Abstract
The Su(Hw) insulator found in the gypsy retrotransposon is the most potent enhancer blocker in Drosophila melanogaster. However, two such insulators in tandem do not prevent enhancer-promoter communication, apparently because of their pairing interaction that results in mutual neutralization. Furthering our studies of the role of insulators in the control of gene expression, here we present a functional analysis of a large set of transgenic constructs with various arrangements of regulatory elements, including two or three insulators. We demonstrate that their interplay can have quite different outcomes depending on the order of and distance between elements. Thus, insulators can interact with each other over considerable distances, across interposed enhancers or promoters and coding sequences, whereby enhancer blocking may be attenuated, cancelled, or restored. Some inferences concerning the possible modes of insulator action are made from collating the new data and the relevant literature, with tentative schemes illustrating the regulatory situations in particular model constructs.
As soon as we admit the long-range action of transcription-modulating elements such as enhancers/silencers, we inevitably face the problem of selectivity (4, 16, 18, 68, 69). Into play come insulators (7, 14, 25, 40, 49, 63), thought to protect promoters from either influence in a simple position-dependent manner, i.e., to stand in the way of positive or negative signaling (10, 17, 19, 27, 31, 34, 38, 44, 50, 54, 57, 60), as well as to create chromatin boundaries, i.e., to obstruct the spread of either state of chromatin (2, 6, 8, 35, 37, 48, 49, 53, 55). Owing to these two main features, a genome region (as well as an integrated transgene) flanked by insulators may behave as a functionally independent gene regulation unit.
The chromatin-bounding function of the insulators will not be considered in this work; regarding their enhancer-blocking function, various models have been comprehensively reviewed (4, 7, 14, 16, 25, 40, 49, 63, 68, 69) and will be mentioned here only when directly pertinent to the matter at hand.
To avoid ambiguity, all interactions among DNA-based regulatory elements, such as enhancers, promoters, and insulators, considered here should by default be understood as functional interactions, not just in the sense that they affect the corresponding function but also in the sense that they do not require direct DNA-DNA contact but rather are mediated by certain (most probably protein) factors functionally associated with the respective DNA sequences. However, such events require certain spatial proximity of the interacting nucleoprotein complexes. Physical approach in enhancer-promoter communication has been directly demonstrated for, e.g., β-globin genes (15, 64) and is supposed to involve special organizer proteins (15, 16, 52, 64, 65, 69). By analogy, long-range functional interactions of the insulators also imply reasonable vicinity.
Among the variety of sequences with an insulator function present in the Drosophila melanogaster genome (for reviews, see references 14, 25, 40, and 68), we chose the well-studied and perhaps the strongest insulator consisting of reiterated binding sites for the Su(Hw) protein, first found in the gypsy retrotransposon (27, 34). The Su(Hw) insulator is a versatile modulator of regulatory interactions, blocking more than a score of different enhancers (10, 11, 12, 13, 17, 20, 21, 27, 32, 33, 34, 41, 47, 50, 54, 57, 58, 66, 70). Recently, an endogenous Su(Hw)-dependent but structurally distinct insulator has been found between the yellow and achaete genes (29, 51).
As the main functional test system, we chose the yellow gene, which has been extensively employed for studies of the enhancer-blocking activity of insulators (24, 27, 29, 41, 47, 51). The yellow gene is responsible for dark pigmentation of larval and adult cuticle and its derivatives. Two upstream enhancers provide for its activation in the body cuticle and wing blades (26, 45). In most cases, the transgenic lines carrying enhancerless yellow constructs have yellow color of wings and body cuticle (26, 41, 45, 47), which indicates that incidental activation of yellow by nearby enhancers located close to the site of transposon insertion is a very rare event.
It was demonstrated that a single copy of the Su(Hw) insulator completely blocked the yellow enhancers when it was inserted at any site between the corresponding enhancers and promoter (27, 41, 47).
On the other hand, such transgenic studies with the yellow and other systems soon revealed a peculiar phenomenon of interaction between Su(Hw) insulators, recognized as “mutual neutralization,” or insertion of two insulators between an enhancer and promoter, which allowed the enhancer to bypass the insulators and activate transcription (12, 41, 47).
In previous works, the enhancer-blocking activity of one or two copies of insulators was examined in constructs with the enhancers, promoters, and insulators close to each other (10, 11, 12, 13, 17, 20, 21, 27, 32, 33, 34, 41, 47, 50, 54, 57, 58, 66, 70). However, endogenous insulators might be located at greater distances from each other and from enhancers and promoters. It is also possible that several insulators are present in the regulatory region of a complex locus. Here we report a functional analysis of the interplay of two and three Su(Hw) insulators in various yellow expression constructs and offer some inferences on the possible modes of insulator action and the ensuing regulatory schemes.
MATERIALS AND METHODS
Plasmid construction.
The 430-bp gypsy sequence (I for insulator) containing the Su(Hw)-binding region was PCR amplified from the gypsy retrotransposon. To confirm its identity, the product after sequencing was subcloned in pSK plasmid in one and two copies (pSK-I and pSK-I-I), between lox sites [lox(I)], together with a 1.9-kb or 2.2-kb fragment of lamba DNA (pSK-I-λ). A fragment of the yellow coding region, 1.5 kb in length (S for spacer), was added to pSK-I-I, yielding pSK-ISI. Plasmid p7K containing the gypsy retrotransposon was received from Y. Ilyin. The 6-kb NcoI-XhoI fragment (Sg) containing the gypsy coding region was subcloned into pSK-I (pSK-I-Sg).
For cloning, we created the pCaSpeR* plasmid obtained by inactivation of the EcoRI site in the polylinker of pCaSpew15. As a result, pCaSpeR* (C*) had the unique EcoRI site downstream of the white gene.
The lox(I) fragments were then subcloned into EcoRI of pCaSpeR* to obtain C*-lox(I). The 5-kb BamHI-BglII fragment containing the yellow coding region (yc) was subcloned into CaSpeR2 (C2-yc), or C*-lox(I) [C*-lox(I)-yc]. The white gene was deleted from pCaSpew15 by digestion with EcoRI to produce the CΔ plasmid.
