Abstract
Cdc48 (p97/VCP) is an AAA-ATPase molecular chaperone whose cellular functions are facilitated by its interaction with ubiquitin binding cofactors (e.g., Npl4-Ufd1 and Shp1). Several studies have shown that Saccharomyces cerevisiae Doa1 (Ufd3/Zzz4) and its mammalian homologue, PLAA, interact with Cdc48. However, the function of this interaction has not been determined, nor has a physiological link between these proteins been demonstrated. Herein, we demonstrate that Cdc48 interacts directly with the C-terminal PUL domain of Doa1. We find that Doa1 possesses a novel ubiquitin binding domain (we propose the name PFU domain, for PLAA family ubiquitin binding domain), which appears to be necessary for Doa1 function. Our data suggest that the PUL and PFU domains of Doa1 promote the formation of a Doa1-Cdc48-ubiquitin ternary complex, potentially allowing for the recruitment of ubiquitinated proteins to Cdc48. DOA1 and CDC48 mutations are epistatic, suggesting that their interaction is physiologically relevant. Lastly, we provide evidence of functional conservation within the PLAA family by showing that a human-yeast chimera binds to ubiquitin and complements doa1Δ phenotypes in yeast. Combined, our data suggest that Doa1 plays a physiological role as a ubiquitin binding cofactor of Cdc48 and that human PLAA may play an analogous role via its interaction with p97/VCP.
Cdc48 (p97/VCP) is a member of the AAA-ATPase family of molecular chaperones (12, 31, 48). As is characteristic of this family, Cdc48 exists as a hexamer that utilizes the hydrolysis of ATP to achieve its cellular functions. These functions include several ubiquitin-dependent processes: endoplasmic reticulum (ER)-associated degradation (ERAD), membrane fusion, transcription factor activation, and spindle disassembly (3, 5, 12, 16, 26, 35, 36). Recent work has demonstrated that Cdc48 involvement in these processes requires the cofactors Ufd1-Npl4, Shp1 (p47), Ufd2, Rad23, and VCIP135, or one of several ubiquitin-related domain (UBX) proteins (8, 15, 16, 24, 28, 29, 43, 48). The two most striking commonalities among many of these cofactors are that they possess a UBX domain and that they interact with ubiquitin (30, 37). The accepted paradigm is that Cdc48 (p97/VCP) interacts with its cofactors via their UBX domains and uses their ubiquitin binding properties to facilitate mobilization or modification of ubiquitinated substrates. Shp1 (p47) possesses a UBA ubiquitin binding domain in its N terminus and forms a complex with Cdc48 to facilitate membrane fusion and possibly proteasomal degradation (15, 25, 39). Also involved in the membrane fusion process is VCIP135, a deubiquitinating enzyme that interacts with the p97-p47 complex and is necessary for Golgi membrane reassembly (43, 46). Ufd1-Npl4 binds to ubiquitin via the NZF ubiquitin binding domain present in Npl4 (30, 45). This subcomplex interacts with Cdc48 to facilitate ERAD and activation of certain membrane-bound transcription factors (16, 36). Ufd2 interacts with ubiquitin via its U-box domain and acts to extend the polyubiquitin chains of proteins bound by the Cdc48-Ufd1-Npl4 complex (24). Rad23 possesses two UBA domains and appears to mediate the transfer of ubiquitinated substrates from Cdc48 to the proteasome via its interaction with Cdc48-bound Ufd2 (37). Lastly, several UBX domain proteins possess UIM and/or UBA domains and appear to participate in proteasomal substrate degradation (8, 15).
Doa1 (Ufd3/Zzz4) and its homologues have also been shown to bind to Cdc48 or p97/VCP, respectively, though only by indirect methods (e.g., immunoprecipitation or yeast two-hybrid assay) (8, 13, 14, 33, 40). Doa1 was first linked to the ubiquitin proteasome pathway by its identification in a screen designed to find Saccharomyces cerevisiae mutants that fail to degrade the short-lived MATα2 transcriptional repressor (17). Subsequently, this gene was shown to affect ubiquitin fusion protein degradation (UFD3) and resistance to volatile anesthetics (ZZZ4) (20, 22). Although the function of Doa1 remains unknown, its loss in yeast results in the depletion of cellular mono- and polyubiquitin, thereby accounting for its identification in these screens (20). In Saccharomyces cerevisiae and Schizosaccharomyces pombe, this depletion appears to be due to aberrant ubiquitin degradation, since ubiquitin message levels remain unchanged; however, the mechanism of this degradation remains elusive (8, 33).
The human homologue of Doa1 has 48% sequence similarity (31% identity) and has been named phospholipase A2 activating protein (PLAA), in part because a 15-amino-acid stretch bears distant resemblance to mellitin (4). Doa1 and other members of the PLAA family have an N-terminal domain containing seven WD40 repeats (∼300 amino acids) and a C-terminal domain (∼400 to 500 amino acids) of unknown function. WD40 domains are protein-protein interaction domains that bind a wide variety of proteins. No mechanistic data exist regarding PLAA function in higher eukaryotes. Therefore, understanding the function(s) of the yeast homologue in terms of its role in the ubiquitin-proteasome pathway may lead to a better understanding of human PLAA function.
Herein, we identify a novel ubiquitin binding domain within Doa1 that we have named the PFU domain (PLAA family ubiquitin binding domain). We show that the C-terminal PUL domain of Doa1 directly binds to Cdc48 and that this interaction facilitates the recruitment of Cdc48 to ubiquitin (19). Moreover, we demonstrate that DOA1 and CDC48 are genetically linked. Last, we reveal functional conservation of the PLAA family of proteins by demonstrating that a yeast-human chimera (containing the WD40 domain of Doa1 and the PFU and PUL domains of human PLAA) binds to ubiquitin and complements Doa1 deletion in yeast. Together, our data provide the first direct evidence that Doa1 functions to mediate Cdc48 binding to ubiquitinated proteins. Furthermore, these data suggest that human PLAA functions in a pathway(s) directly analogous to that of Doa1, allowing for a better understanding of PLAA in higher eukaryotes.
MATERIALS AND METHODS
General.
