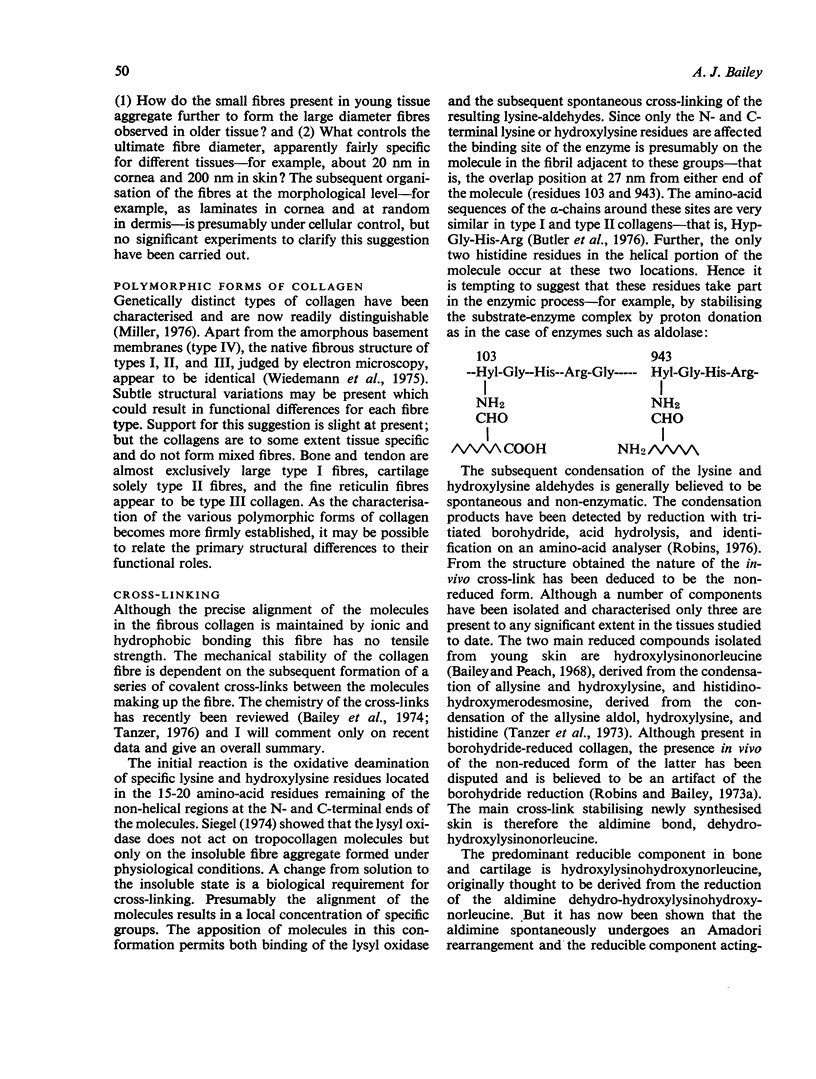
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Peach C. M. Isolation and structural identification of a labile intermolecular crosslink in collagen. Biochem Biophys Res Commun. 1968 Dec 9;33(5):812–819. doi: 10.1016/0006-291x(68)90233-7. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Prockop D. J. Synthesis of elastin in aortas from chick embryos. Conversion of newly secreted elastin to cross-linked elastin without apparent proteolysis of the molecule. Biochemistry. 1977 Apr 5;16(7):1406–1412. doi: 10.1021/bi00626a026. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Miller E. J., Finch J. E., Jr The covalent structure of cartilage collagen. Amino acid sequence of the NH2-terminal helical portion of the alpha 1 (II) chain. Biochemistry. 1976 Jul 13;15(14):3000–3006. doi: 10.1021/bi00659a010. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Click E. M., Harper E., Bornstein P. Interchain disulfide bonds in procollagen are located in a large nontriple-helical COOH-terminal domain. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3009–3013. doi: 10.1073/pnas.72.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B. A., Starcher B. C., Urry D. W. Coacervation of alpha-elastin results in fiber formation. Biochim Biophys Acta. 1973 Jul 12;317(1):209–213. doi: 10.1016/0005-2795(73)90215-8. [DOI] [PubMed] [Google Scholar]
- Davidson J. M., McEneany L. S., Bornstein P. Intermediates in the conversion of procollagen to collagen. Evidence for stepwise limited proteolysis of the COOH-terminal peptide extensions. Eur J Biochem. 1977 Dec 1;81(2):349–355. doi: 10.1111/j.1432-1033.1977.tb11958.x. [DOI] [PubMed] [Google Scholar]
- Davis N. R., Anwar R. A. On the mechanism of formation of desmosine and isodesmosine cross-links of elastin. J Am Chem Soc. 1970 Jun 17;92(12):3778–3782. doi: 10.1021/ja00715a040. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Nimni M. E. Characterization of the aldehydes present on the cyanogen bromide peptides from mature rat skin collagen. Biochemistry. 1971 Apr 27;10(9):1640–1647. doi: 10.1021/bi00785a022. [DOI] [PubMed] [Google Scholar]
- Faris B., Salcedo L. L., Cook V., Johnson L., Foster J. A., Franzblau C. The synthesis of connective tissue protein in smooth muscle cells. Biochim Biophys Acta. 1976 Jan 5;418(1):93–103. doi: 10.1016/0005-2787(76)90330-0. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Morris N. P., Fessler J. H. Procollagen: biological scission of amino and carboxyl extension peptides. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4905–4909. doi: 10.1073/pnas.72.12.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. A., Mecham R., Imberman M., Faris B., Franzblau C. A high molecular weight species of soluble elastin-proelastin. Adv Exp Med Biol. 1977;79:351–369. doi: 10.1007/978-1-4684-9093-0_31. [DOI] [PubMed] [Google Scholar]
- Foster J. A., Rubin L., Kagan H. M., Franzblau C., Bruenger E., Sandberg L. B. Isolation and characterization of cross-linked peptides from elastin. J Biol Chem. 1974 Oct 10;249(19):6191–6196. [PubMed] [Google Scholar]
- Fujii K., Corcoran D., Tanzer M. L. Isolation and structure of a cross-linked tripeptide from calf bone collagen. Biochemistry. 1975 Oct 7;14(20):4409–4413. doi: 10.1021/bi00691a011. [DOI] [PubMed] [Google Scholar]
- Fujimoto D. Isolation and characterization of a fluorescent material in bovine achilles tendon collagen. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1124–1129. doi: 10.1016/0006-291x(77)90972-x. [DOI] [PubMed] [Google Scholar]