The 3-kb SalI-BamHI fragment containing the yellow regulatory region (yr) was subcloned into the pGEM7 plasmid digested with BamHI and XhoI. The I, I-I, or ISI fragments were subcloned into the Eco47III site of yr plasmids to produce yr-I-I and yr-ISI.
EyI(S)(Ey)IYW and EyI(Py)(Ey)IYW.
The 1.5-kb spacer (S) derived from the yellow coding region was cloned between two lox sites [lox(S)]. The HindIII-BamHI fragment from the yellow gene containing the promoter region was flanked by lox sites [lox(Py)]. The SalI-Eco47III fragment including the yellow enhancer region was cloned between frt sites [frt(Ey)]. Two fragments, lox(S) and frt(Ey) or lox(Py) and frt(Ey), were subsequently cloned into yr-I-I between the Su(Hw) insulator sequences to produce yr-I-lox(S)-frt(Ey)-I and yr-I-lox(Py)-frt(Ey)-I. To obtain the final constructs, the yr-I-lox(S)-frt(Ey)-I and yr-I-lox(Py)-frt(Ey)-I fragments were cloned into C2-yc digested with XbaI and BamHI.
IλEyISIYW.
The yr-ISI fragment was subcloned into C2-yc digested with XbaI and BamHI (yr-ISI-C2-yc). The I-λ fragment was cloned into yr-ISI-C2-yc digested with XbaI.
EyISIY(I) and EyISIYλ(I).
The lox(I) fragment was cloned into the CΔ plasmid digested with EcoRI [CΔ-lox(I)]. The CΔ-lox(I) fragment was cloned with 5.1 kb of lambda DNA [CΔ-λ-lox(I)]. The CΔ-lox(I) and CΔ-λ-lox(I) fragments were cloned into yr-ISI digested with XbaI and BamHI.
EyISIYW(I).
The yr-ISI fragment was subcloned into C*-lox(I)-yc digested with XbaI and BamHI.
(I)EyISIYW and EyISIY(I)W.
The yr-ISI fragment was cloned into C2-yc digested with XbaI and BamHI (yr-ISI-C2-yc). The lox(I) fragment was cloned into yr-frt(Ee)-ISI-C2-yc digested either with XbaI or with BglII.
EyIYIW(I).
The yr-I fragment was cloned into C*-lox(I)-yc. The I fragment was inserted at the BglII site between the yellow and white genes.
EyISIIYW.
The I fragment was cloned into the yr plasmid digested with KpnI (yr-I). The ISI fragment was cloned into the yr-I plasmid digested with Eco47III (yr-I-ISI). The yr-I-ISI fragment was subcloned into C2-yc digested with XbaI and BamHI.
In the following constructs, we deleted the white gene (to reduce the construct size) and used yellow expression in bristles as a transformation marker for screening the transformed flies. The 5-kb BamHI-BglII fragment containing the coding region (yc) was inserted in direct orientation into the CΔ plasmid digested with BamHI (CΔ-yc). Then the XbaI-BamHI fragment containing the yellow regulatory region (yr) was subcloned into CΔ-yc digested with XbaI and BamHI (CΔ-y).
EyISgY(I) and EySgIY(I).
lox(I) was inserted into the CΔ-y plasmid cleaved with SmaI [CΔ-y-lox(I)]. Then, the Sg-I fragment was inserted in both orientations into the CΔ-y-lox(su) plasmid cleaved with Eco47III at position −893 from the yellow transcription start site.
IλEySgIY and IλEyISgY.
The I-λ fragment was inserted in direct orientation into the CΔ-y plasmid cleaved with XbaI (CΔ-y-I-λ). Then, the Sg-I fragment was inserted in both orientations into the CΔ-y-I-λ plasmid cleaved with Eco47III.
IλEySgIY(I).
The lox(I) fragment was inserted into the IλEySgIY plasmid cleaved with SmaI at the 3′ side of the yellow gene.
IλΔEySgIY.
The yrΔEy plasmid containing the yellow regulatory region with the enhancers deleted was obtained from A. Golovnin. The yrΔEy fragment was cloned into the CΔ-yc plasmid digested with XbaI and BamHI (CΔ-ΔEy-y). The I-λ fragment was inserted in direct orientation into the CΔ-ΔEy-y plasmid cleaved with XbaI (CΔ-ΔEy-y-I-λ). Then, the Sg-I fragment was inserted into the CΔ-ΔEy-y-I-λ plasmid cleaved with Eco47III.
Generation and analysis of the transgenic lines.
All flies were maintained at 25°C on standard yeast medium. The mutant alleles and chromosomes used in this work and the balancer chromosomes are described elsewhere (42). The construct, together with a P element having defective inverted repeats used as a transposase source, P25.7wc (36), was injected into y ac w1118 preblastoderm embryos as described previously (56, 62). The resulting flies were crossed with y ac w1118 flies, and the transgenic progeny were identified by their eye and/or cuticle structure color. Chromosome localization of various transgene insertions was determined by crossing the transformants with the y ac w1118 balancer stock carrying dominant markers: In(2RL),CyO for chromosome two and In(3LR)TM3,Sb for chromosome three. The transformed lines were tested for transposon integrity and copy number by Southern blot hybridization.
The selected lines were crossed into the su(Hw)v/su(Hw)f mutant background (21) to establish the contribution of the Su(Hw) insulator to the yellow and white phenotypes.
The lines with excisions were obtained by crossing the flies bearing the transposons with Flp (w1118; S2 CyO hsFLP ISA/Sco; +) or Cre (y1, wi; CyO, P[w+, cre]/Sco; +) recombinase-expressing lines. A high level of FLP recombinase was produced by heat shock of late embryos and second or third instar larvae for 2 h at 37°C. The excisions were confirmed by Southern blot hybridization and/or PCR analysis. The details of the crosses used for genetic analysis and for excision of functional elements are available upon request.