Matrix-assisted laser desorption ionization (MALDI) mass spectrometry was performed by the microchemical facility at Emory University. All primers were synthesized by Operon Biotechnologies, Inc. (Huntsville, AL). Canavanine and anisomycin (Sigma, St. Louis, MO) were used in the yeast drug sensitivity assays. Ponceau S protein stain (Sigma, St. Louis, MO) was used when noted to confirm equal protein loading. Complete protease inhibitors (Roche Applied Science, Indianapolis, IN) were added to all lysates. Antibodies to the following epitopes were used: Flag (clone M2; Sigma-Aldrich Corp., St. Louis, MO), ubiquitin (clone P4D1; Santa Cruz Biotechnology, Santa Cruz, CA), hexahistidine (clone H-15; Santa Cruz Biotechnology, Santa Cruz, CA), PGK (Molecular Probes, Inc., Eugene, OR), and hemagglutinin (HA; clone 12CA5; Maine Biotechnology Services, Inc., Portland, ME). Flag epitope immunoprecipitations were carried out using anti-Flag agarose (clone M2; Sigma-Aldrich Corp., St. Louis, MO).
Gene disruptions, truncations, and point mutations.
The wild-type MHY501 and DBY469 and mutant DBY1247 (cdc48-1) yeast strains were used for all deletions and truncations (31, 41). Deletions and truncations of chromosomal copies of DOA1 were created using plasmids and techniques described by Longtine et al. (2, 27). Briefly, PCR primers were synthesized having 5′-end homology to the gene of interest and 3′ homology to the required Longtine vector. PCR products were transformed using the LiCl method, and transformants were isolated using selective agar plates and verified using PCR. DOA1 point mutants were created via PCR-based site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Gene-specific primers were used to amplify wild-type Doa1 and the F417D/F434D point mutant, in tandem with the tryptophan selection marker, for transformation into doa1Δ yeast. This created yeast strains expressing either wild-type, Flag-tagged Doa1 or the point mutant, Flag-tagged Doa1 under the endogenous Doa1 promoter. The sequences of all primers used are available upon request.
Plasmid construction.
Standard molecular biology techniques were used for all cloning procedures. The bacterial expression vectors for HisFlag-Doa1Cterm and HisFlag-Doa1PUL were constructed by PCR amplification of the DOA1 gene sequence encoding amino acids 288 through 715 and 453 through 715, respectively, using yeast genomic DNA as template. Individually, these PCR products were digested with NdeI and HindIII and ligated into the NdeI and HindIII sites of pRSET-B (Invitrogen, Carlsbad, CA). Ligation into the NdeI site removes the epitope tags encoded by the pRSET-B vector. The hexahistidine and Flag epitope tags are encoded within the forward primers. The bacterial expression vector for HisFlag-Doa1WD40 was constructed by PCR amplification of the DOA1 gene sequence encoding amino acids 1 through 298 using yeast genomic DNA as template. This PCR product was digested with NheI and XhoI and ligated into the NheI and XhoI sites of pRSET-B. The Flag epitope tag is encoded within the forward primer. Ligation into the NheI site inserts the PCR product directly after the hexahistidine tag encoded by pRSET-B. The bacterial expression vector for His-Cdc48 was constructed by PCR amplification of the CDC48 gene sequence using yeast genomic DNA as template. This PCR product was digested with NdeI and SacI and ligated into the NdeI and SacI sites of pRSET-B (Invitrogen, Carlsbad, CA). Ligation into the NdeI site removes the epitope tags encoded by the pRSET-B vector. The hexahistidine epitope tag is encoded within the forward primer. The yeast expression vector for the hemagglutinin (HA)-tagged Doa1-PLAA chimera was constructed by a two-step ligation. First, the WD40 domain (amino acids 1 through 298) encoding sequence of the DOA1 gene was amplified from yeast genomic DNA, digested with EcoRI and SacI, and ligated into the EcoRI and SacI sites of pYEPGAP to generate pYEPGAP-DOA1WD40. Second, the C-terminal domain (amino acids 311 through 795) encoding sequence of the human PLAA gene was amplified from pBS-PLAA, digested with SacI and XhoI, and ligated into the SacI and XhoI sites of pYEPGAP-DOA1WD40. The N-terminal HA epitope is encoded within the forward primer. The pBS-PLAA and pYEPGAP vectors were described previously (4, 6). The yeast expression vector for HA-tagged Doa1 was constructed by PCR amplification of the DOA1 gene sequence using yeast genomic DNA as template. This PCR product was digested with SacI and XhoI and ligated into the SacI and XhoI sites of pYEPGAP. The HA epitope tag is encoded within the forward primer.
Ubiquitin and Cdc48 binding experiments.
Control, monoubiquitin, and 29-linked tetraubiquitin analogue-Sepharose resins were synthesized as previously described (38). For analysis of ubiquitin binding by Doa1 truncations from whole-cell yeast lysates, 300 μg of yeast whole-cell lysate (150 mM NaCl, 50 mM Tris, pH 7.4, 1 mM dithiothreitol, Complete protease inhibitors, 0.05% Triton X-100) was incubated with 100 μl of ubiquitin resin (8 mg/ml ubiquitin) or control resin for 3 h at 4°C, washed (three times) with lysis buffer, and eluted by boiling in 1.5× sodium dodecyl sulfate (SDS) loading buffer. For analysis of ubiquitin binding by purified, recombinant Doa1, 1 μg of Doa1 was incubated with ubiquitin or control resins for 3 h at 4°C and washed and eluted as above. To analyze the direct interaction of Doa1 and Cdc48 using purified recombinant proteins, 0.5 μg of His-Cdc48 was preincubated with or without 1 μg of HisFlag-Doa1, 1 μg of bovine serum albumin (BSA), or ATP (5 mM ATP with 20 mM MgCl2) for 10 min at 4°C, followed by incubation with Flag resin (as above) for 30 min at 4°C. The HisFlag-Doa1 vector was described previously (14). For assays to analyze the recruitment of Cdc48 to monoubiquitin-Sepharose by Doa1, 0.5 μg of Cdc48 was preincubated with or without 1 μg of Doa1, 1 μg of BSA, or ATP (5 mM ATP with 20 mM MgCl2) for 10 min at 4°C, followed by incubation with ubiquitin resin (as above) for 30 min at 4°C.
Determination of ubiquitin levels and drug sensitivity.
