Abstract
Focal adhesion kinase (FAK) is activated following integrin engagement or stimulation of transmembrane receptors. Autophosphorylation of FAK on Tyr-397 is a critical event, allowing binding of Src family kinases and activation of signal transduction pathways. Tissue-specific alternative splicing generates several isoforms of FAK with different autophosphorylation rates. Despite its importance, the mechanisms of FAK autophosphorylation and the basis for differences between isoforms are not known. We addressed these questions using isoforms of FAK expressed in brain. Autophosphorylation of FAK+, which is identical to that of “standard” FAK, was intermolecular in transfected cells, although it did not involve the formation of stable multimeric complexes. Coumermycin-induced dimerization of gyrase B-FAK+ chimeras triggered autophosphorylation of Tyr-397. This was independent of cell adhesion but required the C terminus of the protein. In contrast, the elevated autophosphorylation of FAK+6,7, the major neuronal splice isoform, was not accounted for by transphosphorylation. Specifically designed immune precipitate kinase assays confirmed that autophosphorylation of FAK+ was intermolecular, whereas autophosphorylation of FAK+6,7 or FAK+7 was predominantly intramolecular and insensitive to the inhibitory effects of the N-terminal domain. Our results clarify the mechanisms of FAK activation and show how alternative splicing can dramatically alter the mechanism of autophosphorylation of a protein kinase.
The nonreceptor tyrosine kinase focal adhesion kinase (FAK) plays a key role at focal adhesion sites in promoting spreading, migration, and transmission of anchorage-dependent antiapoptotic signals (50). The important physiological role of FAK is demonstrated by the lethality of its null mutation around midgestation, with severe developmental defects (33). FAK is phosphorylated on tyrosine in response to integrin engagement, G protein-coupled receptor activation, and growth factor receptor stimulation (20, 57). In cultured cells, integrin engagement promotes FAK recruitment to focal adhesions and its phosphorylation on multiple tyrosine residues (26, 46). Tyrosine phosphorylation of FAK results from autophosphorylation and from phosphorylation by other tyrosine kinases, including Src family kinases (4, 11) and insulin receptor (1). In contrast to many other kinases (see reference 32), autophosphorylation of FAK does not occur in the activation loop of the catalytic domain (A-loop), but on a tyrosine located at position 397, in the linker region between the N-terminal domain and the catalytic domain.
Autophosphorylation at Tyr-397 is a key event for FAK biological function, since it creates a high-affinity binding site for proteins with SH2 domains, including Src family kinases (c-Src, Fyn) (11) that play a major role in integrin signaling (6). Following their binding to phospho-Tyr-397, Src family kinases phosphorylate FAK on other tyrosine residues (4, 5, 48). Phosphorylation of Tyr-576 and 577, located in the A-loop, increases FAK activity (4), whereas phosphorylation of Tyr-925 provides a binding site for the SH2 domain of Grb2, which can mediate ERK 1/2 activation through the Ras pathway (48). Phospho-Tyr-397 is also a docking site for phosphatidylinositide 3′-OH-kinase (8), which activates the antiapoptotic Akt pathway (53). Thus, the available evidence indicates that FAK functions essentially as an adapter protein regulated by autophosphorylation that triggers the activation of various signal transduction pathways.
Alternative RNA splicing results in the formation of several FAK isoforms, which are highly conserved among vertebrates (2, 3, 28). The “standard” isoform, here referred to as FAK°, is devoid of additional exons, whereas FAK molecules expressed in neurons and also in some other cell types, including astrocytes, contain a 3-amino-acid insertion in the C-terminal region and are referred to as FAK+ (3, 14, 37). (FAK isoform nomenclature: FAK° refers to the standard isoform without additional peptides. FAK+ corresponds to the presence of three additional residues inserted after residue 903; FAK6 corresponds to six additional residues inserted after residue 392; FAK7 corresponds to seven additional residues after position 411 and a frameshift mutation that causes a Thr to Ala residue substitution. FAK+6,7 corresponds to the isoform with these three insertions. FAK+6,7,28 contains an additional 28 residues immediately N-terminal to box 6. To avoid confusion, in this report we always use the numbering of residues corresponding to the sequence of rat FAK°, not taking into account the change in numbering resulting from the presence of additional exons or additional polypeptides in fusion proteins.)
