Abstract
Low intracellular K+ concentration ([K+]i) promotes apoptosis and blocking K+ loss prevents apoptosis, but the mechanism of action of low [K+]i remains unclear. Here, we show that low [K+]i increases NF-κB transcriptional activity by enhancing its binding to the promoter of target genes without affecting its activation and nuclear translocation in cortical neurons deprived of serum. Low K+ concentration promotes NF-κB/DNA binding through direct effects on the interaction of NF-κB dimers with DNA. Up-regulation of proapoptotic protein Bcl-XS and neuronal apoptosis induced by serum deprivation are blocked by inhibition and/or down-regulation of NF-κB and by prevention of K+ loss. Thus, a direct action of K+ on NF-κB/DNA binding regulates gene transcription related to neuronal apoptosis.
Low intracellular K+ concentration ([K+]i) is believed to be a permissive step for apoptotic progression. In many cell types, blocking intracellular K+ loss by tetraethylammonium (TEA), a nonselective potassium channel blocker, or high levels of extracellular K+ prevents apoptosis induced by a number of cellular insults (21, 47, 50, 51). Serum deprivation (SD), staurosporine, and excitotoxic overstimulation cause loss of intracellular K+ and subsequent apoptosis of cultured cortical neurons (50, 51). Intracellular K+ loss is thought to be mediated by enhanced K+ efflux through the delayed rectifier potassium (designated IK) channels (51). Under physiological conditions, normal [K+]i suppresses endonuclease activity and restrains the activation of caspase-3, a critical enzyme in apoptosis (21, 22, 33, 47). However, low [K+]i itself does not activate caspase-3 or endonuclease (21, 22, 33, 47). Rather, synthesis of new proteins appears to be required because the neuronal death related to low [K+]i is inhibited by cycloheximide, an inhibitor of protein synthesis (50, 51). Taken together, these observations suggest that gene transcription is regulated by K+ loss in apoptosis of cortical neurons.
The mitochondrial pathway is important to apoptosis in the nervous system (35). Expression of proapoptotic members of Bcl-2 family is increased by apoptotic stimuli. As a result, cytochrome c is released from mitochondria into cytosol to activate caspase-9, which in turn initiates the caspase cascade to promote apoptosis. Bcl-XL, a member of the Bcl-2 family, protects mitochondrial membrane in most cell types in the developing brain. Deletion of Bcl-XL causes widespread death of immature neurons (34), while deletion of Bcl-2 only causes postnatal neuronal degeneration of the peripheral nervous system (32). Differential splicing of bcl-x mRNA yields antiapoptotic Bcl-XL and proapoptotic Bcl-XS under different conditions (7). Bcl-XS, but not Bcl-XL, is up-regulated in mature neurons by apoptotic insults (10, 34, 45).
We investigated whether the transcription of Bcl-X is regulated by K+ loss to promote neuronal death. Bcl-X transcription in neurons is driven by NF-κB, a transcriptional factor sensitive to a variety of stressors (10, 14, 45). NF-κB, which is always in dimeric form, is implicated in multiple neuronal functions including neuronal apoptosis (4, 9, 30, 36). Our results demonstrated that NF-κB/DNA binding is regulated by [K+]i and that this regulation is critical for low-[K+]i-induced apoptosis in mature cortical neurons.
MATERIALS AND METHODS
Cell culture and treatments.
The cortical neurons isolated from 18-day-old fetal rats were cultured in Eagle's minimal essential medium (MEM; Gibco) with 10% fetal bovine serum and 10% horse serum (49). Cultures were used for experiments 10 to 13 days after plating in vitro (DIV). By culturing rat cortical neurons at a high density and using mitotic inhibitors to suppress the proliferation of glial cells, we got 93.4% ± 1.7% (n = 3) neurons (TuJ1-positive cells) and 6.7% ± 0.57% (n = 3 experiments) astrocytes (glial fibrillary acidic protein-positive cells) in DIV12 cultures (data not shown). For SD treatment, total medium was replaced by MEM without serum after being rinsed twice with MEM. The cells were treated with the following drugs from Sigma: TEA, H89, and nifedipine. 1-Pyrrolidinecarbodithioic acid (PDTC) was purchased from Calbiochem.
Measurement of [K+]i.
Cortical neurons were cultured in coverslips coated with poly-d-lysine for 12 days. After treatment, the cells were loaded with either the potassium- or sodium-sensitive fluorescent dyes, potassium-binding benzofuran isophthalate (5 μm), and sodium-binding benzofuran isophthalate (5 μm) (Molecular Probes), which were freshly prepared by combining them with equal volumes of a 25% (wt/vol) Pluronic F-127 (Molecular Probes) for 20 min at 37°C. The medium was then replaced by MEM without phenol red and incubated for another 20 min at room temperature. Samples were then placed in fresh MEM without phenol red, and images were taken with a Nikon TE2000E microscope with a Till Photonics polychrome IV rapidly tunable excitation source that provides excitation wavelengths at 340 nm or 380 nm. The change in 340 nm/380 nm ratio that represents the alteration of [K+]i was analyzed with the Meta Fluor Imaging system (Universal Imaging Corporation). About 200 neurons were analyzed for each experiment, and data from three independent experiments exhibited the same profile.
EMSAs.
Electrophoretic mobility shift assays (EMSAs) were conducted with 20 μg nuclear proteins (or 0.5 μg recombinant proteins) isolated from the cortical neurons as described previously (1). Briefly, the nuclear proteins extracted by the buffer (20 mM HEPES [pH 7.9], 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], and 0.5 mM dithiothreitol) from neuronal nuclei were incubated in a binding buffer [20 mM HEPES (pH 7.9), 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM PMSF, 0.5 dithiothreitol, 0.1 μg · μl−1 poly(dI-dC), 0.4 μg · μl−1 bovine serum albumin] containing (unless stated; see Fig. 4 and 6) indicated K+ concentrations plus 15 to 30 mM NaCl for 15 min before incubation with 32P-labled oligonucleotides at room temperature for 20 min. The DNA-protein complex was separated on a nondenatured 6% polyacrylamide gel electrophoresis (PAGE) gel and visualized by autoradiography. Signal densities were scanned with Typhoon9410 and quantified with Image Quant software (Amersham). The probes used in EMSAs were NF-κB, 5′-AGTTGAGGGGACTTTCCCAGGC-3′; mutated NF-κB, 5′-AGTTGAGGCGACTTTCCCAGGC-3′ (the site of mutation is underlined; Forkhead, 5′-AATAGATCTTAAATAAATAGATCTTTA-3′; STAT, 5′-AGCTTCATTTCCCGTAAATCCCTA-3′; E2F, 5′-GATCCACTAGTTTCGCGCGCTTTCTA-3′; and Sp1, 5′-ATTCGATCGGGGCGGGGCGAC-3′.
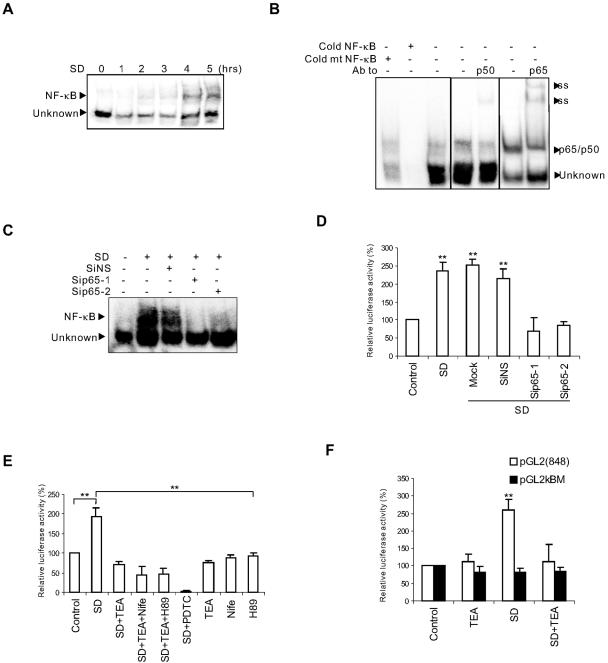
FIG. 4.