Yeast cultures were grown to late log phase (optical density at 600 nm [OD600] of 1.5) in minimal synthetic dextrose (SD) medium at 30°C. Samples were normalized by optical density and boiled in SDS loading buffer prior to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Equal loading was verified by staining the resulting nitrocellulose membrane with Ponceau S protein stain and/or by using antibodies to PGK. The nitrocellulose membrane was boiled in distilled deionized water for 10 min prior to blocking. For drug sensitivity assays, yeast cultures were grown to an OD600 of 1.0 in SD and then 2 μl of cells was spotted directly, as well as diluted 1:10, 1:100, and 1:1000, onto SD agar plates with or without canavanine (0.6 μg/ml) or anisomycin (20 μg/ml). The yeast ubiquitin expression vector used to determine the effect of ubiquitin overexpression on drug sensitivity was described previously (11).
Identification of Doa1 binding proteins.
The yeast HisFlag-Doa1 expression vector was used to transform doa1Δ yeast (14). doa1Δ yeast expressing HisFlag-Doa1 or wild-type yeast were grown to log phase (OD600 of 1.0) in synthetic raffinose-galactose medium. Yeast were harvested and lysed by the liquid nitrogen/mortar and pestle method as previously described (38). Yeast lysates were subjected to Ni2+ affinity chromatography to isolate Doa1 and Doa1 binding proteins. Prominent bands from the resulting Sypro Ruby-stained SDS-PAGE gel were excised and submitted for MALDI mass spectrometry as previously described (38).
RESULTS
Doa1 possesses a novel ubiquitin binding domain.
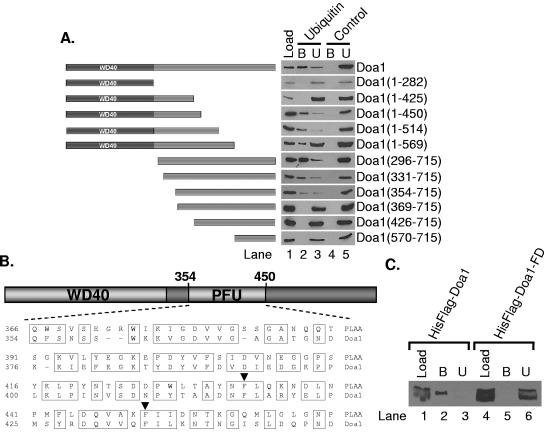
We have previously identified Doa1 as a ubiquitin binding protein and have shown that it binds to polyubiquitin analogues directly (38); however, no ubiquitin binding domain consensus sequence was recognizable within Doa1. We therefore set out to localize the ubiquitin binding domain by creating truncations of the chromosomal copy of DOA1 in yeast. Whole-cell extracts from yeast expressing these truncations were incubated with 29-linked tetraubiquitin analogue-Sepharose or control resin to analyze Doa1 binding (Fig. 1A, lane 2 versus lane 4). We saw similar levels of binding to resins having 11-, 48-, and 63-linked tetraubiquitin analogues as well as to monoubiquitin (data not shown). These data allowed the identification of a minimal ubiquitin binding domain residing between residues ∼350 and ∼450. This domain is conserved in human PLAA (Fig. 1B). We propose to name this domain the PFU domain (for PLAA family ubiquitin binding domain). To further confirm that the PFU domain is responsible for binding to ubiquitin, we mutated two conserved residues within this domain (Fig. 1B, F417D and F434D) and expressed the recombinant double mutant and wild-type Doa1 in Escherichia coli. These residues were chosen for mutagenesis because they are highly conserved and are predicted to reside within two adjacent α-helices (data not shown) and were therefore thought likely to disrupt the structure of the PUL domain (23). These mutations abrogate binding of Doa1 to monoubiquitin-Sepharose (Fig. 1C, lane 5 versus lane 2). Future structural studies will be needed to determine if the residues chosen for mutation directly interact with ubiquitin or are simply required for overall PFU domain integrity.
FIG. 1.
Localization of a novel ubiquitin binding domain in Doa1, the PFU domain. (A) Whole-cell lysates from yeast strains expressing various HA-tagged Doa1 truncations were incubated with 29-linked tetraubiquitin analogue-Sepharose (ubiquitin) or control-Sepharose (control). Load, bound, and unbound Doa1 were detected immunochemically using antibodies to the HA epitope. B, bound; U, unbound. (B) Schematic depicting the domain architecture of the PLAA family above a sequence alignment comparing the primary structure of the PFU domains from human PLAA and S. cerevisiae Doa1. Arrowheads point to conserved residues mutated in panel C. (C) Whole-cell lysates containing recombinantly expressed, His-Flag-tagged wild-type Doa1 (HisFlag-Doa1) or Doa1(F417D, F434D) (HisFlag-Doa1-FD) were incubated with monoubiquitin-Sepharose. Load, bound, and unbound Doa1 were detected immunochemically using antibodies to the Flag epitope. B, bound; U, unbound.
Ubiquitin binding is necessary for Doa1 function.
Deletion of DOA1 or disruption of its function is known to cause a depletion of mono- and polyubiquitin in yeast (Fig. 2A) (20). Deletion of DOA1 also causes sensitivity to several stresses, including misfolding of protein and translation inhibition, as is shown by treating yeast with canavanine and anisomycin, respectively (Fig. 2B, doa1Δ plus vector versus DOA1 plus vector). Overexpression of ubiquitin restores wild-type growth, suggesting that these phenotypes are caused by ubiquitin depletion (Fig. 2B, doa1Δ plus vector versus doa1Δ plus UBI). To determine whether ubiquitin binding by Doa1 is necessary for its function, yeast strains were generated that replaced the chromosomal DOA1 gene with altered genes expressing Flag-Doa1 or Flag-Doa1(F417D, F434D). Yeast expressing Flag-Doa1(F417D, F434D) have ubiquitin depletion (Fig. 2C, lane 2 versus lane 1) and partial drug sensitivity (Fig. 2D, Flag-DOA1-FD versus Flag-DOA1) phenotypes compared to yeast expressing Flag-Doa1, confirming that the ubiquitin binding domain is at least in part necessary for Doa1 function.
FIG. 2.
Ubiquitin binding by Doa1 is necessary for its function of maintaining ubiquitin homeostasis. (A) Whole-cell lysates from wild-type and doa1Δ yeast strains grown on SD medium were analyzed immunochemically for monoubiquitin and high-molecular-weight ubiquitin conjugates using ubiquitin antibodies. (B) Serial dilutions of wild-type (DOA1) and doa1Δ yeast, which were transformed with empty vector or a ubiquitin expression vector (UBI), were used to determine the effect of ubiquitin overexpression on sensitivity to canavanine and anisomycin. Growth on defined glucose medium (control) is shown to demonstrate equal loading. (C) Whole-cell lysates expressing Flag-tagged wild-type Doa1 (Flag-Doa1) or Doa1(F417D, F434D) (Flag-Doa1-FD) grown on SD medium were analyzed immunochemically for ubiquitin to determine the effect of PFU domain mutations on Doa1 function in vivo as measured by monoubiquitin levels. (D) Serial dilutions of Flag-Doa1-, doa1Δ-, and Flag-Doa1-FD-expressing yeast strains were used to determine the effect of PFU mutations on Doa1 function in vivo as measured by sensitivity to canavanine and anisomycin. Growth on defined glucose medium (control) is shown to demonstrate equal loading.
