Abstract
Adipose differentiation-related protein (ADFP; also known as ADRP or adipophilin), is a lipid droplet (LD) protein found in most cells and tissues. ADFP expression is strongly induced in cells with increased lipid load. We have inactivated the Adfp gene in mice to better understand its role in lipid accumulation. The Adfp-deficient mice have unaltered adipose differentiation or lipolysis in vitro or in vivo. Importantly, they display a 60% reduction in hepatic triglyceride (TG) and are resistant to diet-induced fatty liver. To determine the mechanism for the reduced hepatic TG content, we measured hepatic lipogenesis, very-low-density lipoprotein (VLDL) secretion, and lipid uptake and utilization, all of which parameters were shown to be similar between mutant and wild-type mice. The finding of similar VLDL output in the presence of a reduction in total TG in the Adfp-deficient liver is explained by the retention of TG in the microsomes where VLDL is assembled. Given that lipid droplets are thought to form from the outer leaflet of the microsomal membrane, the reduction of TG in the cytosol with concomitant accumulation of TG in the microsome of Adfp−/− cells suggests that ADFP may facilitate the formation of new LDs. In the absence of ADFP, impairment of LD formation is associated with the accumulation of microsomal TG but a reduction in TG in other subcellular compartments.
Adipose differentiation-related protein (ADFP) was first isolated by differential hybridization screening of 1246 cells during their differentiation to adipocytes (29). Its mRNA is induced 100 fold during the process. Using 3T3L1 cells, Brasaemle et al. (3) showed that Adfp gene expression is induced early, at day 1 of adipocyte differentiation, and that mRNA levels are maintained throughout differentiation. In contrast, ADFP protein levels, initially upregulated, gradually go down after day 4 (3), suggesting that these levels are subject to significant translational or posttranslational regulation. At the same time, upregulation of perilipin (PLIN), another lipid droplet (LD) protein, is observed at day 4 of differentiation; it has been postulated that perilipin and ADFP might compete for LD localization during the differentiation of 3T3L1 cells (3, 36). ADFP protein is localized to the surface of the LD, though it also has been detected in the LD core by freeze fracture electron microscopy (49, 50).
ADFP shares sequence homology with other LD proteins including perilipin and Tip47, collectively known as PAT domain-containing proteins (36, 43), as well as with another LD protein, S3-12 (53). S3-12 shares a 11-amino-acid repeat motif with ADFP, in addition to another region of homology to both ADFP and Tip47 at its carboxyl terminus (6, 24, 36). With the exception of perilipin, which is expressed only in fat and steroidogenic tissues, the other LD proteins are detected in a variety of cells and tissues (22). Perilipin is phosphorylated by protein kinase A during lipolysis, resulting in an altered conformation to allow hormone-sensitive lipase (10, 58, 61) and other lipases (73) to act on the LD. Consistent with this finding, perilipin-deficient mice exhibit elevated basal lipolysis, reduced fat mass, and resistance to diet-induced and genetic obesity (40, 62). In contrast, ADFP appears not to be phosphorylated (15) but is acylated (23), which may contribute to its association with LDs.
Although the function of ADFP is not fully understood, some studies have suggested that it plays a role in fatty acid (FA) transport. Gao and colleagues transfected Adfp to COS-7 cells, which do not normally express Adfp, and observed that induced ADFP expression stimulated long-chain FA uptake (16, 17). Similarly, Imamura et al. showed that adenovirus-mediated overexpression of ADFP induced lipid accumulation in murine fibroblasts without changes in the level of expression of lipogenic genes (26). Serrero's group found that ADFP was associated with the plasma membrane of COS-7 cells (16, 17), a physical location consistent with its putative role in fatty acid transport, whereas others have observed an intracellular instead of a plasma membrane location of ADFP (26, 42). On the other hand, freeze fracture electron microscopy, combined with immunogold labeling, has demonstrated that ADFP and other PAT family proteins are an integral part of the plasma membrane (49) and the LD core in cells that are cultured under high-lipid conditions.
Adfp expression is induced during differentiation of adipocytes and other specialized cells such as keratinocytes and trophoblasts (1, 55), upon lipid loading, or under pathological conditions in other tissues and cells (5, 11, 13, 22, 54, 60, 66). Adfp expression is upregulated in 1246 cells by long-chain but not short-chain FAs (17). Since FAs have been implicated as ligands for members of the nuclear receptor transcription factor family, the induction of the Adfp gene may be mediated through one or more of these pathways. Indeed, all three peroxisome proliferator-activated receptor (PPAR) subtypes (alpha, delta, and gamma) have been reported to increase murine and human Adfp expression (1, 9, 31, 63, 65).
To study the function of the Adfp gene in adipocyte differentiation, fatty acid transport, and LD formation, we have created ADFP-deficient mice by gene targeting. We found that absence of ADFP reduces the amount of triglyceride (TG) in the liver without affecting plasma lipid profile. It also protects against diet-induced fatty liver. Significantly, however, it does not affect adipocyte differentiation, fat mass, or body weight.
MATERIALS AND METHODS
Chemicals and reagents.
G418 and tissue culture media were purchased from Invitrogen. Ganciclovir (Cytovene-IV) was a gift from Roche. Lipid standards for thin-layer chromatography (TLC) analysis were obtained from Avanti Polar Lipids, Inc. Intralipid was purchased from Sigma. [3H]oleic acid was purchased from BP Biomedicals, [14C]palmitic acid was purchased from Pharmacia, and [14C]oleoyl-coenzyme A (CoA) was purchased from Perkin-Elmer, Inc. All other chemicals were purchased from Sigma Chemical Co. Primary antibodies were purchased from BD Biosciences (FA synthase [FAS]), Chemicon (histone and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]), Progen (adipophilin and Tip47), and RDI (perilipin). Mouse microsomal triglyceride transfer protein (MTP) antibody was one that we have used previously (8).
Generation of Adfp-targeted mice.
We isolated mouse genomic Adfp DNAs by screening a 129-strain lambda genomic library (Stratagene). Two overlapping clones encompassing exons 1 to 4 were used to construct a replacement targeting vector. A neo cassette (β-geo) was inserted into a genomic region which replaced exons 2 and 3 and part of the intronic regions of Adfp (Fig. 1A). A thymidine kinase cassette was ligated to the 3′ end of the construct for selection against random insertion events.
FIG. 1.
ADFP gene targeting. (A) ADFP partial genomic structure, gene targeting construct, and expected homologous recombinant allele. Restriction enzyme sites: A, AccI; B, BamHI; E, EcoRI; X, XbaI; and Xh, XhoI. (B) Southern blot genotyping of the wild type (+/+) and heterozygous (+/−), and homozygous (−/−) knockouts. (C) Northern blot analysis of RNA from four different tissues (L, liver; M, muscle; (B) brain; and W, white adipose tissue) in wild-type and homozygous knockouts. (D) Western blot of total protein extracted from wild-type and homozygous knockout livers.
We used an R1 embryonic stem (ES) cell-line (from Andras Nagy at the University of Toronto), passage 14, to generate Adfp-inactivated mice as described previously (21). Nine ES cell clones were injected into blastocysts of C57BL/6J mice to generate recombinant chimeric mice. After germ line transmission was confirmed by Southern blot analysis, Adfp-targeted mice were backcrossed to C57BL/6J mice for 8 generations.
Animals.
Mice were maintained in a temperature-controlled facility with fixed 12-h-light and 12-h-dark cycles and free access to regular chow and water. Some experiments were done on animals fed with a high-fat diet (HFD; Teklad TD88137; 42% kcal from fat and 4.54 kcal/g of body weight) for 4 weeks. Animals of 8 to 12 weeks old were used throughout this study unless otherwise indicated.
MEF cell experiments.
We isolated five to eight 13.5-day-old mouse embryos, removed the heads and viscera, washed and minced them, and collected the minced pieces into a syringe containing 3 ml of 0.25% trypsin. We forced the tissues through an 18-gauge needle into a 10-cm tissue culture plate. After a 5-min incubation at 37°C, we collected the mouse embryonic fibroblast (MEF) cells by centrifugation and seeded them to six-well plates at a density of 5 × 105 cells/well. We initiated the differentiation protocol 2 days after cells reached 100% confluence, as described previously (26).
Oil Red O staining of differentiated EF cells was done by fixing the day 8 cells in formalin for 5 min before staining them with filtered Oil Red O solution prepared in triethyl phosphate (Fluka Chemie) solution (30).
Lipolysis in vitro and in vivo.
We determined the concentration of triglyceride, glycerol, and nonesterified FAs (NEFA) in day 8 differentiated MEF cells with enzymatic kits (Wako). To measure triglyceride, we extracted total lipid using methanol and chloroform according to Bligh and Dyer (2). To determine lipolysis in vitro, after washing the cells twice with phosphate-buffered saline (PBS), we incubated them in serum-free medium in the presence or absence of 10 μM isoprotenerol. A total of 100 μl of media was collected at various time points for glycerol and NEFA levels. For in vivo lipolysis, we fasted the mice for 4 h and treated them with an intraperitoneal injection of the β3 adrenergic receptor agonist CL 316,243 (0.1 mg per kg of body weight) or isoproterenol (10 mg per kg). We collected blood from the orbital plexus before and 15 min after injection for determination of NEFA and glycerol levels.