In order to determine the yellow phenotypes, we visually estimated the degree of pigmentation in the abdominal cuticle (B) and wings (W) of 3- to 5-day-old males developing at 25°C. A five-grade scale was used, with grade 1 corresponding to pigmentation levels characteristic of flies with complete or nearly complete loss of yellow gene expression and grade 5 corresponding to pigmentation in flies with a wild-type level of yellow gene expression. The flies with the yellow alleles characterized previously were used to determine pigmentation levels. Grade 2 corresponded to the pigmentation level associated with the gypsy-induced yellow mutation, y2; this pigmentation level is expected upon a complete block of the wing and body enhancers. Differences in pigmentation by at least one grade were interpreted as evidence for significant differences in level of yellow gene expression.
RESULTS
Su(Hw) insulators can interact across yellow enhancers and promoter.
We have previously demonstrated that when two Su(Hw) insulators are inserted in tandem (with a short spacer) between the yellow enhancers and the promoter, they both lose their enhancer-blocking activity (47). To explain the blocking activity of the Su(Hw) insulators surrounding either an enhancer or a promoter, in contrast to that of the tandem insulators, it was proposed that an enhancer or promoter between two Su(Hw) insulators disrupted their local mutually neutralizing interaction (46, 68).
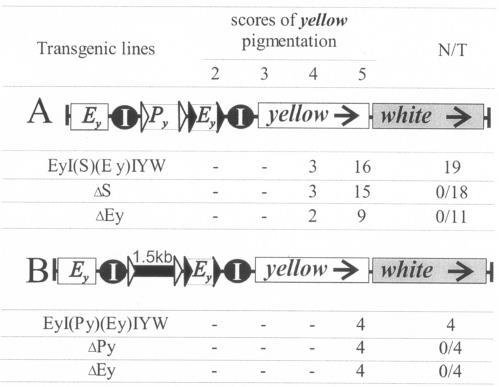
To test this assertion, we made two constructs (Fig. 1) with insertions between the yellow enhancers and promoter, at position −893 relative to the yellow transcription start site (this will be the standard position for all insulator inserts unless stated otherwise). In EyI(S)(Ey)IYW (Fig. 1A), two Su(Hw) insulators (I) were separated by an additional pair of yellow enhancers (Ey) and a 1.5-kb spacer (S). The other construct, EyI(Py)(Ey)IYW (Fig. 1B), had an additional yellow promoter (Py) instead of the spacer. Throughout the paper, parentheses in construct designations mark the elements enclosed in frt or lox sites for in vivo excision by crossing (28, 59), as outlined in Materials and Methods.
FIG. 1.
Schemes of the transgenic constructs and analysis of transgenic lines for the ability of Su(Hw) insulators to interact across interposed elements. The Su(Hw) insulators are shown as black circles marked I. The yellow enhancers (Ey) and the additional promoter (Py) are shown as white boxes. The 1.5-kb DNA spacer fragment (S) is shown as a thick black line. The yellow and white genes are framed, with arrows indicating the direction of transcription. The frt and lox sites are shown as black and white triangles, respectively, in the schemes and are denoted by parentheses in construct names. The data below the schemes give the numbers of transgenic lines with the specified (top) y pigmentation levels in the abdominal cuticle (reflecting the activity of the body enhancer); in most of the lines, the pigmentation levels in wing blades (reflecting the activity of the wing enhancer) closely correlated with these scores. N is the number of lines in which flies acquired a new y phenotype upon deletion (Δ) of the specified DNA fragment; T is the total number of lines examined for each particular construct.
The flies of all 19 EyI(S)(Ey)IYW and all 4 EyI(Py)(Ey)IYW transgenic lines had almost wild-type levels of wing and body pigmentation (Fig. 1). Elimination of the additional enhancers, spacer, or additional promoter or both excisable elements from transgenic flies did not change their pigmentation. As shown previously (47), yellow enhancers that are followed by an Su(Hw) insulator or surrounded by two insulators, similarly to the Fig. 1 constructs, cannot stimulate yellow expression (see also Fig. 2F below). Hence, it appears that the above assertion (46, 68) is too simplistic and that the two Su(Hw) insulators do somehow interact around an enhancer. In this manner, the enhancer is blocked but the activity of the insulators is exhausted, so that the upstream yellow enhancers in EyI(S)(Ey)IYW and EyI(Py)(Ey)IYW can stimulate the target gene over the insulator pair (whether or not the latter encloses enhancers, promoter, or neutral spacer).
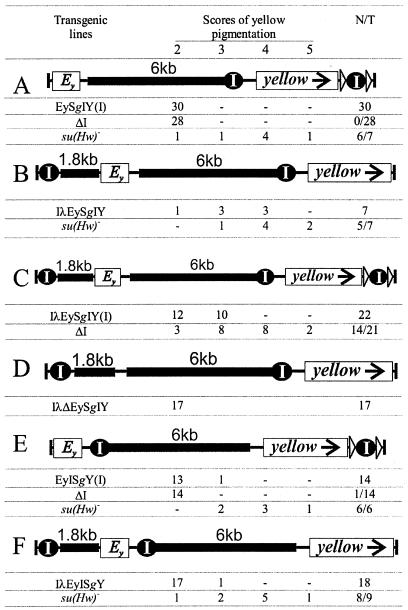
FIG. 2.
Tests for the ability of Su(Hw) insulators to interact at various distances and positions in the transgenic constructs. Designations are described in the legend for Fig. 1. Lengths for the lambda- and gypsy-derived spacers (λ and Sg in construct names) are indicated. su(Hw)− refers to the su(Hw)v/su(Hw)f mutations, and N is the number of transgenic lines in which flies acquired a new y phenotype in the background devoid of the Su(Hw) protein.
Role of the distance between Su(Hw) insulators in blocking enhancer-promoter communication.
Considering the above data, one can reasonably suppose that multiple interacting proteins [Su(Hw), Mod(mdg4) (22, 23), etc.] bound to the insulators around a transcriptionally important element may obstruct the propagation of the regulatory signal or the operation of the transcription machinery. If this is the case, the feasibility of enhancer-promoter communication may depend on the distance between the insulators as well as on the distance from the insulator to the regulatory element.