Human PLAA binds ubiquitin and is a functional homologue of Doa1.
Recently, DOA1 was shown to complement the deletion of one of its homologues, LUB1, in S. pombe (33). We were interested in determining if the PLAA family is functionally conserved in humans. To this end, we attempted to express human PLAA in S. cerevisiae. However, expression of the full-length human protein was very low compared to Doa1, and its expression imparted a minor growth defect (data not shown). Since these phenotypes may be due to defective binding or misfolding of one of the domains of PLAA, we designed an HA-tagged chimeric protein consisting of the WD40 domain from Doa1 fused to the C terminus of human PLAA, which includes the putative PFU and PUL domains (Fig. 3A, schematic diagram). The HA-Doa1-PLAA chimera is expressed in doa1Δ yeast at levels comparable to HA-Doa1 expressed from the same vector (Fig. 3A, Western blot) and does not cause any apparent growth defect (data not shown). Like HA-Doa1, the HA-Doa1-PLAA chimera binds to monoubiquitin and 29-linked tetraubiquitin analogues (Fig. 3B, lanes 2 and 4 versus lane 6), suggesting that the PFU domain is a conserved ubiquitin binding domain (note that the WD40 domain of Doa1 does not bind to ubiquitin under these conditions) [Fig. 1A, Doa1 (1-282)]. In support of this finding, we have observed that in whole-cell extracts, murine PLAA also binds to monoubiquitin (unpublished results). In addition, expression of the HA-Doa1-PLAA chimera in doa1Δ yeast results in complementation of the ubiquitin depletion phenotype (Fig. 3C, lane 4 versus lane 2) and the drug sensitivity phenotype (Fig. 3D, doa1Δ plus HA-chimera versus doa1Δ plus vector).
FIG. 3.
The function of the human PLAA C-terminal domain is conserved. (A) Top: schematic depicting the chimera of the yeast Doa1 WD40 domain and the human PLAA C-terminal domain. Bottom: relative size and expression levels of HA-Doa1 and the HA-Doa1-PLAA chimera in yeast whole-cell lysates. Both proteins were detected immunochemically using antibodies to the HA epitope. (B) Whole-cell lysate from yeast strains expressing HA-tagged Doa1-PLAA were incubated with monoubiquitin-, 29-linked tetraubiquitin analogue-, or control-Sepharose. Load, bound, and unbound Doa1-PLAA were detected immunochemically using antibodies to the HA epitope. B, bound; U, unbound. (C) Whole-cell lysates from wild-type parental (WT) and doa1Δ cell lines transformed with empty vector, HA-DOA1, or HA-DOA1-PLAA (chimera) were analyzed immunochemically for monoubiquitin and high-molecular-weight ubiquitin conjugates using antibodies to ubiquitin. (D) Serial dilutions of wild-type parental (DOA1 + vector) and doa1Δ cell lines transformed with empty vector (doa1Δ + vector), HA-DOA1 (doa1Δ + HA-DOA1), or HA-DOA1-PLAA (doa1Δ + HA-chimera) were used to demonstrate that HA-Doa1-PLAA complements doa1Δ sensitivity to canavanine and anisomycin. Growth on defined glucose medium (control) is shown to demonstrate equal loading.
Doa1 interacts directly with Cdc48 and recruits Cdc48 to ubiquitin.
Several groups have demonstrated that Doa1 and Cdc48 interact by using indirect assays such as the yeast two-hybrid assay or affinity chromatography (8, 13, 14, 33, 40). We confirmed the interaction between these proteins in yeast via Ni2+ affinity resin chromatography of whole-cell extracts from yeast expressing HisFlag-Doa1, followed by MALDI mass spectrometry of the resulting excised protein bands (Fig. 4A, lane 3). Given that the varied cellular functions of Cdc48 appear to be mediated through its direct interactions with other ubiquitin binding proteins, (e.g., Shp1 and Ufd1/Npl4), we investigated whether there is a direct physical interaction between Doa1 and Cdc48. To accomplish this, recombinant HisFlag-Doa1 and His-Cdc48 were separately expressed in E. coli and purified using Ni2+ affinity resin chromatography (data not shown). Immunoprecipitation of recombinant HisFlag-Doa1 with anti-Flag antibody coprecipitates recombinant His-Cdc48 in a manner independent of the presence of ATP (Fig. 4B, lanes 4 and 6 versus lane 2), suggesting that Doa1 is not simply acting as a substrate of Cdc48. This interaction appears to be specific, as BSA does not compete for this interaction (Fig. 4B, lane 8 versus lane 4 or 6). The domain responsible for interaction with Cdc48 appears to reside within the C-terminal domain of Doa1, as His-Cdc48 coimmunoprecipitates with HisFlag-Doa1Cterm but not HisFlag-Doa1WD40 (Fig. 4C, lane 8 versus lanes 6 and 2). We further localized this binding interaction to a previously predicted globular domain within the C terminus of Doa1 termed the PUL domain (see Discussion) (Fig. 4C, lane 10 versus lanes 4 and 2) (19).
FIG. 4.