Plasma lipid analysis.
We collected blood from the orbital plexus under isoflurane (Vedco) anesthesia. Serum was frozen in aliquots and stored at −20°C. We used enzymatic assay kits for determination of serum NEFA, glycerol, glucose, cholesterol, and total triglyceride (Sigma) levels. Serum insulin was measured by enzyme-linked immunosorbent assay (Crystal Chem). Serum β-hydroxybutyrate was measured by enzymatic assay in a buffer containing 100 mM Tris-HCl (pH 8.5), 30 mM MgCl2, 20 mM oxalic acid, 2 mM EDTA, 0.4 mg β-NAD, and 0.025 U of β-hydroxybutyrate dehydrogenase. The change in optical density at 340 nm due to reduction of NAD to NADH was monitored in a 96-well plate reader under kinetic measurement setting for 10 readings at 1-min intervals at 37°C. d,l-Hydroxybutyrate standards were used for standardization.
Liver lipid analysis.
We homogenized liver tissues in 5 to 10 ml of standard PBS buffer per gram of tissue, extracted lipids from half of the homogenate according to Bligh and Dyer (2), and determined the total lipid content gravimetrically. To fractionate the different lipid species, we extracted total lipids from the remaining homogenate after addition of known amounts of l-α-phosphatidyl choline diheptadecanoyl (C17:0 phospholipid [PL]), triheptadecanoin (C17:0 triglyceride), heptadecanoic acid (C17:0 free fatty acid [FFA]), and cholesteryl heptadecanoate (C17:0 cholesteryl ester) as internal standards. Total lipids were dissolved in 0.2 ml chloroform and the PL, TG, free fatty acid, and cholesteryl ester fractions were separated by one-dimensional thin-layer chromatography (Silica Gel 60), using hexane-diethyl ether-glacial acetic acid (75:35:1). Methyl esters of all fractions, including the total lipid fraction, were prepared with boron trifluoride-methanol (44) and quantified by gas liquid chromatography (Hewlett-Packard 5890 gas chromatograph) on a DB-225 capillary column (J&W Scientific). Fatty acids were identified by comparison to the retention times of fatty acid methyl ester standards.
Determination of rate of VLDL secretion in vivo.
We quantified the rate of very-low-density lipoprotein (VLDL) secretion in vivo as described previously (8). Briefly, we injected Triton WR1339, a lipoprotein lipase inhibitor, intravenously and monitored the plasma triglycerides at 1, 2, 3, and 4 h afterwards.
Triglyceride clearance assay.
We injected 0.25 ml of intralipid (Sigma) through the tail vein of mice and measured the plasma TG concentration every 30 min for 4 h.
Fatty acid uptake by isolated hepatocytes in vitro.
We measured the rate of FA uptake in isolated hepatocytes as previously described (47). Mice were anesthetized and perfused through the portal vein with 10 ml of perfusion medium (Invitrogen), followed by 20 ml of collagenase (0.1 mg/ml; type IV from Sigma). We then dispersed the hepatocytes in a sterile petri dish and washed them with 20 ml of hepatocyte wash medium (Invitrogen). Dead and aggregated cells were separated from isolated cells by Percoll (Amersham Biosciences) density gradient centrifugation. The pelleted cells were weighed and resuspended in PBS (0.1 g/ml), and viable cells were counted by trypan blue exclusion assay. This procedure routinely resulted in a >95% viability of hepatocytes.
To determine oleate acid uptake, we added 200 μl of cell suspension to 200 μl oleate-bovine serum albumin (BSA) buffer solution containing 40 μM of oleic acid (ICN), 10 μM BSA, and 5 μCi/ml [3H]oleate in PBS. At 0, 15, 30, 60, and 120 s afterward, we added to the solution 5 ml of buffer containing 0.1% of BSA and 500 μM phloretin in PBS to the mixture to stop the assay. The entire mixture was immediately run through a glass fiber filter under low vacuum (<10 mm Hg) to collect the cells. The glass fiber was placed in a vial with scintillation fluid to count radioactivity. A blank vial and a vial with an aliquot of oleate-BSA buffer solution were used as negative and positive controls, respectively. Duplicate samples were collected and measured. The assays done at time zero were used as the background uptake activity and subtracted from the activity measured at each time point. The final values were corrected for cell viability, determined after hepatocyte isolation.
β-Oxidation in isolated hepatocytes.
We measured β-oxidation in freshly isolated heptocytes as previously described (52). Cells (5 × 105) were incubated for 1 h in buffer containing carnitine, NAD, ATP, cytochrome c, MgCl2, coenzyme A, and [14C]palmitic acid complexed with fatty acid-free bovine serum albumin. The reaction was stopped with 60% perchloric acid, and the released CO2 was trapped by hyamine hydroxide at the top of a hanging center well. We measured the radioactivity in the CO2 with a β-scintillation counter. We added epinephrine (1 μg/ml) to a portion of the samples for maximum stimulation of β-oxidation.
Lipogenesis in vivo.
In vivo lipogenesis was measured by tritiated water labeling as previously described (37, 59). Briefly, mice were trained to feed during 3-h periods from 8 to 11 a.m. every day for 2 weeks. On the day of the experiment, after a 3-h feeding, they were fasted for 1 h before receiving an intraperitoneal injection of 2 mCi of tritiated water. We sacrificed them after they were maintained without food or water for another hour. We removed the liver and used a 0.3-g piece for lipid extraction. The tissue was minced to small pieces and incubated in 2 ml of saponification solution containing 45% KOH-H2O-70% ethanol (2:1:5) at 80°C for 3 h. After saponification, 5 ml of water was added, and the mixture was extracted three times with 8 ml of petroleum ether. The top petroleum-soluble phase was pooled and dried under a fume hood before digitonin was added to precipitate cholesterol for quantitative analysis. The bottom alkaline aqueous phase contained saponified FA. It was acidified by the addition of 1.25 ml of 10 N H2SO4 followed by three extractions with petroleum ether. The pooled petroleum-soluble phase was dried in a hood and dissolved in chloroform for analysis by TLC and radioactivity measurement.
Lipogenic enzyme assays.
We determined FAS activity by measuring the decrease in NADPH absorption at 340 nm as previously described (28). Briefly, liver tissue was homogenized in a buffer containing 0.25 M sucrose, 20 mM potassium phosphate (pH 7.0), 1 mM EDTA, 1 mM dithiothreitol, and a protease inhibitor cocktail (Roche). After centrifugation, the proteins in the supernatant were precipitated with 40% ammonium sulfate, and the pellet was reconstituted with buffer for the assay with 100 μg of protein.
Diacylglycerol acyltransferase (DGAT) activities were determined as previously described (7). sn-1,2-Dioleoylglycerol was used as acceptor and 1-[14C]oleoyl-CoA was used as an acyl donor (25,000 cpm/nmol). The reaction mixture contained 175 mM Tris-HCl (pH 7.5), 1-mg/ml BSA (fatty acid free), 16 mM MgCl2, 0.4 mM diacylglycerol, 40 μM oleoyl-CoA, and 100 μg microsomal protein in a volume of 0.25 ml. The reaction mixture was incubated for 5 min at 37°C. The lipids were extracted as described above, dried, and then dissolved in chloroform containing trioleoylglycerol as the standard. The samples were subjected to thin-layer chromatography and developed in hexan-diethylether-acetic acid (70:29:1; vol/vol/vol) for visualization and quantitation of the radioactive TG spots.
Northern blot and Western blot analysis.
We isolated RNA using Trizol reagent (Invitrogen) treatment of freshly isolated tissues or tissues that had been snap frozen in liquid N2. The RNA (20 μg) was separated by denaturing formamide gel electrophoresis and transferred to a nylon membrane (Amersham Biosciences) for UV cross-linking and Northern blot analysis. We generated hybridization probes by reverse transcription-PCR. Hybridization was performed in Ultrahyb solution (Ambion, Inc.), and a fluorographic image was obtained using a Cyclone membrane (Packard). For Western blotting, equivalent amounts of protein homogenate were resolved by 4 to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE), transferred to a polyvinylidene difluoride membrane, and probed with specific antibodies for visualization by enhanced chemiluminescence (SuperSignal kit; Pierce).
Histology.
We removed the liver and fixed a piece in formalin overnight before dehydration and paraffin embedding. Five-micrometer-thin sections were obtained, and the tissue was stained with hematoxylin and eosin. LD size distributions were obtained with SigmaScan Pro 8.0 software after contrast enhancement with Adobe Photoshop 7.0.
Statistical analysis.
The Student t test was used for statistical analysis. Differences were considered significant when P values were <0.05. Results were expressed as means ± standard deviations.
RESULTS
Generation of ADFP-deficient mice.