To test this idea, we increased the distance between the Su(Hw) insulators surrounding either the enhancers or the yellow gene itself. In the series described in Fig. 2, the spacer (Sg) was a 6-kb fragment of the gypsy coding sequence devoid of a promoter or other identified elements that could influence the activity of the enhancers and insulator.
In the EySgIY(I) (Fig. 2A) and IλEySgIY (Fig. 2B) constructs, the 6-kb spacer was inserted between the yellow enhancers and the Su(Hw) insulator at −893. In EySgIY(I), the second excisable insulator was separated from the first one by the 6-kb span of the yellow gene. In 30 independent EySgIY(I) transgenic lines, the flies had a y2-like phenotype reflecting complete blockage of the yellow enhancers (Fig. 2A). Against the su(Hw)− background, cuticle pigmentation was partly restored in six of seven tested transgenic lines, indicating that the yellow enhancers themselves were able to activate the yellow promoter over a 7-kb distance in most construct insertion sites (Fig. 2A). This was in line with earlier observations (24). Removal of the downstream insulator in 28 EySgIY(I) lines did not change the cuticle pigmentation (Fig. 2A), i.e., one Su(Hw) insulator efficiently blocked the enhancers that were ∼6 kb upstream.
In the IλEySgIY construct (Fig. 2B), the second Su(Hw) element was inserted 1.8 kb before the yellow enhancers. Thus, two insulators surrounded the enhancers but were 10 kb away from each other. Six of seven IλEySgIY transgenic lines had intermediate levels of wing and body pigmentation, which further increased against the su(Hw)− background in five of seven lines (Fig. 2B), suggesting partial insulation. To check whether it was the yellow enhancers that stimulated yellow expression in the IλEySgIY transgenic lines, we made a control construct from which the yellow enhancers were deleted (Fig. 2D). Indeed, in all 17 IλΔEySgIY transgenic lines the flies had a y2-like phenotype. We could conclude that some enhancer-promoter communication over the intervening Su(Hw) insulator became possible when there was a second insulator 10 kb upstream.
Under these intriguing circumstances, we decided to combine the constructs shown in Fig. 2A and B into one array with three widely spaced insulators (Fig. 2C). In 22 IλEySgIY(I) lines, the flies had yellow or slightly pigmented body and wing tissues. In vivo excision of the downstream insulator considerably increased pigmentation in 14 of 21 lines. Thus, a third insulator downstream of the target gene can markedly influence the interplay of the two upstream insulators around the enhancer. There may be several explanations to this effect, including simultaneous interaction of the three copies or differential outcome of insulator pairing around an enhancer and around a gene, but perhaps a simpler idea is that the outermost insulators interact to neutralize each other and virtually restore the classical enhancer/insulator/promoter pattern in the middle of the construct.
Next, we tested an arrangement (Fig. 2E) similar to that shown in Fig. 2A but with the spacer and adjoining insulator swapped over, whereby the distance between the insulators around the yellow gene was doubled to 12 kb. All results with EyISgY(I) proved to be much the same as those with EySgIY(I) (cf. Fig. 2E and A).
To round out the series, the same spacer-insulator swap was made in the arrangement shown in Fig. 2B, thereby placing the insulator closely after the enhancer, with the upstream insulator only 4 kb away (IλEyISgY) (Fig. 2F). In this case, we observed practically complete Su(Hw)-mediated blocking of yellow expression, in contrast to the partial blocking in the case of the arrangement in Fig. 2B. Thus, the distance between the Su(Hw) insulators around the yellow enhancers is essential for their blocking efficacy.
Interplay between a tandem pair and a third Su(Hw) insulator.
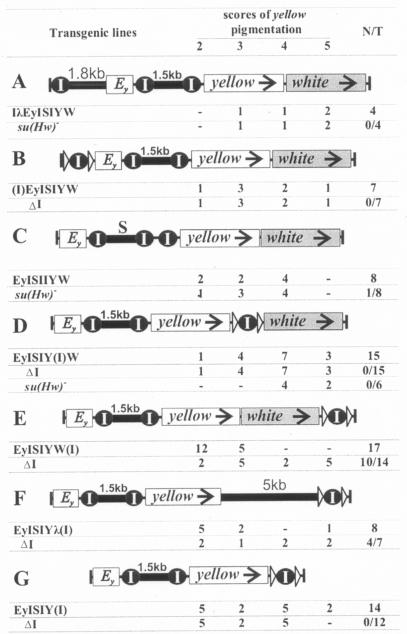
Knowing that two consecutive Su(Hw) insulators between an enhancer and the target gene promoter do not block gene activation (12, 41, 47) and having just witnessed an interesting interplay of two and three appreciably spaced insulators (Fig. 2B and C), we further examined the functional outcome of placing a mutually neutralizing tandem pair of insulators (ISI) in the standard position [actually an analog of the construct shown in Fig. 1A with (Ey) excised] and a third insulator in various places throughout the construct: 1.8 kb upstream of (IλEyISIYW [Fig. 3A ]) or directly before [(I)EyISIYW (Fig. 3B)] the yellow enhancers, between the enhancers and promoter at −343 (i.e., ∼0.5 kb after the tandem) (EyISIIYW [Fig. 3C]), after the yellow gene [EyISIY(I)W (Fig. 3D)], and after the white gene [EyISIYW(I) (Fig. 3E)].
FIG. 3.
Analysis of the functional outcome of interaction between a tandem pair and a third Su(Hw) insulator at various positions. Designations are described in the legend for Fig. 1.
In all four IλEyISIYW transgenic lines (Fig. 3A) and six of seven (I)EyISIYW lines (Fig. 3B), the yellow gene was markedly activated. Neither the su(Hw)− background (Fig. 3A) nor excision of the upstream insulator (Fig. 3B) had any effect. Comparing these data with the results from Fig. 2F, one may conclude that tandem pairing prevails over interaction with an upstream insulator.
Likewise, appreciable yellow expression was observed (though no wild-type levels were attained) when the third insulator was placed closely after the tandem (Fig. 3C). The su(Hw)− background slightly increased pigmentation in only one of eight lines, suggesting that three consecutive copies of the Su(Hw) insulator failed to efficiently block yellow enhancers.