FAK+6,7, the major isoform of FAK expressed in neurons, contains two additional short peptides (boxes 6 and 7) on either side of Tyr-397 (3, 15). A minor brain isoform, FAK+6,7,28, contains an additional 28-residue peptide immediately C-terminal to box 6 (3). All isoforms undergo autophosphorylation in cells and in vitro and are able to recruit Src family kinases when Tyr-397 is phosphorylated (3, 55). However, alternative splicing dramatically alters the autophosphorylation rate: FAK+ has the same low autophosphorylation capacity as FAK°, whereas FAK+6,7 and FAK+6,7,28 display an increased autophosphorylation in transfected cells and in immune precipitate kinase assays (3, 55). Boxes 6 and 7 both contribute to this enhanced autophosphorylation (55). Insertion of boxes 6 and 7 in chicken FAK replicates the enhanced autophosphorylation (19). In rodent hippocampus, the autophosphorylation of FAK+6,7 is stimulated by several neurotransmitters and neuromodulators (13-15, 52), and this isoform of FAK may play an important role in neuronal development and synaptic plasticity (see reference 20 for a discussion).
Thus, autophosphorylation is a critical aspect of FAK function and is facilitated by alternative RNA splicing in neurons. However, in spite of its functional importance, the precise mechanisms leading to FAK autophosphorylation on Tyr-397 and the basis for the differences between isoforms are not known. Previous studies with CD2-FAK fusion proteins have shown that constitutive recruitment of FAK to the plasma membrane results in its permanent tyrosine phosphorylation (7), but the mechanism of this effect was not established. Interestingly, with mutants of the ATP-binding site, it has been shown that transphosphorylation of FAK (16) and of CD2-FAK chimeras is possible (7). Therefore, by analogy with what is known about the activation of receptor tyrosine kinases (56), it is generally assumed without further evidence that phosphorylation of FAK on Tyr-397 may result from intermolecular autophosphorylation (50).
The present study was designed to examine the mechanisms of autophosphorylation of FAK and to elucidate the basis for the differences between isoforms. We used the isoforms expressed in brain, which can be specifically distinguished from COS-7 endogenous FAK° by their 3-residue C-terminal insert, which is recognized by specific antibodies. Our results show that the autophosphorylation of FAK+ on Tyr-397 results from an intermolecular reaction and that FAK+ dimerization is sufficient to induce Tyr-397 phosphorylation. FAK+6,7 has the additional capacity to undergo intramolecular autophosphorylation and is less sensitive to inhibition by the N-terminal domain. These results provide mechanistic insights into the mechanism of FAK activation and explanations for the high capacity of autophosphorylation of the neuronal isoform.
MATERIALS AND METHODS
Materials.
Antiserum SL 41 was raised against a synthetic peptide encompassing residues 901 to 911 of FAK+ (52). Anti-phospho-Tyr-397 antibodies were either affinity-purified SL625857 (55) or commercially available antibodies from Biosource Inc. (1:2,000 for immunoblotting). These antibodies have been fully characterized, react specifically with FAK phosphorylated on Tyr-397, and do not cross-react with the unphosphorylated form or with other phosphorylated residues of FAK (44, 55). Antibodies reacting specifically with FAK phosphorylated on Tyr-577 or Tyr-925 were from Biosource Inc. (1:2,000 for immunoblotting). Anti-FAK polyclonal antibodies directed against residues 2 to 18 (1:250 for immunoblotting), were from Santa Cruz Biotechnology Inc. (A-17) or produced by Agro-Bio (La Ferté, Saint Aubin, France). Antiphosphotyrosine monoclonal antibody 4G10 (1:4,000 for immunoblotting) was from Upstate Biotechnologies Inc. Anti-vesicular stomatitis virus (VSV) antibodies (1:250 for immunoblotting) were raised in a rabbit, and antihemagglutinin (HA) mouse monoclonal antibody (1:500 for immunoblotting) was from Boehringer. Coumermycin, novobiocin, and dimethyl sulfoxide were from Sigma, and PP2 [4-amino-5-(chlorophenyl)-7-(t-butyl)pyrazolol(3,4-d)pyrimidine)] was from Calbiochem.
Subcloning and construction of FAK+ mutants and of Gyr-FAK+
In all experiments, we used rat FAK+ (2) or isoforms FAK+6,7, FAK+7, and FAK+6,7,28 (3, 55), which have in common a 3-amino-acid insertion (Pro-Trp-Arg) in the C-terminal region of the protein. The presence of this insertion does not alter the localization or autophosphorylation of FAK and allows its identification with specific antibodies without the necessity for peptide tagging (55). The kinase-dead mutants (K454R) and the autophosphorylation site mutants (Y397F) were generated with the Quick-Change site-directed mutagenesis kit (Stratagene). The double mutant (K454R, Y397F) was created by introducing the fragment containing Y397F in the kinase-dead mutant at the SphI and ClaI restriction sites.
