Abstract
Heregulins are a family of ligands for the ErbB3/ErbB4 receptors that play important roles in breast cancer cell proliferation and tumorigenesis. Limited information is available on the contribution of Rho GTPases to heregulin-mediated signaling. In breast cancer cells, heregulin β1 (HRG) causes a strong activation of Rac; however, it does so with striking differences in kinetics compared to epidermal growth factor, which signals through ErbB1 (epidermal growth factor receptor [EGFR]). Using specific ErbB receptor inhibitors and depletion of receptors by RNA interference (RNAi), we established that, surprisingly, activation of Rac by HRG is mediated not only by ErbB3 and ErbB2 but also by transactivation of EGFR, and it is independent of ErbB4. Similar receptor requirements are observed for HRG-induced actin cytoskeleton reorganization and mitogenic activity via extracellular signal-regulated kinase (ERK). HRG-induced Rac activation was phosphatidylinositol 3-kinase dependent and Src independent. Furthermore, inactivation of Rac by expression of the Rac GTPase-activating protein β2-chimerin inhibited HRG-induced ERK activation, mitogenicity, and migration in breast cancer cells. HRG mitogenic activity was also impaired by depletion of Rac1 using RNAi. Our studies established that Rac is a critical mediator of HRG mitogenic signaling in breast cancer cells and highlight additional levels of complexity for ErbB receptor coupling to downstream effectors that control aberrant proliferation and transformation.
The human ErbB/Her receptor family comprises four tyrosine kinase receptors (Her1/ErbB1 or epidermal growth factor receptor (EGFR), Her2/ErbB2, Her3/ErbB3, and Her4/ErbB4) that play important roles in the progression of various types of cancers, including breast, prostate, and colon cancer. It is well established that dysregulation of ErbB receptor signaling leads to enhanced cell proliferation, migration, and malignant transformation (22). Overexpression of ErbB2 is often associated with breast cancer progression, metastasis, and poor prognosis, and a blocking antibody for ErbB2 is widely used for breast cancer therapy. Overexpression of EGFR or ErbB3 is also correlated with reduced survival of breast cancer patients (35, 51, 54). In contrast, studies show that ErbB4 mediates antiproliferative and differentiation responses in breast cancer cells (42), and its expression is correlated with better survival in breast cancer patients (51). One of the features of ErbB receptors is their diverse coupling to signaling pathways that control mitogenicity as well as the progression and maintenance of the malignant phenotype. This is exemplified by the EGFR, which, upon binding of a specific ligand (such as epidermal growth factor [EGF] or transforming growth factor alpha), becomes activated by homodimerization and autophosphorylation and couples to multiple SH2 domain-containing adaptor molecules and effectors, including PLCγ, phosphatidylinositol 3-kinase (PI3K), Shc, and Grb2 (43). The four ErbB receptors differ in their pattern of phosphorylation sites (55) and thus couple to distinct (but overlapping) sets of downstream effectors. Diversity in ErbB signaling activation is further enhanced by combinatorial heterodimerization of the various receptors (55).
Heregulins (also called neuregulins) are a group of EGF-like ligands for the ErbB3 and ErbB4 receptors (13) and are often expressed in breast cancer tissues (11). Accumulating evidence indicates that heregulins increase breast cancer cell proliferation and promote tumorigenesis, aggressive and invasive phenotypes (3, 13). Moreover, blockade of heregulin expression inhibits tumorigenicity and metastasis of breast cancer cells (49). Heregulins activate PI3K-Akt and Erk mitogen-activated protein kinase (MAPK) in breast cancer cells (14, 34, 50), pathways that are critical in the mitogenic and tumorigenic effects of heregulins. The individual ErbB receptors and effectors responsible for MAPK activation by heregulins are a subject of intense investigation. Heregulins also promote marked changes in cytoskeleton reorganization accompanied by the formation of membrane ruffles, filopodia, and stress fibers, and they confer a motile phenotype (2). Thus, it is predictable that heregulin stimulation leads to the activation of Rho G proteins known to cause such phenotypic changes. Rac, one Rho family member, plays a major role in control of actin cytoskeleton but also controls cyclin expression, cell cycle progression, and malignant transformation (37, 39, 53). Some reports have shown Rac to be overexpressed or hyperactivated in breast cancer tissues (16, 44), and one Rac guanine nucleotide exchange factor (GEF) (Tiam1) is overexpressed in highly invasive breast tumors (1). Moreover, recent studies from our laboratory have demonstrated that inactivation of Rac by the Rac GTPase-activating protein (GAP) β2-chimerin inhibits breast cancer cell migration and proliferation, as well as actin cytoskeleton reorganization in response to growth factors (30, 53). The finding that Tiam1 activation by heregulin leads to a motile phenotype further points to Rac as a downstream player in heregulin signaling (1). However, while it is well established that EGF signaling activates Rho, Cdc42, and Rac, there is no direct evidence that heregulins activate Rho GTPases or of what the functional consequences of such activation might be. Given the complexities in ErbB receptor coupling to downstream effectors, one might expect differences in Rac regulation by heregulin relative to well-established paradigms, such as the EGFR- or PDGFRmediated activation of Rho GTPases.
In this paper we explore the activation of Rac by heregulin β1 (HRG) in breast cancer cell lines. Our objectives in this study were threefold. First, we wanted to determine whether HRG indeed promotes Rac activation. Our results reveal that HRG is a strong activator of Rac and show that the time course of this activation is markedly different from that seen with EGF. Second, we determined which ErbB receptors are involved in Rac activation by HRG. Studies using a wide range of pharmacological and molecular approaches revealed that ErbB2, ErbB3, and EGFR (but not ErbB4) are required for HRG-induced activation of Rac. Last, we established a functional link between HRG-induced activation of Rac and mitogenic signaling.
MATERIALS AND METHODS
Materials.
HRG was purchased from Lab Vision (Fremont, CA). EGF was obtained from Sigma (St. Louis, MO). Wortmannin and AG1478 were from LC Laboratories (Woburn, MA). PP2, U0126, and SP600125 were from Calbiochem (San Diego, CA). The EGFR blocking monoclonal antibody C225/cetuximab (Erbitux) was a generous gift from Kathryn M. Ferguson (University of Pennsylvania). Blocking antibodies for ErbB3 and ErbB4 were from Upstate Biotechnology (Lake Placid, NY).
Cell lines and cell culture.
Human breast cancer cell lines MCF-7 and T-47D were purchased from ATCC. MCF-7-Tet-on cells were purchased from Clontech (Palo Alto, CA) and cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and G418 (100 μg/ml) at 37°C in a humidified 5%- CO2 atmosphere. Generation of MCF-7 V12Rac1 cells was described elsewhere (53).
Generation of adenoviruses.
The generation of adenoviruses (AdVs) for β2-chimerin and LacZ (β2-chimerin-AdV and LacZ-AdV) was described elsewhere (53). Serum-starved (8 h) MCF-7 cells were infected with AdVs for 16 h. AdVs were then removed by extensive washing. Maximum expression was observed at 24 h and remained stable for at least three additional days. Experiments were performed 24 or 48 h after infection.
RNA interference (RNAi).
Small interfering RNA (siRNA) duplexes were purchased from Dharmacon Research, Inc. (Chicago, IL). The target sequences were as follows: AACCCCGAGGGCAAATACAGC (EGFR), AAGGTGCTTGGATCTGGCGCT (ErbB2), AAGAGACAGAGCTAAGGAAGC (ErbB3), and AATCCAGTGGAGGAGAACCCT (ErbB4). The siRNA sequences for Rac1 were as follows: AAGGAGATTGGTGCTGTAAAA (Rac1-RNAi1) and AACCTTTGTACGCTTTGCTCA (Rac1-RNAi2). As a control, duplexes used were either a green fluorescent protein duplex (Dharmacon, Inc.) or a random control siRNA duplex (AACATCGCTGTAGCATCGTCT). siRNA duplexes (100 to 200 nM) were transfected using Oligofectamine (Invitrogen) in serum-free medium, and after 4 h the medium was supplemented with 10% FBS. Twenty hours later, cells were subjected to 48 h of serum starvation followed by various designated treatments.