A third insulator placed directly after the yellow gene produced no effect on the mutual neutralization of the tandem pair (Fig. 3D), just as it had no effect on enhancer blocking by a single intervening insulator (Fig. 2A and E). By contrast, clear Su(Hw)-mediated enhancer blocking was observed when the third insulator was moved farther downstream [EyISIYW(I) (Fig. 3E)]. This was not associated with any specific influence of white-related sequences, because replacement of the latter with a neutral λ spacer gave nearly the same results (Fig. 3F), whereas the third insulator after yellow remained ineffective upon deletion of all white sequences [EyISIY(I) (Fig. 3G)].
DISCUSSION
As already established (27, 41, 47) and again confirmed here, a single Su(Hw) insulator at any position between the yellow enhancer and promoter completely precludes gene activation in cis, whereas tandem pairing of the insulators results in “mutual neutralization” (41, 47) or “cancellation” (12, 43). Here we see that the insulators can also pair across interposed regulatory elements (enhancers or promoter) (Fig. 1); likewise, two Su(Hw) insulators were reported to interact across a strong scs insulator, which thereby was also rendered incapable of enhancer blocking (41) (i.e., was locked in [see below]).
Previously, we have shown that a pair of closely spaced insulators does not in itself stimulate transcription of yellow in wings and body cuticle (47). Here, collation of the arrangements shown in Fig. 1A and 2F shows that the very act of putting together a couple of insulators and an enhancer (or other element) does not create an artificial enhancer-like region that could have smeared the results.
Further, we see that downstream gene activation is completely blocked when two insulators surround the enhancer quite closely (within 4 kb of each other) (Fig. 2F) but only partly blocked when the insulators are 10 kb apart (Fig. 2B). We also see that in a system with a “cancelled” tandem pair of insulators, enhancer blocking can be almost completely (Fig. 3E and F) restored by a third downstream insulator.
We do not yet know how the insulator disrupts enhancer-promoter communication, but we can already make some educated guesses, trying to reconcile common knowledge and new data in accordance with the schematic cases compiled in Fig. 4.
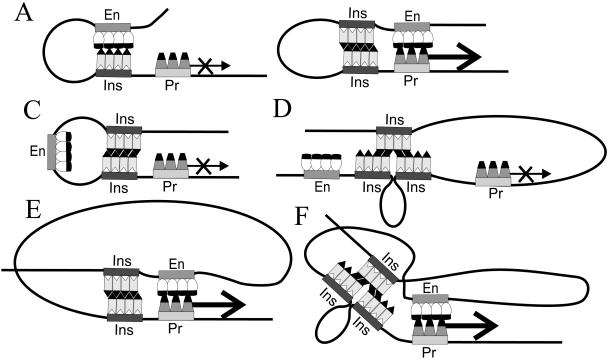
FIG. 4.
Schematic cases of possible interplay among the elements in the regulation of gene expression, as related to the literature data and models and the results of this work. En, Pr, and Ins mark the nucleoprotein complexes of the enhancer, promoter, and insulator, respectively; the thick arrows denote promoter stimulation by the enhancer with ensuing transcription, and the thin, crossed arrows denote lack of stimulation (blocked enhancer-promoter communication). (A) “Basic” arrangement, in which a single intervening insulator fully blocks enhancer-promoter communication. There is ample experimental support, but enhancer trapping as implied in the common “promoter decoy” model has not been demonstrated, and only transient association is consistent with the definition of insulator function. (B) Another basic case, in which pairing of two intervening insulators allows free enhancer-promoter communication, commonly known as insulator mutual neutralization, cancellation, and bypass. There is ample experimental support for this case. (C) Pairing of two insulators around the enhancer locks it in a loop and topologically isolates it from the promoter, commonly known as the loop domain model (corresponds to Fig. 2F). (D) Reverse case, where interaction between a tandem pair and a third downstream insulator topologically isolates the gene promoter from the enhancer (Fig. 3E and F). The actual positioning of the three copies here and in the case shown in panel F is unknown. (E) Pairing of broadly spaced insulators around the enhancer gives the latter enough freedom to approach the promoter region beyond the loop (Fig. 2B). Note the spatial similarity to the “linear” case shown in panel B. (F) Analogous case for an intervening tandem and a third upstream insulator (Fig. 3A). For more detailed comments and references, see the text.
Though the “promoter decoy” model shown in Fig. 4A is most common (19, 25, 40, 50, 66), to our knowledge there is no actual evidence that an insulator complex binds to an enhancer complex to neutralize it (as in insulator pairing) or traps its vital component(s), as is inherent in the model; on the contrary, the definitive position dependence of insulator action implies that insulators do not inactivate enhancers, silencers, or promoters (10, 27, 50, 57, 66). Furthermore, we have very recently demonstrated that enhancer blocking by an intervening Su(Hw) insulator in cis does not prevent the enhancer action in trans (39); this makes insulator-enhancer pairing quite unlikely, because such neutralization should have affected both modes of gene activation. In the aggregate, this gives us grounds to suppose that the insulator interacts with the enhancer only when and inasmuch as the enhancer tries to negotiate the insulator. One can immediately see that the decoy model as amended for transient interaction (25, 40, 69) represents a particular case of this general issue.
If “enhancer-promoter communication” actually means the mutual approach of the transcription factory and target gene (25, 40, 69), the insulator(s) may obstruct this process (i) sterically (and in a sense nonspecifically, by the overall bulk of insulator-associated proteins), i.e., “one stands in the way, two lock it in,” and/or (ii) functionally owing to the binding capacities of the associated protein set, i.e., by creating a “viscid” region that the enhancer machinery cannot overcome. “Viscidity” may be reduced or abolished when two such sets merge on paired insulators (Fig. 4B shows a common case of cancellation, which is additionally exemplified by ΔEy in Fig. 1A).
When two insulators surround an enhancer, their pairing locks the enhancer in a loop (Fig. 4C corresponds to the known loop domain model [3, 5, 9, 40, 43, 69]; also see Fig. 4E), and this is where we can observe the role of the distance between the insulators. If the loop is large enough, the enclosed enhancer can still interact with a promoter-carrying DNA region beyond (Fig. 4E, which in terms of element positioning is equivalent to Fig. 4B); if the loop is quite small (close flanking [Fig. 4C]), there may be a “tight knot” with conformational and/or steric hindrances to enhancer function. This can well explain the difference between Fig. 2B and F.