Cdc48 is recruited to ubiquitin via direct interaction with the C-terminal PUL domain of Doa1. (A) Whole-cell lysates from wild-type parental (WT) or doa1Δ yeast expressing HisFlag-Doa1 were subjected to Ni2+ agarose affinity chromatography. Eluted proteins were separated by SDS-PAGE, visualized by staining with Sypro Ruby, and excised for identification by mass spectrometry. Arrows indicate the two most prominent proteins identified, Doa1 and Cdc48. (B) Antibodies to the Flag epitope were used to coimmunoprecipitate purified, recombinant His-Cdc48 in the presence or absence of purified, recombinant HisFlag-Doa1, BSA, and/or ATP. Load, bound, and unbound Cdc48 and Doa1 were detected immunochemically using antibodies to the His6 epitope. (C) Antibodies to the Flag epitope were used to coimmunoprecipitate purified, recombinant His-Cdc48 in the presence or absence of purified, recombinant His-Flag-tagged full-length Doa1 (HisFlag-Doa1), WD40 domain (HisFlag-Doa1WD40), C-terminal domain (HisFlag-Doa1Cterm), or PUL domain (HisFlag-Doa1PUL). The schematic depicts the location of the PUL domain within Doa1. Load, bound, and unbound Cdc48 and Doa1 were detected immunochemically using antibodies to the His6 epitope. Asterisks denote a weak cross-reaction to Flag antibody heavy and light chains. (D) Purified, recombinant His-Cdc48 and HisFlag-Doa1 were incubated individually or in combination, with or without ATP, and analyzed for binding to monoubiquitin-Sepharose. Load, bound, and unbound Cdc48 and Doa1 were detected immunochemically using antibodies to the His6 epitope. L, load; B, bound; U, unbound.
We next set out to determine if the direct interaction between Doa1 and Cdc48 was sufficient to recruit Cdc48 to ubiquitin. It has been shown that Cdc48, itself, can interact with ubiquitin; however, the presence of specific ubiquitin binding cofactors enhances this interaction (37, 39, 48, 49). Under the conditions of our assay we did not observe binding of Cdc48 alone to ubiquitin-Sepharose (Fig. 4D, lane 2). However, when Doa1 was added to Cdc48, Cdc48 was then present in the ubiquitin-bound fraction along with Doa1, suggesting that Doa1 recruits Cdc48 to ubiquitin (Fig. 4D, lane 6 versus lane 2). Like the Doa1-Cdc48 interaction, Doa1-mediated recruitment of Cdc48 to ubiquitin is independent of the presence of ATP (Fig. 4D, lane 6 versus lane 8).
CDC48 has an epistatic genetic relationship with DOA1.
Given that our binding data imply a functional relationship between Doa1 and Cdc48, we set out to test this assertion by examining the genetic linkage between these proteins. We achieved this by comparing the phenotypes of DOA1 and CDC48 single and double mutants. Since CDC48 is an essential gene, we utilized an impaired CDC48 variant, cdc48-1, that exhibits a cold-sensitive growth phenotype (31). Yeast with impaired Cdc48 have a defect in proteasomal degradation caused by the improper retention of ubiquitinated ERAD substrates in the ER (i.e., impaired Cdc48 fails to function normally to deliver substrates to the proteasome) (10). This defect is observed in cdc48-1 strains even at the permissive temperature (14). As shown previously, doa1Δ yeast cells also have a defect in the ubiquitin-proteasome pathway, which is manifested as depletion of cellular ubiquitin levels and sensitivity to canavanine (Fig. 2). We determined that CDC48 and DOA1 are epistatic by comparing the canavanine sensitivities of single mutant cdc48-1 and doa1Δ yeast with double mutant cdc48-1/doa1Δ yeast (Fig. 5A, cdc48-1 and doa1Δ versus cdc48-1/doa1Δ). doa1Δ yeast are sensitive to canavanine, while neither the cdc48-1 single mutant nor the cdc48-1/doa1Δ double mutant display sensitivity to canavanine. That is, the doa1Δ phenotype is suppressed by mutations in CDC48. These results may be a reflection of total cellular ubiquitin levels, since the double mutant cdc48-1/doa1Δ yeast have more polyubiquitin than do the single mutant doa1Δ yeast (Fig. 5B, lane 4 versus lane 3). This epistatic relationship suggests that Doa1 and Cdc48 have physiological roles in the same cellular pathway(s).
FIG. 5.
DOA1 is genetically linked to CDC48. (A) Serial dilutions were used to determine the effects of inactivating Cdc48 (cdc48-1) on canavanine sensitivity in parental wild-type (WT), doa1Δ, and doa4Δ yeast cells cultured at 30°C. Growth on defined glucose medium (control) is shown to demonstrate equal loading. (B) Whole-cell lysates from yeast strains described for panel A and cultured at 20°C were analyzed immunochemically for monoubiquitin and high-molecular-weight ubiquitin conjugates using ubiquitin antibodies. Lowest panel: immunochemical analysis using antibodies to PGK, used as a loading control.
To demonstrate that the epistatic effect of cdc48-1 is specific to ubiquitin loss via a pathway involving Doa1, we analyzed the effect of Cdc48 impairment in yeast having a ubiquitin loss phenotype due to the deletion of DOA4. We achieved this by comparing canavanine sensitivity in DOA4 and CDC48 single and double mutants. doa4Δ yeast have a ubiquitin loss phenotype due to aberrant vacuolar degradation of ubiquitin (i.e., involving a process that has not been linked to Cdc48 function) (1, 41). In contrast to the epistatic relationship between cdc48-1 and doa1Δ, the double mutant cdc48-1/doa4Δ has a stronger growth defect compared to either cdc48-1 or doa4Δ single mutants, even in the absence of canavanine (Fig. 5A, cdc48-1 and doa4Δ versus cdc48-1/doa4Δ). This greater-than-additive effect suggests that this defect is due to a combination of Doa4-dependent and Cdc48-dependent pathways. These results diminish the possibility that CDC48 and DOA1 genetically interact simply due to the neutralizing effects of ubiquitin accumulation (cdc48-1) and ubiquitin loss (doa1Δ).
DISCUSSION
The finding that interaction of Cdc48 and Doa1 is direct has important implications for understanding the function of both of these proteins. Although several groups have demonstrated that Doa1 and Cdc48 interact, none have shown this to be direct; whole-cell or whole-cell lysate methods such as yeast two-hybrid and immunoprecipitation/affinity chromatography were employed (8, 13, 14, 33, 40). The major caveat with these data is that, given that Doa1 and Cdc48 both bind to ubiquitin, it remained possible that the apparent Cdc48-Doa1 interaction was an artifact of their common affinities for ubiquitin, a molecule omnipresent in whole cells/cell lysates, or some other protein. Our direct binding data show that the Cdc48-Doa1 interaction can take place independently of other binding partners. Furthermore, indirect binding data from other groups conflict as to which Doa1 domain mediates the putative Doa1-Cdc48 interaction. Ghislain et al. showed that the Doa1 WD40 domain alone does not interact with Cdc48 (14). In agreement with this finding, they isolated a WD40 domain mutant (Ufd3-2; C237Y) that causes a null phenotype in yeast yet retains its ability to interact with Cdc48 (14). This suggests that the WD40 domain is indispensable for function but dispensable for the Doa1-Cdc48 interaction. Consistent with this, Decottignies et al. presented data suggesting that it is the C terminus of Doa1, and not the WD40 domain, that mediates the interaction between these two proteins (8). In contrast to these findings, however, data from Ogiso et al. suggest that the S. pombe homologue of Doa1, Lub1, binds to Cdc48 via the WD40 domain (33). While we cannot rule out species differences, our data, based on use of purified recombinant Cdc48 and Doa1, concur with the former two observations, i.e., that full-length Doa1 and a C-terminal domain of Doa1 (the PUL domain; see below) bind directly to Cdc48. A possible explanation for data suggesting a WD40 domain-Cdc48 interaction is that, in whole-cell extracts, the WD40 domain of Doa1 interacts with yet another protein, which in turn interacts with Cdc48. This potential emphasizes the importance of demonstrating direct binding between Cdc48 and Doa1.