Western blot.
Cells were lysed using Tris-sodium dodecyl sulfate (SDS) as described by Yang et al. (53) and subjected to SDS-polyacrylamide gel electrophoresis (10 to 40 μg of protein/lane). The following antibodies were used: anti-Rac, anti-Cdc42, anti-ErbB3, and anti-ErbB4 (Upstate Biotechnology); anti-RhoA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); anti-hemagglutinin (anti-HA) tag, anti-phospho-tyrosine (p-Tyr-100), anti-EGFR, anti-phospho-EGFR (Tyr992), anti-Her2, anti-phospho-ErbB2 (Tyr1248), anti-phospho-ErbB3 (Tyr1289), anti-Src, anti-phospho-Src (Tyr416), anti-Akt, anti-phospho-Akt (Ser473), anti-Erk1/2, anti-phospho-Erk1/2 (Thr202/Tyr204), anti-c-Jun N-terminal protein kinase (JNK), anti-phospho-JNK (Thr183/Tyr185), and anti-phospho-ATF2 (Thr71) (Cell Signaling Technology, Beverly, MA); anti-β2-chimerin (53); and anti-β-actin (Sigma).
Pull-down assays.
After serum starvation (48 h), cells were stimulated with either HRG or EGF for different times. Rac GTP and Cdc42 GTP levels were determined with a pull-down assay using the p21-binding domain (PBD) of p21-activated kinase, as described previously (53), and using either anti-Rac or anti-Cdc42 antibodies for Western blot detection, respectively. RhoA-GTP levels were determined with a pull-down assay using the rhotekin binding domain (40) and an anti-RhoA antibody for Western blot detection.
IP assay.
After 48 h of serum starvation, cells were stimulated with HRG (10 ng/ml) for different times. Cells were lysed at 4°C for 10 min in 500 μl of buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, and protease inhibitor cocktail (1:500) (Sigma). After preclearing with Gammabind G-Sepharose (Pharmacia Biotech) for 2 h at 4°C, the supernatant was used for immunoprecipitation (IP) using 4 μg of anti-ErbB4 polyclonal antibody (2 h, 4°C). Gammabind G-Sepharose (40 μl) was then added (overnight, 4°C). Samples were resolved in an 8% SDS-polyacrylamide gel and analyzed by Western blotting.
Phalloidin staining.
After 48 h of serum starvation, cells cultured in cover slides were pretreated with various inhibitors or blocking antibodies for 1 h and then stimulated with either EGF (100 ng/ml, 5 min) or HRG (10 ng/ml, 10 min). Cells were washed twice with phosphate-buffered saline (PBS), fixed in 4% formaldehyde in PBS for 10 min, and then permeabilized using 0.1% Triton X-100 in PBS for 3 min. Cells were stained with phalloidin (Molecule Probes) in PBS containing 1% bovine serum albumin (BSA) (20 min, room temperature) and then counterstained with 4′,6′-diamidino-2-phenylindole (1 μg/ml, 20 min, 4°C). Cells were visualized with a Nikon TE2000-U fluorescence microscope.
Cell migration.
HRG-induced cell migration was determined using a Boyden Chamber (Neuro Probe, Inc., Gaithersburg, MD) according to instructions from the manufacturer. Briefly, after 8 h of serum starvation, cells were infected with AdVs at different multiplicities of infections (MOIs), washed, and serum starved for 24 h. Cells were then trypsinized and suspended in serum-free DMEM supplemented with 0.1% BSA. A polycarbonate filter with 12-μm pores (NeuroProbe) coated overnight with type IV collagen in cold PBS was placed on the lower-chamber wells filled with serum-free DMEM with or without HRG (20 ng/ml), and 2 × 105 cells were loaded into the upper-chamber wells. After incubation at 37°C in 5% CO2 for 5 h, the upper side of the filter was wiped free of cells and the filter was fixed and stained with Wright Giemsa staining buffer (Sigma). Migrating cells were counted under a phase-contrast microscope. For each treatment, at least four randomly selected high-magnification fields (10 × 20) were counted. Each treatment was performed in quadruplicate. To evaluate the effect of Rac1 depletion on HRG-induced cell migration, siRNA duplexes (100 nM) were transfected into MCF-7 cells as described above. After 48 h of serum starvation, cells were trypsinized and suspended in serum-free DMEM supplemented with 0.1% BSA for the cell migration experiment, as described above. Parallel samples were used to determine Rac1 expression by Western blotting.
BrdU incorporation.
5-Bromo-2′-deoxyuridine (BrdU) incorporation was determined using flow cytometry (53) 24 h after incubation with HRG (10 ng/ml).
Statistical analysis.
Data are presented as means ± standard deviations and were analyzed using either a Student t test or one-way analysis of variance with Scheffe's test. A P value of <0.05 was considered statistically significant.
RESULTS
Differential temporal activation of Rac by HRG and EGF in breast cancer cells.
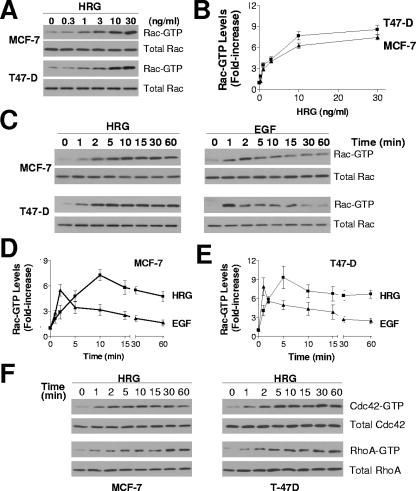
Two breast cancer cell lines, MCF-7 and T-47D, were used to examine if HRG activates Rac. Figure 1A shows that HRG triggered Rac activation in a dose-dependent manner. At 30 ng/ml, HRG caused 7.5-fold ± 0.6-fold and 8.6-fold ± 1.1-fold increases in Rac GTP levels in MCF-7 and T-47D cells, respectively (Fig. 1B). A time course analysis with MCF-7 cells revealed significant Rac activation at 2 min and maximum stimulation at 10 min. Rac GTP levels remained high (64% of maximum) 60 min after HRG stimulation (Fig. 1C and D). Sustained Rac activation by HRG was also observed in T-47D cells. In this case, maximum Rac activation was observed at 5 min, and Rac GTP levels remained elevated (72% of maximum) 60 min after stimulation (Fig. 1C and E). A comparison with EGF revealed striking differences in the kinetics of Rac activation. EGF-induced maximum activation of Rac occurred at earlier times than with HRG (1 min in T-47D cells and 2 min in MCF-7 cells). The effect of EGF was not as persistent as that caused by HRG, with EGF-induced Rac GTP levels dropping by ∼50% at 5 min and returning to near-basal levels at 30 to 60 min. The sustained activation of Rac by HRG was long lasting even after extensive washing with serum-free medium (data not shown). Thus, while both EGF and HRG strongly activate Rac in breast cancer cells, the activation by HRG is slightly slower and much more sustained. HRG also caused sustained activation of Cdc42 and RhoA (Fig. 1F).
FIG. 1.
HRG and EGF induce Rac/Cdc42/RhoA activation in MCF-7 and T-47D cells. (A) Dose-dependent activation of Rac by HRG. MCF-7 and T-47D cells were serum starved for 48 h and then stimulated with HRG (0 to 30 ng/ml) for 10 or 5 min, respectively. Rac GTP levels were determined using a PBD “pull-down” assay. (B) Densitometric analysis of Rac activation, normalized to the corresponding total Rac levels. Data are presented as means ± standard deviations (n = 3). (C) Time-dependent activation of Rac by HRG and EGF. Rac activation was determined in serum-starved cells after HRG (10 ng/ml) or EGF (100 ng/ml) treatment. (D and E) Densitometric analysis of time-dependent activation of Rac. Data are presented as means ± standard deviations (n = 3). (F) Cdc42 and RhoA activation by HRG (10 ng/ml) in MCF-7 and T-47D cells. Similar results were observed in three independent experiments.