There are two recent studies in line with the loop lock idea. An insulator-like element was constructed in vitro using a sequence-specific DNA-binding protein (lac repressor) known to cause stable DNA looping (5). The insulator function was entirely dependent on the formation of a DNA loop that topologically isolated the enhancer from the promoter. Similarly, enclosing a simian virus 40 enhancer in a DNA loop could block activation of gene expression in HeLa cells (1). We cannot yet say to what extent the patterns emerging for the Su(Hw) insulator interplay in yellow regulation can be extended to other elements. Anyway, in our hands no insulator pairing is required for enhancer blocking (see above), though something of this kind is perhaps required for DNA looping; hence, DNA looping as such is not obligatory for enhancer blocking. On the contrary, it may help circumvent the block (e.g., Fig. 4E).
Representation of the three-insulator systems (Fig. 4D and F) is even more schematic because we do not know whether there is triple interaction or preferential pairing and displacement, but this is not essential at the moment. Anyhow, engagement of the third downstream insulator may create an obstacle (Fig. 4D and 3E and F) for the enhancer that otherwise would have bypassed the “cancelled” tandem pair (Fig. 4B). On the other hand, an upstream third insulator may have little effect even if it interacts with the tandem pair but thereby brings the enhancer closer to the promoter (Fig. 4F and 3A). Again, a third insulator after the pair does not cause pronounced blocking (Fig. 3C). This appears to be at variance with an earlier report (41) of enhancer blocking by a triple tandem of Su(Hw) insulators. We should, however, bear in mind that when the elements are put quite close to each other, even small differences in spacing may affect the outcome. Thus, it may well be that in our construct shown in Fig. 3C the two outer copies preferentially pair to lock in and incapacitate or shield the middle one, just as they did with the scs insulator in the previously cited work (41).
We must admit that an attempt to envisage a general picture immediately encounters an exception. Thus, collation of the data in Fig. 2A, C, and E and Fig. 3D and G suggests that the insulator directly after the yellow gene has practically no influence on the standard (intervening) insulator(s) regardless of the distance thereto; this is perhaps a most distinct case of context dependence, though its causes are obscure. On the other hand, this agrees nicely with the reinforcement of enhancer blocking in Fig. 2C as interpreted in Results.
One can see that the cases outlined in Fig. 4 and corroborated by the results of this work reasonably combine certain features of the “transcriptional” and “structural” models of insulator function (3, 5, 9, 14, 22, 23, 25, 40, 41, 43, 68, 69), which are indeed nonexclusive. Note that the first four cases (Fig. 4A to D) are simple “linear” schemes, whereas the case shown in Fig. 4E and its three-insulator analog shown in Fig. 4F imply spatial interaction to override topological isolation, in a sense equivalent to trans action. Therewith, we do not introduce any new participants into the system. Our experiments are realistic in the sense that we did not create any extravagant arrangements hardly encountered in nature. Yet certainly the real situation is far more complex; suffice it to say that the effectiveness of the Su(Hw) insulator depends on the enhancer and promoter strength (13, 41, 58, 66) and that in certain contexts the Su(Hw) insulator can even stimulate transcription (30, 61, 67). However, we can say that insulators are not just barriers to interaction between other elements but rather versatile agents that may be widely involved in regulation of complex genetic loci.
Acknowledgments
We are very grateful to Y. Ilyin for providing us with the p7K plasmid.
This work was supported by the Molecular and Cellular Biology Program of the Russian Academy of Sciences, the Russian Foundation for Basic Research (project MD-268-2003-04), a stipend from the Center for Medical Studies, Oslo University (to E.S.), and an International Research Scholar Award from the Howard Hughes Medical Institute (to P.G.).
REFERENCES
- 1.Ameres, S. L., L. Drueppel, K. Pfleiderer, A. Schmidt, W. Hillen, and C. Berens. 2005. Inducible DNA-loop formation blocks transcriptional activation by an SV40 enhancer. EMBO J. 24:358-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi, X., and J. R. Broach. 1999. UASrpg can function as a heterochromatin boundary element in yeast. Genes Dev. 13:1089-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanton, J., M. Gaszner, and P. Schedl. 2003. Protein:protein interactions and the pairing of boundary elements in vivo. Genes Dev. 17:664-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondarenko, V. A., Y. V. Liu, Y. I. Jiang, and V. M. Studitsky. 2003. Communication over a large distance: enhancers and insulators. Biochem. Cell Biol. 81:241-251. [DOI] [PubMed] [Google Scholar]
- 5.Bondarenko, V. A., Y. I. Jiang, and V. M. Studitsky. 2003. Rationally designed insulator-like elements can block enhancer action in vitro. EMBO J. 22:4728-4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonifer, C., M. Vidal, F. Grosveld, and A. E. Sippel. 1990. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 9:2843-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasset, E., and C. Vaury. 2005. Insulators are fundamental components of the eukaryotic genomes. Heredity 94:571-576. [DOI] [PubMed] [Google Scholar]
- 8.Burgess-Beusse, B., C. Farrell, M. Gaszner, M. Litt, V. Mutskov, F. Recillas-Targa, M. Simpson, A. West, and G. Felsenfeld. 2002. The insulation of genes from external enhancers and silencing chromatin. Proc. Natl. Acad. Sci. USA 99:16433-16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd, K., and V. G. Corces. 2003. Visualization of chromatin domains created by the gypsy insulator of Drosophila. J. Cell Biol. 162:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai, H., and M. Levine. 1995. Modulation of enhancer-promoter interactions by insulators in the Drosophila embryo. Nature 376:533-536. [DOI] [PubMed] [Google Scholar]