HA-FAK+ was obtained by placing the FAK+ sequence 3′ of the HA tag in a pECE expression vector, with PvuII and XbaI restriction sites. To create VSV-FAK+, the SacI site was eliminated from pBlueScript.SK+ (pBS.SK+) (Stratagene), and the VSV tag was introduced by SmaI and BamHI digestion. A FAK PCR fragment produced with the primers MF9 (5′-TCTGCAGCATCAGCAGCC-3′) and FAKSTOPMOD1 (5′-TCAACCCGGGCCGTGTCTGCCCTAGCAT-3′), which contains an XbaI site placed just before the stop codon, was introduced in pBS.SK+, 5′ of the VSV tag by XbaI-PstI digestion. The SacI-XbaI fragment was then transferred into FAK+ sequence.
For expression in COS-7 cells, the various constructs were placed in pBK-CMV2, derived from pBK-CMV (Stratagene) by deletion of an NheI-SalI fragment corresponding to the bacterial promoter. The chimeric protein Gyr-FAK+ was generated with the 24-kDa N-terminal fragment of the B subunit of bacterial DNA gyrase (gyrase B) (17), kindly given by M. Farrar. The corresponding sequence was introduced in an SpeI site created in rat FAK+ at the position of amino acid 18 with the Quick-Change site-directed mutagenesis kit (Stratagene).
C-terminal deletion mutations (841 to 1054, with the numbering of residues in FAK°) of FAK+ and FAK+6,7,28, were obtained by transferring the XbaI-XhoI fragment of rat FAK cDNA (3) from pBS.SK+ into an SpeI- and XhoI-digested pBK-CMV2 vector. To construct Gyr-FAK+Δ841-1054, the 1.8-kb XhoI (FAK 2950)-MluI (vector 463) fragment of Gyr-FAK+ was replaced by a 0.6-kb XhoI-MluI fragment from FAKΔ841-1054 bearing a stop codon immediately after the XhoI site. The 1 to 386 deletion of FAK+ and FAK+6,7,28 was created through multiple steps: elimination of the SacI restriction site in the pBS.SK+ vector; insertion of the XbaI-EcoRI PCR fragment containing an ATG in a Kozak sequence identical to that of the natural FAK initiation codon; insertion of the EcoRI-XhoI fragment from pBK-FAK+ (805 to 2950) or pBK FAK+6,7,28 (805 to 3040); elimination of the SacI-SphI (linker 1583) fragment; and insertion of the XbaI-XhoI (linker 2950 or 3040) fragments into SpeI- and XhoI-digested (linker 2950) pBK-CMV2 FAK+. All mutated forms were verified by sequencing.
Cell culture and transfection.
COS-7 cells were grown on 100-mm-diameter culture dishes at 37°C in a humidified atmosphere with 5% CO2. The transfections were carried out in the presence of polyethyleneimine, as described previously (55), with a final amount of 8 μg of DNA per dish. For experiments with Gyr-FAK+, cells were serum starved in Dulbecco's modified Eagle's medium (DMEM) for 18 h the day after transfection and treated with 10 μM coumermycin, 10 μM novobiocin, or vehicle (dimethyl sulfoxide) for the indicated times. PP2 (10 μM) was applied 20 min before and during coumermycin or novobiocin treatment. All drugs were diluted in dimethyl sulfoxide and mixed with DMEM before addition to the culture dishes. Suspended cells were prepared by incubating the cultures in 2 ml of HEPES containing trypsin (2.5 mg/ml) and EDTA (1 mM; Gibco-BRL). When cells were detached, soybean trypsin inhibitor (Sigma) was added to a final concentration of 0.5 mg/ml in DMEM containing 2% (wt/vol) bovine serum albumin, and cells were washed once in DMEM-2% bovine serum albumin. Cells were gently resuspended in DMEM-2% bovine serum albumin, and 14 ml of the suspension was incubated in 15-ml polypropylene tubes on a shaking platform at 37°C.
Immunoprecipitation and immunoblotting.