We used a replacement targeting construct (Fig. 1A), to transfect R1 ES cells (46) as previously described (8). After G418 and ganciclovir selection, 93 cell clones were screened by Southern blot analysis, and about 20% were found to be targeted cell clones. Nine of the clones were amplified and injected into blastocysts isolated from C57BL/6J mice, and these were implanted into foster C57BL/6J female mice. Chimeric male mice were bred to C57 BL/6J females to generate F1 offspring. Heterozygous F1 mice were identified by Southern blot analysis (Fig. 1B). Complete knockout of ADFP in homozygous mice was confirmed by Northern (Fig. 1C) and Western (Fig. 1D) blotting. Comparison of the phenotype revealed no difference between lines; one of the lines was maintained and backcrossed to C57BL/6J mice through eight generations.
Adfp knockout mice were born live and were no different from wild-type mice in terms of their body weight, growth curves, and food consumption (Table 1). They also had normal plasma glucose, insulin, and lipid profiles (Table 2 and Table 3).
TABLE 1.
Body and organ weights (in grams)
| Mouse line | Gender (n) | Body wt | Liver | Perigonadal WAT | Interscapular BAT |
|---|---|---|---|---|---|
| Adfp+/+ | Male (11) | 20.51 ± 0.75 | 0.93 ± 0.16 | 0.22 ± 0.06 | 0.057 ± 0.009 |
| Female (4) | 19.78 ± 1.53 | 0.88 ± 0.10 | 0.18 ± 0.05 | 0.063 ± 0.004 | |
| Adfp−/− | Male (11) | 21.13 ± 1.62 | 1.03 ± 0.17 | 0.25 ± 0.07 | 0.052 ± 0.016 |
| Female (4) | 20.33 ± 1.22 | 0.87 ± 0.06 | 0.24 ± 0.02 | 0.054 ± 0.013 |
TABLE 2.
Basic glucose and lipid metabolic variables in Adfp+/+ and Adfp−/− mice
| Mouse line (n) | Plasma (4-h fasting)
|
|||
|---|---|---|---|---|
| Glucose (mg/dl) | Insulin (ng/ml) | Cholesterol (mg/dl) | Triglyceride (mg/dl) | |
| Adfp+/+ (6) | 141.5 ± 17.8 | 0.82 ± 0.45 | 80 ± 8 | 73 ± 24 |
| Adfp−/− (6) | 153.8 ± 24.3 | 1.13 ± 0.95 | 77 ± 8 | 67 ± 27 |
TABLE 3.
Hepatic lipid metabolic variables in Adfp+/+ and Adfp−/− mice
| Mouse line (n) | Liver variable (mg/g tissue)
|
||
|---|---|---|---|
| Triglyceridea | NEFA | Phospholipids | |
| Adfp+/+ (4) | 14.71 ± 0.22* | 4.19 ± 0.69 | 31.94 ± 1.13 |
| Adfp−/− (4) | 6.28 ± 1.18* | 3.47 ± 0.75 | 32.77 ± 1.01 |
*, significantly different between Adrp+/+ and Adrp−/− mice (P < 0.01).
Absence of ADFP does not affect adipocyte differentiation in vitro or adipogenesis in vivo.
To determine if ADFP plays a significant role in fat cell differentiation as suggested by multiple investigators and implied by its namesake (29, 43, 71), we analyzed adipocyte differentiation in vitro using MEFs. At day 2 of the differentiation scheme, numerous lipid droplets appeared in cells from both wild-type and knockout mice. There was no discernible difference in the timing of LD appearance or the size of the LDs.
We used Northern blot analysis to monitor the expression profile of known markers of adipocyte differentiation. Wild-type and Adfp−/− cells shared similar timing of appearance of transcripts for aP2, PPAR-γ, and CCAAT/enhancer binding protein alpha (C/EBP-α) (Fig. 2A). At day 0, all three transcripts were undetectable, but PPAR-γ and C/EBP-α mRNAs were detected at day 2, reaching a peak at day 5. aP2 mRNA did not appear until after day 2. C/EBP-δ expression was detected throughout the course of differentiation and was highest at day 2 (Fig. 2A).
FIG. 2.
Adipocyte differentiation of primary MEFs and in vitro lipolysis. Primary fibroblasts were isolated from embryos (embryonic day, 13.5) of Adfp+/+ and Adfp−/− mice for induction of adipocyte differentiation, as described in Materials and Methods. (A) Northern blot analysis of expression of various adipocyte differentiation marker genes using RNA isolated from day 0 (D0) to day 8 (D8) of differentiation. (B) Cellular triglyceride content of fully differentiated cells isolated at day 8. (C and D) Basal [ISO(−)]- and isoprotenerol [ISO(+)]-induced in vitro lipolysis measured by NEFA (C) and glycerol (D) in the media from fully differentiated MEF-derived adipocytes. (E and F) In vivo lipolysis measured using Adfp+/+ and Adfp−/− mice (three mice per group) as plasma NEFA (E) or glycerol (F) concentrations under basal (CL−) or CL-316243 (β3-adrenergic receptor agonist; CL+)-stimulated conditions.
We measured the TG content of the fully differentiated adipocytes at day 8 and found no difference between wild-type cells (0.134 ± 0.067 mg/dl/gram of cellular protein) and Adfp−/− cells (0.125 ± 0.041 mg/dl/gram of cellular protein) (Fig. 2B). These in vitro data suggest that absence of ADFP does not affect adipocyte differentiation.
Finally, we observed no difference in the mass of white adipose tissue (WAT) and brown adipose tissue (BAT) in Adfp−/− and Adfp+/+ mice (Table 1) or in the histological appearance of fat cells and size of the LDs in WAT and BAT of the two genotypes (data not shown), indicating that the absence of ADFP does not affect adipose tissue differentiation and growth in vivo.
Absence of ADFP does not affect basal and isoproterenol-stimulated lipolysis in in vitro-differentiated adipocytes.
The PAT domain protein PLIN is known to regulate lipolysis, but it is not clear if ADFP shares a similar function. We measured lipolysis under basal conditions and after β-adrenergic stimulation of differentiated MEF adipocytes. Under basal conditions, there was low but steady secretion of NEFA and glycerol from wild-type and Adfp−/− adipocytes (Fig. 2C and D). Upon isoproterenol stimulation, NEFA release increased linearly until it plateaued at 2 h (Fig. 2C). The release of glycerol had a similar pattern, although with greater variability (Fig. 2D). There was no significant difference in either basal or isoproterenol-stimulated lipolysis between wild-type and Adfp−/− adipocytes, both showing an approximately threefold increase in rate of NEFA release following isoproterenol treatment.
The absence of ADFP does not affect basal and β-adrenergic agonist-stimulated lipolysis in Adfp−/− mice in vivo.
We examined the plasma NEFA and glycerol as a measure of lipolysis in wild-type and Adfp−/− mice under basal conditions and 15 min after β-adrenergic stimulation (40). Fasting basal plasma NEFA and glycerol levels were similar in Apfp−/− mice and their wild-type littermates. Administration of CL 316,243, a β3-adrenergic agonist, led to a two- to threefold simulation of NEFA and glycerol, without any significant difference in response between wild-type and Adfp−/− mice (Fig. 2E). Taken together, these results from MEF-derived adipocytes in vitro and from whole animals in vivo indicate that ADFP does not play a significant role in lipolysis in adipose tissue.
Expression of other lipid droplet protein genes in Adfp−/− mice.
Silencing of a gene may elicit compensatory cellular responses through upregulation of genes sharing overlapping functions. We examined the expression of other LD protein genes in response to the loss of Adfp by Northern blot analysis. In adipose tissue, the level of perilipin mRNA was similar in wild-type and Adfp−/− mice (Fig. 3A). We did not detect perilipin mRNA in liver or muscle tissues following overnight autoradiography, although trace but detectable expression in muscle, heart, thymus, and liver after prolonged exposure has been reported by another group (12).
FIG. 3.
PAT domain protein gene expression in Adfp+/+ (+/+) and Adfp−/− (−/−) mice. (A) Northern blot analysis. A total of 20 μg of total RNA isolated from WAT, BAT, muscle, and liver was separated on a 1% agarose gel; the RNAs were transferred to a nylon membrane and probed with cDNA of perilipin (PlinA), Tip47, S3-12, and Adfp. (B) Western blot analysis. A total of 20 μg of total protein extracts isolated from WAT, BAT, muscle, and liver was separated by polyacrylamide gel electrophoresis (PAGE) using a 4 to 15% gradient gel; the proteins were transferred to a nylon membrane and detected using antisera raised against perilipin and Tip47 and enhanced chemiluminescence kits.
We examined Tip47 mRNA expression in WAT, BAT, liver, and muscle by Northern blotting but were unable to detect any significant difference in the level of expression in Adfp−/− mice compared to wild-type mice (Fig. 3A). S3-12 mRNA expression was essentially undetectable in liver and muscle but was evident in adipose tissue. As with Tip47, the S3-12 transcript expression level was not different between wild-type and Adfp−/− mice (Fig. 3A).