NF-κB p65/p50 responsible for Bcl-XS transcription was activated by SD but suppressed by high [K+]i in neurons. (A) Time courses of activation of NF-κB induced by SD were analyzed by EMSA. Each EMSA reaction contained the same ionic strength (30 mM Na+ and 60 mM K+) as that found in apoptotic cells (3). (B) Supershift analysis with anti-p50 and -p65 antibodies (Ab) and the competition experiments with indicated probes. The nuclear extract used for supershift and competition analysis came from the neurons treated by SD for 6 h. Cold mt, cold mutated NF-κB probe; ss, supershifting complex. (C) Down-regulation of p65 by siRNAs reduced the binding of NF-κB to its consensus sequence induced by SD for 8 h, as analyzed by EMSA. (D) Down-regulation of p65 by siRNAs inhibited pGL2(848) reporter activity induced by SD for 8 h. Mock, transfection of neurons without siRNAs. **, P < 0.01 compared to control. (E) TEA and PDTC (100 μM) inhibited the elevation of κB-luciferase activity in neurons treated by SD for 6 h. Nifedipine (Nife; 5 μM) and H89 (10 μM) did not block the effects of TEA on κB-luciferase activity. **, P < 0.01 compared to SD. (F) SD induced elevation of pGL2(848) reporter activity but not that of pGL2κBM. TEA blocked SD-induced pGL2(848) reporter activity. **, P < 0.01 compared to control. Data were means ± SEM of three to five independent experiments (D to F).
FIG. 6.
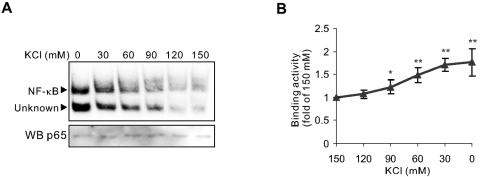
High K+ concentration directly inhibits the DNA binding of SD-activated NF-κB. (A) DNA-binding activities of NF-κB in the same nuclear extract isolated from the neurons after SD for 6 h were analyzed by EMSAs with the binding buffer containing K+ of the indicated concentrations. (B) Quantitative data represented means ± SEM of three independent experiments and demonstrated the threshold of the direct effect of K+ on DNA-binding activity of neuronal activated NF-κB. *, P < 0.05; **, P < 0.01 (versus 150 mM K+).
For supershifting, antibodies against NF-κB p65 and p50 (Santa Cruz) were incubated with the mixture of nuclear extracts and the probes for 1 h prior to separation on the nondenatured PAGE gel.
Reporter assays.
Three micrograms of luciferase reporter genes per 60-mm petri dish was transfected into neurons with Lipofectamine 2000 (Invitrogen). The κB-luciferase reporter gene contains an upstream consensus NF-κB-binding sequence. pGL2(848) is a basic pGL2 vector containing a luciferase reporter gene (Promega, Madison, Wis.) with upstream insertion of a promoter fragment at the base position spanning −848 to −75 from the translation initiation ATG of the mouse bcl-x gene. pGLκBM is a mutant of pGL2(848), carrying CC-to-GG mutations at positions −841 and −840 within the NF-κB-binding site. Neurons were subjected to different treatments 3 h after transfection. Two micrograms of β-galactosidase genes was cotransfected to normalize the transfection efficiency. After treatments, cells were lysed and luciferase activities were assayed with the Luciferase Assay system (Promega).
Reverse transcription-PCR (RT-PCR).
Total RNA was isolated from the neurons using Trizol reagent (Invitrogen). cDNA was made using RevertAid M-MuLV reverse transcriptase (MBI) in a total 20-μl reaction volume containing 5 μg of total RNA and 0.5 μg oligo(dT18) primer. PCR was performed in a 25-μl reaction volume using Taq (Promega) and 0.5 μM primers (for bcl-XL/S, 5′-AGGCTGGCGATGAGTTTGAA-3′ and 5′-CGGCTCTCGGCTGCTGCATT-3′; for β-actin, 5′-GGATTGGTCTGTTGTGACTTGC-3′ and 5′-TGGCTTGTACAGACACCAGACA-3′). The PCR was conducted for 30 cycles for bcl-XL/S and for 20 cycles for β-actin (each cycle consisting of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s) with 4 μl and 1 μl cDNA as template, respectively. PCR products were electrophoresed on a 2.5% agarose gel and scanned with Typhoon9410.
Western blotting and immunocytochemistry.
Whole-cell or nuclear extracts containing equal amount of proteins were separated and electrotransferred to Hybond-P (Amersham), which was then probed and reprobed with the indicated antibodies: IκBα, NF-κB p65, NF-κB p50/p105, Bcl-XS/L, Actin and Lamin B (Santa Cruz). The bands were visualized by an Amersham ECLplus system. Immunocytochemistry was performed as previously described (39). Goat anti-rabbit immunoglobulin G conjugated with Texas Red-X (Molecular Probes) was used as a secondary antibody, and the images were analyzed by confocal microscopy (Zeiss LSM510). If costained with Hoechst stain, the samples were again incubated with 5 μg · ml−1 of Hoechst 33258 stain for 5 min. For propidium iodide (PI) staining, the neurons were incubated with 2.5 μg · ml−1 of PI for 20 min and observed.
Generation of recombinant NF-κB.
Recombinant p65 and p50 were expressed as glutathione S-transferase (GST) fusion forms from pGEX-KG vector (17) and purified with the GST Purification kit (Clontech). GST tags were removed by thrombin digestion (17). After incubation of Δp65/Δp65 and p50/p50 at equal molar ratios in denaturing buffer (25 mM Tris-HCl [pH 7.5], 500 mM NaCl, 7 M urea, 0.5 mM EDTA, 0.1 mM PMSF, and 10 mM β-mercaptoethanol), Δp65/p50 heterodimers were obtained in renaturing buffer (25 mM Tris-HCl [pH 7.5], 500 mM NaCl, 1.0 mM EDTA, 0.2 mM PMSF, 10 mM β-mercaptoethanol) and then desalted by dialysis into 25 mM Tris-HCl (pH 7.5)-20 mM NaCl (8).
RNA interference.