ErbB3, ErbB2, and EGFR are all required for HRG-induced Rac activation.
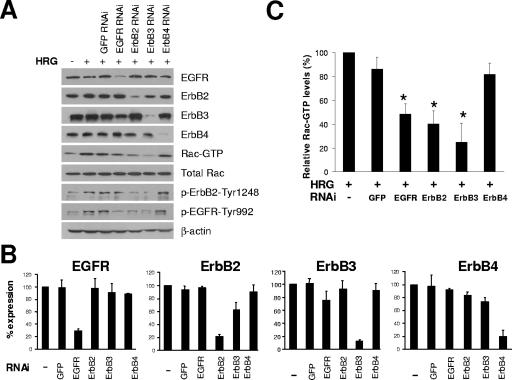
HRG is a specific ligand for ErbB3 and ErbB4, but it can also transactivate ErbB2 and/or EGFR through receptor heterodimerization (55). To define which ErbB receptors are involved in HRG-induced Rac activation, we used multiple approaches that include pharmacological inhibitors, ErbB receptor blocking antibodies, and RNAi for individual ErbB receptors. As shown in Fig. 2A and B, each ErbB receptor was successfully knocked down by >70% upon delivery of specific siRNA duplexes into T-47D cells. Interestingly, HRG-induced Rac activation was significantly blunted by depletion of ErbB3, ErbB2, or EGFR (Fig. 2A and C). RNAi depletion of either ErbB3 or ErbB2 led to a reduction in ErbB2 phosphorylation (activation), as expected if ErbB2/ErbB3 heterodimers form a major HRG receptor (9). More surprisingly, knock-down of ErbB2 as well as ErbB3 or EGFR reduced HRG-induced EGFR phosphorylation, whereas a reduction in EGFR expression only reduced the activation of EGFR, without obvious effect on ErbB2 phosphorylation. Remarkably, ErbB4 depletion had no effect on HRG-induced activation of Rac, ErbB2, or EGFR (Fig. 2A and C). Moreover, Rac activation by HRG could be blocked by an ErbB3 blocking antibody but not by an ErbB4 blocking antibody (data not shown). These results argue that ErbB2, ErbB3, and EGFR are all involved in HRG-induced Rac activation, whereas ErbB4 is not.
FIG. 2.
Effect of siRNA knock-down individual ErbB receptors on Rac activation by HRG. (A) siRNA duplexes for each ErbB receptor were transfected into T-47D cells. Twenty-four hours later, cells were serum starved for 48 h, and Rac activation was determined after stimulation with HRG (10 ng/ml, 5 min). ErbB2 and EGFR phosphorylation after HRG (10 ng/ml, 10 min) were analyzed by Western blotting using specific anti-phospho-ErbB2-Tyr1248 or anti-phospho-EGFR-Tyr992 antibody. Similar results were observed in three independent experiments. (B and C) Densitometric analysis of the effect of individual ErbB receptor knock-down and its effect on HRG-induced Rac activation, respectively, shown as percentages of expression relative to that for control (nontransfected) cells. Data are presented as means ± standard deviations (n = 3). *, P < 0.05, compared to results with nontransfected HRG-stimulated cells.
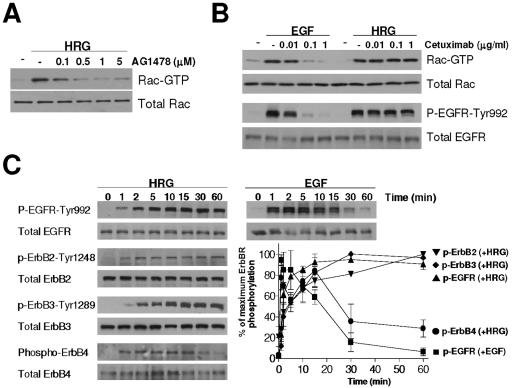
To further explore the EGFR requirement for HRG-induced Rac activation, we used the specific EGFR inhibitor AG1478. As shown in Fig. 3A, AG1478 impaired HRG-triggered Rac activation in a dose-dependent manner. Another EGFR tyrosine kinase inhibitor, Iressa (ZD1839), also completely blocked HRG-induced Rac activation (data not shown). Thus, inhibition of EGFR kinase activity blocks the ability of HRG to activate Rac. There are several possible explanations for this finding, which will be considered further in the Discussion.
FIG. 3.
Requirement of EGFR for HRG-induced Rac activation. (A) T-47D cells were serum starved for 48 h, incubated with different concentrations of AG1478 for 1 h, and then stimulated with HRG (10 ng/ml) for 5 min. Rac GTP levels were then assayed. (B) T-47D cells were serum starved for 48 h, pretreated with different concentrations of cetuximab for 1 h, and then stimulated with EGF (100 ng/ml for 1 min for the Rac GTP assay and 2 min for EGFR phosphorylation analysis) or HRG (10 ng/ml, 5 min for the Rac GTP assay and 10 min for EGFR phosphorylation analysis). Similar results were observed in three independent experiments. Panel C. After 48 h of serum starvation, T47D cells were treated with HRG (10 ng/ml) or EGF (100 ng/ml). Cell extracts were subjected to Western blot analysis using the indicated antibodies. ErbB4 phosphorylation was detected by IP. Inset. Densitometric analysis of ErbB receptor phosphorylation, expressed as a percentage of the maximum response in each case. Data are presented as means ± standard deviations (n = 3).
Immunoblotting with antibodies against phosphorylated forms of the ErbB receptors showed that HRG treatment induces phosphorylation of all family members (Fig. 3C), so understanding its signaling consequences requires consideration of the entire ErbB network. Importantly, the kinetics of HRG-induced receptor phosphorylation matched that of Rac activation only for ErbB2, ErbB3, and EGFR. Despite not playing any clear role in HRG-induced Rac activation, ErbB4 became significantly autophosphorylated upon HRG treatment but with a time course that was significantly less sustained than that of any other ErbB receptor (Fig. 3C). Interestingly, the kinetics of EGFR phosphorylation following HRG treatment was strikingly different from that seen following EGF stimulation. Whereas EGF promotes rapid and transient EGFR phosphorylation, HRG treatment of cells leads to slower and more sustained phosphorylation of the EGFR (Fig. 3C). To exclude the possibility that EGF agonists are responsible for (indirect) EGFR activation by HRG (e.g., through an autocrine loop), we showed that cetuximab, an anti-EGFR antibody that blocks the ligand binding site on the receptor (29), could not prevent HRG-induced EGFR and Rac activation, even at concentrations that completely block EGFR and Rac activation by saturating levels of EGF (Fig. 3B).
HRG-induced Rac activation is Src independent and PI3K dependent.
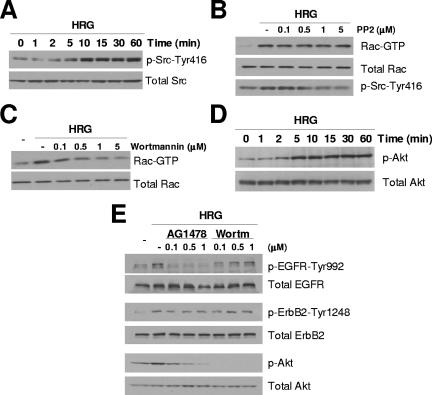
To understand the mechanism of Rac activation by HRG in breast cancer cells, we next assessed the roles of Src and PI3K, which are well-established effectors of the ErbB receptor network. Figure 4A shows that HRG triggers robust Src activation in T-47D cells, as determined using a specific anti-phospho-Src antibody. However, we find that this effect on Src is not required for Rac activation. Indeed, whereas HRG-induced Rac activation peaks at 5 min after HRG treatment (Fig. 1), the effect of HRG on Src activity is minimal at this time, peaking instead at 10 min after HRG addition. Moreover, the Src inhibitor PP2 had no effect on HRG-induced Rac activation even at concentrations that completely block Src phosphorylation (Fig. 4B). On the other hand, the PI3K inhibitor wortmannin completely blocked the HRG-induced elevation in Rac GTP levels (Fig. 4C). In T-47D cells, HRG strongly activates Akt (a PI3K effector), an effect that persisted for at least 60 min (Fig. 4D). EGF strongly activates Akt, although the effect lasted for less than 60 min (data not shown). As shown in Fig. 4E, wortmannin abolished the activation of Akt by HRG. Interestingly, the EGFR tyrosine kinase inhibitor AG1478 also impaired Akt activation by HRG. Neither AG1478 nor wortmannin significantly affected phosphorylation of ErbB2; and wortmannin did not inhibit EGFR activation (Fig. 4E). The inhibitors of EGFR and PI3K also prevented HRG-triggered Akt and Rac activation in MCF-7 cells (data not shown). Together, these results indicate that HRG activation of Rac in breast cancer cells is dependent on PI3K and also requires EGFR kinase activity but is independent of Src.
