Abstract
Respiratory syncytial virus (RSV) is a major cause of severe lower respiratory tract disease in infants and the elderly, but no safe and effective RSV vaccine is yet available. For reasons that are not well understood, RSV is only weakly immunogenic, and reinfection occurs throughout life. This has complicated the search for an effective live attenuated viral vaccine, and past trials with inactivated virus preparations have led to enhanced immunopathology following natural infection. We have tested the hypothesis that weak stimulation of innate immunity by RSV correlates with ineffective adaptive responses by asking whether expression of the fusion glycoprotein of RSV by Newcastle disease virus (NDV) would stimulate a more robust immune response to RSV than primary RSV infection. NDV is a potent inducer of both alpha/beta interferon (IFN-α/β) production and dendritic cell maturation, while RSV is not. When a recombinant NDV expressing the RSV fusion glycoprotein was administered to BALB/c mice, they were protected from RSV challenge, and this protection correlated with a robust anti-F CD8+ T-cell response. The effectiveness of this vaccine construct reflects the differential abilities of NDV and RSV to promote dendritic cell maturation and is retained even in the absence of a functional IFN-α/β receptor.
Respiratory syncytial virus (RSV), a Pneumovirus of the family Paramyxoviridae, infects the majority of individuals in their first year of life. RSV is the major cause of bronchiolitis and pneumonia in infants and young children and accounts for approximately 85,000 pediatric hospitalizations per year in the United States alone (48). Many pediatric viral diseases have been eradicated or lessened in severity by successful vaccination programs, but no safe and effective vaccine yet exists for RSV despite exploitation of multiple approaches (12, 13). Difficulties in RSV vaccine development relate to (i) the poor immunogenicity of RSV (reinfection occurs throughout life [26]) and (ii) the ability of viral proteins to elicit a Th2, or allergic-type, memory response in some contexts (23, 28, 32, 46, 61). Although primary infection with RSV promotes Th1 cell differentiation, early trials of an inactivated viral vaccine (FI-RSV) led to incomplete immunity and exacerbated eosinophilic disease in vaccinated children after natural infection (10, 16, 35, 36). These results have led RSV researchers to proceed with caution and to look beyond conventional methods and adjuvants in exploring new RSV vaccine strategies (27, 45).
We have hypothesized that poor stimulation of innate immune defenses by RSV might be the basis for the inadequate adaptive immune response to this infection. One indication of this inhibition of, or escape from, innate host responses is the minimal alpha/beta interferon (IFN-α/β) response to RSV infection. IFN-α/β are synthesized by virus-infected cells and by virus-stimulated plasmacytoid dendritic cells (pDCs) within hours postinfection in many viral systems (1). Importantly, it has been shown that RSV infection blocks production of IFN-α/β in a variety of infected cell types, including the pDCs (54, 55), which are the major source of serum IFN. While the direct antiviral effects of IFN-α/β are their best-characterized function, their ability to influence dendritic cell (DC) activation has suggested a role for these cytokines in shaping adaptive immune responses to virus infection (14, 38, 57). In this proof-of-principle study, we tested whether mucosal immunization with a Newcastle disease virus (NDV) vector (44) expressing an RSV antigen would result in protective antiviral immunity without exacerbated immunopathology. NDV, a member of the Avulavirus genus in the Paramyxoviridae family, replicates poorly in mammalian cells but is known for its ability to induce significant amounts of IFN-α/β and to provide a strong stimulus for DC maturation (6, 30). In vitro we observed a marked difference in the ability of NDV and RSV to stimulate DC maturation, with greatly enhanced activation of DC cultures by NDV.
To test whether delivery of RSV proteins by NDV could augment the antiviral response induced by primary infection, we constructed a strain of NDV expressing the RSV fusion (F) glycoprotein (NDV-F) and used this to prime naïve BALB/c mice. The NDV-F-treated animals were partially protected from RSV challenge with decreased viral loads and minimal disease. Specifically, NDV-F-induced immunity was mediated by a more potent RSV F-specific CD8+ T-cell response than that seen following a typical primary RSV infection. These results supported the prediction that increased levels of IFN-α/β would augment both DC maturation and CD8+ T-cell priming but did not prove that the effects of NDV were in fact IFN-α/β dependent. This question was asked using a genetic approach and by comparing the efficacy of NDV-F immunization in wild-type (WT) and IFN-α/β receptor knockout (IFNAR−/−) mice. Surprisingly, the adjuvant effect of the NDV vector was not IFN-α/β dependent, and a similar boost in the number of antigen-specific memory T cells was obtained in WT or knockout animals. While it is generally accepted that maturation of myeloid DCs, the antigen-presenting cells thought to be most important for CD8+ T-cell priming, requires IFN-α/β (38), we asked whether this was true for NDV-infected cells. Where large differences were observed in the ability of RSV and NDV to promote maturation of WT myeloid dendritic cells (mDCs), this was not true for cells obtained from IFNAR−/− animals. In receptor-deficient mice, neither virus stimulated the appearance of maturation markers in mDCs generated from bone marrow (BM) in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF). Interestingly, the absolute requirement for IFN-α/β signaling did not extend to BM-derived, Flt-3L-cultured DCs. In this more heterogeneous DC population (19), activation of IFNAR−/− cells by virus was somewhat decreased in comparison to WT but was not ablated. Thus, stimulation of DCs by the NDV vector occurs by both IFN-α/β-dependent and -independent pathways.
MATERIALS AND METHODS
Cells, virus stocks, and plasmids.
Vero (ATCC CCL-81) and HEp-2 (ATCC CCL-23) cells were maintained in Dulbecco modified Eagle's medium (DMEM) containing 10% fetal bovine serum and 1% penicillin-streptomycin (Gibco). DC studies were carried out using cultures derived from BM of WT or IFNAR−/− BALB/c mice. BM cells were washed in RPMI and plated at 2 × 106 cells/10-cm plate in RPMI containing 10% fetal bovine serum and 20 ng/ml GM-CSF (R&D). Human RSV, A2 strain, was obtained from ATCC (VR-1540) and grown in HEp-2 cells using previously described methods (17). Recombinant NDV was prepared as described by Nakaya et al. (44). Plasmid LF1 containing the F gene of the RSV Long strain was kindly provided by Jose Antonio Melero (Instituto de Salud Carlos III, Madrid, Spain).
RSV titers.
The lungs of mice euthanized 5 and 8 days postchallenge were quick-frozen and stored at −80°C until assayed for viral titers. After homogenization in 1 ml of phosphate-buffered saline, the samples were used in a plaque assay on murine STAT1−/− fibroblast monolayers (15). Titers are expressed as the log10 PFU per gram of lung tissue.
Generation of monoclonal antibodies against RSV.