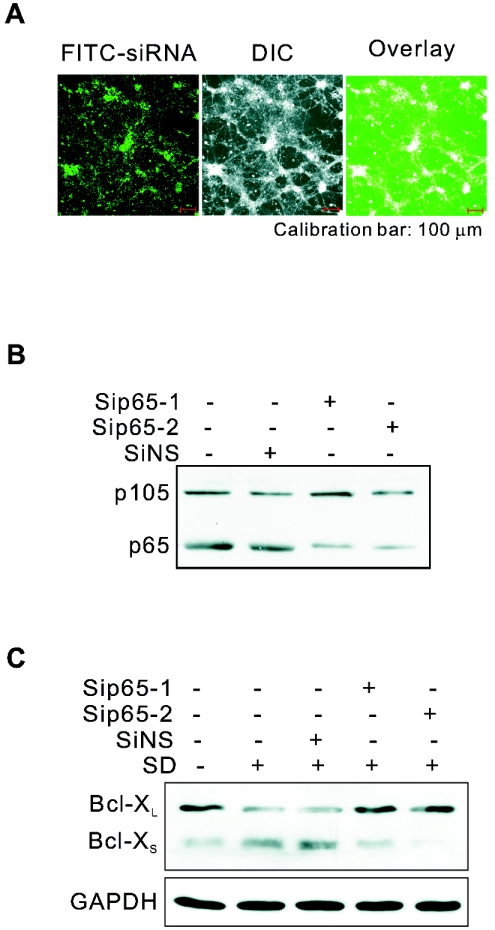
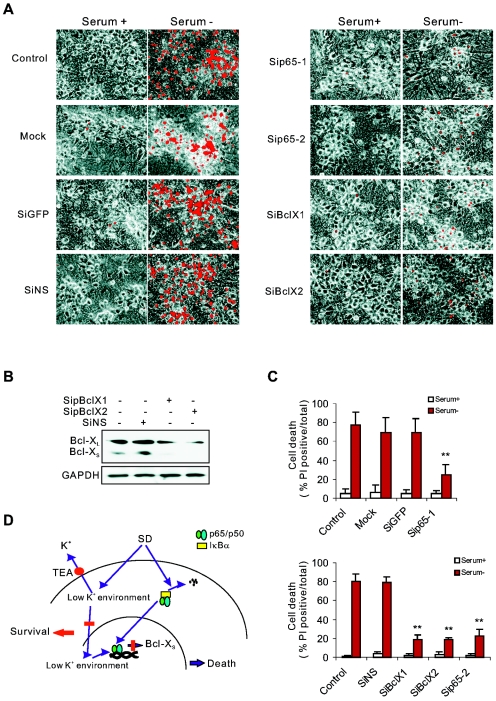
Small interfering RNA (siRNA) for p65 (SiP65-1, sense, 5′-GAA GCG AGA CCU GGA GCA AdTdT-3′; antisense, 5′-UUG CUC CAG GUC UCG CUU CdTdT-3′; Sip65-2, sense, 5′-CUC AGA GUU UCA GCA GCU UdTdT-3′; antisense, 5′-AAG CUG CUG AAA CUC UGA GdTdT-3′), siRNA for green fluorescent protein (GFP) (SiGFP, sense, 5′-GCA AGC UGA CCC UGA AGU UCA T-3′; antisense, 5′-GAA CUU CAG GGU CAG CUU GCC G-3′), and a nonsilencing siRNA used as nonspecific siRNA control (SiNS, sense, 5′-UUC UCC GAA CGU GUC ACG UdTdT-3′; antisense, 5′-ACG UGA CAC GUU CGG AGA AdTdT-3′) were synthesized by Shanghai GeneChem Co., Ltd. siRNA for Bcl-X (SiBclX1, sense, 5′-AGC AUU CAG UGA UCU AAC AdTdT-3′; antisense, 5′-UGU UAG AUC ACU GAA UGC UCdT-3′; SiBclX2, sense, 5′-GAC UGU GGC UGG UGU AGU UdTdT-3′; antisense, 5′-AAC UAC ACC AGC CAC AGU CAdT-3′) were synthesized by Shanghai GenePharma Co., Ltd. Transfections of siRNA were carried out with TransMessenger transfection reagent (QIAGEN, Chatsworth, CA) according to the manufacturer's instructions and the protocol (26) previously described with some modifications. Briefly, the oligonucleotides (1.6 μg per well in a 12-well plate) were condensed with 3.2 μl Enhancer R and formulated with 4 μl of TransMessenger reagent. Cortical neurons at DIV7 were exposed directly to the transfection complex diluted in 900 μl of MEM without any antibiotics for 3 h. The cells were changed to the original culture medium collected before transfection and incubated for 48 to 72 h to down-regulate p65 or Bcl-X. SD was then performed. The transfection efficiency was determined by transfecting neurons with a nonsilencing siRNA conjugated with fluorescein isothiocyanate (FITC-siRNA). The knockdown efficiency of siRNA of p65 or Bcl-X was determined by Western blot analysis. Cell death was measured by the ratio of the number of PI-positive cells to the total number of cells.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed according to the protocol provided by D. Baltimore with some modifications (27). Neurons (DIV12) incubated immediately after treatment in 3% formaldehyde in medium at 37°C for 10 min were scraped and resuspended in L1 buffer (50 mM Tris-HCl [pH 8.0], 2 mM EDTA, 0.1% NP-40, 10% glycerol, 1 mM PMSF, 10 μg · ml−1 leupeptin, and 10 μg · ml−1 aprotinin), following by vigorous vortexing to break the cytoplasmic membrane. Collected nuclei were lysed with L2 buffer containing 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 1% sodium dodecyl sulfate, 1 mM PMSF, 10 μg · ml−1 leupeptin, and 10 μg · ml−1 aprotinin. Nuclear extracts were sonicated to shear chromosome DNA to 500 to 1,000 bp in length. After centrifugation at 13,000 rpm at 4°C for 15 min, supernatant with equal amounts of protein was diluted 10 fold with ChIP dilution buffer containing 50 mM Tris-HCl (pH 8.0), 0.5% NP-40, 5 mM EDTA, and 200 mM NaCl. The mixture was precleared with salmon sperm DNA-protein A-Sepharose beads (80 μl) at 4°C overnight. The resulting supernatant was incubated with the antibody against p65 (sc-372; Santa Cruz) at 4°C for 12 h and then with salmon sperm DNA-protein A-Sepharose beads (50 μl) for another hour at 4°C. The beads collected were washed according to the protocol from Upstate Biotechnology (Lake Placid, NY) and incubated with proteinase K buffer (50 mM Tris-Cl [pH 8.0], 0.5% sodium dodecyl sulfate, 1 mM EDTA, 100 mM NaCl) for 6 h at 65°C to break the DNA-protein binding, followed by treatment with 100 μg · ml−1 proteinase K for 3 h at 55°C. The DNA was recovered by phenol-chloroform extraction and ethanol precipitation. PCRs were conducted with the eluted DNA as a template and the following primers. For the IκBα 5′ flanking region (166 bp), the sense primer was +89 (5′-AGT TGG GTG GCG AGT TCA-3′), and the antisense primer was −77 (5′-CCC TGG GTT TAG GCT TCT-3′). For the Bcl-X 5′ flanking region (169 bp), the sense primer was −987 (5′-GAC AAC TAG CGG TGT TTG TGG GG-3′), and the antisense primer was −818 (5′-TGA GGC TTC CGA AAC CCT AAA GG-3′). The primers for genomic actin as the input control were the same as those used for RT-PCR. PCR products were electrophoresed on a 2 to 2.5% agarose gel and pictures were taken with the Tanon GIS-1000 Image system. All the PCR products have been confirmed by sequencing.
RESULTS
Preventing intracellular K+ loss inhibits SD-induced expression of Bcl-XS.
To determine whether de novo gene transcription is involved in the apoptotic process following intracellular K+ loss, we examined expression of Bcl-XL/S in cultured cortical neurons deprived of serum, a condition that leads to intracellular K+ loss in these neurons (51). Consistent with previous work (51), cortical neurons underwent apoptosis 24 to 48 h after SD and TEA prevented cell death (data not shown). Similarly, SD caused chronic loss of intracellular K+ in a TEA-sensitive manner (Fig. 1). Furthermore, potassium chloride and cesium chloride (Cs+), two agents known to reduce the efflux of K+, also protected neurons against apoptosis induced by SD (data not shown). As shown in Fig. 2A and B, SD enhanced both mRNA and protein levels of Bcl-XS but not those of Bcl-XL, a splice variant of Bcl-X. Treatment with TEA inhibited Bcl-XS enhancement induced by SD. In addition to preventing K+ loss, TEA may depolarize neurons to induce Ca2+ influx (15, 51) and activate protein kinase A (PKA) (15, 40), both of which can modulate gene transcription. To determine whether Ca2+ influx or PKA activation is involved, we studied effects of H89, a specific inhibitor of PKA, and nifedipine, a blocker of L-type calcium channels that are activated by TEA treatment (20, 38). Both had no effect on TEA's inhibition of increased Bcl-XS expression (Fig. 2C), suggesting that TEA inhibition of SD-induced Bcl-XS is due to TEA action on prevention of intracellular K+ loss. To further test this notion, we examined the effect of Cs+ on SD-induced Bcl-XS expression. As shown in Fig. 2D, Bcl-XS enhancement induced by SD was suppressed in Cs+-treated neurons. However, TEA or Cs+ alone did not change Bcl-XS expression (Fig. 2C and D). These results identify Bcl-XS as a potential effector in SD-induced apoptosis and reveal an important role of intracellular K+ in regulating Bcl-XS expression.
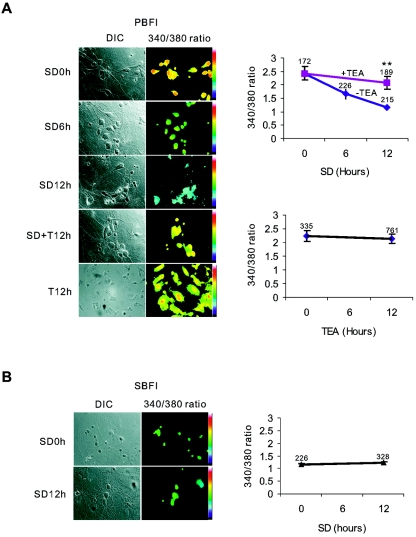
FIG. 1.