We further examined the expression of PLIN and Tip47 protein by Western blot analysis. PLIN A and B protein levels are similar in WAT and BAT between wild-type and Adfp−/− mice (Fig. 3B). Although Tip47 is lower in BAT, liver, and muscle of Adfp−/− mice, Tip47 protein expression overall was only slightly affected by the absence of ADFP in most of the tissues examined, with a slight increase in the WAT of the Adfp−/− mice (Fig. 3B).
Lack of ADFP markedly reduces intrahepatic lipid content.
Fatty liver induction causes ADFP to accumulate in the liver (22); however, it is unclear if the appearance of ADFP represents a compensatory response or an unrelated paraphenomenon or if the protein plays an active role in the process. In an attempt to further understand the role of ADFP in lipid homeostasis in the liver, we compared the weight and lipid content of the liver isolated from Adfp−/− and Adfp+/+ mice. We found that liver weight was similar between the two groups of mice fed a regular chow (Table 1). Under these conditions, the liver of the Adfp−/− mice and that of wild-type littermates on a chow diet appeared normal on histological examination (data not shown). We extracted total lipids from the livers of these animals and found that the total lipid mass was much lower (P < 0.01) in the Adfp−/− (47.85 ± 2.73 mg/g of tissue) than in the wild-type (58.80 ± 0.83 mg/g of tissue) liver. This difference was accounted for mainly by a 57% (P < 0.01) reduction of TG in the Adfp−/− liver (Table 3). There was no significant difference in hepatic NEFA, cholesterol, cholesteryl ester, or PL content (Table 3; see also Fig. 5A). Gas chromatography analysis of fatty acid composition in hepatic NEFA and PL did not show any difference between wild-type and Adfp−/− (see Fig. S1 and S2 in the supplemental material). The concentration of almost every species of FA in TG of Adfp−/− was significantly lower than those of the wild-type mice (Fig. 4A and B). Importantly, however, the proportion of each FA to total FA in TG remained the same between Adfp−/− and wild-type mice (Fig. 4C and D), indicating that every species of fatty acid in the TG was reduced proportionately in the Adfp−/− mouse liver. Interestingly, plasma TG concentrations were similar in Adfp−/− and Adfp+/+ mice (Table 2).
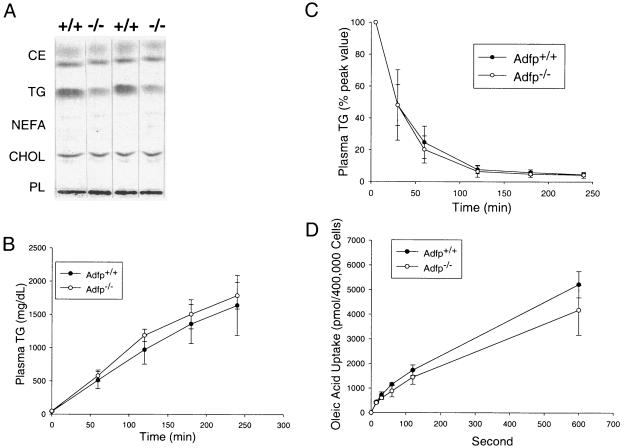
FIG. 5.
Hepatic lipid analysis and lipid metabolism. (A) Thin-layer chromatography analysis of lipids isolated from Adfp+/+ (+/+) and Adfp−/− (−/−) mouse livers. Lipids were extracted using 0.2 g of liver from an individual mouse, dried under nitrogen, reconstituted in chloroform, and loaded on to a TLC plate. Lipid standards were used to identify each lipid band. Lipid fractions were visualized using iodine vapor. (B) VLDL secretion after inhibition of lipoprotein lipase by Triton WR1339 treatment. Eight-week-old mice (male; n = 5) were fasted for 4 h, and plasma samples were taken as baseline (time zero). Plasma TG was measured at time zero and every hour for 4 h after Triton WR1339 treatment. (D) Intralipid clearance. Eight-week-old mice (n = 6) were infused with intralipid through the tail vein. Plasma TG levels are expressed as a percentage of the peak value measured 5 min after intralipid injection. (D) Oleic acid uptake in primary hepatocytes. Hepatocytes were isolated from male mice (n = 4). Individually isolated cells were separated into tubes for fatty acid uptake assay (Materials and Methods) in the presence of [3H]oleic acid; the reaction was stopped by the addition of phloretin solution at different time points. Cells were washed and pelleted by centrifugation, and radioactivity was determined by scintillation counting.
FIG. 4.
Liver triglyceride fatty acid composition. Liver triglyceride fatty acids from Adfp+/+ and Adfp−/− mice (four mice per group) were analyzed by gas chromatography after methyl esterification, as described in Materials and Methods. Major species of fatty acids are presented (A), with classes grouped (B) as follows: Sat, saturated; Mono, monunsaturated; Poly, polyunsaturated; n-6, n-6 polyunsaturated; and n-3, n-3 polyunsaturated fatty acids. The relative percentage of each individual (C) or grouped (D) fatty acids out of the total fatty acids is also presented. *, significant difference (P < 0.01) between Adfp+/+ and Adfp−/− mice.
Hepatic triglyceride production and clearance in Adfp−/− mice.
There are several possible mechanisms that could account for the reduction in hepatic TG content in Adfp−/− mice compared with their wild-type littermates: (i) increased hepatic TG secretion as VLDL, (ii) decreased rates of hepatic lipid uptake, (iii) increased levels of FA oxidation, or (iv) reduction in TG synthesis or any combination of these mechanisms. To test for the first possibility, we infused Triton WR1339 after a 4-h fast to block lipoprotein lipase activity in vivo and determined the rate of VLDL accumulation, a measure of TG secretion from the liver. We found that the rate of VLDL secretion of Adfp+/+ and Adfp−/− mice was similar, though results for Adfp−/− mice showed a minimal insignificant trend toward an increased rate of VLDL secretion (Fig. 5B).
To measure TG uptake in the liver, we infused intralipid (250 μl), a lipid emulsion of TG enveloped by phospholipids, into mice and measured the fall in the plasma TG concentrations over the next 4 h. We found that 80% of the infused TG was cleared by the first hour in both wild-type and Adfp−/− mice, with the plasma TG level returning to preinfusion levels by 2 h. The data were very comparable to those of a previous study (51). Thus, there was no difference between Adfp−/− and wild-type mice in TG clearance (Fig. 5C).
Hepatic uptake of long-chain fatty acids in Adfp−/− and Adfp+/+ mice.
ADFP has been implicated in the facilitation of long-chain FA uptake (16). We therefore determined the uptake of [3H]oleic acid in primary hepatocytes and found that the rate of uptake was similar in hepatocytes isolated from Adfp−/− and Adfp+/+ mice (Fig. 5D).
Hepatic β-oxidation in Adfp−/− and Adfp+/+ mice.
To quantify hepatic β-oxidation, we incubated primary hepatocytes in the presence of [14C]palmitic acid and measured [14C]CO2 as the product of FA oxidation. As shown in Fig. 6A, there was no difference in rate of β-oxidation between primary hepatocytes isolated from wild-type mice and those isolated from Adfp−/− mice. We next measured the plasma level of β-hydroxybutyrate, a ketone body produced during β-oxidation with overnight fasting. Again, there was no difference in plasma β-hydroxybutyrate levels between wild-type and Adfp−/− mice (Fig. 6B). These data indicate that increased FA utilization is not the mechanism for the reduced TG content in the liver in the absence of ADFP.
FIG. 6.
Hepatic β-oxidation parameters. (A) β-Oxidation in primary hepatocytes. Primary hepatocytes were isolated from Adfp+/+ and Adfp−/− mice (three mice per group). Viable cells were counted, and 5 × 105 viable cells were isolated from each mouse and used for the assay. (B) Plasma β-hydroxybutyrate concentrations in Adfp+/+ and Adfp−/− mice (four mice per group) after 12-h fasting.
Hepatic lipogenesis in Adfp−/− and Adfp+/+ mice.
Fatty acid synthesis was measured in the livers of Adfp+/+ and Adfp−/− mice using [3H]H2O as a label (37). At 1 h after injection of [3H]H2O, we isolated the liver and extracted and saponified the lipids to recover the total fatty acids, which includes NEFA and acyl groups removed from other lipids. By this analysis, wild-type and Adfp−/− mice had a similar amount of newly synthesized FA (Fig. 7A). We next measured the total FAS activity of liver homogenates but found no difference in hepatic FAS activity between wild-type and Adfp−/− mice (Fig. 7B). Western blotting of two key enzymes of fatty acid synthesis, AccI (for acetyl-CoA carboxylase I) and FAS, also did not reveal any difference in protein expression level (Fig. 7C). Real-time quantitative reverse transcription-PCR also showed no significant difference in the mRNA concentrations for the lipogenic genes AccI, Fas, and ATP-citrate lyase gene between wild-type and Adfp−/− mice (data not shown). The absence of detectable changes in lipogenesis in the absence of ADFP was in keeping with the observations of Imamura et al., who found that adenovirus-mediated ADFP overexpression induced lipid accumulation in murine fibroblasts without changes in the level of lipogenic gene expression (26).