The RSV A2 strain was grown in HEp-2 cells and purified as described previously (52). Fifty micrograms of purified RSV per mouse was used to inoculate BALB/c mice intraperitoneally. Three days before the fusions, mice were injected intravenously with 25 μg of purified RSV. Three weeks after the first inoculation, one mouse was euthanized and the splenocytes were used to make fusions to generate monoclonal antibodies. The first screening of hybridomas was performed by enzyme-linked immunosorbent assay (ELISA) using lysates from HEp-2 cells uninfected or infected with RSV. Hybridoma clones were subsequently screened by immunostaining on mock- and RSV-infected cells. Eight monoclonal antibodies specific for RSV F protein (9B1, 9E4, 18B6, 18D5, 22A4, 22G6, 34H5, and 75D1) were obtained, and all of them were characterized by immunostaining and immunofluorescence (data not shown). The F-specific monoclonal antibodies 47F, 22A4, and 22G6 were used to characterize the NDV virion expressing the RSV F glycoprotein.
Construction, rescue, and growth of recombinant NDVs expressing the RSV F glycoprotein.
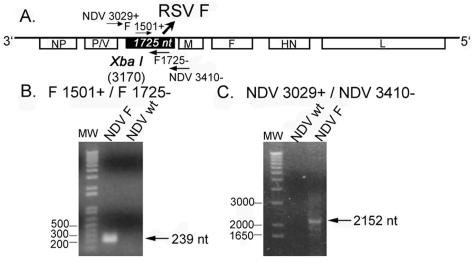
The open reading frame (ORF) from the RSV Long strain F glycoprotein was amplified by PCR from the LF1 plasmid and cloned between the P and M genes of the NDV Hitchner B1 cDNA (Fig. 1A) (44). Primer NDV-RSVF 5′/XbaI (5′-CGCGTCTAGATTAGAAAAAATACGGGTAGAACCGCCACCATGGAGTTGCCAATCCTCAAAGCAAAT-3′), containing the XbaI site (underlined), NDV gene stop (bold), intergenic region, transcription start (italics), and the Kozak sequence (CCGCCACC), and primer NDV-RSVF 3′/NheI (5′-CGCGGCTAGCGTTATTAGTTACTAAATGCAATATTATTTAT-3′) containing a NheI restriction site (underlined) and four extra nucleotides (bold) after the stop codon to comply with the rule of six, were used to PCR amplify the F ORF from the LF1 plasmid. The virus was rescued from cDNA as described elsewhere (44), and the presence in the viral genome of the inserted ORF was confirmed by reverse transcription-PCR (RT-PCR) and immunofluorescence. The NDV-F virus was grown in 10-day-old embryonated chicken eggs. Virus titers were determined by immunofluorescence using a polyclonal antibody against NDV.
FIG. 1.
The ORF encoding the RSV F protein was cloned between the P and M genes of the NDV cDNA to create the NDV-F vaccine construct (A). After the recombinant virus was rescued from the cDNA, proper insertion of the ORF was confirmed by RT-PCR (B and C).
Immunofluorescence assays.
Confluent monolayers of Vero cells were infected with WT NDV and NDV-F. After 1 h of adsorption, the virus was removed and cells were incubated in 10% DMEM. Vero cells infected with RSV A2 were used as a positive control. At 48 h postinfection, cells were fixed in 2.5% paraformaldehyde, permeabilized with 0.1% Triton X-100 and incubated with the monoclonal antibodies 22A4 and 22G6 (specific for RSV-F). Rabbit polyclonal antibody against NDV (1:100) was used as a control for NDV infection. Infected cells were then incubated with a secondary anti-mouse fluorescein isothiocyanate (FITC)-conjugated antibody (1:100) or anti-rabbit-FITC antibody (1:100) and visualized under a fluorescence microscope at 20× magnification. RSV-F-specific monoclonal antibody 47F, a gift from J. A. Melero, was used as a positive control for the immunofluorescence. The monoclonal antibody against the NDV-HN glycoprotein (7B1) was generated by the Mount Sinai School of Medicine hybridoma facility.
Immunization and challenge.
WT BALB/c mice, 3 to 4 weeks of age, were purchased from Harlan (Indianapolis, IN). IFNAR−/− mice (43) backcrossed for nine generations onto the BALB/c background were housed in the specific-pathogen-free barrier facility at Columbus Children's Research Institute. The mice were immunized intranasally (i.n.) with 5 × 105 PFU in 50 μl of NDV-WT, NDV-F, or RSV under light anesthesia (Avertin; Sigma-Aldrich). The mock immunization group received allantoic fluid. Four weeks postimmunization, the mice were challenged i.n. with 107 PFU RSV in 50 μl. The mice were sacrificed at 5 and 8 days postchallenge for collection of bronchoalveolar lavage fluid (BAL), lung tissues, and splenocytes. All procedures involving mice were performed in compliance with federal and institutional guidelines.
Lung histopathology.
Lungs harvested for microscopic examination were inflated with 10% formalin at harvest, fixed in formalin, and processed for paraffin embedding. Four-micron sections were either stained with hematoxylin and eosin or deparaffinized for immunohistochemical staining. The slides were treated with 3% hydrogen peroxide for 15 min and blocked with Superblock (ScyTek) for 5 min at room temperature. Immunohistochemistry was performed using goat anti-RSV serum (1:500 dilution) followed by biotinylated anti-goat immunoglobulin G (ScyTek). After soaking in Optimax wash buffer (BioGenex) for 10 min, the slides were treated with streptavidin-horseradish peroxidase (ScyTek) for 20 min at room temperature. A red color reaction was observed following aminoethylcarbazole (ScyTek) substrate addition, and hematoxylin was used as a counterstain. Images were captured using the Zeiss AxioCam HRc with AxioVision software.
BAL.
Mice were euthanized by CO2 inhalation. The trachea was cannulated, and the lungs were washed with 1 ml phosphate-buffered saline. Lavage fluid from individual animals was centrifuged, and the supernatants were stored at −80°C. The BAL pellets were resuspended, and total cell counts were recorded based on trypan blue exclusion. Cytospins of the BAL cells were stained with Wright Giemsa and used to determine differential cell counts.
IFN-α/β bioassay.
One lung from each mouse was homogenized in 1 ml serum-free DMEM and clarified by centrifugation. Lung samples were acid treated to a pH of 2 to inactivate any input virus as well as other cytokines. Samples were then neutralized with sodium bicarbonate, and twofold dilutions of each were added to murine fibroblast monolayers in 96-well plates. After overnight incubation at 37°C, 1.25 × 105 PFU of vesicular stomatitis virus (VSV) were added to each well. An IFN-α/β standard was used in parallel to generate a standard curve (Access Biomedical). Additional controls included untreated monolayers with and without VSV infection. After 2 days of incubation, wells were fixed with 2% formaldehyde and stained with crystal violet. Test sample IFN-α/β concentrations were determined by comparison of protection from VSV-induced cell killing with that seen with known amounts of IFN-α/β.
In vitro stimulation of memory splenocytes.