The implications of a direct binding interaction between Doa1 and Cdc48 became apparent with our discovery that Doa1 also possesses a novel ubiquitin binding domain, the PFU domain. This domain appears to be unique to the PLAA family of proteins and has no homology to several known ubiquitin binding domains (e.g., UIM, NZF, UBA, UEV, UBP, or CUE domains) (9, 18, 21, 34, 42, 45). However, secondary structure predictions of the PFU domain suggest the presence of an extensive length of β-sheet, N-terminal to an α-helical region, and tertiary structure predictions using 3D-PSSM (three-dimensional position-specific scoring matrix) give a strong correlation to the Mms2 UEV domain (data not shown) (23, 32, 44). Although speculative at this point, it will be interesting to see if future structural studies concur with this prediction, since the precedent of similar structures having little sequence homology has already been established with other ubiquitin binding domains (e.g., UBA and CUE domains).
Using a bioinformatics approach, Iyer et al. identified a conserved domain (the PUL domain, present in PLAA, Ufd3, and Lub1) within the PLAA family (Doa1 residues 465 to 715) and speculated that it is a ubiquitin binding domain (19). In part, they based this analysis on previous biochemical data from our lab that also suggested the presence of a ubiquitin binding domain in Doa1 (38). The experimental results given herein differ from the bioinformatics prediction in that we have localized the PLAA family ubiquitin binding domain to the central portion of these proteins (Doa1 residues 354 to 450). Our data demonstrate that the PUL domain actually encompasses the Cdc48 (p97/VCP) interaction domain of this protein family.
Several of the known functions of Cdc48 (e.g., ER-associated degradation and membrane fusion) are mediated through ubiquitin binding cofactors [e.g., Ufd1/Npl4, Shp1 (p47), VCIP, and Ufd2] (16, 24, 28, 29, 43, 48). The prevailing model is that Cdc48 couples the ubiquitin binding properties of specific cofactors with its ATP-driven mechanical force, thus mobilizing ubiquitinated substrates. It is not known which Cdc48-mediated process involves Doa1. Decottignies et al. stated that doa1Δ yeast exhibit no defect in ER membrane fusion (8). Also, doa1Δ yeast do not show sensitivity to the ERAD-inducing drug tunicamycin (unpublished data) and, to our knowledge, Doa1 has not been identified in screens for components of the ERAD pathway. In contrast, Cdc48 impairment (or Npl4-Ufd1 impairment) leads to the accumulation of polyubiquitinated substrate proteins on ER membranes (7, 37, 47).
The above findings do not a priori rule out the involvement of Doa1 in proteolysis, however. It is possible that Doa1 acts in conjunction with Cdc48 at a step that does not preclude substrate degradation; for example, it may be necessary to facilitate the release of polyubiquitin from the proteasome, thus preventing its proteasomal degradation. Although at this point speculative, this model is particularly attractive for the following reasons. Ubiquitin appears to be abnormally degraded in doa1Δ yeast, and results from our laboratory suggest that this degradation is not taking place in the vacuole (unpublished results) (8, 33). Furthermore, Cdc48 has a well-established role in proteasomal degradation yet has never been linked either to vacuolar degradation or autophagy (16, 36, 37). It seems unlikely that Doa1 plays a role with Cdc48 either during ERAD substrate extraction from the ER or during substrate recruitment to the proteasome, since under these circumstances doa1Δ yeast would be expected to present an ERAD phenotype. Last, this model is consistent with our genetic interaction data, in that impairment of Cdc48-mediated substrate delivery to the proteasome (cdc48-1) prevents ubiquitin loss and drug sensitivity in doa1Δ yeast.
Our results also demonstrate the functional conservation of the PLAA protein family. Ogiso et al. convincingly showed that PLAAs from two closely related lower eukaryotes, S. cerevisiae and S. pombe, were functional orthologues. Deletion of the S. pombe homologue, Lub1, results in a depletion of ubiquitin phenotype similar to that seen in S. cerevisiae, and DOA1 can compensate for LUB1 loss in S. pombe (33). Yet, aside from Cdc48/p97 interaction data, little mechanistic data exist regarding the functional conservation of the PLAA family of proteins in higher eukaryotes (40). A single member of this family of proteins exists in every eukaryotic species examined. Each of these homologues possesses identical domain structure: an N-terminal WD40 domain, a central PFU domain, and a C-terminal PUL domain. The fact that the members of the PLAA family share extensive sequence homology throughout these domains suggests each domain is under selective pressure. Our findings that the Doa1-PLAA chimera binds to ubiquitin and complements doa1Δ yeast suggest that functional conservation may extend throughout all eukaryotic lineages.
In conclusion, our results indicate that Doa1 directly binds to Cdc48 and possesses a novel ubiquitin binding domain, the PFU domain, therefore suggesting that Doa1 mediates an interaction between Cdc48 and some ubiquitinated protein(s). We offer genetic interaction data that support the hypothesis that this physical interaction is physiologically relevant. Furthermore, our data suggest functional conservation within the PLAA family of proteins. Given that we can now assign a function to every Doa1 domain except the WD40 domain (Fig. 6), understanding what protein(s) binds to its WD40 domain is critical to understanding the role of Doa1. Future studies designed to address this question may also shed some light onto the decade-old question of how and why ubiquitin is destabilized in doa1Δ yeast.
FIG. 6.