Cells were used 48 h after transfection. For immunoprecipitation, COS-7 cells were homogenized in modified radioimmunoprecipitation assay (RIPA) buffer (1% Triton X-100, 0.5% [wt/vol] deoxycholate, 0.1% [wt/vol] SDS, 50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM sodium orthovanadate, and complete proteases inhibitors [Boehringer]), as described previously (55). The homogenates were precleared with 120 μl of Sephacryl beads, and immunoprecipitation was carried out with 30 μl of rabbit antiserum SL41 coupled to protein A-Sepharose, as described previously (38).
For direct immunoblot analysis, cells were lysed in 1% sodium dodecyl sulfate (SDS) and 1 mM sodium orthovanadate. Immunoblotting after electrophoresis in a 7% (wt/vol) polyacrylamide gel in the presence of SDS and peroxidase chemiluminescence detection of antibodies were carried out as described previously (55). Quantification was achieved by computer-assisted densitometry scanning of autoradiograms.
In vitro kinase assays.
For in vitro kinase assays, COS-7 cells were homogenized in ice-cold nondenaturing buffer (20 mM Tris-HCl [pH 7.4], 2 mM EDTA) and protease inhibitors (Boehringer) in the absence of sodium orthovanadate. Prior to immunoprecipitation, homogenates were incubated for 10 min with the catalytic domain of receptor-like protein tyrosine phosphatase β (RPTP-β), a receptor-like protein tyrosine phosphatase, in fusion with glutathione S-transferase (GST-RPTP-β; a generous gift of Janine Ragab, INSERM, Toulouse, France), coupled to glutathione-Sepharose beads. This treatment permitted removal of endogenous phosphate from tyrosine residues. Beads were removed by centrifugation, and FAK+ was immunoprecipitated from the supernatants. Immune precipitate kinase assays were carried out for 5 min at 25°C in 50 μl of buffer containing 50 mM HEPES (pH 7.4), 10 mM MnCl2, 1 μM ATP, and 5 μCi of [γ-32P]ATP (3,000 Ci/mmol; NEN Life Science Products).
To study the kinase activity of FAK, 50 μg of poly(Glu, Tyr) 4:1 (Sigma) was added, and samples were incubated for 4 min at 25°C prior to the addition of ATP. In kinase assays designed to test the inter- or intramolecular nature of FAK autophosphorylation (cis/trans immunoprecipitate kinase [CITIK] assay; see Results), the lysate from one 100-mm-diameter culture dish of transfected COS-7 cells was diluted, dispatched in seven tubes, and used for immunoprecipitation with the same amount of protein A but increasing amounts of serum. The immune precipitates were further treated for in vitro kinase assays as described above. Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), 7% (wt/vol) acrylamide or 10% for truncated forms, and transferred to nitrocellulose. Quantification of 32P incorporation was achieved by direct measurement of the radioactivity with an Instant Imager (Packard). The levels of FAK on the membranes were determined by immunoblotting.
RESULTS
FAK autophosphorylation occurs in trans in intact cells.
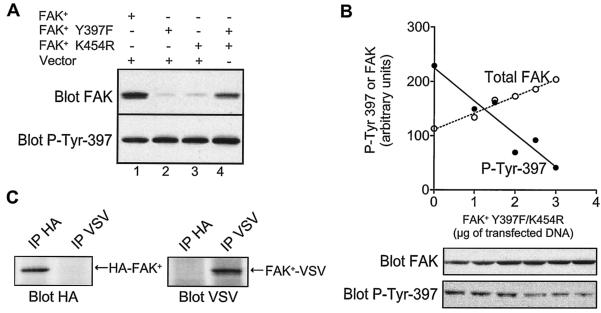
To determine whether phosphorylation of Tyr-397 can be intermolecular, COS-7 cells were transfected with wild-type or kinase-dead (FAK+ K454R) and autophosphorylation site point-mutated (FAK+ Y397F) forms of FAK+, alone or in combination. The phosphorylation of Tyr-397 was measured by immunoblotting with a phosphorylation state-specific antibody, which reacts specifically with FAK phosphorylated on Tyr-397 (55). As expected, wild-type FAK+ was phosphorylated on Tyr-397 in adherent COS-7 cells (Fig. 1A, lane 1), whereas neither mutated form transfected alone was phosphorylated (Fig. 1A, lanes 2, 3). In contrast, when the two mutated forms of FAK+ were transfected together, a significant phosphorylation of FAK+ K454R on Tyr-397 was observed (Fig. 1A, lane 4). No transphosphorylation of FAK+ K454R by FAK+ Y397F was observed in suspended cells (data not shown) demonstrating that the intermolecular phosphorylation observed in transiently transfected cells corresponded to the normal, adhesion-dependent mechanism of phosphorylation of FAK.