- Gerber G. E., Anwar R. A. Comparative studies of the cross-linked regions of elastin from bovine ligamentum nuchae and bovine, porcine and human aorta. Biochem J. 1975 Sep;149(3):685–695. doi: 10.1042/bj1490685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B. Kinetics of processing of type I and type III procollagens in fibroblast cultures. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3322–3325. doi: 10.1073/pnas.74.8.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosline J. M. The physical properties of elastic tissue. Int Rev Connect Tissue Res. 1976;7:211–249. doi: 10.1016/b978-0-12-363707-9.50011-3. [DOI] [PubMed] [Google Scholar]
- Gotte L., Giro M. G., Volpin D., Horne R. W. The ultrastructural organization of elastin. J Ultrastruct Res. 1974 Jan;46(1):23–33. doi: 10.1016/s0022-5320(74)80019-5. [DOI] [PubMed] [Google Scholar]
- Gray W. R., Sandberg L. B., Foster J. A. Molecular model for elastin structure and function. Nature. 1973 Dec 21;246(5434):461–466. doi: 10.1038/246461a0. [DOI] [PubMed] [Google Scholar]
- Hoeve C. A., Flory P. J. The elastic properties of elastin. Biopolymers. 1974 Apr;13(4):677–686. doi: 10.1002/bip.1974.360130404. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. P., Olsen B. R., Chen H. T., Prockop D. J. Segment-long-spacing aggregates and isolation of COOH-terminal peptides from type I procollagen. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4304–4308. doi: 10.1073/pnas.73.12.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley T., Tanzer M. L., Henson E., Gallop P. M. Collagen crosslinking: isolation of hydroxyaldol-histidine, a naturally-occurring crosslink. Biochem Biophys Res Commun. 1975 Nov 17;67(2):824–830. doi: 10.1016/0006-291x(75)90887-6. [DOI] [PubMed] [Google Scholar]
- Kang A. H. Studies on the location of intermolecular cross-links in collagen. Isolation of a CNBr peptide containing -hydroxylysinonorleucine. Biochemistry. 1972 May 9;11(10):1828–1835. doi: 10.1021/bi00760a015. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Lenaers A., Lapiere C. M. Type III procollagen and collagen in skin. Biochim Biophys Acta. 1975 Jul 21;400(1):121–131. doi: 10.1016/0005-2795(75)90132-4. [DOI] [PubMed] [Google Scholar]
- Lent R., Franzblau C. Studies on the reduction of bovine elastin: evidence for the presence of delta-6,7-dehydrolysinonorleucine. Biochem Biophys Res Commun. 1967 Jan 10;26(1):43–50. doi: 10.1016/0006-291x(67)90250-1. [DOI] [PubMed] [Google Scholar]
- Lyerla J. R., Jr, Torchia D. A. Molecular mobility and structure of elastin deduced from the solvent and temperature dependence of 13C magnetic resonance relaxation data. Biochemistry. 1975 Nov 18;14(23):5175–5183. doi: 10.1021/bi00694a024. [DOI] [PubMed] [Google Scholar]
- Miller A., Wray J. S. Molecular packing in collagen. Nature. 1971 Apr 16;230(5294):437–439. doi: 10.1038/230437a0. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Robertson P. B. The stability of collagen cross-links when derived from hydroxylsyl residues. Biochem Biophys Res Commun. 1973 Sep 5;54(1):432–439. doi: 10.1016/0006-291x(73)90940-6. [DOI] [PubMed] [Google Scholar]
- Minor R. R., Clark C. C., Strause E. L., Koszalka T. R., Brent R. L., Kefalides N. A. Basement membrane procollagen is not converted to collagen in organ cultures of parietal yolk sac endoderm. J Biol Chem. 1976 Mar 25;251(6):1789–1794. [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C., Kuzan F. Studies on the action of lysyl oxidase on soluble elastin. Adv Exp Med Biol. 1977;79:491–508. doi: 10.1007/978-1-4684-9093-0_42. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE S. M., DAVIS H. F., ADAIR G. S. The chemistry of connective tissues. 2. Soluble proteins derived from partial hydrolysis of elastin. Biochem J. 1955 Sep;61(1):11–21. doi: 10.1042/bj0610011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S. M. Biosynthesis and nature of elastin structures. Fed Proc. 1966 May-Jun;25(3):1023–1029. [PubMed] [Google Scholar]
- Partridge S. M., Whiting A. H. The coacervate-sol transition observed with alpha-elastin and its N-formyl O-methyl derivative. Adv Exp Med Biol. 1977;79:715–723. doi: 10.1007/978-1-4684-9093-0_60. [DOI] [PubMed] [Google Scholar]