FIG. 7.
(A) In vivo hepatic fatty acid synthesis using the [3H]H2O labeling method (39, 40). Male Adfp+/+ and Adfp−/− mice (four mice per group) were synchronized to 3-h (8 to 11 a.m.) feeding each day for 2 weeks before experiments. Livers were removed 1 h after [3H]H2O injection. Total lipids were extracted, saponified, and separated by TLC; fatty acid fractions were scraped into scintillation vials to determine radioactivity. (B) Liver FAS activity of liver extracts from Adfp+/+ and Adfp−/− mice (four mice per group). (C) Representative Western blot of liver FAS and ACCI from Adfp+/+ and Adfp−/− mice. (D) Liver microsomal DGAT activity assay (Materials and Methods) of microsome preparations from Adfp+/+ and Adfp−/− mice (four mice per group). The reaction products from the DGAT assay were separated by TLC, the TG fraction was scrapped, and radioactivity was determined by scintillation counting.
Hepatic DGAT activity in Adfp−/− and Adfp+/+ mice.
DGAT1 knockout mice were previously reported to display reduced hepatic TG content (57). We therefore measured hepatic microsomal DGAT activity in wild-type and Adfp−/− mice as previously described (7) but found that this final step of TG synthesis was similar in the two genotypes (Fig. 7D).
Increased TG content in the microsomes of Adfp−/− liver.
To further characterize the difference in hepatic TG content between wild-type and Adfp−/− mice, we analyzed the subcellular distribution of lipids in the different cellular compartments separated by cell fractionation. We separated liver homogenates isolated from Adfp+/+ and Adfp−/− mice into cytosolic, microsomal, and nuclear fractions by differential high-speed centrifugation, using the following proteins as markers: a large subunit of MTP for the microsomal fraction, GAPDH for the cytosolic fraction, and histones for the nuclear fraction. As shown in Fig. 8B, the cytosol and microsome fractions were distinctive and well separated in homogenates from both wild-type and Adfp−/− mice; there was some overlap in microsomal (MTP) and nuclear (histone) marker distribution, due to the continuity of the microsomal and nuclear membranes, but these markers did show preferential distribution in their respective intracellular fractions. GAPDH is known to be present in both cytosol and nucleus (41, 56), but the relative abundance is higher in the cytosolic fraction. Thus, the markers show the expected subcellular distribution.
FIG. 8.
TLC analysis of cytosolic and microsomal lipid distribution. A total of 0.4 g of liver from Adfp+/+ and Adfp−/− mice (four mice per group) was homogenized and separated into nuclear, microsomal, and cytosolic compartments. Lipids were extracted from each compartment and analyzed by TLC. Two lipid standards were loaded in the center lanes as a control. (B) Western blot analysis of subcellular fractions. A total of 20 μg of proteins from each subcellular fraction (prepared as described for panel A) was analyzed by PAGE, transferred to a nylon membrane, and immunoblotted with anti-MTP (microsomal marker), anti-GAPDH (cytosolic marker), and antihistone (nuclear marker). T, total liver protein extract; C, cytosolic fraction; N, nuclear fraction; M, microsomal fraction. +/+, Adfp+/+ mice; −/−, Adfp−/− mice. (C) Intracellular lipid partitioning in Adfp−/− and Adfp+/+ mice. Lipid (TG, NEFA, and PL) abundance was analyzed by TLC, quantified by densitometry, and expressed according to each lipid's subcellular compartment.
The reconstituted lipids of each fraction were analyzed by TLC. The relative abundance of each fraction found in the nuclear fraction was similar to that of the total lipids in that only the TG level was reduced in Adfp−/− mice (Fig. 5A and data not shown). In contrast, TG, NEFA, and PL content was all reduced in the cytosolic fraction of Adfp−/− mice compared to that of Adfp+/+ mice (Fig. 8A). Furthermore, in the microsomal fraction, the TG content was almost twofold higher and FA content was almost threefold higher, while PL content remained similar in Adfp−/− liver compared to the microsomal fraction isolated from Adfp+/+ liver (Fig. 8C). In summary, only in the microsomal fraction, as opposed to the cytosolic or nuclear compartments, was TG and PL content higher in the livers of Adfp−/− mice in the face of significant and substantial reduction in total TG content in the entire liver.
The absence of ADFP protects against diet-induced fatty liver development.
We showed that lack of ADFP reduces total intrahepatic TG content in mice fed a regular chow, which is associated with normal liver histology whether ADFP is present or not. To determine if the absence of ADFP would also protect against hepatic TG accumulation in association with fatty liver development, we fed Adfp−/− and wild-type mice an HFD to induce fatty liver formation. Total body and liver weights after 4 weeks of HFD feeding were not different between Adfp+/+ and Adfp−/− mice (see Fig. S3 in the supplemental material). Histological examination revealed that HFD feeding led to fatty liver changes in both Adfp+/+ and Adfp−/− mice. There was, however, a marked difference in the severity of fatty infiltration with the HFD, causing the appearance of many more LDs in the wild-type liver than the Adfp−/− liver (Fig. 9A and B). Morphometric analysis revealed that the LD density (number of LDs per unit area) was significantly reduced in Adfp−/− liver compared to wild-type liver sections (3,247.3 ± 1,228.2 versus 8,472.8 ± 2,157.6 per mm2). Moreover, there was a shift in the LD size distribution towards significantly smaller droplets in Adfp−/− mice (Fig. 9C and D). The decrease in the number and size of LDs translates into a significant (∼40%) decrease in hepatic TG content in the Adfp−/− mice (102.9 ± 19.9 versus 170.8 ± 29.1 mg/dl). These experiments indicate that absence of ADFP protects against diet-induced fatty liver development in mice.
FIG. 9.
Liver histology and LD number and size distribution in HFD-fed mice. Adfp+/+ and Adfp−/− mice (8 weeks old; four mice per group) were fed with HFD for 4 weeks. (A and B) Photomicroscopy of liver section from HFD-fed Adfp+/+ (A) and Adfp−/− (B) mice. (C and D) Average number and size distribution of LDs in liver sections of HFD-fed Adfp+/+ (C) and Adfp−/− (D) mice.
DISCUSSION
We have generated Adfp-deficient mice and demonstrated that the targeted mice are devoid of Adfp mRNA and protein (Fig. 1 and 2). The loss of Adfp does not affect body weight or plasma lipid or glucose levels. Nor does it cause any gross morphological or anatomical changes when the mice are fed a regular chow.
Induction of adipocyte differentiation using cultured MEFs revealed no significant difference in the expression pattern of key regulators or markers of adipocyte differentiation during the transition from fibroblast to adipocyte morphology and no difference in total intracellular TG content after differentiation (Fig. 2). In addition, Adfp-deficient mice have the same fat pad mass as their wild-type littermates (Table 1), indicating that absence of ADFP does not lead to a significant impairment in adipogenesis in vivo. Furthermore, Adfp−/− mice display normal lipolysis under basal conditions and after β-adrenergic agonist stimulation (Fig. 2E). Together, these data support the conclusion that Adfp is not essential for adipocyte differentiation or lipolysis. Hence, the burst of Adfp gene transcription during early adipocyte differentiation appears to be the consequence of increasing lipid accumulation and not the driving force of adipocyte differentiation. In this regard, adipophilin (22) may be a more appropriate name than ADFP for this gene and protein.
The appearance of Adfp mRNA during adipocyte differentiation precedes that of PLIN mRNA and protein (3). LDs are initially coated by ADFP during early adipocyte differentiation, being replaced later by PLIN, although Adfp mRNA persists after the disappearance of ADFP protein (3). In this regard, Xu et al. recently showed that ADFP is regulated posttranslationally by proteasomes at the onset of PLIN expression during adipocyte differentiation (70). We and others showed that Plin−/− mice display upregulated basal lipolysis in vivo and in isolated adipocytes (40, 62); an increase of ADFP protein was found in Plin−/− mice, although functionally, it failed to compensate for the loss of PLIN. Interestingly, we observed no reciprocal increase in Plin gene expression in adipocytes of Adfp−/− mice. There was also no compensatory increase in mRNA expression of other PAT domain-containing genes (the Tip47 or S3-12 gene) in these animals (Fig. 3A), although there seemed to be a small compensatory upregulation of Tip47 protein in WAT of Adfp−/− mice. It is interesting that the mild Tip47 protein upregulation in the WAT of the Adfp−/− mice is not associated with an increase in its mRNA, indicating that Tip47 may be regulated posttranslationally in Adfp−/− mice. It is not clear if the presence of Tip47 compensates for the lack of a WAT phenotype in ADFP deficiency, but it is a possibility that warrants further study. Thus, ADFP does not appear essential for the production or normal functioning of adipocytes; alternatively, the presence of other LD proteins may compensate for the loss of ADFP. It is also possible that ADFP serves a subtle and nonessential adipocyte function for which we have not tested in this study.