FIG. 1.
Transphosphorylation of FAK+ Tyr-397 in COS-7 cells. (A) COS-7 cells were transfected with 4 μg of plasmids coding for FAK+, FAK+ Y397F, FAK+ K454R (kinase dead), or empty pBK-CMV2 vector (vector), as indicated. Immunoblotting of cell lysates was carried out with antibodies recognizing specifically FAK phosphorylated on Tyr-397 (SL625857, blot P-Tyr 397) or independently of its phosphorylation state (A-17, blot FAK). Results are representative of three independent experiments. (B) COS-7 cells were transfected with 2 μg of plasmid coding for wild-type FAK+ and increasing amounts of the FAK+ K454R/Y397F double mutant. The total amount of DNA was kept constant by the addition of vector. The phospho-Tyr 397 immunoreactivity (blot P-Tyr-397) of transfected FAK+ and the total immunoreactivity of FAK+ and FAK+ K454R/Y397F (blot FAK) were quantified from immunoblots by densitometry scanning of autoradiograms and analysis with NIH Image software. Plotted data are the means of two independent experiments. (C) To determine whether stable complexes comprising several molecules of FAK were formed in cells, COS-7 cells were cotransfected with HA-FAK+ and FAK+-VSV. After 48 h, cells were lysed, and the lysates were subjected to immunoprecipitation with anti-HA (IP HA) or anti-VSV (IP VSV) antibodies. The samples were immunoblotted with anti-HA (blot HA) or anti-VSV (blot VSV) antibodies.
To determine the contribution of an intermolecular mechanism in the autophosphorylation of wild-type FAK, we transfected COS-7 cells with FAK+ and increasing amounts of the FAK+ Y397F/K454R double mutant (Fig. 1B). We observed a dose-dependent inhibition of FAK+ phosphorylation on Tyr-397, as expected if the double mutant was competing in the intermolecular autophosphorylation reaction.
If FAK autophosphorylation occurs as an intermolecular reaction in intact cells, it could result from the oligomerization of two or more FAK molecules by themselves or in combination with other proteins. To test whether complexes containing several molecules of FAK could exist, we cotransfected COS-7 cells with two differently tagged FAK+ molecules (HA-FAK+ and FAK+-VSV) and examined whether they coimmunoprecipitated. Neither anti-HA nor anti-VSV immunoprecipitation pulled down the other tagged molecule (Fig. 1C). As a control, we verified that p130-Cas, a protein known to be associated with FAK (27), coimmunoprecipitated with FAK+ in the same experimental conditions (data not shown). Other approaches attempted to detect possible stable intermolecular interactions between FAK domains (coimmunoprecipitation experiments with truncated mutants of FAK and yeast two-hybrid assays) were also negative (data not shown). Altogether, these results show that FAK autophosphorylation occurs as an intermolecular reaction in intact cells but that FAK molecules do not form stable complexes.
Induced dimerization of Gyr-FAK+ fusion proteins strongly increases Tyr-397 phosphorylation in intact cells.
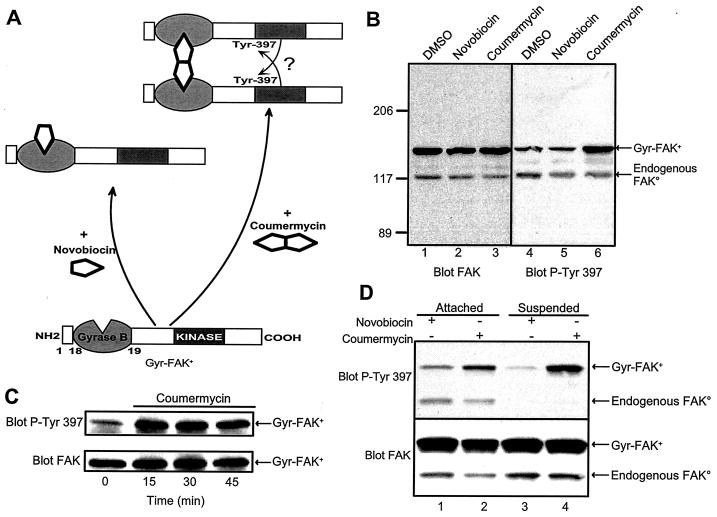
The results reported above confirm and extend previous reports (7, 16) and indicate that FAK autophosphorylation occurs as an intermolecular reaction in cells. To determine whether bringing together two molecules of FAK is sufficient to induce phosphorylation of Tyr-397, we used the procedure described by Farrar et al. (17), in which fusion of the protein of interest to the N-terminal domain of the B subunit of bacterial DNA gyrase (gyrase B) allows its drug-induced dimerization in intact cells, as depicted in Fig. 2A. Coumermycin, a small, divalent, cell-permeating molecule, binds two gyrase B moieties and induces the dimerization of the fusion protein. The use of novobiocin, a related monovalent molecule that binds to the same site but is unable to induce dimerization, provides an optimal control condition ensuring that coumermycin effects are due to its ability to promote dimerization. Gyr-FAK+ molecules migrated as a 150-kDa band and were easily distinguished from endogenous FAK, which has an apparent molecular mass of ≈125 kDa (Fig. 2B, lanes 1 to 3).