- 11.Cai, H. N., and M. Levine. 1997. The gypsy insulator can function as a promoter-specific silencer in the Drosophila embryo. EMBO J. 16:1732-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai, H. N., and P. Shen. 2001. Effects of cis arrangement of chromatin insulators on enhancer-blocking activity. Science 291:493-495. [DOI] [PubMed] [Google Scholar]
- 13.Cai, H. N., Z. Zhang, J. R. Adams, and P. Shen. 2001. Genomic context modulates insulator activity through promoter competition. Development 128:4339-4347. [DOI] [PubMed] [Google Scholar]
- 14.Capelson, M., and V. G. Corces. 2004. Boundary elements and nuclear organization. Biol. Cell 96:617-629. [DOI] [PubMed] [Google Scholar]
- 15.Carter, D., L. Chakalova, C. S. Osborne, Y. F. Dai, and P. Fraser. 2002. Long-range chromatin regulatory interactions in vivo. Nat. Genet. 32:623-626. [DOI] [PubMed] [Google Scholar]
- 16.Chambeyron, S., and W. A. Bickmore. 2004. Does looping and clustering in the nucleus regulate gene expression? Curr. Opin. Cell Biol. 16:256-262. [DOI] [PubMed] [Google Scholar]
- 17.Dorsett, D. 1993. Distance-independent inactivation of an enhancer by the suppressor of Hairy-wing DNA-binding protein of Drosophila. Genetics 134:1135-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorsett, D. 1999. Distant liaisons: long-range enhancer-promoter interactions in Drosophila. Curr. Opin. Genet. Dev. 9:505-514. [DOI] [PubMed] [Google Scholar]
- 19.Dunaway, M., J. Y. Hwang, M. Xiong, and H.-L. Yuen. 1997. The activity of the scs and scs′ insulator elements is not dependent on chromosomal context. Mol. Cell. Biol. 17:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gause, M., P. Morcillo, and D. Dorsett. 2001. Insulation of enhancer-promoter communication by a gypsy transposon insert in the Drosophila cut gene: cooperation between suppressor of hairy-wing and modifier of mdg4 proteins. Mol. Cell. Biol. 21:4807-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgiev, P., and M. Kozycina. 1996. Interaction between mutations in the suppressor of Hairy wing and modifier of mdg4 genes of Drosophila melanogaster affecting the phenotype of gypsy-induced mutations. Genetics 142:425-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerasimova, T. I., K. Byrd, and V. G. Corces. 2000. A chromatin insulator determines the nuclear localization of DNA. Mol. Cell 6:1025-1035. [DOI] [PubMed] [Google Scholar]
- 23.Gerasimova, T. I., and V. G. Corces. 1998. Polycomb and trithorax group proteins mediate the function of a chromatin insulator. Cell 92:511-521. [DOI] [PubMed] [Google Scholar]
- 24.Geyer, P. K., C. Spana, and V. Corces. 1986. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 5:2657-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geyer, P. K., and I. Clark. 2002. Protecting against promiscuity: the regulatory role of insulators. Cell. Mol. Life Sci. 59:2112-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geyer, P. K., and V. G. Corces. 1987. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1:996-1004. [DOI] [PubMed] [Google Scholar]
- 27.Geyer, P. K., and V. G. Corces. 1992. DNA position-specific repression of transcription by a Drosophila zinc finger protein. Genes Dev. 6:1865-1873. [DOI] [PubMed] [Google Scholar]
- 28.Golic, K. G., and S. Lindquist. 1989. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59:499-509. [DOI] [PubMed] [Google Scholar]
- 29.Golovnin, A., I. Birukova, O. Romanova, M. Silicheva, A. Parshikov, E. Savitskaya, V. Pirrotta, and P. Georgiev. 2003. An endogenous Su(Hw) insulator separates the yellow gene from the Achaete-scute gene complex in Drosophila. Development 130:3249-3258. [DOI] [PubMed] [Google Scholar]
- 30.Golovnin, A., E. Melnick, A. Mazur, and P. Georgiev. 2005. Drosophila Su(Hw) insulator can stimulate transcription of a weakened yellow promoter over a distance. Genetics 170:1133-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gyurkovics, H., J. Gausz, J. Kummer, and F. Karch. 1990. A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation. EMBO J. 9:2579-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagstrom, K., M. Muller, and P. Schedl. 1997. A Polycomb and GAGA dependent silencer adjoins the Fab-7 boundary in the Drosophila bithorax complex. Genetics 146:1365-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogga, I., J. Mihaly, S. Barges, and F. Karch. 2001. Replacement of Fab-7 by the gypsy or scs insulator disrupts long-distance regulatory interactions in the Abd-B gene of the bithorax complex. Mol. Cell 8:1145-1151. [DOI] [PubMed] [Google Scholar]
- 34.Holdridge, C., and D. Dorsett. 1991. Repression of hsp70 heat shock gene transcription by the suppressor of hairy-wing protein of Drosophila melanogaster. Mol. Cell. Biol. 11:1894-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishii, K., G. Arib, C. Lin, G. Van Houwe, and U. K. Laemmli. 2002. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell 109:551-562. [DOI] [PubMed] [Google Scholar]
- 36.Karess, R. E., and G. M. Rubin. 1984. Analysis of P transposable element functions in Drosophila. Cell 38:135-146. [DOI] [PubMed] [Google Scholar]
- 37.Kellum, R., and P. Schedl. 1991. A position-effect assay for boundaries of higher order chromosomal domains. Cell 64:941-950. [DOI] [PubMed] [Google Scholar]
- 38.Kellum, R., and P. Schedl. 1992. A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol. Cell. Biol. 12:2424-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kravchenko, E., E. Savitskaya, O. Kravchuk, A. Parshikov, P. Georgiev, and M. Savitsky. 2005. Pairing between gypsy insulators facilitates the enhancer action in trans throughout the Drosophila genome. Mol. Cell. Biol. 25:9283-9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhn, E. J., and P. K. Geyer. 2003. Genomic insulators: connecting properties to mechanism. Curr. Opin. Cell Biol. 15:259-265. [DOI] [PubMed] [Google Scholar]
- 41.Kuhn, E. J., M. M. Viering, K. M. Rhodes, and P. K. Geyer. 2003. A test of insulator interactions in Drosophila. EMBO J. 22:2463-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsley, D. L., and G. G. Zimm. 1992. The genome of Drosophila melanogaster. Academic Press, New York, N.Y.