In contrast to its nonessential function in lipid accumulation in adipocytes, loss of ADFP in the liver downregulates hepatic TG content (by ∼60%). This study establishes an important function of ADFP in the liver that appears not to be replaceable by other LD proteins. The apparently nonoverlapping and different effects of PLIN in adipocytes and of ADFP in hepatocytes suggest that LD proteins may serve unique functions in a tissue-specific manner.
From our experiments, ADFP appears to be important in maintenance of hepatic TG homeostasis because the knockout animals store less TG without changes in cholesterol, NEFA, or PL content in the liver. One possible explanation is that the Adfp−/− liver has an impaired ability to take up FA. FA uptake has been thought to occur through passive diffusion because it is hydrophobic and could potentially pass through plasma membranes with ease. However, recent studies indicate that the transport of FA, particularly long-chain FA, is a regulated process involving translocases and transporters (19). A study using transiently transfected COS-7 cells revealed the localization of ADFP to the plasma membrane (16). More recently, Robenek et al. used freeze fracture electron microscopy and localized ADFP and other PAT family proteins to the cytoplasmic face of the plasma membrane in cultured cells treated with acetylated low-density lipoprotein (49), consistent with a possible role in FFA uptake. However, we found no significant difference in oleic acid uptake in hepatocytes isolated from Adfp−/− mice versus those isolated from wild-type mice (Fig. 5D).
We also found that the Adfp−/− mice have a TG clearance similar to that of the wild-type. In this study, we used intralipid to circumvent the factors associated with intestinal lipid absorption, chylomicron production, and secretion. It is generally believed that about 60% of the infused intralipid is taken up by rat liver (48), although the reported hepatic clearance rate has ranged from 20% to 88% (for a review, see reference 25). Given the 60% reduction of hepatic TG in the Adfp−/− mice and even if we assume that the hepatic contribution to TG clearance is only 20%, we would still expect to see a difference in the TG clearance curve in the Adfp−/− mice if TG clearance was the cause for the reduced hepatic content. The identical clearance curve between Adfp−/− and wild-type mice (Fig. 5C) makes it highly unlikely that reduced TG clearance by the liver is the cause of low hepatic TG content in Adfp−/− mice. We cannot exclude the unlikely scenario caused by the presence of compensatory upregulation of muscle TG uptake in the face of a reduced hepatic uptake that is not detected by this experiment.
In one report, L-Fabp−/− (liver-type fatty acid binding protein knockout) mice under prolonged starvation were also reported to exhibit reduced TG content (47). We note, however, that another group reported a substantially different phenotype for L-Fabp−/− mice (39), with increased hepatic cholesterol, cholesteryl ester, and PL levels thought to be due to a compensatory upregulation of the Scp-2 gene. In contrast to either report, we did not observe a change in hepatic cholesterol and phospholipid levels under normal feeding conditions; the reduction in hepatic TG was readily observed without prolonged fasting. Moreover, there was no increase in expression of the L-Fabp gene in Adfp−/− mice (data not shown). As such, perturbation of intracellular TG homeostasis in Adfp deficiency appears not to be mediated through L-Fabp.
Liver TG reduction was also observed with aP2/mac-1 double-knockout mice (38) and mitochondrial glycerol-3-phosphate acyltransferase-deficient mice (20), but these mice have complicated alterations in multiple components of metabolism, such as high energy expenditures, changes in plasma lipids, and increased fatty acid β-oxidation, none of which was evident in Adfp−/− mice.
With a substantial reduction of intracellular TG, one would expect that VLDL secretion would be reduced because studies have shown that degradation of apolipoprotein B (apoB), the obligatory constituent apolipoprotein of VLDL, is enhanced when lipid supply is limiting (14, 18, 33, 34, 69, 72). However, in the analysis of lipid distribution in different intracellular compartments, we discovered that while TG was reduced in cytosol, it was actually increased in the liver microsome fraction of samples from Adfp−/− mice (Fig. 8A). Importantly, there was a concomitant increase in the concentration of MTP in Adfp−/− mice compared to Adfp+/+ mice (see Fig. S4 in the supplemental material). Increased MTP availability may have contributed to the normal folding of apoB, protecting apoB from degradation and ensuring a normal VLDL secretion rate, despite the presence of reduced hepatic TG content in Adfp−/− hepatocytes (Fig. 5B). It is known that TG exists in two different intracellular pools in HepG2 cells, a microsomal pool that is coupled to VLDL secretion and a cytosolic pool that is not (68). For example, ob/ob mice, which have a severely steatotic liver, have downregulated VLDL secretion (32, 67) probably because of TG deficiency in the microsomal pool. Our analysis suggests that ADFP plays a crucial role in the balance between the cytosolic and microsomal TG pools in the liver.
Intracellular lipid partitioning in the Adfp−/− liver is quite different from that of the wild-type liver (Fig. 8). In the cytosolic fraction, TG, NEFA, and PL were all reduced. Owing to its strong hydrophobicity, cytosolic fat has to be sequestered in LDs which are generally thought to originate from the outer leaflet of the microsomal membranes, with the polar PL on the surface enclosing neutral lipids, such as TG and cholesteryl esters, in the core (45). Although NEFA can be carried by the cytosolic fatty acid binding proteins, TG and PL must exist in LDs in the cytosol, and their reduction in the Adfp−/− (in the cytosol) is an indication of reduced numbers (and size) of LDs, which can be inferred from what we have observed with HFD-treated Adfp−/− mice (Fig. 9 and see below). The reduction of cytosolic NEFA in Adfp−/− mice could be a reflection of redistribution of NEFA or reduction of NEFA uptake, although the latter is not supported by direct measurement in this study. In the microsomal fraction, both TG and NEFA were increased in the Adfp−/− liver. We hypothesize that the absence of ADFP somehow hinders LD formation, leading to the accumulation of TG and NEFA in the microsomal compartment where TG is synthesized.
Under normal chow conditions in C57BL/6 mice, LDs are not readily detectable in the liver even under high-power light microscopy. However, ADFP is upregulated and LDs become readily visible in the fatty liver (22). We used HFD treatment to induce fatty liver and observed the appearance of LDs in the livers of the wild-type mice and the Adfp−/− mice. The density of LDs was much reduced in Adfp−/− mice, being 38% per unit area in these mice compared to that (100%) in wild-type animals. Moreover, LDs in Adfp−/− livers were also significantly smaller than those in wild-type mice (Fig. 9C and D), supporting a role for ADFP in LD formation. There is a reduction of hepatic TG but not PL in Adfp−/− mice. This is similar to the hepatic fat distribution observed with the chow-fed mice (Table 3), suggesting that a similar molecular mechanism operates to reduce TG in the HFD-treated Adfp−/− mice.
LDs received much attention recently with the discovery of many proteins physically localized to these intracellular particles (4, 27, 35, 64). LD proteins are involved in important biological functions such as lipid metabolism, lipid transport, membrane trafficking, molecular chaperoning, and apoptosis. Nonalcoholic fatty liver disease (NAFLD) is a common ailment in developed countries (21), manifesting histologically as excessive LD accumulation. This study documents that ADFP deficiency confers resistance to fatty liver development in the presence of normal lipid metabolism. It underscores the use of Adfp−/− mice as a unique model to study the molecular pathogenesis of NAFLD, while highlighting ADFP as a possible drug target for the prevention and treatment of NAFLD.
Supplementary Material
Acknowledgments
We thank Ilke Wagner of NHLBI, NIH; Richard Lehner of the University of Alberta; and Salih Wakil of the Baylor College of Medicine for providing us with protocols to measure lipogenesis and helping with the discussion. We thank Sue Samson for critical readings of the manuscript.