Splenocytes from mice immunized i.n. with mock, NDV-WT, NDV-F, or RSV and then challenged with RSV i.n. were harvested 8 days postchallenge. Pooled splenocytes from each group of five mice were cultured in RPMI 1640 with 5% fetal calf serum and 5% rat T-STIM (Collaborative Biomedical Products, Bedford, MA) in the presence of an H-2Kd-restricted peptide (10 μg/ml) for 1 week. The major histocompatibility complex (MHC) class I-restricted peptide KYKNAVTEL from the RSV F protein (F85-93) was synthesized by Research Genetics, Inc. (Invitrogen) (9). On day 7, cell culture supernatants were collected from each treatment group before the cells were restimulated with peptide for 6 h using peptide-pulsed gamma-irradiated splenocytes harvested from naïve wild-type BALB/c mice. The stimulated cells were lysed in TRIzol (Life Technologies, Rockville, MD), and cytokine transcripts from 10 to 20 μg of RNA were assayed by RNase protection using the RiboQuant mck1 multiprobes (BD Pharmingen) according to the manufacturer's instructions. Quantitation was carried out using the Storm PhosphorImager (Molecular Dynamics) and ImageQuant software. Data were graphed following normalization of values to the intensity of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) signal in each sample.
ELISA and ELISPOT assays.
BALs, lymphocyte culture supernatants, and lung homogenate supernatants were tested for IFN-γ protein by ELISA (Becton-Dickinson) as per the directions included in each kit. IFN-γ ELISPOTS were carried out using kits purchased from U-CYTECH (Utrecht, Holland)
Dendritic cell studies.
DC studies were carried out using cultures derived from BM of BALB/c WT or IFNAR−/− mice. BM cells were washed in RPMI and plated at 2 × 106 cells/10-cm plate in RPMI containing 10% fetal bovine serum and 20 ng/ml murine GM-CSF (R&D) or 100 ng/ml human Flt-3L (Peprotech) according to the method of Gilliet et al. (19). This protocol routinely gives >90% pure populations of CD11c+ DCs after 8 days of culture in either cytokine and mixtures of (80 to 85%) CD11c+ CD11b+ B220− mDCs and (15 to 20%) CD11c+ CD11b− B220+ pDCs after 8 days of culture in Flt-3L. Cells were infected with virus at a multiplicity of infection of 5 to 10 and harvested for analysis after 48 h. DCs were stained with phycoerythrin (PE)-labeled biotinylated antibody to CD11c plus PE-conjugated streptavidin as well as FITC-labeled antibodies to CD40, CD80, CD86, and MHC class II. All antibodies were purchased from eBioscience. Stained cells were analyzed using a FACSCalibur flow cytometer (Becton-Dickinson) and FlowJo software.
Statistical analysis.
Data are expressed as means ± standard errors of the means. Statistical significance was determined by the Student t test as calculated using SigmaPlot 8.0 for Windows software. P values of <0.05 were considered statistically significant.
RESULTS
A recombinant NDV expressing the F protein from RSV was constructed.
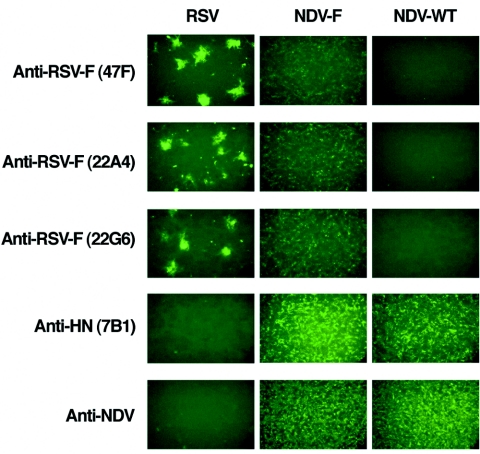
Our group has previously reported the development of a reverse genetic system for the generation of recombinant NDV viruses, and these methods were used in the preparation of NDV-F (44). The F gene from RSV was inserted between the P and M genes of the NDV cDNA (Fig. 1A). NDV-F was rescued from the cDNA as previously described, and the presence of the RSV F gene was confirmed by RT-PCR. The recombinant virus grew to titers similar to wild-type NDV in embryonated chicken eggs (data not shown). Expression of the RSV F protein by NDV-F was confirmed by immunostaining of infected Vero cells 48 h postinfection (Fig. 2). As expected, cells infected with wild-type NDV were not stained by monoclonal antibodies specific for the RSV F protein, but they were positive for immunostaining using rabbit polyclonal antisera against NDV as well as the monoclonal antibody 7B1, specific for the NDV-HN glycoprotein. In contrast, cells infected with NDV-F were stained by three different monoclonal antibodies against the RSV F protein (47F, 22A4, and 22G6) as well as the anti-NDV antiserum (Fig. 2).
FIG. 2.
Expression of the RSV fusion protein by NDV-F was confirmed by immunofluorescence studies. Vero cells were infected with RSV, NDV-F, and NDV-WT to confirm infection and production of the RSV F glycoprotein from the NDV vector. Monoclonal antibodies specific for the RSV F protein (47F, 22A4, and 22G6) were used to visualize RSV F-specific fluorescent staining. Polyclonal antibodies against NDV and a monoclonal antihemagglutinin antibody (7B1) were used as a control for NDV infection.
NDV-F is a more potent inducer of IFN-α/β than RSV.
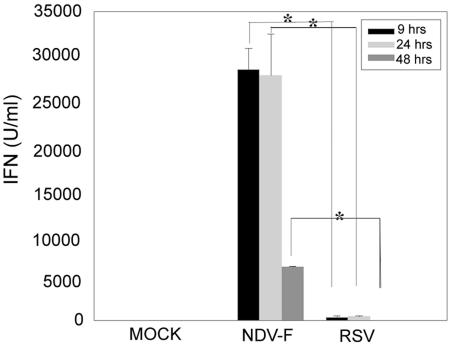
NDV was chosen as a viral vector because of its ability to induce a strong IFN-α/β response (2, 6). As IFN-α/β are known to enhance antigen presentation through the MHC class I pathway (30, 37, 53, 57), we predicted that RSV proteins would be more immunogenic if presented in the context of stronger IFN-α/β induction. To measure the IFN-inducing capacity of our chimeric viral construct, we used NDV-F and RSV (A2 strain) to infect BALB/c mice i.n. and determined IFN-α/β activity in bronchoalveolar lavage supernatants harvested 9, 24, and 48 h postinoculation with 5 × 105 PFU of each. Although the kinetics of IFN-α/β induction measured in BAL fluids was similar between NDV-F- and RSV-inoculated mice, the magnitude of the IFN response was 1,000-fold greater in NDV-F compared to RSV-treated animals at various time points postinfection (Fig. 3). Similar results were obtained when levels of IFN-α/β bioactivity in lung homogenates from NDV-WT-, NDV-F-, and RSV-treated mice were compared at the same time points (data not shown). By 48 h IFN levels decreased in all samples, but the response induced by NDV-F was sustained over a longer time period (Fig. 3). The control group lacked any detectable IFN-α/β response 9, 24, or 48 h post-intranasal instillation of allantoic fluid.
FIG. 3.
NDV-F is a potent inducer of IFN-α/β in vivo. An IFN-α/β bioassay was performed on BAL supernatants to determine the level of induction 9, 24, and 48 h post-intranasal inoculation with NDV-F or RSV. Installation of allantoic fluid was used as a negative control. The asterisk indicates a statistically significant (P < 0.05) difference between the NDV-F- and RSV-treated animals.