FIG. 4.
HRG-induced Rac activation is Src independent and PI3K dependent. (A and D) Time course of Src and Akt activation by HRG. T-47D cells were serum starved for 48 h and then treated with HRG (10 ng/ml). Cell extracts were subjected to Western blot analysis using specific anti-phospho-Src and anti-phospho-Akt antibodies. (B and C) After 48 h of serum starvation, T-47D cells were treated either with PP2 (0 to 5 μM) or wortmannin (0 to 5 μM) for 1 h and stimulated with HRG (10 ng/ml) for 5 min, and Rac GTP levels were then determined. (E) Effect of AG1478 and wortmannin (Wortm) pretreatment on the activation of EGFR, ErbB2, and Akt. After 48 h of serum starvation, T-47D cells were treated with either AG1478 or wortmannin for 1 h and stimulated with HRG (10 ng/ml) for 10 min. Cell extracts were subjected to Western blotting. Similar results were observed in three independent experiments.
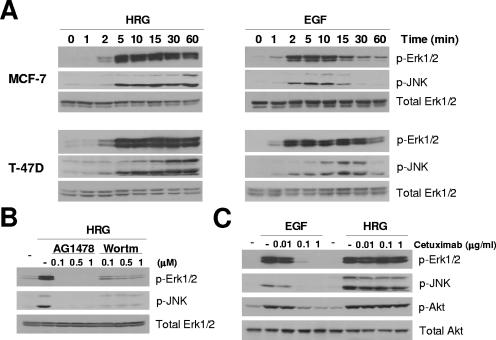
Rac is required for HRG-induced activation of Erk1/2 and JNK.
HRG activates MAPK cascades (14, 34), which are known effectors of Rac (12). As shown in Fig. 5A, HRG caused a marked activation of Erk1/2 and JNK, which was detectable within 2 to 5 min and remained sustained for at least 60 min in both MCF-7 and T-47D cells. On the other hand, EGF-induced activation of Erk1/2 and JNK was transient, peaking at 2 to 10 min and returning to basal levels within 30 to 60 min. The characteristic kinetics of Erk1/2 and JNK activation by each ligand strongly resembled the time course of Rac activation for that ligand shown in Fig. 1.
FIG. 5.
Differential kinetics of Erk1/2 and JNK activation by HRG and EGF. (A) Cells were serum starved for 48 h and then treated with either HRG (10 ng/ml) or EGF (100 ng/ml). Cell extracts were subjected to Western blot analysis using the indicated antibodies. (B) Effect of AG1478 and wortmannin (Wortm) on HRG-induced Erk1/2 and JNK activation in T-47D cells. Cells were treated as described in the legend to Fig. 4E, and cell extracts were subjected to Western blot analysis. (C) T-47D cells were serum starved for 48 h, pretreated with different concentrations of cetuximab for 1 h, and then stimulated with EGF (100 ng/ml, 2 min) or HRG (10 ng/ml, 10 min). Cell extracts were subjected to Western blot analysis. Similar results were observed in three independent experiments.
Activation of Erk1/2 and JNK by HRG was completely blocked by the EGFR kinase inhibitors AG1478 (Fig. 5B) and Iressa (data not shown), suggesting that the HRG-induced EGFR activation shown in Fig. 2A and 3B is required for this response. On the other hand, cetuximab did not affect HRG-induced activation of ERK, JNK, and Akt, although it completely impaired their activation by EGF (Fig. 5C). Wortmannin blocked Erk1/2 and JNK activation by HRG very efficiently (Fig. 5B), indicating that HRG-induced MAPK activation is also PI3K dependent. Similar results were observed with MCF-7 cells (data not shown).
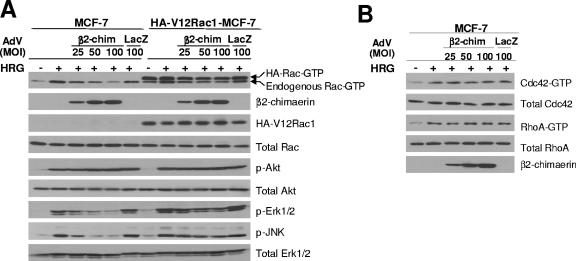
To further investigate the role of Rac in HRG-induced MAPK activation, we employed β2-chimerin, a Rac-specific GAP that inactivates Rac both in cell-free systems and in cells, including breast cancer cells (7, 53). HA-tagged β2-chimerin was delivered into MCF-7 cells using an adenoviral approach. As shown in Fig. 6A (lanes 1 to 6), expression of β2-chimerin in MCF-7 cells inhibited Rac activation by HRG. The effect was proportional to the expression level of β2-chimerin achieved by varying the MOIs of the β2-chimerin-AdV. A LacZ-AdV, on the other hand, was ineffective. Overexpressed β2-chimerin did not affect the levels of phospho-Akt induced by HRG treatment, arguing that PI3K/Akt activation by HRG occurs upstream of Rac. On the other hand, β2-chimerin significantly impaired HRG-induced activation of JNK and Erk1/2, arguing that these events are downstream of Rac activation. To confirm that the effects of β2-chimerin are Rac specific, we also assessed activation of Cdc42 and RhoA by HRG and found that these were unchanged even at high levels of β2-chimerin expression (Fig. 6B).
FIG. 6.
Inhibition of β2-chimerin on HRG-induced Rac, Erk1/2, and JNK activation. (A) MCF-7 and HA-V12Rac1-MCF-7 cells were serum starved for 8 h and then infected with either HA-β2-chimerin-AdV (β2-chim) or LacZ-AdV (LacZ) for 16 h in serum-free DMEM. After extensive washing, cells were grown for 24 h in serum-free DMEM and then stimulated with HRG (10 ng/ml) for 10 min. Activation of Rac, Akt, Erk1/2, and JNK was then determined. Expression of HA-β2-chimerin and HA-V12Rac1 was examined by Western blotting using an anti-HA antibody. (B) MCF-7 cells were treated as described for panel A. Cdc42-GTP and RhoA-GTP levels were determined using pull-down assays.
To further assess the Rac-MAPK link in our experimental model, we used MCF-7 cells stably expressing a constitutively active Rac1 mutant (HA-tagged V12Rac1). We hypothesized that cells retaining high levels of this active Rac mutant should be insensitive to the effect of the Rac GAP. Although we could readily detect V12Rac1 expression using an anti-HA antibody, total Rac levels remained basically unchanged, arguing that V12Rac1 was expressed at low levels compared with the endogenous wild-type protein, although it comprised the majority of the Rac GTP. As expected, HA-tagged Rac GTP levels remained high even after infection with the β2-chimerin-AdV (note that endogenous Rac GTP levels were reduced by β2-chimerin). Remarkably, expression of HA-tagged V12Rac1 largely overcame the inhibitory effect of β2-chimerin on HRG-induced Erk1/2 and JNK activation (Fig. 6A, lanes 7 to 12). Thus, Rac appears to be required for HRG-induced activation of Erk1/2 and JNK. An additional important observation is that Rac activation does not appear to be sufficient for Erk1/2 or JNK signaling, since levels of phospho-Erk or phospho-JNK were not elevated in cells expressing V12Rac1 in the absence of HRG treatment. Thus, our studies suggest that in breast cancer cells, Rac activation is necessary but not sufficient for the activation of these signaling pathways.