Loss of intracellular K+ caused by SD is blocked by TEA. (A) [K+]i alteration in cortical neurons induced by SD were examined by the K+-sensitive dye potassium-binding benzofuran isophthalate (PBFI). TEA significantly suppressed intracellular K+ loss induced by SD. TEA alone had no effect on [K+]i. **, P < 0.01 compared to the neurons treated by SD for 12 h. (B) Sodium-binding benzofuran isophthalate (SBFI), an Na+-sensitive dye, was used as a control to demonstrate that the loading of the fluorescence dye was not affected by the treatments. The numbers above the data points in panels A and B are the numbers of the neurons analyzed. The concentration of TEA used was always 5 mM.
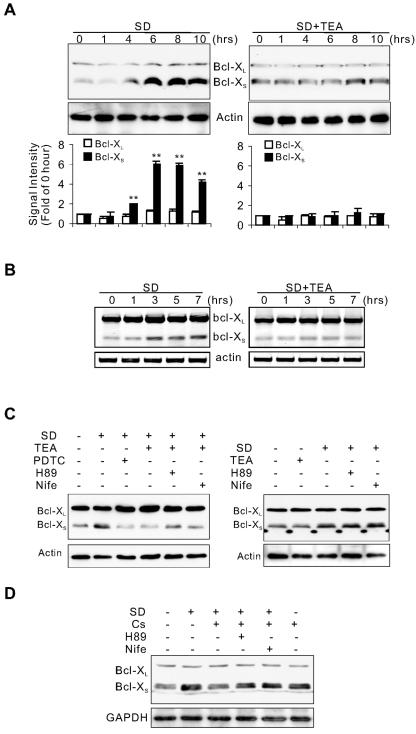
FIG. 2.
High [K+]i suppresses the SD-induced transcription of Bcl-XS. Effects of SD and TEA on the expression of Bcl-XL/Bcl-XS were assayed by Western blotting (A) and RT-PCR (B). (A) Signal intensities of Bcl-XL/Bcl-XS normalized with actin were means ± standard error of the mean (SEM) of three independent experiments. **, P < 0.01 compared to 0 h. (C) Western blot analysis of effects of H89 or nifedipine on TEA-mediated inhibition of the expression of Bcl-XS induced by SD for 8 h. PDTC (100 μM) blocked the expression of Bcl-XS. (D) Western blot analysis of effects of cesium chloride (Cs; 2 mM) with or without H89 or nifedipine on SD-induced expression of Bcl-XS. H89, 10 μM; nifedipine (Nife), 5 μM. Actin and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) served as loading controls.
NF-κB is one of transcription factors known to regulate neuronal Bcl-XS expression (10, 14, 45). To determine whether NF-κB mediates SD-induced Bcl-XS expression, we treated neurons with PDTC, an NF-κB inhibitor. As shown in Fig. 2C, SD-induced augmentation of Bcl-XS was reduced by PDTC. Further, we used siRNA to selectively reduce the expression of p65, a major subunit of NF-κB in neurons. With the high transfection efficiency of siRNA (Fig. 3A), Western blot analysis revealed that the p65 protein level in neurons transfected with two different siRNAs against p65, Sip65-1 and Sip65-2, was reduced to 30.5% ± 0.96% and 33.0% ± 1.3%, respectively, of that in neurons transfected with nonsilencing siRNA in three experiments (Fig. 3B). Down-regulation of p65 in the cultured neurons (from DIV7 to DIV12) inhibited SD-induced expression of Bcl-XS without effect on the constitutive expression of Bcl-XL, confirming that SD-increased Bcl-XS expression was indeed regulated by NF-κB (Fig. 3C).
FIG. 3.
Down-regulation of p65 blocks the enhanced Bcl-XS expression induced by SD. (A) Transfection efficiency was illustrated by transfecting cultured cortical neurons (from DIV7 to DIV12) with FITC-siRNA with TransMessenger transfection reagent. Western blot analysis showed the efficiency of knockdown of p65 (B) by two different siRNAs for p65 (Sip65-1 and Sip65-2) and the effect of knockdown of p65 on SD-induced Bcl-XS expression (C). SiNS was a negative control for siRNA. The p50 precursor, p105, which exists in cultured rat cortical neurons, served as an unaffected protein to determine the specificity of siRNA-p65. GAPDH served as a loading control.
NF-κB transcriptional activity is increased by SD but suppressed by high [K+]i.
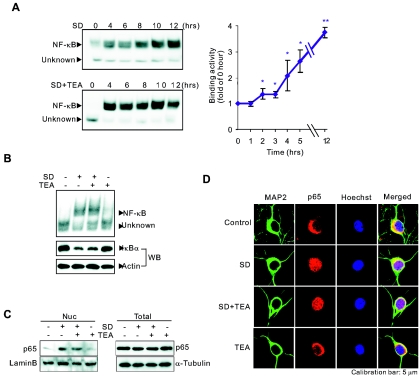
To determine whether NF-κB is involved in SD-induced neuronal apoptosis, we examined the DNA-binding activity of nuclear NF-κB by EMSAs with the nuclear extracts isolated from neurons deprived of serum. Neuronal NF-κB has both constitutive and inducible activity (36). Its activation requires degradation of the inhibitory proteins, IκBs that sequester NF-κB in the cytoplasm, and possible posttranslational modifications of NF-κB subunits that endow NF-κB with DNA-binding and transactivational ability (13, 28). The activated NF-κB then translocates into the nucleus and binds to DNA (13, 28). EMSA is an approach to examine the amount of transcription factors in the nucleus that has possessed the ability to bind to their consensus DNA sequences. As shown in Fig. 4A, two prominent complexes from the nuclear extracts of SD-treated neurons bound to the consensus DNA sequence for NF-κB. The upper band shown in Fig. 4A appeared 2 h after SD, while the lower band was unaffected. Further analysis illustrated that the upper band (NF-κB) was supershifted by the antibodies against p65 and p50, two major members of the NF-κB family found in neurons. The lower band (Fig. 4A, Unknown) appeared to be nonspecific, since it was not supershifted by the antibodies and also bound to a mutated NF-κB consensus sequence (Fig. 4B). The antibodies against three other members (c-Rel, RelB, and p52) of the NF-κB family did not change the migration of the two complexes (data not shown). Thus, the upper band induced by SD in cortical neurons was an inducible p65/p50 heterodimer, the general form in the nervous system. Further, down-regulation of p65 by siRNAs suppressed binding of NF-κB to the promoter of its target genes (Fig. 4C) and decreased SD-induced expression of the luciferase reporter gene (Fig. 4D), pGL2(848), which contains the NF-κB-binding sequence found in the bcl-x promoter region (48). Therefore, SD-activated p65/p50 was responsible for Bcl-X transcription.
Since blocking K+ loss inhibited an increase in mRNA levels of Bcl-X, an NF-κB target gene, we then asked whether the change in [K+]i regulates NF-κB transcriptional activity. As shown in Fig. 4E and 4F, preventing K+ loss by the treatment with TEA abolished SD-induced elevation of κB-luciferase and pGL2(848) activity in neurons, whereas TEA treatment alone had no effect on the activity of κB-luciferase or pGL2(848). Moreover, treatment with H89 or nifedipine had no effect on TEA's inhibition of κB-luciferase activity induced by SD (Fig. 4E). In contrast, SD did not increase the activity of the luciferase reporter with the mutated bcl-x promoter (pGLκBM) in neurons (Fig. 4F). Since the mutations in pGLκBM promoter specifically disrupted its binding to NF-κB (48), this result confirmed that SD-induced and [K+]i-sensitive transcription of Bcl-X was indeed mediated by NF-κB. Taken together, our results support the notion that the loss of intracellular K+ caused by SD up-regulates the transcriptional activity of NF-κB in intact neurons, whereas blockage of the K+ loss suppresses this activity.
Increase in intracellular K+ level inhibits NF-κB binding to cis elements.