- 43.Majumder, P., and H. N. Cai. 2003. The functional analysis of insulator interactions in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 100:5223-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallin, D. R., J. S. Myung, J. S. Patton, and P. K. Geyer. 1998. Polycomb group repression is blocked by the Drosophila suppressor of Hairy-wing [Su(Hw)] insulator. Genetics 148:331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin, M., Y. B. Meng, and W. Chia. 1989. Regulatory elements involved in the tissue-specific expression of the yellow gene of Drosophila. Mol. Gen. Genet. 218:118-126. [DOI] [PubMed] [Google Scholar]
- 46.Mongelard, F., and V. G. Corces. 2001. Two insulators are not better than one. Nat. Struct. Biol. 8:192-194. [DOI] [PubMed] [Google Scholar]
- 47.Muravyova, E., A. Golovnin, E. Gracheva, A Parshikov, T. Belenkaya, V. Pirrotta, and P. Georgiev. 2001. Loss of insulator activity by paired Su(Hw) chromatin insulators. Science 291:495-498. [DOI] [PubMed] [Google Scholar]
- 48.Mutskov, V. J., C. M. Farrell, P. A. Wade, A. P. Wolffe, and G. Felsenfeld. 2002. The barrier function of an insulator couples high histone acetylation levels with specific protection of promoter DNA from methylation. Genes Dev. 16:1540-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oki, M., and R. T. Kamakaka. 2002. Blockers and barriers to transcription: competing activities? Curr. Opin. Cell Biol. 14:299-304. [DOI] [PubMed] [Google Scholar]
- 50.Parnell, T. J., and P. K. Geyer. 2000. Differences in insulator properties revealed by enhancer blocking assays on episomes. EMBO J. 19:5864-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parnell, T. J., M. M. Viering, A. Skjesol, C. Helou, E. J. Kuhn, and P. K. Geyer. 2003. An endogenous Suppressor of Hairy-wing insulator separates regulatory domains in Drosophila. Proc. Natl. Acad. Sci. USA 100:13436-13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patrinos, G. P., M. de Krom, E. de Boer, A. Langeveld, A. M. Imam, J. Strouboulis, W. de Laat, and F. G. Grosveld. 2004. Multiple interactions between regulatory regions are required to stabilize an active chromatin hub. Genes Dev. 18:1495-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Recillas-Targa, F., A. C. Bell, and G. Felsenfeld. 1999. Positional enhancer-blocking activity of the chicken beta-globin insulator in transiently transfected cells. Proc. Natl. Acad. Sci. USA 96:14354-14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roseman, R. R., V. Pirrotta, and P. K. Geyer. 1993. The su(Hw) protein insulates expression of the Drosophila melanogaster white gene from chromosomal position-effects. EMBO J. 12:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roseman, R. R., E. A. Johnson, C. K. Rodesch, M. Bjerke, R. N. Nagoshi, and P. K. Geyer. 1995. A P element containing suppressor of Hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster. Genetics 141:1061-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubin, G. M., and A. C. Spradling. 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218:348-353. [DOI] [PubMed] [Google Scholar]
- 57.Scott, K. S., and P. K. Geyer. 1995. Effects of the su(Hw) insulator protein on the expression of the divergently transcribed Drosophila yolk protein genes. EMBO J. 14:6258-6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott, K. S., A. D. Taubman, and P. K. Geyer. 1999. Enhancer blocking by the Drosophila gypsy insulator depends upon insulator anatomy and enhancer strength. Genetics 153:787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siegal, M. L., and D. L. Hartl. 2000. Application of Cre/loxP in Drosophila. Site-specific recombination and transgene coplacement. Methods Mol. Biol. 136:487-495. [DOI] [PubMed] [Google Scholar]
- 60.Sigrist, C. J. A., and V. Pirrotta. 1997. Chromatin insulator elements block the silencing of a target gene by the Drosophila polycomb response element (PRE) but allow trans interactions between PREs on different chromosomes. Genetics 147:209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith, P. A., and V. G. Corces. 1995. The suppressor of Hairy-wing protein regulates the tissue-specific expression of the Drosophila gypsy retrotransposon. Genetics 139:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spradling, A. C., and G. M. Rubin. 1982. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218:341-347. [DOI] [PubMed] [Google Scholar]
- 63.Sun, F. L., and S. C. Elgin. 1999. Putting boundaries on silence. Cell 99:459-462. [DOI] [PubMed] [Google Scholar]
- 64.Tolhuis, B., R. J. Palstra, E. Splinter, F. Grosveld, and W. de Laat. 2002. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell 10:1453-1465. [DOI] [PubMed] [Google Scholar]
- 65.Vakoc, C. R., D. L. Letting, N. Gheldof, T. Sawado, M. A. Bender, M. Groudine, M. J. Weiss, J. Dekker, and G. A. Blobel. 2005. Proximity among distant regulatory elements at the β-globin locus requires GATA-1 and FOG-1. Mol. Cell 17:453-462. [DOI] [PubMed] [Google Scholar]
- 66.Wei, W., and M. D. Brennan. 2000. Polarity of transcriptional enhancement revealed by an insulator element. Proc. Natl. Acad. Sci. USA 97:14518-14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei, W., and M. D. Brennan. 2001. The gypsy insulator can act as a promoter-specific transcriptional stimulator. Mol. Cell. Biol. 21:7714-7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.West, A. G., M. Gaszner, and G. Felsenfeld. 2002. Insulators: many functions, many mechanisms. Genes Dev. 16:271-288. [DOI] [PubMed] [Google Scholar]
- 69.West, A. G., and P. Fraser. 2005. Remote control of gene transcription. Hum. Mol. Genet. 14:101-111. [DOI] [PubMed] [Google Scholar]
- 70.Zhou, J., H. N. Cai, S. Ohtsuki, and M. Levine. 1997. The regulation of enhancer-promoter interactions in the Drosophila embryo. Cold Spring Harbor Symp. Quant. Biol. 62:307-312. [PubMed] [Google Scholar]