Rac dependence of HRG-induced cell migration and proliferation.
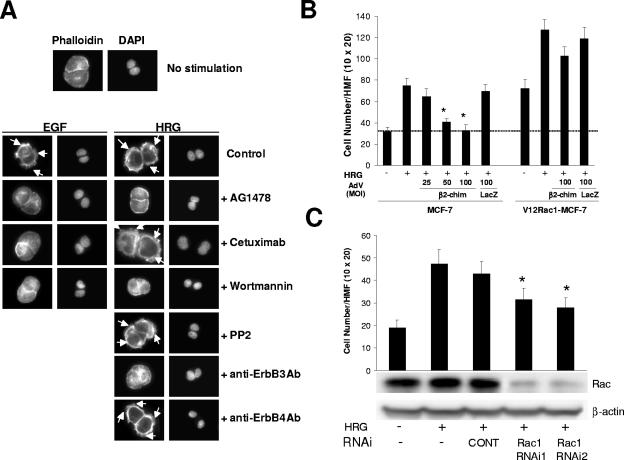
HRG is known to cause morphological changes, including the formation of filopodia and membrane ruffles (2). When T47-D cells were treated with either HRG or EGF, characteristic ruffles were observed (Fig. 7A), which is consistent with the activation of Rac. Inhibition of EGFR with AG1478 impaired not only ruffle formation caused by EGF but also the effect of HRG. On the other hand, cetuximab inhibited only the morphological changes caused by EGF, without affecting the HRG effect. Consistent with our signaling studies, the effect of HRG was also blocked by the anti-ErbB3 blocking antibody but was not affected by the anti-ErbB4 blocking antibody or PP2. Wortmannin completely inhibited ruffle formation caused by EGF and HRG. Thus, these results parallel those in which Rac GTP levels were determined. To further examine the biological significance of HRG-induced Rac activation, we also examined cell migration and proliferation. Consistent with its potent and sustained effect on Rac activation, HRG significantly enhanced MCF-7 cell migration, as determined with a Boyden chamber (Fig. 7B). Expression of the Rac GAP β2-chimerin significantly inhibited this effect. This inhibition was proportional to the expression levels of β2-chimerin. As expected, V12Rac1 significantly enhanced migration of MCF-7 cells. The expression of constitutively active Rac significantly overcame the inhibitory effect of β2-chimerin. Furthermore, HRG-induced cell migration was impaired by depletion of Rac1 using two different Rac1 siRNA duplexes (Fig. 7C).
FIG. 7.
β2-Chimerin inhibits HRG-induced MCF-7 cell migration. (A) Effects of inhibitors and blocking antibodies on EGF/HRG-induced ruffle formation. After 48 h of serum starvation, T-47D cells were treated with AG1478 (1 μM), Cetuximab (1 μg/ml), wortmannin (1 μM), PP2 (1 μM), ErbB3, or ErbB4 blocking antibody (10 μg/ml) for 1 h, stimulated with EGF (100 ng/ml, 5 min) or HRG (10 ng/ml, 10 min), and then stained with phalloidin. Ruffles are indicated by arrows. Similar results were observed in three independent experiments. (B) Effect of β2-chimerin on HRG-induced cell migration. Migration of MCF-7 or HA-V12Rac1-MCF-7 cells infected with HA-β2-chimerin-AdV or LacZ-AdV (see Fig. 6) was determined using a Boyden chamber. Data are presented as means ± standard deviations (n = 4). *, P < 0.05, compared to results for non-AdV-infected HRG-stimulated cells. (C) Effect of Rac1 depletion on HRG-induced cell migration. MCF-7 cells were transfected with siRNA duplexes for Rac1 (RNAi1 or RNAi2) or a control duplex (CONT), and cell migration was determined 72 h after transfection. Data are presented as means ± standard deviations (n = 4). *, P < 0.05, compared to results for nontransfected, HRG-stimulated cells. Rac expression is shown in a representative Western blot. HMF, high-magnification field.
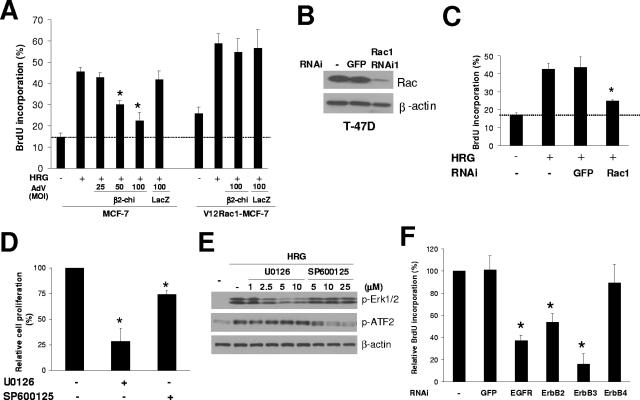
Rac is involved in proliferation control through the regulation of G1/S progression (37). We found that it plays an essential role in HRG stimulation of MCF-7 cell proliferation, as determined by BrdU incorporation (Fig. 8A). Expression of β2-chimerin dose-dependently inhibited the ability of HRG to stimulate proliferation. V12Rac1-expressing cells showed slightly higher levels of BrdU incorporation than control cells, and this was greatly further enhanced by treatment with HRG, in agreement with the finding in Fig. 6A that Rac activation is necessary but not sufficient for proliferative signaling. With V12Rac1 overexpression, β2-chimerin had no detectable inhibitory effect (Fig. 8A). The requirement for Rac in HRG-stimulated proliferation was further demonstrated using RNAi, with HRG-induced cell proliferation being significantly inhibited when Rac1 levels were depleted (Fig. 8B and C).
FIG. 8.
Effect of β2-chimerin, MAPK inhibitors, and siRNA knock-down of Rac1 and individual ErbB receptors on MCF-7 and T-47D cell proliferation. (A) HA-β2-chimerin-AdV- or LacZ-AdV-infected MCF-7 or HA-V12Rac1-MCF-7 cells were stimulated with HRG (10 ng/ml) for 24 h, and then BrdU incorporation was determined. Data are presented as means ± standard deviations (n = 3). *, P < 0.05, compared to results for non-AdV-infected HRG-stimulated cells. (B, C, and F) siRNA duplexes for Rac1 or each ErbB receptor were transfected into T-47D cells. Twenty-four hours later, cells were serum starved for 48 h and stimulated with HRG (10 ng/ml). BrdU incorporation was determined 24 h later. Data are presented as means ± standard deviations (n = 3). *, P < 0.05, compared to results for nontransfected HRG-stimulated cells. Panel B shows a representative Western blot for Rac1 knock-down. (D) After 48 h of serum starvation, T-47D cells were incubated with U0126 (5 μM) or SP600125 (25 μM) for 1 h and then stimulated with HRG (10 ng/ml) in the presence of the inhibitors. BrdU incorporation was determined 48 h later. (E) Phospho-Erk and phospho-ATF2 were analyzed by Western blotting after treatment with HRG (10 ng/ml, 10 min) in the presence of UO126 and SP600125.
In order to determine the contribution of MAPKs in HRG-induced mitogenesis, we used a MEK1 inhibitor (U0126) and a JNK inhibitor (SP600125). Figure 8D shows that U0126 inhibited HRG-induced cell proliferation in T-47D cells by 72%, while SP600125 had little effect. The activity and selectivity of the inhibitors for each pathway was confirmed by their ability to inhibit Erk1/2 phosphorylation (U0126) or ATF2 phosphorylation (SP600125) in response to HRG (Fig. 8E).
Last, we examined the effect of ErbB receptor siRNA on the proliferative response of HRG in T-47D cells. This analysis revealed that the HRG effect was dependent on ErbB3, ErbB2, and EGFR (Fig. 8F), which are the receptors required for Rac activation (Fig. 2). Taken together, these results suggest a critical role for multiple ErbB receptors and Rac activation in HRG-stimulated cell proliferation.