NDV-F immunization protects against RSV challenge without enhanced pathology.
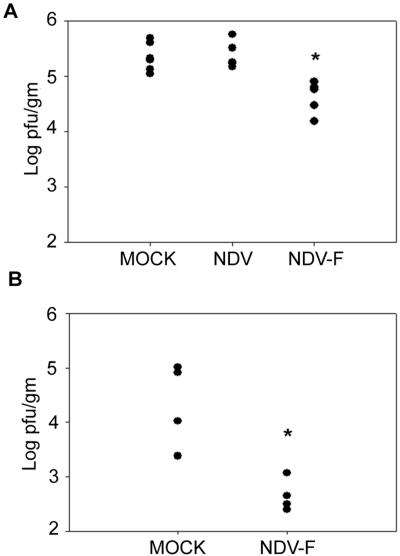
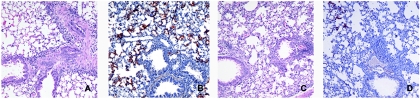
To determine whether mucosal immunization with NDV-F would be protective, cohorts of 10 BALB/c mice were mock infected or infected i.n. with 5 × 105 PFU of recombinant NDV-WT or NDV-F. Controls received equal volumes of allantoic fluid. Four weeks after priming, the immunized animals were challenged with 107 PFU of RSV, also delivered i.n. At day 5 postchallenge, when RSV replication was at or near its peak (24), lungs from five immunized mice of each group were assayed for viral titers. Virus loads in mock-immunized mice were on the order of 105 PFU per g of lung tissue, similar to the levels detected in mice immunized with wild-type NDV (Fig. 4). In contrast, mice immunized with NDV-F consistently showed, over three experiments, a 10-fold decrease in RSV lung titers (Fig. 4). The significance of this 1-log decrease in virus titer following immunization with NDV-F was best appreciated by immunohistochemical staining of RSV antigens in lung sections taken from each experimental group of animals. This study is shown in Fig. 5 in photomicrographs taken using a low-power, 10× objective (×100 magnification). Lungs from mock-immunized mice (Fig. 5B) showed diffuse, RSV-specific immunostaining on day 5 after RSV challenge which was brightest in the pneumocytes lining the alveolar spaces. RSV antigen was also detected in the lungs of NDV-F-primed mice (Fig. 5D), but in those animals the staining was very focal and reduced to approximately 10% of that seen in mock-immunized controls. Animals primed with NDV-WT were similar to mock-infected controls and are not pictured. By day 8 after challenge, no viral antigen could be detected in any animal.
FIG. 4.
NDV-F-treated mice were protected against RSV challenge. Four weeks after mock immunization or immunization with the NDV vector or NDV-F, WT (A) and IFNAR−/− (B) mice were challenged with 107 PFU of RSV A2 and lung virus titers were determined 5 days later by plaque assay. An asterisk denotes a significant difference between the control-immunized mice (mock and NDV) and the NDV-F-treated mice (P = 0.05 for WT mice; P = 0.025 for IFNAR−/− mice). Data in panel A were replicated in two additional experiments.
FIG. 5.
RSV infection of lung parenchyma is decreased by 10 times in immunized mice. Microscopic examination of lung tissue shows a marked reduction in the extent of RSV infection in NDV-F-immunized animals. Lung sections from mock-infected (A and B) or NDV-F-primed (C and D) mice sacrificed day 5 postchallenge were stained with hematoxylin and eosin (A and C) or polyclonal anti-RSV rabbit serum (B and D). Cells positive for the presence of RSV antigens appear red.
In corresponding hematoxylin-eosin-stained sections taken from the same tissue blocks (Fig. 5A and C), no notable differences in severity of inflammation were seen between cohorts. In addition to the microscopic examination, BAL specimens from similarly treated mice were used to obtain a quantitative estimate of the number and type of inflammatory cells seen after challenge. Cell counts were similar for mock- and NDV-F-primed mice, as well as for animals experiencing a secondary RSV infection (data not shown). At day 8 postchallenge, differential cell counts were also similar between these groups (data not shown). Infiltrates consisted of lymphocytes and macrophages distributed primarily around airways and blood vessels in mock-, NDV-WT-, and NDV-F-immunized animals. Therefore, NDV-F-immunized animals were not predisposed to enhanced immunopathology after RSV challenge, suffering only the relatively mild lymphocytic inflammation typical of primary RSV infection
NDV-F promotes CD8+ T-cell activation.
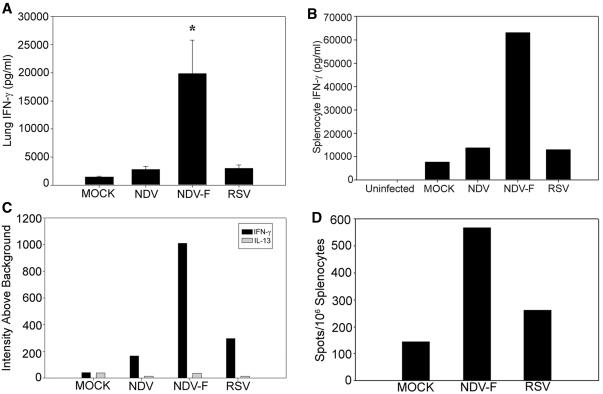
Having observed that NDV-F priming provides protection against RSV challenge, we wished to know what type of immunity was primed in the immunized animals. Neutralizing antibodies were not detected in the sera of any group day 8 postchallenge (data not shown), and so we were interested to see whether RSV-specific CD4+ and/or CD8+ T-cell responses could be found in immunized animals and how these responses differed from those in mice previously infected with RSV. Mice were mock infected or infected i.n. with 5 × 105 PFU of NDV-WT, NDV-F, or RSV. We observed that mice immunized with NDV-F had a fourfold greater increase in pulmonary IFN-γ levels 4 days following virus challenge than did mock-immunized, vector-immunized, or previously RSV-infected animals (Fig. 6A). At this time point, lung IFN-γ levels in previously RSV-infected mice were not significantly different from those found in mock- or NDV-immunized controls.
FIG. 6.
NDV-F induces an enhanced CD8+ T-cell response without enhanced Th2 cytokine secretion. NDV-F-immunized mice produce significantly more IFN-γ in response to RSV challenge than mice previously infected with RSV. IFN-γ levels in lung homogenates 4 days post-RSV challenge were determined by ELISA. (A) A significant (P < 0.05) increase was observed in NDV-F-immunized mice in comparison to all other treatment groups. A similar result was found with splenocytes harvested 8 days after RSV challenge of mock-infected, NDV-WT-primed, NDV-F-primed, or RSV-immune mice. (B) Cells were stimulated in vitro with the MHC class I-restricted F85-93 peptide, and day 7 lymphocyte culture supernatants were collected and analyzed by ELISA. Again, IFN-γ production by cells from NDV-F-immunized mice was increased. Uninfected cultures represent splenocytes taken directly from mice that were neither immunized nor challenged. (C) Comparison of cytokine responses to the viral F protein in differently immunized animals. Eight days after RSV challenge, splenocytes were harvested and cultured with peptide, as above. The relative abundance of IFN-γ and IL-13 transcripts in each culture was compared by analysis of RNase protection assay data normalized to levels of GAPDH mRNA. Black bars, IFN-γ levels; gray bars, IL-13. (D) Correlative ELISPOT data. In this experiment, splenocytes from each cohort were combined on day 8 after live virus challenge and assayed directly.