We next asked whether this inhibitory effect of TEA on NF-κB transcriptional activity was due to inhibition of NF-κB activation or nuclear translocation. As shown in Fig. 5A and B, treatment of neurons with TEA had no effect on either the increase in nuclear accumulation of NF-κB as analyzed by EMSAs or the degradation of IκBα, a critical step for NF-κBp65/p50 activation, as analyzed by Western blotting. The treatment of neurons with TEA for up to 12 h did not block SD-induced nuclear accumulation of p65/p50 (Fig. 5A). Consistent with EMSA results, immunolocalization and Western blot experiments showed that nuclear translocation of p65 induced by SD was not blocked by the TEA treatment (Fig. 5C and D). These results demonstrated that the intracellular K+ loss associated with SD did not affect the activation and nuclear translocation of NF-κB. Thus, it is possible that intracellular K+ regulates NF-κB transcriptional activity through action on its binding to the promoter regions of target genes.
FIG. 5.
TEA treatment does not affect NF-κB activation or nuclear translocation induced by SD. (A) Time course of activation of NF-κB induced by SD and the effect of TEA on the activation of NF-κB in neurons were analyzed by EMSAs as described in the legend to Fig. 4A. (Right) Data represent means ± SEM of three independent experiments. The asterisks indicate data significantly different from that at 0 h: *, P < 0.05; **, P < 0.01. (B) Effects of SD and TEA for 6 h on IκBα degradation (Western blotting [WB]) and nuclear NF-κB activity (by EMSAs) were illustrated together. Actin served as a loading control. (C) Effects of SD and TEA for 12 h on nuclear p65 level (Nuc) were analyzed by Western blotting. Results from whole lysates of treated neurons demonstrated that the total p65 was not altered (Total). Lamin B and α-tubulin served as loading controls. (D) Confocal images of indirect immunofluorescence of p65 in neurons after different treatments for 12 h.
To test the notion, we assayed binding activity by a stepwise decrease in the K+ concentration in EMSAs with the same nuclear extracts from neurons deprived of serum. As shown in Fig. 6, the DNA-binding activity of SD-activated NF-κB was significantly enhanced, as the K+ concentration was decreased from the physiological level of 150 mM to 90 mM. The threshold concentration, 90 mM, for promoting NF-κB/DNA binding is higher than that finally attained during apoptosis (∼50 mM) (3, 21, 50, 51). Western blot analysis using an antibody against p65 showed that there were similar amounts of NF-κB protein in these assays (Fig. 6), precluding the possibility that NF-κB was differently degraded by proteases during incubation of nuclear extract in different K+ concentrations in EMSAs and suggesting that enhanced activity was indeed due to the elevation of NF-κB/DNA binding.
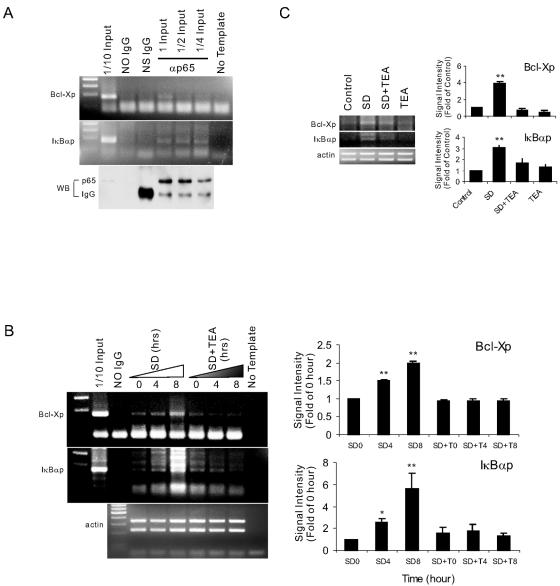
To provide direct evidence that the alteration of [K+]i affects NF-κB/DNA binding in cells, we performed ChIP assays. The inhibitory protein of NF-κB, IκBα, was the NF-κB target gene in autoregulation of NF-κB signaling (43, 46). Its expression following an initial degradation was again elevated 8 h after SD, and the elevation was blocked by TEA (data not shown). We found that p65 specifically bound to the cis elements of IκBα and Bcl-X in response to SD, confirming that IκBα and Bcl-X were NF-κB target genes in neurons (Fig. 7A). Moreover, the SD-induced increase in binding of p65 to the promoters was time dependent and suppressed by TEA treatment (Fig. 7B). However, TEA treatment alone without changing [K+]i (Fig. 1A) did not alter the p65 interaction with DNA in control neurons (Fig. 7C). Therefore, the results obtained from ChIP experiments and EMSAs point to the conclusion that SD-activated NF-κB/DNA binding was regulated by K+ concentration in intact neurons.
FIG. 7.
High [K+]i inhibits in vivo DNA binding of NF-κB. Neurons were cultured under the indicated conditions. PCR analysis was conducted with coimmunoprecipitated DNA using IκBα promoter primers (IκBαp) and Bcl-X promoter primers (Bcl-Xp). (A) The specificity of binding of p65 to IκBα and Bcl-X promoters. No Ig, ChIP without antibody; NS Ig, ChIP with 2 μg normal rabbit IgG; α-p65, ChIP with 2 μg antibody to p65. The inputs for lanes labeled No Ig, NS Ig, and α-p65 with 1 input were similar. (B) Time course of the binding of p65 at IκBα and Bcl-X promoters in neurons induced by SD in the presence or absence of TEA. A sample from SD for 0 h was also used for the ChIP assay without antibody (No Ig). (C) Effects of SD for 8 h with or without TEA on p65 binding at IκBα and Bcl-X promoters. Quantitative data normalized with genomic actin represented means ± SEM of three independent experiments (B and C). *, P < 0.05; **, P < 0.01 (versus 0 h).
K+ directly and specifically regulates NF-κB interaction with DNA.
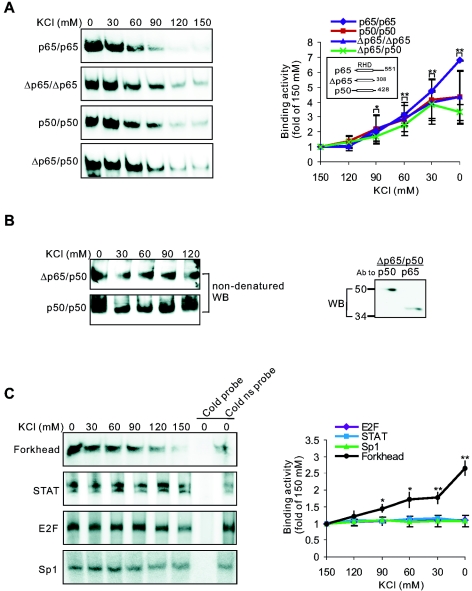
To elucidate that NF-κB/DNA binding regulated by K+ levels depends on direct NF-κB interaction with DNA but not on other factors, we examined K+ effects on purified NF-κB. As shown in Fig. 8A, DNA-binding activity of purified p65/p65 or p50/p50 was also increased by lowering K+ concentrations, and the threshold K+ concentration was found to be 90 mM. A truncated form of p65 (Δp65) containing only the Rel homology domain (RHD), which is essential for dimerization and NF-κB/DNA binding (13, 28), exhibited a sensitivity to K+ that was similar to that of wild-type p65, suggesting that the K+-sensitive site is within the RHD (Fig. 8A). Since RHD mediates both subunit dimerization and DNA binding, we next examined the possibility that the K+ effect on NF-κB/DNA binding was due to the action of K+ on protein-protein interaction between NF-κB subunits. Using nondenatured Western blotting with anti-p50 antibody, we found that under conditions identical to those used in EMSAs, changes in K+ concentration did not affect the dimerization of either p50/p50 or Δp65/p50 (Fig. 8B). These results are consistent with the notion that the K+ effect is due to a direct action on DNA-binding activity of NF-κB dimer rather than on the subunit interaction.
FIG. 8.