- Pope F. M., Nicholls A. C. Molecular abnormalities of collagen. J Clin Pathol Suppl (R Coll Pathol) 1978;12:95–104. [PMC free article] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. Relative stabilities of the intermediate reducible crosslinks present in collagen fibres. FEBS Lett. 1973 Jul 1;33(2):167–174. doi: 10.1016/0014-5793(73)80184-x. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. The chemistry of the collagen cross-links. The characterization of fraction C, a possible artifact produced during the reduction of collagen fibres with borohydride. Biochem J. 1973 Dec;135(4):657–665. doi: 10.1042/bj1350657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg L. B., Weissman N., Gray W. R. Structural features of tropoelastin related to the sites of cross-links in aortic elastin. Biochemistry. 1971 Jan 5;10(1):52–56. doi: 10.1021/bi00777a008. [DOI] [PubMed] [Google Scholar]
- Siegel R. C. Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4826–4830. doi: 10.1073/pnas.71.12.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Abraham P. A., Carnes W. H. Crosslinkage of salt-soluble elastin in vitro. Biochem Biophys Res Commun. 1975 Oct 6;66(3):893–899. doi: 10.1016/0006-291x(75)90724-x. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Brown D. M., Carnes W. H. Preparation and properties of salt-soluble elastin. J Biol Chem. 1972 Apr 25;247(8):2427–2432. [PubMed] [Google Scholar]
- Smith J. W. Molecular pattern in native collagen. Nature. 1968 Jul 13;219(5150):157–158. doi: 10.1038/219157a0. [DOI] [PubMed] [Google Scholar]
- Sykes B. C., Partridge S. M. Salt-soluble elastin from lathyritic chicks. Biochem J. 1974 Aug;141(2):567–572. doi: 10.1042/bj1410567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B., Hawker S. Some investigations of elastin biosynthesis in vitro using an immunoprecipitant. Adv Exp Med Biol. 1977;79:453–459. doi: 10.1007/978-1-4684-9093-0_39. [DOI] [PubMed] [Google Scholar]
- THOMAS J., ELSDEN D. F., PARTRIDGE S. M. PARTIAL STRUCTURE OF TWO MAJOR DEGRADATION PRODUCTS FROM THE CROSS-LINKAGES IN ELASTIN. Nature. 1963 Nov 16;200:651–652. doi: 10.1038/200651a0. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Housley T., Berube L., Fairweather R., Franzblau C., Gallop P. M. Structure of two histidine-containing crosslinks from collagen. J Biol Chem. 1973 Jan 25;248(2):393–402. [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Nowack H., Wiedemann H., Fietzek P. P., Kühn K. Isolation, chemical and electron microscopical characterization of neutral-salt-soluble type III collagen and procollagen from fetal bovine skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1783–1792. doi: 10.1515/bchm2.1975.356.2.1783. [DOI] [PubMed] [Google Scholar]
- Traub W., Piez K. A. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Long M. M. Conformations of the repeat peptides of elastin in solution: an application of proton and carbon-13 magnetic resonance to the determination of polypeptide secondary structure. CRC Crit Rev Biochem. 1976 Jun;4(1):1–45. doi: 10.3109/10409237609102557. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Starcher B., Partridge S. M. Coacervation of solubilized elastin effects a notable conformational change. Nature. 1969 May 24;222(5195):795–796. doi: 10.1038/222795a0. [DOI] [PubMed] [Google Scholar]
- Weis-Fogh T., Anderson S. O. New molecular model for the long-range elasticity of elastin. Nature. 1970 Aug 15;227(5259):718–721. doi: 10.1038/227718a0. [DOI] [PubMed] [Google Scholar]
- Wiedemann H., Chung E., Fujii T., Miller E. J., Kühn K. Comparative electron-microscope studies on type-III and type-I collagens. Eur J Biochem. 1975 Feb 21;51(2):363–368. doi: 10.1111/j.1432-1033.1975.tb03936.x. [DOI] [PubMed] [Google Scholar]