Proposed model of Doa1's functional domains and relevance to Cdc48 function. In this model, the Doa1 PFU domain binds to ubiquitinated substrates which are acted upon by Cdc48 via its interaction with the PUL domain of Doa1. Binding to the WD40 domain of Doa1 by an as-of-yet-unidentified protein may either contribute to the actions of Cdc48 or act as a regulator of the Cdc48-Doa1 interaction.
Acknowledgments
We thank members of the Wilkinson Lab for critical reading of the manuscript, Michel Ghislain (Universite Catholique de Louvain) for the HisFlag-Doa1 constructs, Arun Seth (University of Toronto) for the human PLAA clone, David Botstein (Stanford University School of Medicine) for the DBY469 and DBY1247 yeast cell lines, Mark Hochstrasser (Yale University) for the MHY501 yeast cell line, Dan Finley (Harvard University) for the ubiquitin expression vector, Judith Fridovich-Keil (Emory University School of Medicine) for the pYEPGAP vector, and Mark Longtine (Oklahoma State University) for the gene disruption vectors.
This work was supported by a postdoctoral fellowship from the American Heart Association and NIH grant R01-GM30308.
REFERENCES
- 1.Amerik, A. Y., J. Nowak, S. Swaminathan, and M. Hochstrasser. 2000. The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol. Biol. Cell 11:3365-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943-951. [DOI] [PubMed] [Google Scholar]
- 3.Bays, N. W., S. K. Wilhovsky, A. Goradia, K. Hodgkiss-Harlow, and R. Y. Hampton. 2001. HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol. Biol. Cell 12:4114-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty, B. G., S. Qi, M. Pienkowska, J. A. Herbrick, T. Scheidl, Z. M. Zhang, I. Kola, S. W. Scherer, and A. Seth. 1999. Chromosomal localization of phospholipase A2 activating protein, an Ets2 target gene, to 9p21. Genomics 62:529-532. [DOI] [PubMed] [Google Scholar]
- 5.Cao, K., R. Nakajima, H. H. Meyer, and Y. Zheng. 2003. The AAA-ATPase Cdc48/p97 regulates spindle disassembly at the end of mitosis. Cell 115:355-367. [DOI] [PubMed] [Google Scholar]
- 6.Chernova, T. A., K. D. Allen, L. M. Wesoloski, J. R. Shanks, Y. O. Chernoff, and K. D. Wilkinson. 2003. Pleiotropic effects of Ubp6 loss on drug sensitivities and yeast prion are due to depletion of the free ubiquitin pool. J. Biol. Chem. 278:52102-52115. [DOI] [PubMed] [Google Scholar]
- 7.Dalal, S., M. F. Rosser, D. M. Cyr, and P. I. Hanson. 2004. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol. Biol. Cell 15:637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decottignies, A., A. Evain, and M. Ghislain. 2004. Binding of Cdc48p to a ubiquitin-related UBX domain from novel yeast proteins involved in intracellular proteolysis and sporulation. Yeast 21:127-139. [DOI] [PubMed] [Google Scholar]
- 9.Dieckmann, T., E. S. Withers-Ward, M. A. Jarosinski, C. F. Liu, I. S. Chen, and J. Feigon. 1998. Structure of a human DNA repair protein UBA domain that interacts with HIV-1 Vpr. Nat. Struct. Biol. 5:1042-1047. [DOI] [PubMed] [Google Scholar]
- 10.Elkabetz, Y., I. Shapira, E. Rabinovich, and S. Bar-Nun. 2004. Distinct steps in dislocation of luminal endoplasmic reticulum-associated degradation substrates: roles of endoplasmic reticulum-bound p97/Cdc48p and proteasome. J. Biol. Chem. 279:3980-3989. [DOI] [PubMed] [Google Scholar]
- 11.Finley, D., S. Sadis, B. P. Monia, P. Boucher, D. J. Ecker, S. T. Crooke, and V. Chau. 1994. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Mol. Cell. Biol. 14:5501-5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frohlich, K. U., H. W. Fries, M. Rudiger, R. Erdmann, D. Botstein, and D. Mecke. 1991. Yeast cell cycle protein CDC48p shows full-length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene expression. J. Cell Biol. 114:443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J. M. Rick, A. M. Michon, C. M. Cruciat, M. Remor, C. Hofert, M. Schelder, M. Brajenovic, H. Ruffner, A. Merino, K. Klein, M. Hudak, D. Dickson, T. Rudi, V. Gnau, A. Bauch, S. Bastuck, B. Huhse, C. Leutwein, M. A. Heurtier, R. R. Copley, A. Edelmann, E. Querfurth, V. Rybin, G. Drewes, M. Raida, T. Bouwmeester, P. Bork, B. Seraphin, B. Kuster, G. Neubauer, and G. Superti-Furga. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 14.Ghislain, M., R. J. Dohmen, F. Levy, and A. Varshavsky. 1996. Cdc48p interacts with Ufd3p, a WD repeat protein required for ubiquitin-mediated proteolysis in Saccharomyces cerevisiae. EMBO J. 15:4884-4899. [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann-Petersen, R., M. Wallace, K. Hofmann, G. Koch, A. H. Johnsen, K. B. Hendil, and C. Gordon. 2004. The Ubx2 and Ubx3 cofactors direct Cdc48 activity to proteolytic and nonproteolytic ubiquitin-dependent processes. Curr. Biol. 14:824-828. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock, A. L., H. Krebber, S. Frietze, A. Lin, M. Latterich, and P. A. Silver. 2001. The conserved npl4 protein complex mediates proteasome-dependent membrane-bound transcription factor activation. Mol. Biol. Cell 12:3226-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochstrasser, M., and A. Varshavsky. 1990. In vivo degradation of a transcriptional regulator: the yeast alpha 2 repressor. Cell 61:697-708. [DOI] [PubMed] [Google Scholar]
- 18.Hu, M., P. Li, M. Li, W. Li, T. Yao, J. W. Wu, W. Gu, R. E. Cohen, and Y. Shi. 2002. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 111:1041-1054. [DOI] [PubMed] [Google Scholar]
- 19.Iyer, L. M., E. V. Koonin, and L. Aravind. 2004. Novel predicted peptidases with a potential role in the ubiquitin signaling pathway. Cell Cycle 3:1440-1450. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, E. S., P. C. Ma, I. M. Ota, and A. Varshavsky. 1995. A proteolytic pathway that recognizes ubiquitin as a degradation signal. J. Biol. Chem. 270:17442-17456. [DOI] [PubMed] [Google Scholar]
- 21.Kang, R. S., C. M. Daniels, S. A. Francis, S. C. Shih, W. J. Salerno, L. Hicke, and I. Radhakrishnan. 2003. Solution structure of a CUE-ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell 113:621-630. [DOI] [PubMed] [Google Scholar]
