons. Arch. Virol. 140:529-545. [DOI] [PubMed] [Google Scholar]
- 14.Eberle, R., D. H. Black, T. W. Lehenbauer, and G. L. White. 1998. Shedding and transmission of baboon Herpesvirus papio 2 (HVP2) in a breeding colony. Lab. Anim. Sci. 48:23-28. [PubMed] [Google Scholar]
- 15.Everett, R. D., and M. L. Fenwick. 1990. Comparative DNA sequence analysis of the host shutoff genes of different strains of herpes simplex virus: type 2 strain HG52 encodes a truncated UL41 product. J. Gen. Virol. 71:1387-1390. [DOI] [PubMed] [Google Scholar]
- 16.Gosztonyi, G., D. Falke, and H. Ludwig. 1992. Axonal and transsynaptic (transneuronal) spread of Herpesvirus simiae (B virus) in experimentally infected mice. Histol. Histopathol. 7:63-74. [PubMed] [Google Scholar]
- 17.Hall, J. D., J. S. Gibbs, D. M. Coen, and D. W. Mount. 1986. Structural organization and unusual codon usage in the DNA polymerase gene from herpes simplex virus type 1. DNA 5:281-288. [DOI] [PubMed] [Google Scholar]
- 18.Han, M. G., J. R. Smiley, C. Thomas, and L. J. Saif. 2004. Genetic recombination between two genotypes of genogroup III bovine noroviruses (BoNVs) and capsid sequence diversity among BoNVs and Nebraska-like bovine enteric caliciviruses. J. Clin. Microbiol. 42:5214-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazuda, D. J., H. C. Perry, A. M. Naylor, and W. L. McClements. 1991. Characterization of the herpes simplex virus origin binding protein interaction with OriS. J. Biol. Chem. 266:24621-24626. [PubMed] [Google Scholar]
- 20.Herold, B. C., R. J. Visalli, N. Susmarski, C. R. Brandt, and P. G. Spear. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J. Gen. Virol. 75:1211-1222. [DOI] [PubMed] [Google Scholar]
- 21.Keeble, S. A., G. J. Christofinis, and W. Wood. 1958. Natural virus-B infection in rhesus monkeys. J. Pathol. Bacteriol. 76:189-199. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 23.Levin, J. L., J. K. Hilliard, S. L. Lipper, T. M. Butler, and W. J. Goodwin. 1988. A naturally occurring epizootic of simian agent 8 in the baboon. Lab. Anim. Sci. 38:394-397. [PubMed] [Google Scholar]
- 24.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukashin, A. V., and M. Borodovsky. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLean, C. A., S. Efstathiou, M. L. Elliott, F. E. Jamieson, and D. J. McGeoch. 1991. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J. Gen. Virol. 72:897-906. [DOI] [PubMed] [Google Scholar]
- 27.Malherbe, H., and M. Strickland-Cholmley. 1969. Simian herpesvirus SA8 from a baboon. Lancet 2:1427. [DOI] [PubMed] [Google Scholar]
- 28.Malherbe, H., and M. Strickland-Cholmley. 1969. Virus from baboons. Lancet 2:1300. [DOI] [PubMed] [Google Scholar]
- 29.Martino, M. A., G. B. Hubbard, T. M. Butler, and J. K. Hilliard. 1998. Clinical disease associated with simian agent 8 infection in the baboon. Lab. Anim. Sci. 48:18-22. [PubMed] [Google Scholar]
- 30.McGeoch, D. J., and B. C. Barnett. 1991. Neurovirulence factor. Nature 353:609. [DOI] [PubMed] [Google Scholar]
- 31.McGeoch, D. J., A. Dolan, S. Donald, and D. H. Brauer. 1986. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 14:1727-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGeoch, D. J., A. Dolan, S. Donald, and F. J. Rixon. 1985. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 181:1-13. [DOI] [PubMed] [Google Scholar]
- 33.McGeoch, D. J., C. Cunningham, G. McIntyre, and A. Dolan. 1991. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J. Gen. Virol. 72:3057-3075. [DOI] [PubMed] [Google Scholar]
- 34.McGeoch, D. J., H. W. Moss, D. McNab, and M. C. Frame. 1987. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J. Gen. Virol. 68:19-38. [DOI] [PubMed] [Google Scholar]
- 35.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 36.McGeoch, D. J., S. Cook, A. Dolan, F. E. Jamieson, and E. A. Telford. 1995. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J. Mol. Biol. 247:443-458. [DOI] [PubMed] [Google Scholar]
- 37.McNabb, D. S., and R. J. Courtney. 1992. Characterization of the large tegument protein (ICP1/2) of herpes simplex virus type 1. Virology 190:221-232. [DOI] [PubMed] [Google Scholar]
