In their recent papers on the assembly of alphaherpesviruses, Wild et al. (13) and Leuzinger et al. (3) propose that the egress of herpesvirus capsids from the nucleus and formation of the enveloped herpesvirus virion occur by two alternative routes.
The first route is similar to that described in earlier proposals (7) and involves primary envelopment of intranuclear capsids at the inner nuclear membrane followed by transport of these primary virions through the endoplasmic reticulum (ER) and secretory Golgi pathway to the cell surface. This mode of herpesvirus release has been challenged in the last few years, since it does not account for a wealth of biochemical, genetic, and morphological data (reviewed in reference 5) which clearly demonstrate a lack of identity between primary and mature virus particles. The former contain proteins homologous to the herpes simplex virus UL31 and UL34 products which are not present in any of the hitherto-analyzed mature herpesvirus virions, whereas important constituents of mature virions, among them major tegument proteins, are absent from primary virions (reviewed in reference 4). Moreover, the lipid composition of the mature virus envelope differs strikingly from that of the nuclear membrane (10), and viral envelope proteins engineered to be retained in the ER do not become part of the mature virion (12). These well-documented differences between primary enveloped and mature virions are best explained by an envelopment-deenvelopment-reenvelopment pathway (8) which entails budding of intranuclear DNA-filled capsids at the inner nuclear membrane followed by fusion of the primary envelope with the outer nuclear membrane, thus transferring capsids through the nuclear membrane into the cytoplasm where they acquire their final tegument and envelope by budding into a presumably trans-Golgi compartment (2, 9, 11, 14). While not every aspect of this model has been proven, it explains the relevant observations outlined above, which any alternative model has to take into account as well.
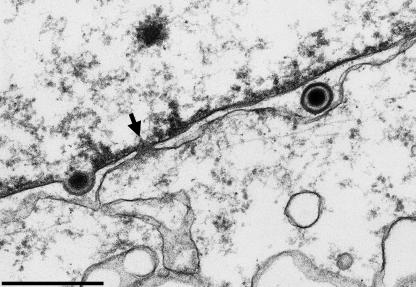
The second route of egress proposed by Wild et al. (13) and Leuzinger et al. (3) involves the dilation of nuclear pores resulting in direct access of capsids to the cytoplasm and envelopment in the Golgi compartment. Indeed, the authors consider that most of the enveloped virions observed in the perinuclear space arise by budding from the cytoplasm into the outer nuclear membrane/ER and hence that naked nucleocapsids are apparently capable of budding into any membrane. These proposals also raise interesting issues. A number of genetic defects (e.g., deletions in UL31/UL34) result in failure of nuclear egress (1, 6), implying that these gene functions may be responsible for nuclear pore dilation. It would be interesting to see whether the authors observe pore dilation in cells infected with mutant viruses lacking these proteins. In extensive studies at relevant time points after infection, we did not observe a similar impairment of nuclear pores (Fig. 1). Furthermore, many mutations that result in the accumulation of capsids in the cytoplasm by a failure of cytoplasmic envelopment (e.g., defects in UL36, UL37, or UL11 and their homologues [reviewed in reference 5)] have been described, yet cells infected with these mutants contain enveloped virions in the perinuclear space. Thus, different functions are clearly required for budding into these different compartments, and this is entirely consistent with the observation that enveloped virions in different compartments have quite different compositions and morphologies.
FIG. 1.
Electron micrograph of BHK21 cells 12 h after infection with HSV-1. The arrow denotes an intact nuclear pore next to a capsid undergoing primary envelopment (left) and a primary enveloped virion in the perinuclear space (right). The nuclear side is on the top, and the cytoplasmic side is on the bottom. Bar, 500 nm. (Electron micrograph courtesy of Harald Granzow, Friedrich-Loeffler-Institut, Insel Riems, Germany).
The electron microscopic observations reported in these two papers challenge current views of the egress and maturation of alphaherpesvirus particles and emphasize the need for further study. However, the models proposed take little account of the weight of biochemical, genetic, and morphological evidence in the literature and cannot be readily reconciled with this evidence.
REFERENCES
- 1.Fuchs, W., B. G. Klupp, H. Granzow, N. Osterrieder, and T. C. Mettenleiter. 2002. The interacting UL31 and Ul34 gene products of pseudorabies virus are involved in egress from the host cell nucleus and represent components of primary enveloped but not of mature virions. J. Virol. 76:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leuzinger, H., U. Ziegler, E. M. Schraner, C. Fraefel, D. L. Glauser, I. Heid, M. Ackermann, M. Mueller, and P. Wild. 2005. Herpes simplex virus type 1 envelopment follows two diverse pathways. J. Virol. 79:13047-13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds, A., B. Ryckman, J. Baines, Y. Zhou, L. Liang, and R. Roller. 2001. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roizman, B., and D. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams and Wilkins, Philadelphia, Pa.
- 8.Skepper, J., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment-deenvelopment-reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turcotte, S., J. Letellier, and R. Lippé. 2005. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol. 79:8847-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Genderen, I. L., R. Brandimarti, M. Torrisi, G. Campadelli-Fiume, and G. van Meer. 1994. The phospholipid composition of extracellular herpes simplex virions differs from that of the host cell nuclei. Virology 200:831-836. [DOI] [PubMed] [Google Scholar]
- 11.Whealy, E. M., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wild, P., M. Engels, C. Senn, K. Tobler, U. Ziegler, E. Schranker, E. Loepfe, M. Ackermann, M. Mueller, and P. Walther. 2005. Impairment of nuclear pores in bovine herpesvirus 1-infected MDBK cells. J. Virol. 79:1071-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu, Z., M. D. Gershon, Y. Hao, R. T. Ambron, C. A. Gabel, and A. A. Gershon. 1995. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J. Virol. 69:7951-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]