K+ directly and selectively affects the interaction between transcription factors and their DNA consensus sequences. (A) Effects of K+ on DNA-binding activities of recombinant NF-κBs were analyzed by EMSAs. (B) Effects of K+ on the dimerization of NF-κB subunits were analyzed with nondenatured Western blotting. Recombinant p50/p50 or Δp65/p50 was incubated with indicated K+ concentrations for 40 min at room temperature, and NF-κB complexes were then separated by a nondenatured 6% PAGE gel and Western blotted using antibody against p50. Denatured Western blot results (right) showed that both Δp65 and p50 were in the Δp65/p50 preparation. (C) Effects of K+ on DNA-binding activities of neuronal Forkhead transcription factor, STAT, E2F, and Sp1. ns, nonspecific. (A and C) Quantitative data represent means ± SEM of three to six independent experiments and are illustrated on the righthand panels. *, P < 0.05; **, P < 0.01 (versus 150 mM K+).
To determine the specificity in the regulatory effect of K+ on NF-κB/DNA binding, we further examined the DNA-binding activity of four other transcription factors: Forkhead transcription factor, STAT, E2F, and Sp1. We found that reduction in K+ concentrations also resulted in a significant enhancement in the DNA-binding activity of Forkhead transcription factor, with a similar threshold K+ concentration (Fig. 8C). In contrast, the DNA-binding activity of STAT, E2F, and Sp1 was unaffected by changes in K+ concentration (Fig. 8C). Thus, there is specificity in the effect of K+ environment on DNA binding of transcription factors, and the effect is not unique to NF-κB.
Suppression of NF-κB target gene expression blocks SD-induced cell death.
To examine the role of the NF-κB pathway in neuronal apoptosis associated with K+ loss, we selectively down-regulated the expression of p65 or Bcl-X in cultured neurons (from DIV7 to DIV12) by siRNA. Down-regulation of p65 and Bcl-X protected neurons against SD but did not affect neuronal morphology and viability in control neurons (Fig. 9A). Western blot analysis revealed that the Bcl-XL protein level in neurons transfected with two different siRNAs against Bcl-X, SiBclX1 and SiBclX2, was reduced to 40.3% ± 0.19% and 22.9% ± 0.22%, and Bcl-XS expression was reduced to 13.2% ± 0.56% and 8.66% ± 0.62%, respectively, of that in neurons transfected with nonsilencing siRNA (Fig. 9B shows the results of three experiments). The percentage of cells that survived after SD for 24 h was about three times higher for either p65-siRNA- or Bcl-X-siRNA-treated neurons than those treated with control siRNAs (Fig. 9C). The NF-κB inhibitor, PDTC, similar to the knockdown of p65 (Fig. 3C, 9A, and 9C), also suppressed expression of Bcl-XS (Fig. 2C) and reduced SD-induced neuronal death (data not shown). Thus, NF-κB transcriptional activity elevated by SD-induced K+ loss is required for the expression of the proapoptotic gene Bcl-XS and neuronal apoptosis.
FIG. 9.
NF-κB/Bcl-XS activation mediates neuronal death induced by SD. (A) Representative images of the effects of knockdown of p65 or Bcl-X on neuronal survival induced by SD. PI staining was illustrated as red to display the cell death. Mock, transfection without siRNA. SiGFP and SiNS were used as negative control siRNAs. (B) RNA interference efficiency of different siRNAs for Bcl-X (SiBclX1, SiBclX2) was determined by Western blotting. GAPDH served as an unaffected protein and a loading control. (C) Protective effects of knockdown of p65 or Bcl-X were quantitatively analyzed by the percentage of PI-positive neurons. Data are means ± SEM of three to six independent experiments. **, P < 0.01 compared to nontransfected neurons treated by SD. (D) The schematic diagram illustrates our hypothesis that K+ regulation of NF-κB/DNA interaction is involved in neuronal apoptosis. SD activates NF-κB and independently causes [K+]i reduction. Low [K+]i enhances p65 binding to DNA and increases the transcription of Bcl-XS, leading to apoptosis. Blocking intracellular K+ loss by TEA inhibits NF-κB transcriptional efficiency and prevents neuronal death.
DISCUSSION
NF-κB activity stimulated by SD promotes neuronal apoptosis through transcription of proapoptotic Bcl-XS.
Depending on the cell type, cellular context, developmental stage, different NF-κB dimers, and the nature of insults, NF-κB activity could be involved in either neural protection or damage (4, 9, 30, 36). Constitutively active NF-κB locates in the neuronal nucleus and is developmentally regulated (2, 6, 24, 42). This form of NF-κB is possibly essential for survival of developing neurons under stressful conditions (6, 11, 19), although the deletion of NF-κB subunits or functional ablation of NF-κB activity from the beginning of neurogenesis does not result in any visible morphological defects in the mouse central nervous system (5, 11, 25, 29, 44). The inducible form of NF-κB, mainly p65/p50 (18, 31, 36), locates in the cytoplasm of neurons and is activated in many neurodegenerative disorders, in rodent models of seizure and stroke (9, 12, 23, 30, 36), and by excitotoxic stimulation (16, 39). In ischemia and other injuries to the adult brain, inhibition of inducible NF-κB activation protects neurons (9). Our findings demonstrated that in cultured mature cortical neurons, NF-κB predominantly located in cytoplasm and SD led to NF-κB activation and nuclear translocation (Fig. 4 and 5). Furthermore, three lines of evidence are consistent with the notion that activation of the inducible form of NF-κB is responsible for SD-induced neuronal apoptosis. First, SD-induced expression of Bcl-XS was mediated by NF-κB (Fig. 3, 4, and 7), and suppression of increased expression of Bcl-XS led to neuronal survival (Fig. 2 and 9). Second, inhibition of NF-κB activation by NF-κB inhibitors suppressed the enhancement of Bcl-XS and prevented cell death (Fig. 2 and data not shown). Third, down-regulation of NF-κB protein (from DIV7 to DIV12) by siRNAs blocked SD-induced enhancement of Bcl-XS and protected neurons against apoptosis (Fig. 3C and 9). Thus, the activation of NF-κB (p65/p50) by SD is detrimental to the mature cortical neurons.
The mechanism of how Bcl-XS, but not Bcl-XL, is up-regulated remains unclear at the present time. Since SD increased mRNA levels of Bcl-XS, but not those of Bcl-XL, differential transcription is possible. The optimal promoter in front of exons for different splicing forms of Bcl-X is not the same (37). In normal embryonic and adult brains, Bcl-XL is the predominant splicing form of Bcl-X; Bcl-XL-deficient mice exhibit massive death of immature neurons in the developing brain and increased susceptibility of telencephalic neurons to some apoptotic stimuli (34, 41, 52). Knockdown of p65 or Bcl-X did not disrupt the morphology and viability of mature cortical neurons (Fig. 9), supporting the notion that Bcl-XL is not indispensable for the survival of mature cells (34). In contrast, Bcl-XS is elevated in ischemic adult brain and in toxin-treated mature neurons in response to NF-κB activation (10, 45). Down-regulation of Bcl-XS prevented neurons against SD (Fig. 9). Moreover, siRNAs against p65 and an inhibitor of NF-κB both suppressed the increased expression of Bcl-XS and prevented neurons against apoptosis induced by SD. Taken together, the NF-κB/Bcl-XS pathway is necessary for SD-induced apoptosis of mature neurons.
Low [K+]i enhances NF-κB transcriptional activity, leading to neuronal apoptosis through direct and specific action of K+ on NF-κB/DNA binding.
The EMSA results shown in Fig. 5A and B demonstrated that nuclear accumulation of freed NF-κB was increased by SD that induced intracellular K+ loss and that these increases were unaffected by TEA treatment. Consistently, Western blotting and immunocytochemistry demonstrated that the degradation of IκBα and nuclear presence of p65 induced by SD were not changed by TEA treatment (Fig. 5B to D). Thus, NF-κB activation and/or nuclear translocation is independent of the change in [K+]i.