DISCUSSION
Our results reveal that HRG causes a strong and sustained activation of Rac and provide strong evidence that Rac is a critical component of the mitogenic and motile responses induced by HRG in breast cancer cells. While substantial evidence exists in support of a role for ErbB3 and ErbB2 as mediators of the mitogenic and oncogenic activities of heregulins, our results introduce a more complex paradigm, since HRG-mediated proliferation via Rac also depends on the EGFR (ErbB1) receptor. Remarkably, the ErbB4 receptor is not involved in Rac activation and mitogenicity, despite becoming significantly activated by HRG.
Multiple ErbB receptors mediate HRG-induced activation of Rac.
It is generally believed that heterodimers formed by ErbB2 and ErbB3 constitute the primary receptor for HRG and elicit potent mitogenic and oncogenic signals (9). These two members of the ErbB receptor family are unique. ErbB2 has no known direct ligand, and ErbB3 appears to have a catalytically inactive or substantially impaired tyrosine kinase domain. Thus, ErbB2 and ErbB3 are thought to require heterodimerization for signaling activity. Our results using RNAi and blocking antibodies clearly establish a requirement for both ErbB2 and ErbB3 in HRG-induced Rac activation, as anticipated. On the other hand, ErbB4 was found to be dispensable for HRG-induced Rac activation and proliferation, despite being efficiently activated by HRG (as evidenced by its autophosphorylation). This finding is consistent with reports that ErbB4 has only a weak capacity to mediate proliferative signals or promotes antiproliferative responses to HRG (8, 42).
More surprisingly, our studies suggest an essential (rather than accessory) role for EGFR in HRG signaling in breast cancer cells. EGFR RNAi or inhibition of its tyrosine kinase activity with AG1478 (or Iressa) blocked the ability of HRG to activate Rac, Akt, Erk1/2, and JNK or to promote cell proliferation. The fact that HRG promotes robust tyrosine phosphorylation of EGFR (Fig. 3C) argues that this is a direct effect and that EGFR activation plays a key role in mediating the HRG response. Several other studies have demonstrated EGFR activation by HRG in cells expressing multiple ErbB receptors (10, 41, 56), although there are reports in which this was not seen, including some studies with T-47D cells (18). The mechanism of EGFR involvement in HRG signaling in our studies is of interest. HRG treatment leads to phosphorylation of ErbB2, ErbB3, and EGFR, and each of these receptors plays an important role in Rac activation and cell proliferation. HRG is thought to stabilize (and activate) ErbB2/ErbB3 heterodimers. We initially hypothesized that EGFR activation could result from its heterodimerization with HRG-bound ErbB3. However, cetuximab had no effect at all on HRG-induced EGFR phosphorylation (Fig. 3B), despite the fact that this antibody prevents EGFR from adopting its dimerization (homo- or hetero-)-competent configuration (29). The failure of cetuximab to inhibit HRG-induced EGFR activation also rules out autocrine mechanisms, since this antibody directly occludes the ligand binding site in EGFR. Our results therefore suggest a mechanism for “transactivation” of EGFR in this system that differs from the view of ErbB receptor heterodimerization suggested by recent structural studies (5). It has alternatively been suggested that blockade of ErbB2/ErbB3 heterodimer signaling by EGFR kinase inhibitors can result from sequestration of ErbB2 in inactive EGFR/ErbB2 heterodimers and a consequent dominant-negative effect (33). We suggest that this mechanism is not relevant in our studies. The robust HRG-induced phosphorylation of EGFR (dependent on the presence of ErbB2 and ErbB3) argues for a positive role for EGFR in HRG signaling, as does the fact that RNAi knock-down of EGFR significantly impaired Rac activation and cell proliferation in response to HRG.
One possible mechanism for EGFR activation by HRG is that HRG-activated ErbB2 or ErbB3 forms “secondary” heterodimers with EGFR, as suggested by Gamett et al. (17), and these differ in structure from EGFR dimers observed crystallographically (so are unaffected by cetuximab). Alternatively, HRG could promote the formation of heterotetramers that include EGFR, ErbB2, and ErbB3, leading to phosphorylation of all three receptors through mechanisms that have yet to be defined (21, 43). A third possibility that is consistent with the failure of cetuximab to have an inhibitory effect is that EGFR becomes activated simply as a substrate for phosphorylation by activated ErbB2 in a manner similar to the JAK2-mediated EGFR phosphorylation promoted by growth hormone (52).
Differential kinetics of Rac activation by HRG and EGF.
A particularly striking observation, illustrated in Fig. 1 and 3C, is that HRG stimulates the activation of EGFR and Rac with a different time course from that seen with EGF-induced activation. The sustained Rac activation seen with HRG tracks precisely with the time course of EGFR phosphorylation. By contrast, EGF causes a rapid and short-lived activation of Rac, an effect that we have also observed in other models, including COS-1, HeLa, and colon cancer cells (unpublished studies). The sustained Rac activation by HRG in breast cancer cells predicted a prominent role for this small GTPase in heregulin signaling. Indeed, interfering with Rac signaling, either by Rac RNAi or by the expression of the specific Rac GAP β2-chimerin, significantly impaired HRG-induced activation of Rac as well as Rac-dependent responses. β2-chimerin accelerates GTP hydrolysis from Rac, leading to its inactivation, and markedly impairs Rac-dependent signaling, motility, and proliferation in various cellular models including breast cancer cells (6, 30, 53).
The contrasting time courses of Rac activation in response to HRG and EGF stimulation could reflect the distinct internalization characteristics of different ErbB receptor dimers. EGFR that has been activated by EGF-induced homodimerization is rapidly internalized and targeted to the lysosome, and this explains the transient activation seen in Fig. 3B. By contrast, EGFR that has been activated through heterodimerization with other ErbB receptors (or possibly by other mechanisms) is internalized less efficiently (4) and/or is more readily recycled (28). These effects may be responsible for the different time courses for Rac activation seen in Fig. 1.
Rac as a mediator of HRG mitogenic signaling.
The requirement of Rac and other Rho GTPases for mitogenic signaling has been defined in earlier studies (26, 37). Rac plays a crucial role in G1/S transition through the control of cyclin D1 expression (24, 38). Growth factor-induced activation of cyclin D1 expression in breast cancer cells is dependent on Rac and is highly sensitive to the effect of the Rac GAP β2-chimerin (53). Here we observed that inhibition of Rac by β2-chimerin or Rac depletion using RNAi markedly inhibits BrdU incorporation in response to HRG, thus placing Rac as an essential mediator of HRG mitogenic signaling.
The inhibition of HRG-induced BrdU incorporation by the MEK-1 inhibitor UO126 supports the involvement of ERK activation in the HRG proliferative response, which is in agreement with previous studies (34, 50). While multiple ErbB receptors have been found to activate ERKs through the Ras-Raf pathway (31, 45), a distinctive aspect of our studies is that they underscore the absolute requirement of the EGFR in ERK activation in response to HRG. ERK activation by HRG was completely blocked by treatment with AG1478. While the mechanistic basis by which Rac modulates the activation of MAPK cascades is beyond the goals of these studies, our results using V12Rac1 (Fig. 6A) argue that in breast cancer cells Rac is required but is not sufficient for ERK (and JNK) activation. The fact that HRG treatment is needed even in V12Rac1-expressing cells to cause ERK activation and to promote the maximum proliferative response instead suggests a cooperative role for Rac signaling with inputs from the Ras cascade. Indeed, recent studies with smooth muscle cells show that the association of the Rac effector p21-activated kinase with ERK and Raf facilitates ERK signal transduction (47). Further studies would be required to understand the nature of those events in the context of HRG stimulation in breast cancer cells.