This research is supported by a pilot grant from the NIH-National Institute of Diabetes and Digestive and Kidney Diseases, Digestive Disease Center grant P30 DK56338 (to B.H.-J.C.), and NIH grant HL 51586 (to L.C.). L.C. was also supported by the Rutherford Chair from St. Luke's Episcopal Hospital and the Baylor College of Medicine.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bildirici, I., C. R. Roh, W. T. Schaiff, B. M. Lewkowski, D. M. Nelson, and Y. Sadovsky. 2003. The lipid droplet-associated protein adipophilin is expressed in human trophoblasts and is regulated by peroxisomal proliferator-activated receptor-gamma/retinoid X receptor. J. Clin. Endocrinol. Metab. 88:6056-6062. [DOI] [PubMed] [Google Scholar]
- 2.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 3.Brasaemle, D. L., T. Barber, N. E. Wolins, G. Serrero, E. J. Blanchette-Mackie, and C. Londos. 1997. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38:2249-2263. [PubMed] [Google Scholar]
- 4.Brasaemle, D. L., G. Dolios, L. Shapiro, and R. Wang. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279:46835-46842. [DOI] [PubMed] [Google Scholar]
- 5.Buechler, C., M. Ritter, C. Q. Duong, E. Orso, M. Kapinsky, and G. Schmitz. 2001. Adipophilin is a sensitive marker for lipid loading in human blood monocytes. Biochim. Biophys. Acta 1532:97-104. [DOI] [PubMed] [Google Scholar]
- 6.Bussell, R., Jr., and D. Eliezer. 2003. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J. Mol. Biol. 329:763-778. [DOI] [PubMed] [Google Scholar]
- 7.Cases, S., S. J. Smith, Y. W. Zheng, H. M. Myers, S. R. Lear, E. Sande, S. Novak, C. Collins, C. B. Welch, A. J. Lusis, S. K. Erickson, and R. V. Farese, Jr. 1998. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 95:13018-13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, B. H., W. Liao, L. Li, M. Nakamuta, D. Mack, and L. Chan. 1999. Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J. Biol. Chem. 274:6051-6055. [DOI] [PubMed] [Google Scholar]
- 9.Chawla, A., C. H. Lee, Y. Barak, W. He, J. Rosenfeld, D. Liao, J. Han, H. Kang, and R. M. Evans. 2003. PPARδ is a very low-density lipoprotein sensor in macrophages. Proc. Natl. Acad. Sci. USA. 100:1268-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifford, G. M., F. B. Kraemer, S. J. Yeaman, and R. G. Vernon. 2001. Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation during the lactation cycle of the rat. Metabolism 50:1264-1269. [DOI] [PubMed] [Google Scholar]
- 11.Corsini, E., B. Viviani, O. Zancanella, L. Lucchi, F. Visioli, G. Serrero, S. Bartesaghi, C. L. Galli, and M. Marinovich. 2003. Induction of adipose differentiation related protein and neutral lipid droplet accumulation in keratinocytes by skin irritants. J. Investig. Dermatol. 121:337-344. [DOI] [PubMed] [Google Scholar]
- 12.Dalen, K. T., K. Schoonjans, S. M. Ulven, M. S. Weedon-Fekjaer, T. G. Bentzen, H. Koutnikova, J. Auwerx, and H. I. Nebb. 2004. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes 53:1243-1252. [DOI] [PubMed] [Google Scholar]
- 13.Faber, B. C., K. B. Cleutjens, R. L. Niessen, P. L. Aarts, W. Boon, A. S. Greenberg, P. J. Kitslaar, J. H. Tordoir, and M. J. Daemen. 2001. Identification of genes potentially involved in rupture of human atherosclerotic plaques. Circ. Res. 89:547-554. [DOI] [PubMed] [Google Scholar]
- 14.Fisher, E. A., M. Zhou, D. M. Mitchell, X. Wu, S. Omura, H. Wang, A. L. Goldberg, and H. N. Ginsberg. 1997. The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J. Biol. Chem. 272:20427-20434. [DOI] [PubMed] [Google Scholar]
- 15.Fong, T. H., C. C. Yang, A. S. Greenberg, and S. M. Wang. 2002. Immunocytochemical studies on lipid droplet-surface proteins in adrenal cells. J. Cell. Biochem. 86:432-439. [DOI] [PubMed] [Google Scholar]
- 16.Gao, J., and G. Serrero. 1999. Adipose differentiation related protein (ADRP) expressed in transfected COS-7 cells selectively stimulates long chain fatty acid uptake. J. Biol. Chem. 274:16825-16830. [DOI] [PubMed] [Google Scholar]
- 17.Gao, J., H. Ye, and G. Serrero. 2000. Stimulation of adipose differentiation related protein (ADRP) expression in adipocyte precursors by long-chain fatty acids. J. Cell. Physiol. 182:297-302. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg, H. N. 1997. Role of lipid synthesis, chaperone proteins and proteasomes in the assembly and secretion of apoprotein B-containing lipoproteins from cultured liver cells. Clin. Exp. Pharmacol. Physiol. 24:A29-A32. [DOI] [PubMed] [Google Scholar]
- 19.Hajri, T., and N. A. Abumrad. 2002. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu. Rev. Nutr. 22:383-415. [DOI] [PubMed] [Google Scholar]
- 20.Hammond, L. E., P. A. Gallagher, S. Wang, S. Hiller, K. D. Kluckman, E. L. Posey-Marcos, N. Maeda, and R. A. Coleman. 2002. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol. Cell. Biol. 22:8204-8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haque, M., and A. J. Sanyal. 2002. The metabolic abnormalities associated with non-alcoholic fatty liver disease. Best. Pract. Res. Clin. Gastroenterol. 16:709-731. [DOI] [PubMed] [Google Scholar]
- 22.Heid, H. W., R. Moll, I. Schwetlick, H. R. Rackwitz, and T. W. Keenan. 1998. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 294:309-321. [DOI] [PubMed] [Google Scholar]
- 23.Heid, H. W., M. Schnolzer, and T. W. Keenan. 1996. Adipocyte differentiation-related protein is secreted into milk as a constituent of milk lipid globule membrane. Biochem. J. 320:1025-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hickenbottom, S. J., A. R. Kimmel, C. Londos, and J. H. Hurley. 2004. Structure of a lipid droplet protein; the PAT family member TIP47. Structure (Cambridge) 12:1199-1207. [DOI] [PubMed] [Google Scholar]
- 25.Hultin, M., C. Carneheim, K. Rosenqvist, and T. Olivecrona. 1995. Intravenous lipid emulsions: removal mechanisms as compared to chylomicrons. J. Lipid Res. 36:2174-2184. [PubMed] [Google Scholar]
- 26.Imamura, M., T. Inoguchi, S. Ikuyama, S. Taniguchi, K. Kobayashi, N. Nakashima, and H. Nawata. 2002. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am. J. Physiol. Endocrinol. Metab. 283:E775-E783. [DOI] [PubMed] [Google Scholar]
- 27.Imanishi, Y., V. Gerke, and K. Palczewski. 2004. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J. Cell Biol. 166:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayakumar, A., M. H. Tai, W. Y. Huang, W. al Feel, M. Hsu, L. Abu-Elheiga, S. S. Chirala, and S. J. Wakil. 1995. Human fatty acid synthase: properties and molecular cloning. Proc. Natl. Acad. Sci. USA 92:8695-8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang, H. P., and G. Serrero. 1992. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc. Natl. Acad. Sci. USA 89:7856-7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koopman, R., G. Schaart, and M. K. Hesselink. 2001. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem. Cell Biol. 116:63-68. [DOI] [PubMed] [Google Scholar]
- 31.Lee, C. H., A. Chawla, N. Urbiztondo, D. Liao, W. A. Boisvert, R. M. Evans, and L. K. Curtiss. 2003. Transcriptional repression of atherogenic inflammation: modulation by PPARδ. Science 302:453-457. [DOI] [PubMed] [Google Scholar]
- 32.Li, X., S. M. Grundy, and S. B. Patel. 1997. Obesity in db and ob animals leads to impaired hepatic very low density lipoprotein secretion and differential secretion of apolipoprotein B-48 and B-100. J. Lipid Res. 38:1277-1288. [PubMed] [Google Scholar]
- 33.Liao, W., B. H. Chang, M. Mancini, and L. Chan. 2003. Ubiquitin-dependent and -independent proteasomal degradation of apoB associated with endoplasmic reticulum and Golgi apparatus, respectively, in HepG2 cells. J. Cell. Biochem. 89:1019-1029. [DOI] [PubMed] [Google Scholar]
- 34.Liao, W., S. C. Yeung, and L. Chan. 1998. Proteasome-mediated degradation of apolipoprotein B targets both nascent peptides cotranslationally before translocation and full-length apolipoprotein B after translocation into the endoplasmic reticulum. J. Biol. Chem. 273:27225-27230. [DOI] [PubMed] [Google Scholar]
- 35.Liu, P., Y. Ying, Y. Zhao, D. I. Mundy, M. Zhu, and R. G. Anderson. 2004. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 279:3787-3792. [DOI] [PubMed] [Google Scholar]
- 36.Londos, C., D. L. Brasaemle, C. J. Schultz, J. P. Segrest, and A. R. Kimmel. 1999. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin. Cell Dev. Biol. 10:51-58. [DOI] [PubMed] [Google Scholar]
- 37.Lowenstein, J. M., H. Brunengraber, and M. Wadke. 1975. Measurement of rates of lipogenesis with deuterated and tritiated water. Methods Enzymol. 35:279-287. [DOI] [PubMed] [Google Scholar]