As a more specific measure of RSV immunity, we looked to see whether memory T cells recognizing a known, immunodominant RSV F protein epitope could be detected in the spleens of NDV-F-immunized mice. Splenocytes of mice sacrificed 8 days following RSV challenge were pooled for each experimental group (n = 5) and stimulated with the MHC class I-restricted F85-93 peptide (KYKNAVTEL) (9) in vitro. As with the lung homogenates, IFN-γ levels measured in the medium following peptide stimulation were sixfold greater in T-cell cultures derived from mice immunized with NDV-F than from cultures derived from the other immunization groups (Fig. 6B). RNase protection assay of cytokine transcripts produced by peptide-stimulated lymphocytes showed a similar (fourfold) rise in IFN-γ transcript without an increase in interleukin-13 (IL-13) production by NDV-F-immunized mice (Fig. 6C). IL-13 was chosen as an indicator of Th2 cytokine production, as this cytokine was previously found to predominate following RSV infection in STAT1−/− mice, which lack the ability to generate a Th1 response (33). As observed in the lung samples taken at 4 days postchallenge, IFN-γ production by splenocytes derived from mice previously infected with RSV was not significantly enhanced compared to cultures from mock-immunized animals. An IFN-γ ELISPOT, performed with the same F85-93 peptide, also demonstrated a twofold increase in the number of memory T cells found in NDV-F- versus RSV-primed animals (Fig. 6D). Thus, it appears that NDV-F is able to stimulate an RSV F-protein-specific, CD8+ T-cell-mediated response to challenge that is more robust than that resulting from previous RSV infection.
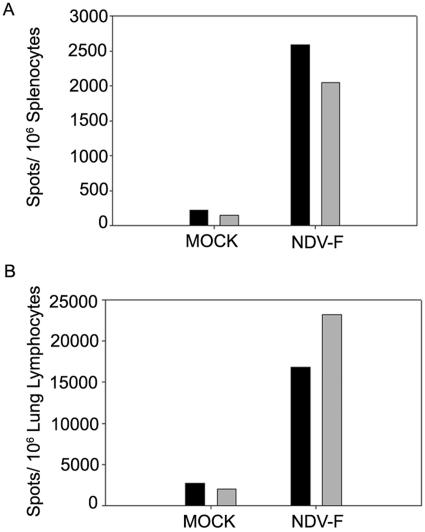
We had initially predicted that the effects of NDV-F would be dependent upon the IFN-α/β generated by the NDV vector, and to test this hypothesis we repeated these studies using BALB/c mice lacking the IFN-α/β receptor (43). Viral titers of infected lung homogenates from NDV-F-primed animals showed the same 10-fold reduction compared to controls (Fig. 4B), and numbers of RSV F protein-specific CD8+ T cells showed the same increase over controls in both wild-type and IFNAR−/− animals (Fig. 7).
FIG. 7.
NDV-F successfully immunizes both WT and IFNAR−/− mice. Numbers of IFN-γ-producing CD8+ T cells were assayed by ELISPOT after priming and challenge of both wild-type (black bars) and IFNAR−/− (gray bars) BALB/c mice. Cohorts of four to five animals were mock primed or infected with NDV-F and then sacrificed on day 36, 8 days after RSV challenge. Splenic (A) and lung (B) lymphocytes from each group of mice were isolated, combined, mixed with the F85-93 peptide, and plated directly onto antibody-coated plates. In mice of either genotype, responses were increased ∼10-fold in NDV-F-immunized versus mock-immunized animals.
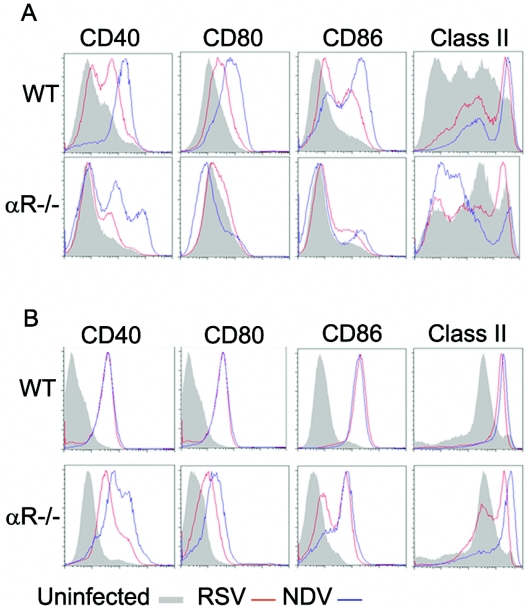
NDV promotes DC maturation much more effectively than RSV.
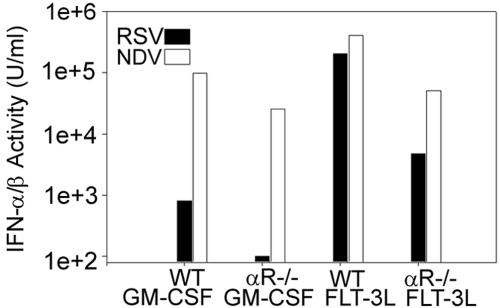
The experiments described above were done to test the hypothesis that strong IFN-α/β induction by the NDV vector would promote increased DC and T-cell activation. However, as demonstrated in Fig. 7, the induction of RSV-specific CD8+ T-cell responses by NDV-F was not, in fact, IFN-α/β dependent. Given this result, we then sought to determine whether the ability of NDV to stimulate DCs was dependent upon IFN-α/β. This question had previously been addressed by Honda et al. (30), who showed that while NDV infection of wild-type DCs, derived from BM of 129SvEv mice and cultured in GM-CSF, did lead to upregulation of the activation markers CD40, CD80, CD86, MHC class I, and MHC class II, cells lacking the IFN-α/β receptor did not mature. To clarify this issue we performed similar in vitro studies with BALB/c mice, but using BM-derived DCs cultured in the presence of either GM-CSF or Flt-3L, to yield mDCs or a mixture of mDCs and pDCs, respectively (19). DCs prepared under each culture condition were inoculated with virus at a multiplicity of infection of 5 to 10 and analyzed 24 h later. Cells were stained with biotinylated anti-CD11c antibody and PE-conjugated streptavidin to mark the dendritic cells and then costained with FITC-conjugated antibodies to activation markers. Gating on the CD11c+ cell population, relative expression of CD40, CD80, CD86, and MHC class II was compared for RSV- and NDV-infected cells. As seen in Fig. 8, WT mDCs infected with either virus upregulated expression of all four markers, although NDV appeared to be much more effective (Fig. 8A). Consistent with the results of Honda et al. (30), mDC activation by either virus was minimal in the absence of the IFN-α/β receptor. However, a very different picture emerged when pDC-containing cultures, grown up in the presence of Flt-3L, were similarly assayed. Preparations of WT cells were stimulated equally well by either virus (Fig. 8B), perhaps related to the very high levels of IFN-α/β activity present in those cultures compared with medium from RSV-infected mDCs (Fig. 9). Nonetheless, in the presence of pDCs, virus-mediated DC activation was much less dependent on IFN-α/β (Fig. 8B), and there was definitive, though decreased, activation of IFNAR−/− DCs by virus. Taken together, results from experiments shown in Fig. 7, 8, and 9 show that NDV stimulation of DCs, in the presence or absence of IFN-α/β signaling, is sufficiently robust to mediate the increased RSV-specific T-cell activation generated by NDV-F.