Recent studies have shown that Src is a mediator of Rac activation in response to various stimuli (25, 46). Consistent with other reports, we also observed Src activation by HRG, although it is delayed by comparison with Rac activation, and inhibition of Src by PP2 did not affect HRG-induced Rac activation. Thus, in this particular cellular context, Src is not involved in Rac activation by HRG. Instead, we found that Rac activation by HRG is PI3K dependent. ErbB receptor coupling to PI3K probably involves multiple mechanisms: while ErbB3 can recruit the p85 regulatory subunit of PI3K directly via an SH2-dependent mechanism (20, 36), PI3K activation by EGFR is primarily mediated by the docking protein Gab1 (32). A role for Gab proteins in ErbB3-mediated activation of PI3K has also been described recently (23). PI3K can activate Tiam1 and Vav Rac GEFs (15, 19), and activated Ras and Tiam1 can cooperate to activate Rac in a PI3K-independent manner (27). To our knowledge the activation of Rac GEFs by ErbB2 and ErbB3 has not been reported. On the other hand, Vav exchange activity is stimulated in response to EGF through a PI3K-dependent mechanism (48), which would fit well with our paradigm of HRG-induced activation of Rac signaling via EGFR. Interestingly, Tiam1 becomes activated in response to HRG, leading to a motile phenotype (1). We determined that HRG not only caused Rac activation but also activated other Rho GTPases, such as Cdc42 and RhoA. Moreover, the PI3K inhibitor wortmannin also dose-dependently and efficiently inhibited Cdc42 activation by HRG (data not shown), suggesting that HRG is capable of activating multiple GEFs and/or GEFs with specificity for multiple Rho GTPases.
Final remarks.
The results presented here establish that HRG is a strong activator of Rac in breast cancer cells and that there is an absolute requirement for Rac in HRG-induced breast cancer cell proliferation. With the exception of ErbB4, all of the ErbB family members are required for HRG-induced Rac activation. Indeed, the requirement of EGFR for HRG-induced Rac activation and Rac-mediated responses, including mitogenesis, argues for an additional level of receptor cross talk in HRG signaling. Given the implications of ErbB receptors in cancer progression, the identification of Rac as a key transducer of HRG mitogenic and motogenic signaling highlights a role for this Rho GTPase in breast tumorigenesis. Since inactivation of ErbB receptors represents a promising strategy for cancer treatment, including treatment for breast cancer, our results may have great implications for understanding the mechanistic basis of the action of targeted ErbB receptor therapy and for considering additional signaling pathways to target in combination.
Acknowledgments
This work was supported by grants RO1-CA74197 (NIH) and RPG-97-092-06-CNE (American Cancer Society) to M.G.K. and RO1-CA096768 (NIH) to M.A.L. C.Y. is supported by a postdoctoral fellowship from the Department of Defense (DAMD17-03-1-0469).
We thank Richard K. Assoian and Margaret M. Chou (University of Pennsylvania) for their generous gifts of plasmids, Kathryn M. Ferguson (University of Pennsylvania) for her kind gift of cetuximab, and Marc Symons (North Shore-LIJ Research Institute, NY) for providing Rac1 RNAi sequence.
REFERENCES
- 1.Adam, L., R. K. Vadlamudi, P. McCrea, and R. Kumar. 2001. Tiam1 overexpression potentiates heregulin-induced lymphoid enhancer factor-1/beta-catenin nuclear signaling in breast cancer cells by modulating the intercellular stability. J. Biol. Chem. 276:28443-28450. [DOI] [PubMed] [Google Scholar]
- 2.Adam, L., R. Vadlamudi, S. B. Kondapaka, J. Chernoff, J. Mendelsohn, and R. Kumar. 1998. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 273:28238-28246. [DOI] [PubMed] [Google Scholar]
- 3.Atlas, E., M. Cardillo, I. Mehmi, H. Zahedkargaran, C. Tang, and R. Lupu. 2003. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol. Cancer Res. 1:165-175. [PubMed] [Google Scholar]
- 4.Baulida, J., M. H. Kraus, M. Alimandi, P. P. Di Fiore, and G. Carpenter. 1996. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem. 271:5251-5257. [DOI] [PubMed] [Google Scholar]
- 5.Burgess, A. W., H. S. Cho, C. Eigenbrot, K. M. Ferguson, T. P. Garrett, D. J. Leahy, M. A. Lemmon, M. X. Sliwkowski, C. W. Ward, and S. Yokoyama. 2003. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell 12:541-552. [DOI] [PubMed] [Google Scholar]
- 6.Caloca, M. J., H. Wang, A. Delemos, S. Wang, and M. G. Kazanietz. 2001. Phorbol esters and related analogs regulate the subcellular localization of beta 2-chimaerin, a non-protein kinase C phorbol ester receptor. J. Biol. Chem. 276:18303-18312. [DOI] [PubMed] [Google Scholar]
- 7.Canagarajah, B., F. Coluccio Leskow, J. Y. Ho, H. Mischak, L. F. Saidi, M. G. Kazanietz, and J. H. Hurley. 2004. Structural mechanism for lipid activation of the Rac-specific GAP, beta2-chimaerin. Cell 119:407-418. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter, G. 2003. ErbB-4: mechanism of action and biology. Exp. Cell Res. 10:66-77. [DOI] [PubMed] [Google Scholar]
- 9.Citri, A., K. B. Skaria, and Y. Yarden. 2003. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp. Cell Res. 10:54-65. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, B. D., J. M. Green, L. Foy, and H. P. Fell. 1996. HER4-mediated biological and biochemical properties in NIH 3T3 cells. Evidence for HER1-HER4 heterodimers. J. Biol. Chem. 271:4813-4818. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, M., P. Sinha, R. Campbell, E. Blackburn, N. Levinson, R. Rampaul, T. Bates, S. Humphreys, and W. J. Gullick. 2004. Co-expression of neuregulins 1, 2, 3 and 4 in human breast cancer. J. Pathol. 203:672-680. [DOI] [PubMed] [Google Scholar]
- 12.Eblen, S. T., J. K. Slack, M. J. Weber, and A. D. Catling. 2002. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell. Biol. 22:6023-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falls, D. L. 2003. Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res. 284:14-30. [DOI] [PubMed] [Google Scholar]
- 14.Fiddes, R. J., P. W. Janes, S. P. Sivertsen, R. L. Sutherland, E. A. Musgrove, and R. J. Daly. 1998. Inhibition of the MAP kinase cascade blocks heregulin-induced cell cycle progression in T-47D human breast cancer cells. Oncogene 16:2803-2813. [DOI] [PubMed] [Google Scholar]
- 15.Fleming, I. N., I. H. Batty, A. R. Prescott, A. Gray, G. S. Kular, H. Stewart, and C. P. Downes. 2004. Inositol phospholipids regulate the guanine-nucleotide-exchange factor Tiam1 by facilitating its binding to the plasma membrane and regulating GDP/GTP exchange on Rac1. Biochem. J. 382:857-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritz, G., I. Just, and B. Kaina. 1999. Rho GTPases are over-expressed in human tumors. Int. J. Cancer 81:682-687. [DOI] [PubMed] [Google Scholar]
- 17.Gamett, D. C., G. Pearson, R. A. Cerione, and I. Friedberg. 1997. Secondary dimerization between members of the epidermal growth factor receptor family. J. Biol. Chem. 272:12052-12056. [DOI] [PubMed] [Google Scholar]
- 18.Graus-Porta, D., R. R. Beerli, J. M. Daly, and N. E. Hynes. 1997. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 16:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han, J., K. Luby-Phelps, B. Das, X. Shu, Y. Xia, R. D. Mosteller, U. M. Krishna, J. R. Falck, M. A. White, and D. Broek. 1998. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279:558-560. [DOI] [PubMed] [Google Scholar]