However, NF-κB-mediated transcription is sensitive to the change in [K+]i. To preclude the pitfall of relying on TEA only, we applied Cs+, an agent also known to block K+ loss. Both TEA and Cs+ suppressed the augmentation of Bcl-XS induced by SD (Fig. 2A to D). TEA can induce depolarization, calcium influx, PKA activation, or other possible effects on both unstimulated and serum-deprived neurons. However, it affected the [K+]i change only in the neurons deprived of serum (Fig. 1A). Correspondingly, TEA or Cs+ alone had no effects on NF-κB activation, nuclear translocation, DNA binding, or target gene transcription (Fig. 2C to D, 4E and F, 5B to D, and 7C). We also eliminated the involvement of possible effects of TEA on calcium influx and PKA activation, which might be induced by depolarization in NF-κB-mediated transcription of Bcl-XS under SD. Therefore, the plausible explanation for the TEA effect on NF-κB-mediated transcription is due to its action on [K+]i change. Ion strength affects protein-protein interactions, such as the interaction between the enzyme and its substrate (22, 33), as well as protein-DNA interactions. Several lines of evidence support that NF-κB transcriptional activity is regulated by [K+]i alteration through the direct regulation of NF-κB/DNA binding. First, maintenance of intracellular [K+]i suppressed the expression of the Bcl-XS and IκBα genes, two target genes of NF-κB, as well as the activity of κB-luciferase and pGL2(848), two NF-κB-driven luciferases, in neurons deprived of serum (Fig. 2, 4E, and 4F). Second, the recruitment of p65 in temporal sequences to the promoters for Bcl-X and IκBα was augmented by SD, and the enhancement in binding was reduced by preventing K+ loss (Fig. 7). Third, changes in K+ concentration specifically affected the interaction of p65/p50 dimers with DNA (Fig. 6 and 8). The direct action of K+ on NF-κB/DNA binding is an undoubtedly important mechanism for altering the transcriptional activity of NF-κB. Thus, the intracellular K+ loss caused by SD affects NF-κB transcriptional activity in intact cells through the direct effect of K+ on NF-κB interactions with DNA. The suppression of the augmentation of Bcl-XS through the inhibition of NF-κB binding to its promoter by preventing intracellular K+ loss indicated that low [K+]i is a permissive condition for proapoptotic consequences of an SD-activated NF-κB/Bcl-XS pathway (Fig. 9D). It is possible that the regulatory effect of [K+]i on a NF-κB/Bcl-X promoter in neurons under apoptotic conditions may also modulate the interaction of NF-κB with other gene promoters throughout the genome (Fig. 9D). However, the essential effect of the K+ environment on NF-κB transcriptional activity in apoptotic neurons does not mean that other stimuli-activated NF-κB forms, such as tumor necrosis factor alpha, cannot yield their transcriptional products in normal [K+]i, although it is possible they would result in much higher transcriptional efficiency in lower [K+]i through enhanced DNA binding by lower ionic strengths.
As the major counterion for intracellular proteins and nucleic acids, a normal K+ environment may be a permissive condition for normal conformational states of these macromolecules and interactions among them. On the other hand, specific interactions of K+ with protein modules can also occur, as exemplified by K+-selective ion channels. Our findings (i) that low concentrations of K+ specifically promote NF-κB/DNA binding (and Forkhead transcription factor), but not STAT, E2F, or Sp1 and (ii) that K+ directly acts on the interaction of NF-κB with DNA, suggesting that some transcription factors are sensitive to a change in ionic strength and possible protein domains might be responsible for the inhibitory effect of high K+ concentrations. An attractive possibility is that some amino acid residues in the DNA-binding domain of p65/p50 are selective K+-binding sites critical for the inhibitory effect.
It is of interest that our finding of K+ regulation shows that the biological activity of a protein is selectively activated by a large deviation of [K+]i from the normal physiological range, a “negative” regulatory mechanism that in principle may be used in the regulation of protein-protein and protein-DNA interactions under pathological conditions. Finally, we note that K+ regulation of NF-κB/DNA binding demonstrated here points to the importance of “posttranslocational” regulation of transcriptional factors during apoptosis, a mechanism that may prove to be general for regulation of gene expression in normal, as well as apoptotic, cells.
Acknowledgments
We thank A. Lin for kindly providing κB-luciferase and p50 plasmids, T. Tsukahara for pGL2 (848) and pGLκBM plasmids, D. Baltimore for ChIP assay protocol, M-m. Poo and L. Mei for helpful suggestions and critical comments, Q. Hu for technical assistance on confocal imaging, Y.-c. Jia for siRNA experiments, and Z.-j. Fan for cortical cell preparations.
This work was supported by grants from the Major State Basic Research Program of China (G200077800) and projects 30321002 and 30225025 from the National Natural Science Foundation of China.
REFERENCES
- 1.Andrews, N. C., and D. V. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakalkin, G., T. Yakovleva, and L. Terenius. 1993. NF-kappa B-like factors in the murine brain. Developmentally-regulated and tissue-specific expression. Brain Res. Mol. Brain Res. 20:137-146. [DOI] [PubMed] [Google Scholar]
- 3.Barbiero, G., F. Duranti, G. Bonelli, J. S. Amenta, and F. M. Baccino. 1995. Intracellular ionic variations in the apoptotic death of L cells by inhibitors of cell cycle progression. Exp. Cell Res. 217:410-418. [DOI] [PubMed] [Google Scholar]
- 4.Barkett, M., and T. D. Gilmore. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18:6910-6924. [DOI] [PubMed] [Google Scholar]
- 5.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376:167-170. [DOI] [PubMed] [Google Scholar]
- 6.Bhakar, A. L., L. L. Tannis, C. Zeindler, M. P. Russo, C. Jobin, D. S. Park, S. MacPherson, and P. A. Barker. 2002. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J. Neurosci. 22:8466-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boise, L. H., M. Gonzalez-Garcia, C. E. Postema, L. Ding, T. Lindsten, L. A. Turka, X. Mao, G. Nunez, and C. B. Thompson. 1993. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597-608. [DOI] [PubMed] [Google Scholar]
- 8.Chen, F. E., D. B. Huang, Y. Q. Chen, and G. Ghosh. 1998. Crystal structure of p50/p65 heterodimer of transcription factor NF-κB bound to DNA. Nature 391:410-413. [DOI] [PubMed] [Google Scholar]
- 9.Denk, A., T. Wirth, and B. Baumann. 2000. NF-κB transcription factors: critical regulators of hematopoiesis and neuronal survival. Cytokine Growth Factor Rev. 11:303-320. [DOI] [PubMed] [Google Scholar]
- 10.Dixon, E. P., D. T. Stephenson, J. A. Clemens, and S. P. Little. 1997. Bcl-Xshort is elevated following severe global ischemia in rat brains. Brain Res. 776:222-229. [DOI] [PubMed] [Google Scholar]
- 11.Fridmacher, V., B. Kaltschmidt, B. Goudeau, D. Ndiaye, F. M. Rossi, J. Pfeiffer, C. Kaltschmidt, A. Israel, and S. Memet. 2003. Forebrain-specific neuronal inhibition of nuclear factor-κB activity leads to loss of neuroprotection. J. Neurosci. 23:9403-9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabriel, C., C. Justicia, A. Camins, and A. M. Planas. 1999. Activation of nuclear factor-κB in the rat brain after transient focal ischemia. Brain Res. Mol. Brain Res. 65:61-69. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 14.Glasgow, J. N., T. Wood, and J. R. Perez-Polo. 2000. Identification and characterization of nuclear factor kappaB binding sites in the murine bcl-x promoter. J. Neurochem. 75:1377-1389. [DOI] [PubMed] [Google Scholar]