FIG. 8.
NDV stimulates DC maturation by both IFN-α/β-dependent and IFN-α/β-independent pathways. The relative abilities of RSV and NDV to induce maturation of WT or IFNAR−/− (αR-/-) DCs were tested in vitro using BM-derived DCs cultured in either GM-CSF or Flt-3L. After 8 days of culture, DCs were infected with either RSV or NDV and analyzed 24 h later. Expression of CD40, CD80, CD86, and MHC class II by CD11c+ cells is shown for uninfected cells (gray-filled histogram) and cells infected with NDV (blue) or RSV (red). Data from one representative experiment are shown; similar results were obtained in four additional experiments at both the 24- and 48-h time points.
FIG. 9.
NDV is a much more potent inducer of IFN-α/β than RSV in GM-CSF-cultured bone marrow-derived dendritic cells. Supernatants from infected and uninfected cultures analyzed in Fig. 8 above were assayed for IFN-α/β activity 24 h after infection. In WT mDC cultures, NDV infection induced approximately 100 times more IFN-α/β than did RSV, and this difference was even greater for IFNAR−/− (αR-/-) DCs. When cultures (grown in FLT-3L) containing both mDCs and pDCs were infected with these viruses, very high IFN levels were produced in response to either. No IFN-α/β could be detected in any uninfected culture. Levels from RSV-infected cells are depicted by black bars, and levels from NDV-infected DC are shown in white.
DISCUSSION
Despite its clinical importance, a safe and effective RSV vaccine has not yet been developed. A number of factors contribute to the difficulty of this problem, including the early age of primary infection, the presence of maternal antibodies in the neonate, and the potential for vaccine-enhanced disease. To circumvent the problems associated with the inactivated viral vaccine, a number of laboratories have pursued the strategy of generating attenuated vaccine strains (13, 51), an approach that has been successful for many other viral pathogens. Unfortunately, separation of disease and immunogenicity has been problematic for RSV, perhaps because the wild-type strains of this virus are themselves so poorly immunogenic. Antibody responses, while protective of the lower airway, are slow to develop in immunocompetent children and do not prevent repeated infection of the upper airway (20, 26, 29, 31, 56). Long lasting T-cell responses are induced by primary RSV infection (47), but their protective efficacy is uncertain (3, 8), and their potential for mediating inflammatory pathology is well established (7, 22, 59).
In this study we have focused on the poor immunogenicity of RSV and asked whether presentation of RSV antigens in the context of strong IFN-α/β induction would induce protective T-cell responses. This hypothesis was based upon the observation that, unlike the strongly immunogenic influenza virus, IFN-α/β is not detected in the serum or nasal secretions of RSV-infected children (25, 41). In addition to the well-known direct antiviral effects of IFN-α/β (1), its role in shaping the adaptive immune responses to viral pathogens has also begun to be appreciated. IFN-α/β have been shown to promote antigen processing and presentation through the MHC class I pathway in DCs (30, 37, 53, 57), as well as the activation, expansion, and survival of CD8+ T cells (34, 40, 50). Not surprisingly, most viruses have evolved mechanisms to limit IFN-α/β production and/or function (18, 21). In the case of RSV, the NS1 and NS2 proteins of both the human and the bovine strains antagonize IFN-α/β production by inhibiting IRF3 activation (4, 5, 55). While it is not clear that antiinduction mechanisms encoded by RSV are more potent than those of other viral pathogens, the very low levels of IFN-α/β found in serum and nasal washings of RSV-infected patients (25, 41) suggest that this may be the case. Nonetheless, there appear to be multiple strategies employed by RSV to avoid immune recognition following primary and secondary infections, and one of these is the inability of this virus to activate the antigen-presenting DC compartment.
We have constructed a chimeric viral vaccine using a reverse genetic approach, inserting the RSV F protein gene into the backbone of NDV, another Paramyxoviridae family member (44). NDV, an avian virus that replicates poorly in mammalian cells or tissues due, at least in part, to its inability to prevent a strong IFN-α/β response in mammalian species, was chosen as a vector for these reasons (2, 42). While the details of IFN-α/β induction by NDV are not completely understood, the IFN-antagonizing functions of the viral V protein appear to be restricted to the avian host (49). The RSV F gene was chosen as the target antigen for two reasons. Firstly, only antibodies directed at the F and G surface glycoproteins of RSV are associated with protection (11), and secondly, of these two proteins, only F contains both CD4+ and CD8+ T-cell epitopes (58). The G protein, which has been associated with the priming of Th2 responses, has no MHC class I-restricted peptide epitopes that are recognized by the BALB/c mouse (60). We predicted that the NDV-F virus would be a much stronger inducer of IFN-α/β in BALB/c mice than RSV following i.n. instillation despite its inability to productively replicate in the mouse, and we found this to be true (Fig. 3). We then looked to see whether NDV-F-immunized mice showed any correlates of protection upon challenge with RSV A2 and, if so, how this protection was mediated. Our objective in performing this experiment was to test the hypothesis that immunization with an IFN-α/β-inducing adjuvant would enhance the adaptive immune response to RSV. While mucosal NDV-F immunization with 5 × 105 PFU did not prevent infection, virus burden was decreased 10-fold without evidence of enhanced inflammation or Th2 cytokine production. RSV F-specific, CD8+ memory T cells were present in greater numbers in NDV-F-primed mice than in animals previously infected with RSV, suggesting that NDV-F may be a more effective immunogen than RSV itself.
The expectation that IFN-α/β induction would have an adjuvant effect was based on the ability of this cytokine to promote DC maturation as well as cross-priming during virus infection (37, 38, 57). Our data show that the effects of both viruses on mDC cultures are largely IFN-α/β dependent; NDV is a much more effective activator but also induces much higher levels of IFN-α/β in the mDC population. Conversely, in Flt-3L-expanded cultures that contain pDCs as well, IFN-α/β levels and extent of maturation are equivalent. This is consistent with our hypothesis but does not explain the ability of NDV-F to protect IFNAR−/− mice from RSV challenge. The in vivo study demonstrating that NDV-F is immunogenic even in the absence of the IFN-α/β pathway (Fig. 7) suggests that NDV can activate DCs by alternate pathways as well. Evidence for this additional pathway is shown in Fig. 8B, which illustrates maturation of IFNAR−/− DCs, particularly by NDV, in BM-derived DCs cultured with Flt-3L. The mechanism underlying this IFN-α/β-independent pathway is beyond the scope of this present study, but we speculate that a population of cells present in the Flt-3L cultures is activated by virus to secrete an additional cytokine(s) that can also mediate DC maturation. Studies aimed at the characterization of these factors are currently in progress.