- 38.McNearney, T. A., C. Odell, V. M. Holers, P. G. Spear, and J. P. Atkinson. 1987. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J. Exp. Med. 166:1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McVoy, M. A., D. E. Nixon, J. K. Hur, and S. P. Adler. 2000. The ends on herpesvirus DNA replicative concatemers contain pac2 cis cleavage/packaging elements and their formation is controlled by terminal cis sequences. J. Virol. 74:1587-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mocarski, E. S., and B. Roizman. 1982. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell 31:89-97. [DOI] [PubMed] [Google Scholar]
- 41.Parris, D. S., A. Cross, L. Haarr, A. Orr, M. C. Frame, M. Murphy, D. J. McGeoch, and H. S. Marsden. 1988. Identification of the gene encoding the 65-kilodalton DNA-binding protein of herpes simplex virus type 1. J. Virol. 62:818-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perelygina, L., L. Zhu, H. Zurkuhlen, R. Mills, M. Borodovsky, and J. K. Hilliard. 2003. Complete sequence and comparative analysis of the genome of herpes B virus (Cercopithecine herpesvirus 1) from a rhesus monkey. J. Virol. 77:6167-6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perry, L. J., and D. J. McGeoch. 1988. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:2831-2846. [DOI] [PubMed] [Google Scholar]
- 44.Read, G. S. 1997. Control of mRNA stability during herpes simplex virus infections, p. 311-321. In J. B. Harford and D. R. Morris (ed.), mRNA metabolism and post-transcriptional gene regulation. Wiley-Liss Inc., New York, N.Y.
- 45.Ritchey, J. W., K. A. Ealey, M. E. Payton, and R. Eberle. 2002. Comparative pathology of infections with baboon and African green monkey alpha-herpesviruses in mice. J. Comp. Pathol. 127:150-161. [DOI] [PubMed] [Google Scholar]
- 46.Ritchey, J. W., M. E. Payton, and R. Eberle. 2005. Clinicopathological characterization of monkey B virus (Cercopithecine herpesvirus 1) infection in mice. J. Comp. Pathol. 132:202-217. [DOI] [PubMed] [Google Scholar]
- 47.Rogers, K. M., K. A. Ealey, J. W. Ritchey, D. H. Black, and R. Eberle. 2003. Pathogenicity of different baboon herpesvirus papio 2 isolates is characterized by either extreme neurovirulence or complete apathogenicity. J. Virol. 77:10731-10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salminen, M. O., J. K. Carr, D. S. Burke, and F. E. McCutchan. 1995. Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning. AIDS Res. Hum. Retrovir. 11:1423-1425. [DOI] [PubMed] [Google Scholar]
- 49.Schachtel, G. A., P. Bucher, E. S. Mocarski, B. E. Blaisdell, and S. Karlin. 1991. Evidence for selective evolution in codon usage in conserved amino acid segments of human alphaherpesvirus proteins. J. Mol. Evol. 33:483-494. [DOI] [PubMed] [Google Scholar]
- 50.Tyler, S. D., G. A. Peters, and A. Severini. 2005. Complete genome sequence of cercopithecine herpesvirus 2 (SA8) and comparison with other simplexviruses. Virology 331:429-440. [DOI] [PubMed] [Google Scholar]
- 51.van Regenmortel, M. H. V., C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.). 2000. Virus taxonomy: seventh report of the International Committee on Taxonomy of Viruses, 1st ed. Academic Press, San Diego, Calif.
- 52.Varmuza, S. L., and J. R. Smiley. 1985. Signals for site-specific cleavage of HSV DNA: maturation involves two separate cleavage events at sites distal to the recognition sequences. Cell 41:793-802. [DOI] [PubMed] [Google Scholar]
- 53.Wang, Z., Z. Liu, G. Zeng, S. Wen, Y. Qi, S. Ma, N. V. Naoumov, and J. Hou. 2005. A new intertype recombinant between genotypes C and D of hepatitis B virus identified in China. J. Gen. Virol. 86:985-990. [DOI] [PubMed] [Google Scholar]
- 54.Weigler, B. J. 1992. Biology of B virus in macaque and human hosts: a review. Clin. Infect. Dis. 14:555-567. [DOI] [PubMed] [Google Scholar]
- 55.Zeng, F. Y., C. W. Chan, M. N. Chan, J. D. Chen, K. Y. Chow, C. C. Hon, K. H. Hui, J. Li, V. Y. Li, C. Y. Wang, P. Y. Wang, Y. Guan, B. Zheng, L. L. Poon, K. H. Chan, K. Y. Yuen, J. S. Peiris, and F. C. Leung. 2003. The complete genome sequence of severe acute respiratory syndrome coronavirus strain HKU-39849 (HK-39). Exp. Biol. Med. (Maywood) 228:866-873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.