- 15.Grewal, S. S., A. M. Horgan, R. D. York, G. S. Withers, G. A. Banker, and P. J. Stork. 2000. Neuronal calcium activates a Rap1 and B-Raf signaling pathway via the cyclic adenosine monophosphate-dependent protein kinase. J. Biol. Chem. 275:3722-3728. [DOI] [PubMed] [Google Scholar]
- 16.Grilli, M., M. Pizzi, M. Memo, and P. Spano. 1996. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science 274:1383-1385. [DOI] [PubMed] [Google Scholar]
- 17.Guan, K. L., and J. E. Dixon. 1991. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262-267. [DOI] [PubMed] [Google Scholar]
- 18.Guerrini, L., F. Blasi, and S. Denis-Donini. 1995. Synaptic activation of NF-kappa B by glutamate in cerebellar granule neurons in vitro. Proc. Natl. Acad. Sci. USA 92:9077-9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamanoue, M., G. Middleton, S. Wyatt, E. Jaffray, R. T. Hay, and A. M. Davies. 1999. p75-mediated NF-κB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol. Cell. Neurosci. 14:28-40. [DOI] [PubMed] [Google Scholar]
- 20.Huber, K. M., M. D. Mauk, and P. T. Kelly. 1995. Distinct LTP induction mechanisms: contribution of NMDA receptors and voltage-dependent calcium channels. J. Neurophysiol. 73:270-279. [DOI] [PubMed] [Google Scholar]
- 21.Hughes, F. M., Jr., C. D. Bortner, G. D. Purdy, and J. A. Cidlowski. 1997. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 272:30567-30576. [DOI] [PubMed] [Google Scholar]
- 22.Hughes, F. M., Jr., and J. A. Cidlowski. 1999. Potassium is a critical regulator of apoptotic enzymes in vitro and in vivo. Adv. Enzyme Regul. 39:157-171. [DOI] [PubMed] [Google Scholar]
- 23.Kaltschmidt, B., M. Uherek, B. Volk, P. A. Baeuerle, and C. Kaltschmidt. 1997. Transcription factor NF-κB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 94:2642-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaltschmidt, C., B. Kaltschmidt, H. Neumann, H. Wekerle, and P. A. Baeuerle. 1994. Constitutive NF-κB activity in neurons. Mol. Cell. Biol. 14:3981-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontgen, F., R. J. Grumont, A. Strasser, D. Metcalf, R. Li, D. Tarlinton, and S. Gerondakis. 1995. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 9:1965-1977. [DOI] [PubMed] [Google Scholar]
- 26.Krichevsky, A. M., and K. S. Kosik. 2002. RNAi functions in cultured mammalian neurons. Proc. Natl. Acad. Sci. USA 99:11926-11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung, T. H., A. Hoffmann, and D. Baltimore. 2004. One nucleotide in a κB site can determine cofactor specificity for NF-κB dimers. Cell 118:453-464. [DOI] [PubMed] [Google Scholar]
- 28.Liou, H. C., and D. Baltimore. 1993. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr. Opin. Cell Biol. 5:477-487. [DOI] [PubMed] [Google Scholar]
- 29.Liou, H. C., Z. Jin, J. Tumang, S. Andjelic, K. A. Smith, and M. L. Liou. 1999. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int. Immunol. 11:361-371. [DOI] [PubMed] [Google Scholar]
- 30.Mattson, M. P., C. Culmsee, Z. Yu, and S. Camandola. 2000. Roles of nuclear factor κB in neuronal survival and plasticity. J. Neurochem. 74:443-456. [DOI] [PubMed] [Google Scholar]
- 31.Meffert, M. K., J. M. Chang, B. J. Wiltgen, M. S. Fanselow, and D. Baltimore. 2003. NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci. 6:1072-1078. [DOI] [PubMed] [Google Scholar]
- 32.Michaelidis, T. M., M. Sendtner, J. D. Cooper, M. S. Airaksinen, B. Holtmann, M. Meyer, and H. Thoenen. 1996. Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron 17:75-89. [DOI] [PubMed] [Google Scholar]
- 33.Montague, J. W., C. D. Bortner, F. M. Hughes, Jr., and J. A. Cidlowski. 1999. A necessary role for reduced intracellular potassium during the DNA degradation phase of apoptosis. Steroids 64:563-569. [DOI] [PubMed] [Google Scholar]
- 34.Motoyama, N., F. Wang, K. A. Roth, H. Sawa, K. Nakayama, I. Negishi, S. Senju, Q. Zhang, and S. Fujii. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506-1510. [DOI] [PubMed] [Google Scholar]
- 35.Nijhawan, D., N. Honarpour, and X. Wang. 2000. Apoptosis in neural development and disease. Annu. Rev. Neurosci. 23:73-87. [DOI] [PubMed] [Google Scholar]
- 36.O'Neill, L. A., and C. Kaltschmidt. 1997. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20:252-258. [DOI] [PubMed] [Google Scholar]
- 37.Pecci, A., L. R. Viegas, J. L. Baranao, and M. Beato. 2001. Promoter choice influences alternative splicing and determines the balance of isoforms expressed from the mouse bcl-X gene. J. Biol. Chem. 276:21062-21069. [DOI] [PubMed] [Google Scholar]
- 38.Pelletier, M. R., and J. J. Hablitz. 1996. Tetraethylammonium-induced synaptic plasticity in rat neocortex. Cereb. Cortex 6:771-780. [DOI] [PubMed] [Google Scholar]
- 39.Pizzi, M., F. Goffi, F. Boroni, M. Benarese, S. E. Perkins, H. C. Liou, and P. Spano. 2002. Opposing roles for NF-κB/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1β. J. Biol. Chem. 277:20717-20723. [DOI] [PubMed] [Google Scholar]
- 40.Reddy, R., D. Smith, G. Wayman, Z. Wu, E. C. Villacres, and D. R. Storm. 1995. Voltage-sensitive adenylyl cyclase activity in cultured neurons. A calcium-independent phenomenon. J. Biol. Chem. 270:14340-14346. [DOI] [PubMed] [Google Scholar]
- 41.Roth, K. A., N. Motoyama, and D. Y. Loh. 1996. Apoptosis of bcl-x-deficient telencephalic cells in vitro. J. Neurosci. 16:1753-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt-Ullrich, R., S. Memet, A. Lilienbaum, J. Feuillard, M. Raphael, and A. Israel. 1996. NF-κB activity in transgenic mice: developmental regulation and tissue specificity. Development 122:2117-2128. [DOI] [PubMed] [Google Scholar]
- 43.Scott, M. L., T. Fujita, H. C. Liou, G. P. Nolan, and D. Baltimore. 1993. The p65 subunit of NF-kappa B regulates I kappa B by two distinct mechanisms. Genes Dev. 7:1266-1276. [DOI] [PubMed] [Google Scholar]
- 44.Sha, W. C., H. C. Liou, E. I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80:321-330. [DOI] [PubMed] [Google Scholar]
- 45.Shou, Y., N. Li, L. Li, J. L. Borowitz, and G. E. Isom. 2002. NF-κB-mediated up-regulation of Bcl-X(S) and Bax contributes to cytochrome c release in cyanide-induced apoptosis. J. Neurochem. 81:842-852. [DOI] [PubMed] [Google Scholar]
- 46.Sun, S. C., P. A. Ganchi, D. W. Ballard, and W. C. Greene. 1993. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science 259:1912-1915. [DOI] [PubMed] [Google Scholar]
- 47.Thompson, G. J., C. Langlais, K. Cain, E. C. Conley, and G. M. Cohen. 2001. Elevated extracellular [K+] inhibits death-receptor- and chemical-mediated apoptosis prior to caspase activation and cytochrome c release. Biochem. J. 357:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsukahara, T., M. Kannagi, T. Ohashi, H. Kato, M. Arai, G. Nunez, Y. Iwanaga, N. Yamamoto, K. Ohtani, M. Nakamura, and M. Fujii. 1999. Induction of Bcl-xL expression by human T-cell leukemia virus type 1 Tax through NF-κB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 73:7981-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Y., and J. P. Durkin. 1995. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, but not N-methyl-d-aspartate, activates mitogen-activated protein kinase through G-protein βγ subunits in rat cortical neurons. J. Biol. Chem. 270:22783-22787. [DOI] [PubMed] [Google Scholar]
- 50.Yu, S. P., C. Yeh, U. Strasser, M. Tian, and D. W. Choi. 1999. NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science 284:336-339. [DOI] [PubMed] [Google Scholar]
- 51.Yu, S. P., C. H. Yeh, S. L. Sensi, B. J. Gwag, L. M. Canzoniero, Z. S. Farhangrazi, H. S. Ying, M. Tian, L. L. Dugan, and D. W. Choi. 1997. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 278:114-117. [DOI] [PubMed] [Google Scholar]
- 52.Zaidi, A. U., C. D'Sa-Eipper, J. Brenner, K. Kuida, T. S. Zheng, R. A. Flavell, P. Rakic, and K. A. Roth. 2001. Bcl-X(L)-caspase-9 interactions in the developing nervous system: evidence for multiple death pathways. J. Neurosci. 21:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]