In addition to its inherently high adjuvant properties, as shown by our studies, NDV is an attractive vaccine viral vector in humans because of several additional characteristics. Importantly, NDV has proven to be safe in humans, as determined in several clinical trials focused on the use of this virus as an oncolytic agent in cancer patients (39). In our experiments, we have used an attenuated viral strain of NDV (Hitchner B1) currently employed as a live attenuated vaccine in poultry against Newcastle disease. The lack of preexisting immunity against this virus in the vast majority of the human population represents another important factor that facilitates the use of NDV as a vaccine vector.
In conclusion, we have provided evidence that the RSV F protein is more immunogenic when presented by NDV-F than by RSV itself and that this correlates with an increased ability of NDV to activate antigen-presenting cells in vitro and to induce high levels of IFN-α/β in vivo. Mucosal NDV-F instillation can effectively prime a population of CD8+ memory T cells that persist and mount a strong IFN-γ response upon reintroduction of antigen. This vectored vaccine therefore offers a measure of protection against RSV challenge, while IFN-γ secreted by F-specific CD8+ T cells promotes a robust Th1 response to viral epitopes not yet seen by the host immune system. The mechanism of this adjuvant effect is largely mediated by the potent IFN induction by the avian viral vector. This approach to RSV immunization, one of expressing RSV antigens in vectors more capable of immune stimulation, may offer a useful alternative to the problems encountered with both live attenuated and inactivated vaccine preparations.
Acknowledgments
We thank Richard Cádagan for excellent technical assistance. We also thank José Melero for providing us with the LF1 plasmid and the 47F monoclonal antibody. Monoclonal antibodies against NDV HN were generated by the Hybridoma Shared Research Facility at the Mount Sinai School of Medicine.
This work was supported by NIH grants to A.G.-S., E.F., and J.E.D.
REFERENCES
- 1.Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14:661-664. [DOI] [PubMed] [Google Scholar]
- 2.Blach-Olszewska, Z. 1970. Interferon induction by Newcastle disease virus in mice. Arch. Immunol. Ther. Exp. 18:418-441. [PubMed] [Google Scholar]
- 3.Bont, L., J. Versteegh, W. T. Swelsen, C. J. Heijnen, A. Kavelaars, F. Brus, J. M. Draaisma, M. Pekellharing-Berghuis, R. A. van Diemen-Steenvoorde, and J. L. Kimpen. 2002. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr. Res. 52:363-367. [DOI] [PubMed] [Google Scholar]
- 4.Bossert, B., and K.-K. Conzelmann. 2002. RSV nonstructural proteins (NS) as host range determinants: a chimeric bovine RSV with NS genes from human RSV is attenuated in interferon-competent bovine cells. J. Virol. 76:4287-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossert, B., S. Marozin, and K.-K. Conzelmann. 2003. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol. 77:8661-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brehm, G., and H. Kirchner. 1986. Analysis of the interferons induced in mice in vivo and in macrophages in vitro by Newcastle disease virus and by polyinosinic-polycytidylic acid. J. Interferon Res. 6:21-28. [DOI] [PubMed] [Google Scholar]
- 7.Cannon, M. J., P. J. M. Openshaw, and B. A. Askonas. 1988. Cytotoxic T lymphocytes clear virus but augment lung pathology in mice infected with RSV. J. Exp. Med. 168:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, J., and T. J. Braciale. 2002. RSV infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ memory in the respiratory tract. Nat. Med. 8:54-60. [DOI] [PubMed] [Google Scholar]
- 9.Chang, J., A. Srikiatkhachorn, and T. J. Braciale. 2001. Visualization and characterization of RSV F-specific CD8+ T cells during experimental virus infection. J. Immunol. 167:4254-4260. [DOI] [PubMed] [Google Scholar]
- 10.Chin, J., R. L. Magoffin, L. A. Shearer, J. H. Schieble, and E. H. Lennette. 1969. Field evaluation of a RSV vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 89:449-463. [DOI] [PubMed] [Google Scholar]
- 11.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 12.Collins, P. L., and B. R. Murphy. 2002. Respiratory syncytial virus: reverse genetics and vaccine strategies. Virology 296:204-211. [DOI] [PubMed] [Google Scholar]
- 13.Crowe, J. E. 2001. Respiratory syncytial virus vaccine development. Vaccine 20(Suppl. 1):S32-S37. [DOI] [PubMed] [Google Scholar]
- 14.Dalod, M., T. Hamilton, R. Salomon, T. P. Salazar-Mather, S. C. Henry, J. D. Hamilton, and C. A. Biron. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 197:885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durbin, J. E., R. Hackenmiller, M. C. Simon, and D. E. Levy. 1996. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84:443-450. [DOI] [PubMed] [Google Scholar]
- 16.Fulginiti, V. A., J. J. Eller, O. F. Sieber, J. W. Joyner, M. Minamitani, and G. Meiklejohn. 1969. Respiratory virus immunization. I: a field trial of two inactivated respiratory virus vaccines, an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 89:435-448. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Barreno, B., C. Palomo, C. Penas, T. Delgado, P. Perez-Brena, and J. A. Melero. 1989. Marked differences in the antigenic structure of human respiratory syncytial virus F and G glycoproteins. J. Virol. 63:925-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Sastre, A. 2004. Identification and characterization of viral antagonists of type I IFN in negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:249-280. [DOI] [PubMed] [Google Scholar]
- 19.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X. L. Xu, G. Trinchieri, A. O'Garrra, and Y. J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glezen, W. P., A. Paredes, J. E. Allison, L. H. Taber, and A. L. Frank. 1986. Risk of primary infection and reinfection with RSV. Am. J. Dis. Child. 140:543-546. [DOI] [PubMed] [Google Scholar]
- 21.Gotoh, B., T. Komatsu, K. Takeuchi, and J. Yokoo. 2002. Paramyxovirus strategies for evading the interferon response. Rev. Med. Virol. 12:337-357. [DOI] [PubMed] [Google Scholar]
- 22.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Reinfection of mice with RSV. J. Med. Virol. 34:7-13. [DOI] [PubMed] [Google Scholar]
- 23.Graham, B. S., G. S. Henderson, Y.-W. Tang, X. Lu, K. M. Neuzil, and D. G. Colley. 1993. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with RSV. J. Immunol. 151:2032-2040. [PubMed] [Google Scholar]
- 24.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary RSV infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 25.Hall, C. B., R. G. J. Douglas, R. L. Simons, and J. M. Geiman. 1978. Interferon production in children with RSV, influenza and parainfluenza virus infections. J. Pediatr. 93:28-32. [DOI] [PubMed] [Google Scholar]