- 20.Hellyer, N. J., M. S. Kim, and J. G. Koland. 2001. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J. Biol. Chem. 276:42153-42161. [DOI] [PubMed] [Google Scholar]
- 21.Huang, G. C., X. Ouyang, and R. J. Epstein. 1998. Proxy activation of protein ErbB2 by heterologous ligands implies a heterotetrameric mode of receptor tyrosine kinase interaction. Biochem. J. 331:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes, N. E., and H. A. Lane. 2005. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5:341-354. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, J. G., P. St Clair, M. X. Sliwkowski, and M. G. Brattain. 2004. Blockade of epidermal growth factor- or heregulin-dependent ErbB2 activation with the anti-ErbB2 monoclonal antibody 2C4 has divergent downstream signaling and growth effects. Cancer Res. 64:2601-2609. [DOI] [PubMed] [Google Scholar]
- 24.Joyce, D., B. Bouzahzah, M. Fu, C. Albanese, M. D'Amico, J. Steer, J. U. Klein, R. J. Lee, J. E. Segall, J. K. Westwick, C. J. Der, and R. G. Pestell. 1999. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J. Biol. Chem. 274:25245-25249. [DOI] [PubMed] [Google Scholar]
- 25.Kawakatsu, T., H. Ogita, T. Fukuhara, T. Fukuyama, Y. Minami, K. Shimizu, and Y. Takai. 2005. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J. Biol. Chem. 280:4940-4947. [DOI] [PubMed] [Google Scholar]
- 26.Lamarche, N., N. Tapon, L. Stowers, P. D. Burbelo, P. Aspenstrom, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87:519-529. [DOI] [PubMed] [Google Scholar]
- 27.Lambert, J. M., Q. T. Lambert, G. W. Reuther, A. Malliri, D. P. Siderovski, J. Sondek, J. G. Collard, and C. J. Der. 2002. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat. Cell Biol. 4:621-625. [DOI] [PubMed] [Google Scholar]
- 28.Lenferink, A. E., R. Pinkas-Kramarski, M. L. van de Poll, M. J. van Vugt, L. N. Klapper, E. Tzahar, H. Waterman, M. Sela, E. J. van Zoelen, and Y. Yarden. 1998. Differential endocytic routing of homo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 17:3385-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, S., K. R. Schmitz, P. D. Jeffrey, J. J. Wiltzius, P. Kussie, and K. M. Ferguson. 2005. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7:301-311. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzano-Menna, P., G. Skilton, F. Coluccio Leskow, D. F. Alonso, D. E. Gomez, and M. G. Kazanietz. 2003. Inhibition of aggressiveness of metastatic mouse mammary carcinoma cells by the beta2-chimaerin GAP domain. Cancer Res. 63:2284-2291. [PubMed] [Google Scholar]
- 31.Marshall, C. J. 1994. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4:82-89. [DOI] [PubMed] [Google Scholar]
- 32.Mattoon, D. R., B. Lamothe, I. Lax, and J. Schlessinger. 2004. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moulder, S. L., F. M. Yakes, S. K. Muthuswamy, R. Bianco, J. F. Simpson, and C. L. Arteaga. 2001. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 61:8887-8895. [PubMed] [Google Scholar]
- 34.Neve, R. M., T. Holbro, and N. E. Hynes. 2002. Distinct roles for phosphoinositide 3-kinase, mitogen-activated protein kinase and p38 MAPK in mediating cell cycle progression of breast cancer cells. Oncogene 21:4567-4576. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson, R. I., J. M. Gee, and M. E. Harper. 2001. EGFR and cancer prognosis. Eur. J. Cancer 37:S9>-S15. [DOI] [PubMed] [Google Scholar]
- 36.Olayioye, M. A., R. M. Neve, H. A. Lane, and N. E. Hynes. 2000. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19:3159-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson, M. F., A. Ashworth, and A. Hall. 1995. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science 269:1270-1272. [DOI] [PubMed] [Google Scholar]
- 38.Page, K., J. Li, J. A. Hodge, P. T. Liu, T. L. Vanden Hoek, L. B. Becker, R. G. Pestell, M. R. Rosner, and M. B. Hershenson. 1999. Characterization of a Rac1 signaling pathway to cyclin D(1) expression in airway smooth muscle cells. J. Biol. Chem. 274:22065-22071. [DOI] [PubMed] [Google Scholar]
- 39.Qiu, R. G., J. Chen, D. Kirn, F. McCormick, and M. Symons. 1995. An essential role for Rac in Ras transformation. Nature 374:457-459. [DOI] [PubMed] [Google Scholar]
- 40.Ren, X. D., and M. A. Schwartz. 2000. Determination of GTP loading on Rho. Methods Enzymol. 325:264-272. [DOI] [PubMed] [Google Scholar]
- 41.Riese, D. J., II, T. M. van Raaij, G. D. Plowman, G. C. Andrews, and D. F. Stern. 1995. The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol. Cell. Biol. 15:5770-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sartor, C. I., H. Zhou, E. Kozlowska, K. Guttridge, E. Kawata, L. Caskey, J. Harrelson, N. E. Hynes, S. Ethier, B. Calvo, and H. S. Earp III. 2001. Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol. Cell. Biol. 21:4265-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 44.Schnelzer, A., D. Prechtel, U. Knaus, K. Dehne, M. Gerhard, H. Graeff, N. Harbeck, M. Schmitt, and E. Lengyel. 2000. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 19:3013-3020. [DOI] [PubMed] [Google Scholar]
- 45.Seger, R., and E. G. Krebs. 1995. The MAPK signaling cascade. FASEB J. 9:726-735. [PubMed] [Google Scholar]
- 46.Servitja, J. M., M. J. Marinissen, A. Sodhi, X. R. Bustelo, and J. S. Gutkind. 2003. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J. Biol. Chem. 278:34339-34346. [DOI] [PubMed] [Google Scholar]
- 47.Sundberg-Smith, L. J., J. T. Doherty, C. P. Mack, and J. M. Taylor. 2005. Adhesion stimulates direct PAK1/ERK2 association and leads to ERK-dependent PAK1 Thr212 phosphorylation. J. Biol. Chem. 280:2055-2064. [DOI] [PubMed] [Google Scholar]
- 48.Tamas, P., Z. Solti, P. Bauer, A. Illes, S. Sipeki, A. Bauer, A. Farago, J. Downward, and L. Buday. 2003. Mechanism of epidermal growth factor regulation of Vav2, a guanine nucleotide exchange factor for Rac. J. Biol. Chem. 278:5163-5171. [DOI] [PubMed] [Google Scholar]
- 49.Tsai, M. S., L. A. Shamon-Taylor, I. Mehmi, C. K. Tang, and R. Lupu. 2003. Blockage of heregulin expression inhibits tumorigenicity and metastasis of breast cancer. Oncogene 22:761-768. [DOI] [PubMed] [Google Scholar]
- 50.Vijapurkar, U., M. S. Kim, and J. G. Koland. 2003. Roles of mitogen-activated protein kinase and phosphoinositide 3′-kinase in ErbB2/ErbB3 coreceptor-mediated heregulin signaling. Exp. Cell Res. 284:291-302. [DOI] [PubMed] [Google Scholar]
- 51.Witton, C. J., J. R. Reeves, J. J. Going, T. G. Cooke, and J. M. Bartlett. 2003. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J. Pathol. 200:290-297. [DOI] [PubMed] [Google Scholar]
- 52.Yamauchi, T., K. Ueki, K. Tobe, H. Tamemoto, N. Sekine, M. Wada, M. Honjo, M. Takahashi, T. Takahashi, H. Hirai, T. Tushima, Y. Akanuma, T. Fujita, I. Komuro, Y. Yazaki, and T. Kadowaki. 1997. Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature 390:91-96. [DOI] [PubMed] [Google Scholar]
- 53.Yang, C., Y. Liu, F. Coluccio Leskow, V. M. Weaver, and M. G. Kazanietz. 2005. Rac-GAP-dependent inhibition of breast cancer cell proliferation by beta2-chimaerin. J. Biol. Chem. 280:24363-24370. [DOI] [PubMed] [Google Scholar]
- 54.Yarden, Y. 2001. Biology of HER2 and its importance in breast cancer. Oncology 61:1-13. [DOI] [PubMed] [Google Scholar]
- 55.Yarden, Y., and M. X. Sliwkowski. 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell. Biol. 2:127-137. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, K., J. Sun, N. Liu, D. Wen, D. Chang, A. Thomason, and S. K. Yoshinaga. 1996. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J. Biol. Chem. 271:3884-3890. [PubMed] [Google Scholar]