- 22.Keil, R. L., D. Wolfe, T. Reiner, C. J. Peterson, and J. L. Riley. 1996. Molecular genetic analysis of volatile-anesthetic action. Mol. Cell. Biol. 16:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley, L. A., R. M. MacCallum, and M. J. Sternberg. 2000. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J. Mol. Biol. 299:499-520. [DOI] [PubMed] [Google Scholar]
- 24.Koegl, M., T. Hoppe, S. Schlenker, H. D. Ulrich, T. U. Mayer, and S. Jentsch. 1999. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell 96:635-644. [DOI] [PubMed] [Google Scholar]
- 25.Kondo, H., C. Rabouille, R. Newman, T. P. Levine, D. Pappin, P. Freemont, and G. Warren. 1997. p47 is a cofactor for p97-mediated membrane fusion. Nature 388:75-78. [DOI] [PubMed] [Google Scholar]
- 26.Latterich, M., K. U. Frohlich, and R. Schekman. 1995. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell 82:885-893. [DOI] [PubMed] [Google Scholar]
- 27.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 28.Medicherla, B., Z. Kostova, A. Schaefer, and D. H. Wolf. 2004. A genomic screen identifies Dsk2p and Rad23p as essential components of ER-associated degradation. EMBO Rep. 5:692-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer, H. H., J. G. Shorter, J. Seemann, D. Pappin, and G. Warren. 2000. A complex of mammalian ufd1 and npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways. EMBO J. 19:2181-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer, H. H., Y. Wang, and G. Warren. 2002. Direct binding of ubiquitin conjugates by the mammalian p97 adaptor complexes, p47 and Ufd1-Npl4. EMBO J. 21:5645-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moir, D., S. E. Stewart, B. C. Osmond, and D. Botstein. 1982. Cold-sensitive cell-division-cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics 100:547-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moraes, T. F., R. A. Edwards, S. McKenna, L. Pastushok, W. Xiao, J. N. Glover, and M. J. Ellison. 2001. Crystal structure of the human ubiquitin conjugating enzyme complex, hMms2-hUbc13. Nat. Struct. Biol. 8:669-673. [DOI] [PubMed] [Google Scholar]
- 33.Ogiso, Y., R. Sugiura, T. Kamo, S. Yanagiya, Y. Lu, K. Okazaki, H. Shuntoh, and T. Kuno. 2004. Lub1 participates in ubiquitin homeostasis and stress response via maintenance of cellular ubiquitin contents in fission yeast. Mol. Cell. Biol. 24:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pornillos, O., S. L. Alam, R. L. Rich, D. G. Myszka, D. R. Davis, and W. I. Sundquist. 2002. Structure and functional interactions of the Tsg101 UEV domain. EMBO J. 21:2397-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabinovich, E., A. Kerem, K. U. Frohlich, N. Diamant, and S. Bar-Nun. 2002. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell. Biol. 22:626-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rape, M., T. Hoppe, I. Gorr, M. Kalocay, H. Richly, and S. Jentsch. 2001. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell 107:667-677. [DOI] [PubMed] [Google Scholar]
- 37.Richly, H., M. Rape, S. Braun, S. Rumpf, C. Hoege, and S. Jentsch. 2005. A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell 120:73-84. [DOI] [PubMed] [Google Scholar]
- 38.Russell, N. S., and K. D. Wilkinson. 2004. Identification of a novel 29-linked polyubiquitin binding protein, Ufd3, using polyubiquitin chain analogues. Biochemistry 43:4844-4854. [DOI] [PubMed] [Google Scholar]
- 39.Schuberth, C., H. Richly, S. Rumpf, and A. Buchberger. 2004. Shp1 and Ubx2 are adaptors of Cdc48 involved in ubiquitin-dependent protein degradation. EMBO Rep. 5:818-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seigneurin-Berny, D., A. Verdel, S. Curtet, C. Lemercier, J. Garin, S. Rousseaux, and S. Khochbin. 2001. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol. Cell. Biol. 21:8035-8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swaminathan, S., A. Y. Amerik, and M. Hochstrasser. 1999. The Doa4 deubiquitinating enzyme is required for ubiquitin homeostasis in yeast. Mol. Biol. Cell 10:2583-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanson, K. A., R. S. Kang, S. D. Stamenova, L. Hicke, and I. Radhakrishnan. 2003. Solution structure of Vps27 UIM-ubiquitin complex important for endosomal sorting and receptor downregulation. EMBO J. 22:4597-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchiyama, K., E. Jokitalo, F. Kano, M. Murata, X. Zhang, B. Canas, R. Newman, C. Rabouille, D. Pappin, P. Freemont, and H. Kondo. 2002. VCIP135, a novel essential factor for p97/p47-mediated membrane fusion, is required for Golgi and ER assembly in vivo. J. Cell Biol. 159:855-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.VanDemark, A. P., R. M. Hofmann, C. Tsui, C. M. Pickart, and C. Wolberger. 2001. Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell 105:711-720. [DOI] [PubMed] [Google Scholar]
- 45.Wang, B., S. L. Alam, H. H. Meyer, M. Payne, T. L. Stemmler, D. R. Davis, and W. I. Sundquist. 2003. Structure and ubiquitin interactions of the conserved zinc finger domain of Npl4. J. Biol. Chem. 278:20225-20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, Y., A. Satoh, G. Warren, and H. H. Meyer. 2004. VCIP135 acts as a deubiquitinating enzyme during p97-p47-mediated reassembly of mitotic Golgi fragments. J. Cell Biol. 164:973-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wojcik, C., M. Yano, and G. N. DeMartino. 2004. RNA interference of valosin-containing protein (VCP/p97) reveals multiple cellular roles linked to ubiquitin/proteasome-dependent proteolysis. J. Cell Sci. 117:281-292. [DOI] [PubMed] [Google Scholar]
- 48.Ye, Y., H. H. Meyer, and T. A. Rapoport. 2001. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414:652-656. [DOI] [PubMed] [Google Scholar]
- 49.Ye, Y., H. H. Meyer, and T. A. Rapoport. 2003. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J. Cell Biol. 162:71-84. [DOI] [PMC free article] [PubMed] [Google Scholar]