- 26.Hall, C. B., C. E. Long, and K. C. Schnabel. 2001. Respiratory syncytial virus infections in previously healthy working adults. Clin. Infect. Dis. 33:792-796. [DOI] [PubMed] [Google Scholar]
- 27.Hancock, G. E., K. M. Heers, K. S. Pryharski, J. D. Smith, and L. Tiberio. 2003. Adjuvants recognized by toll-like receptors inhibit the induction of polarized type 2 T cell responses by natural attachment (G) protein of respiratory syncytial virus. Vaccine 21:4348-4358. [DOI] [PubMed] [Google Scholar]
- 28.Hancock, G. E., D. J. Speelman, K. Heers, E. Bortell, J. Smith, and C. Cosco. 1996. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of RSV. J. Virol. 70:7783-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson, F. W., A. M. Collier, W. A. Clyde, and F. W. Denny. 1979. RSV infections, reinfections, and immunity. A prospective, longitudinal study in young children. N. Engl. J. Med. 300:530-534. [DOI] [PubMed] [Google Scholar]
- 30.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IMpact RSV Study Group. 1998. Reduction of RSV hospitalization among premature infants and infants with bronchopulmonary dysplasia using RSV monoclonal antibody prophylaxis. Pediatrics 102:531-537. [Google Scholar]
- 32.Johnson, T. R., and B. S. Graham. 2004. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during the primary respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 23:S46-S57. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, T. R., S. E. Mertz, N. Gitiban, S. Hammond, R. Legallo, R. K. Durbin, and J. E. Durbin. 2005. Role for innate IFNs in determining RSV immunopathology. J. Immunol. 174:7234-7241. [DOI] [PubMed] [Google Scholar]
- 34.Kamath, A. T., C. E. Sheasby, and D. F. Tough. 2005. Dendritic cells and NK cells stimulate bystander T cell activation in response to TLR agonists through secretion of IFN-alpha/beta and IFN-gamma. J. Immunol. 174:767-776. [DOI] [PubMed] [Google Scholar]
- 35.Kapikian, A. A., R. H. Mitchell, R. M. Chanock, et al. 1969. An epidemiologic study of altered clinical reactivity to RSV infection in children previously vaccinated with an inactivated RSV vaccine. Am. J. Epidemiol. 89:405-421. [DOI] [PubMed] [Google Scholar]
- 36.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. RSV disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 37.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 38.Liu, Y.-J. 2005. IPC: professional type I interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275-306. [DOI] [PubMed] [Google Scholar]
- 39.Lorence, R. M., A. L. Pecora, P. P. Major, S. J. Hotte, S. A. Laurie, M. S. Roberts, W. S. Groene, and M. K. Bamat. 2003. Overview of phase I studies of intravenous administration of PV701, an oncolytic virus. Curr. Opin. Mol. Ther. 5:618-624. [PubMed] [Google Scholar]
- 40.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntosh, K. 1978. Interferon in nasal secretions from infants with viral respiratory tract infections. J. Pediatr. 93:33-36. [DOI] [PubMed] [Google Scholar]
- 42.Mentkevich, L. M., and T. G. Orlova. 1966. Multiplication of Newcastle disease virus strains and interferon production in the mouse, an animal naturally insusceptible to this infection. Acta Virol. 10:226-229. [PubMed] [Google Scholar]
- 43.Muller, U., U. Steinhoff, L. F. L. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional roles of type I and type II interferons in antiviral defense. Science 264:1818-1821. [DOI] [PubMed] [Google Scholar]
- 44.Nakaya, T., J. Cros, M. S. Park, Y. Nakaya, H. Zheng, A. Sagrera, E. Villar, A. Garcia-Sastre, and P. Palese. 2001. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 75:11868-11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuzil, K. M., J. E. Johnson, Y.-W. Tang, J.-P. Prieels, M. Slaoui, N. Gar, and B. S. Graham. 1997. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with RSV FG subunit vaccine. Vaccine 15:597-602. [DOI] [PubMed] [Google Scholar]
- 46.Openshaw, P. J., S. L. Clarke, and F. M. Record. 1992. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int. Immunol. 4:493-500. [DOI] [PubMed] [Google Scholar]
- 47.Ostler, T., and S. Ehl. 2002. Pulmonary T cells induced by respiratory syncytial virus are functional and can make an important contribution to long-lived protective immunity. Eur. J. Immunol. 32:2562-2569. [DOI] [PubMed] [Google Scholar]
- 48.Paramore, L. C., V. Ciuryla, G. Cisela, and L. Liu. 2004. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 22:275-284. [DOI] [PubMed] [Google Scholar]
- 49.Park, M.-S., A. Garcia-Sastre, J. F. Cros, C. F. Basler, and P. Palese. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pien, G. C., K. B. Nguyen, L. Malmgaard, A. R. Satoskar, and C. A. Biron. 2002. A unique mechanism for innate cytokine promotion of T cell responses to viral infections. J. Immunol. 169:5827-5837. [DOI] [PubMed] [Google Scholar]
- 51.Polack, F. P., and R. A. Karron. 2004. The future of respiratory syncytial virus vaccine development. Pediatr. Infect. Dis. J. 23:S65-S73. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Fauquier, A., N. Villanueva, and J. A. Melero. 1987. Isolation of cross-reactive, subtype-specific monoclonal antibodies against influenza virus HA1 and HA2 hemagglutinin subunits. Arch. Virol. 97:251-265. [DOI] [PubMed] [Google Scholar]
- 53.Santini, S. M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K.-K. Conzelmann. 2005. Inhibition of TLR 7 and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by RSV and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spann, K. M., K.-C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta and lambda interferons by the NS1 and NS2 proteins of human RSV in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Talaat, A. M., R. Lyons, and S. A. Johnston. 2001. A combination vaccine confers full protection against co-infections with influenza, herpes simplex and respiratory syncytial viruses. Vaccine 20:538-544. [DOI] [PubMed] [Google Scholar]
- 57.Tough, D. F. 2004. Type I IFN as a link between innate and adaptive immunity through dendritic cell stimulation. Leukemia Lymphoma 45:257-264. [DOI] [PubMed] [Google Scholar]
- 58.Varga, S. M., and T. J. Braciale. 2002. RSV-induced immunopathology: dynamic interplay between the virus and host immune response. Virology 295:203-207. [DOI] [PubMed] [Google Scholar]
- 59.Varga, S. M., X. Wang, R. M. Welsh, and T. J. Braciale. 2001. Immunopathology in RSV infection is mediated by a discrete olgioclonal subset of antigen-specific CD4+ T cells. Immunity 15:637-646. [DOI] [PubMed] [Google Scholar]
- 60.Varga, S. M., E. L. Wissinger, and T. J. Braciale. 2000. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J. Immunol. 165:6487-6495. [DOI] [PubMed] [Google Scholar]
- 61.Waris, M. E., C. Tsou, D. D. Erdman, S. R. Zaki, and L. J. Anderson. 1996. RSV infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like pattern. J. Virol. 70:2852-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]