- 38.Maeda, K., H. Cao, K. Kono, C. Z. Gorgun, M. Furuhashi, K. T. Uysal, Q. Cao, G. Atsumi, H. Malone, B. Krishnan, Y. Minokoshi, B. B. Kahn, R. A. Parker, and G. S. Hotamisligil. 2005. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 1:107-119. [DOI] [PubMed] [Google Scholar]
- 39.Martin, G. G., H. Danneberg, L. S. Kumar, B. P. Atshaves, E. Erol, M. Bader, F. Schroeder, and B. Binas. 2003. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J. Biol. Chem. 278:21429-21438. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Botas, J., J. B. Anderson, D. Tessier, A. Lapillonne, B. H. Chang, M. J. Quast, D. Gorenstein, K. H. Chen, and L. Chan. 2000. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 26:474-479. [DOI] [PubMed] [Google Scholar]
- 41.Mazzola, J. L., and M. A. Sirover. 2003. Subcellular localization of human glyceraldehyde-3-phosphate dehydrogenase is independent of its glycolytic function. Biochim. Biophys. Acta 1622:50-56. [DOI] [PubMed] [Google Scholar]
- 42.McManaman, J. L., W. Zabaronick, J. Schaack, and D. J. Orlicky. 2003. Lipid droplet targeting domains of adipophilin. J. Lipid Res. 44:668-673. [DOI] [PubMed] [Google Scholar]
- 43.Miura, S., J. W. Gan, J. Brzostowski, M. J. Parisi, C. J. Schultz, C. Londos, B. Oliver, and A. R. Kimmel. 2002. Functional conservation for lipid storage droplet association among perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 277:32253-32257. [DOI] [PubMed] [Google Scholar]
- 44.Morrison, W. R., and L. M. Smith. 1964. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 53:600-608. [PubMed] [Google Scholar]
- 45.Murphy, D. J., and J. Vance. 1999. Mechanisms of lipid-body formation. Trends Biochem. Sci. 24:109-115. [DOI] [PubMed] [Google Scholar]
- 46.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newberry, E. P., Y. Xie, S. Kennedy, X. Han, K. K. Buhman, J. Luo, R. W. Gross, and N. O. Davidson. 2003. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 278:51664-51672. [DOI] [PubMed] [Google Scholar]
- 48.Qi, K., M. Al Haideri, T. Seo, Y. A. Carpentier, and R. J. Deckelbaum. 2003. Effects of particle size on blood clearance and tissue uptake of lipid emulsions with different triglyceride compositions. JPEN J. Parenter. Enteral Nutr. 27:58-64. [DOI] [PubMed] [Google Scholar]
- 49.Robenek, H., M. J. Robenek, I. Buers, S. Lorkowski, O. Hofnagel, D. Troyer, and N. J. Severs. 2005. Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J. Biol. Chem. 280:26330-26338. [DOI] [PubMed] [Google Scholar]
- 50.Robenek, H., M. J. Robenek, and D. Troyer. 2005. PAT family proteins pervade lipid droplet cores. J. Lipid Res. 46:1331-1338. [DOI] [PubMed] [Google Scholar]
- 51.Ross, C. J., J. Twisk, J. M. Meulenberg, G. Liu, O. K. van den, E. Moraal, W. T. Hermens, J. Rip, J. J. Kastelein, J. A. Kuivenhoven, and M. R. Hayden. 2004. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPLS447X beneficial mutation. Hum. Gene Ther. 15:906-919. [DOI] [PubMed] [Google Scholar]
- 52.Saha, P. K., H. Kojima, J. Martinez-Botas, A. L. Sunehag, and L. Chan. 2004. Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J. Biol. Chem. 279:35150-35158. [DOI] [PubMed] [Google Scholar]
- 53.Scherer, P. E., P. E. Bickel, M. Kotler, and H. F. Lodish. 1998. Cloning of cell-specific secreted and surface proteins by subtractive antibody screening. Nat. Biotechnol. 16:581-586. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt, S. M., K. Schag, M. R. Muller, T. Weinschenk, S. Appel, O. Schoor, M. M. Weck, F. Grunebach, L. Kanz, S. Stevanovic, H. G. Rammensee, and P. Brossart. 2004. Induction of adipophilin-specific cytotoxic T lymphocytes using a novel HLA-A2-binding peptide that mediates tumor cell lysis. Cancer Res. 64:1164-1170. [DOI] [PubMed] [Google Scholar]
- 55.Schmuth, M., C. M. Haqq, W. J. Cairns, J. C. Holder, S. Dorsam, S. Chang, P. Lau, A. J. Fowler, G. Chuang, A. H. Moser, B. E. Brown, M. Mao-Qiang, Y. Uchida, K. Schoonjans, J. Auwerx, P. Chambon, T. M. Willson, P. M. Elias, and K. R. Feingold. 2004. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J. Investig. Dermatol. 122:971-983. [DOI] [PubMed] [Google Scholar]
- 56.Sirover, M. A. 2005. New nuclear functions of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in mammalian cells. J. Cell. Biochem. 95:45-52. [DOI] [PubMed] [Google Scholar]
- 57.Smith, S. J., S. Cases, D. R. Jensen, H. C. Chen, E. Sande, B. Tow, D. A. Sanan, J. Raber, R. H. Eckel, and R. V. Farese, Jr. 2000. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25:87-90. [DOI] [PubMed] [Google Scholar]
- 58.Souza, S. C., K. V. Muliro, L. Liscum, P. Lien, M. T. Yamamoto, J. E. Schaffer, G. E. Dallal, X. Wang, F. B. Kraemer, M. Obin, and A. S. Greenberg. 2002. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J. Biol. Chem. 277:8267-8272. [DOI] [PubMed] [Google Scholar]
- 59.Spady, D. K., and J. M. Dietschy. 1983. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J. Lipid Res. 24:303-315. [PubMed] [Google Scholar]
- 60.Steiner, S., D. Wahl, B. L. Mangold, R. Robison, J. Raymackers, L. Meheus, N. L. Anderson, and A. Cordier. 1996. Induction of the adipose differentiation-related protein in liver of etomoxir-treated rats. Biochem. Biophys. Res. Commun. 218:777-782. [DOI] [PubMed] [Google Scholar]
- 61.Tansey, J. T., A. M. Huml, R. Vogt, K. E. Davis, J. M. Jones, K. A. Fraser, D. L. Brasaemle, A. R. Kimmel, and C. Londos. 2003. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J. Biol. Chem. 278:8401-8406. [DOI] [PubMed] [Google Scholar]
- 62.Tansey, J. T., C. Sztalryd, J. Gruia-Gray, D. L. Roush, J. V. Zee, O. Gavrilova, M. L. Reitman, C. X. Deng, C. Li, A. R. Kimmel, and C. Londos. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA 98:6494-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Targett-Adams, P., M. J. McElwee, E. Ehrenborg, M. C. Gustafsson, C. N. Palmer, and J. McLauchlan. 2005. A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochim. Biophys. Acta 1728:95-104. [DOI] [PubMed] [Google Scholar]
- 64.Umlauf, E., E. Csaszar, M. Moertelmaier, G. J. Schuetz, R. G. Parton, and R. Prohaska. 2004. Association of stomatin with lipid bodies. J. Biol. Chem. 279:23699-23709. [DOI] [PubMed] [Google Scholar]
- 65.Vosper, H., L. Patel, T. L. Graham, G. A. Khoudoli, A. Hill, C. H. Macphee, I. Pinto, S. A. Smith, K. E. Suckling, C. R. Wolf, and C. N. Palmer. 2001. The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J. Biol. Chem. 276:44258-44265. [DOI] [PubMed] [Google Scholar]
- 66.Wang, X., T. J. Reape, X. Li, K. Rayner, C. L. Webb, K. G. Burnand, and P. G. Lysko. 1999. Induced expression of adipophilin mRNA in human macrophages stimulated with oxidized low-density lipoprotein and in atherosclerotic lesions. FEBS Lett. 462:145-150. [DOI] [PubMed] [Google Scholar]
- 67.Wiegman, C. H., R. H. Bandsma, M. Ouwens, F. H. van der Sluijs, R. Havinga, T. Boer, D. J. Reijngoud, J. A. Romijn, and F. Kuipers. 2003. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes 52:1081-1089. [DOI] [PubMed] [Google Scholar]
- 68.Wu, X., A. Shang, H. Jiang, and H. N. Ginsberg. 1996. Low rates of apoB secretion from HepG2 cells result from reduced delivery of newly synthesized triglyceride to a “secretion-coupled” pool. J. Lipid Res. 37:1198-1206. [PubMed] [Google Scholar]
- 69.Wu, X., A. Shang, H. Jiang, and H. N. Ginsberg. 1997. Demonstration of biphasic effects of docosahexaenoic acid on apolipoprotein B secretion in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 17:3347-3355. [DOI] [PubMed] [Google Scholar]
- 70.Xu, G., C. Sztalryd, X. Lu, J. T. Tansey, J. Gan, H. Dorward, A. R. Kimmel, and C. Londos. Post-translational regulation of ADRP by the ubiquitin/proteosome pathway. J. Biol. Chem., in press. [DOI] [PubMed]
- 71.Ye, H., and G. Serrero. 1998. Stimulation of adipose differentiation related protein (ADRP) expression by ibuprofen and indomethacin in adipocyte precursors and in adipocytes. Biochem. J. 330:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeung, S. J., S. H. Chen, and L. Chan. 1996. Ubiquitin-proteasome pathway mediates intracellular degradation of apolipoprotein B. Biochemistry 35:13843-13848. [DOI] [PubMed] [Google Scholar]
- 73.Zimmermann, R., J. G. Strauss, G. Haemmerle, G. Schoiswohl, R. Birner-Gruenberger, M. Riederer, A. Lass, G. Neuberger, F. Eisenhaber, A. Hermetter, and R. Zechner. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383-1386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.