FIG. 2.
Coumermycin-induced dimerization enhances phosphorylation of gyrase B-FAK+ fusion proteins (Gyr-FAK+) on Tyr-397. (A) Schematic representation of the gyrase B-inducible dimerization system (17) applied to FAK+. The positions of the dimerization domain of the B subunit of bacterial gyrase (gyrase B), the central catalytic domain of FAK+ (kinase), and Tyr-397 are indicated. Addition of coumermycin, a divalent ligand of gyrase B, leads to the formation of Gyr-FAK+ homodimers. Novobiocin, a monovalent molecule also able to bind to gyrase B, is unable to induce its dimerization and was used as a negative control. (B) COS-7 cells transfected with Gyr-FAK+ were treated for 15 min with dimethyl sulfoxide (DMSO, vehicle), 10 μM novobiocin, or 10 μM coumermycin. Cell lysates were analyzed directly by immunoblotting with an antibody reacting with all isoforms of FAK (A-17, blot FAK). The apparent molecular weights of endogenous FAK° and Gyr-FAK+ were approximately 125,000 and 150,000, respectively. Phosphorylation of Tyr-397 was assessed with specific antibodies (SL625857, blot P-Tyr 397). (C) Time course of coumermycin-induced phosphorylation of Tyr-397. COS-7 cells transfected with Gyr-FAK+ were incubated in the presence of 10 μM coumermycin for the indicated times. Gyr-FAK+ immune precipitates were subjected to immunoblotting with anti-phospho-Tyr-397 (SL625857, blot P-Tyr 397) or anti-FAK (A17, blot FAK). Results are representative of three independent experiments. (D) The effects of coumermycin treatment on Gyr-FAK+ were examined in attached or suspended transfected COS-7 cells. After incubation in the presence of 10 μM novobiocin or coumermycin, the cells were lysed in RIPA buffer. An aliquot of the lysate was used for immunoblotting with antibodies specific for phospho-Tyr 397 (Biosource, blot P-Tyr 397), and the total amount of Gyr-FAK+ was determined by immunoblotting with an antibody reacting with FAK independently of its level of phosphorylation (A-17, blot FAK). Gyr-FAK+ Tyr-397 phosphorylation was: novobiocin/attached, 7 ± 1; coumermycin/attached, 75 ± 10; novobiocin/suspended, 3 ± 1; coumermycin/suspended, 94 ± 5 (arbitrary units, mean ± standard error of the mean of three independent experiments) (analysis of variance: P < 0.05).
With this chimeric protein, we were able to test the effects of dimerization on FAK+ phosphorylation independently of any other stimulus. Phosphorylation of Tyr-397 was measured with a specific antibody (Fig. 2B, lanes 4 to 6). Treatment of transfected COS-7 cells with coumermycin markedly increased the phosphorylation of Gyr-FAK+ on Tyr-397 (Fig. 2B, lane 6). In contrast, novobiocin did not alter Gyr-FAK+ phosphorylation (Fig. 2B, lane 5), demonstrating that the effects of coumermycin were due to drug-induced dimerization. Neither coumermycin nor novobiocin altered the total levels of Gyr-FAK+ (Fig. 2B, lanes 1 to 3) or the phosphorylation of endogenous FAK° (Fig. 2B, lanes 4 to 6). The increase in the phospho-Tyr-397 signal of Gyr-FAK+ observed at 15 min was stable at later time points (Fig. 2C). These results demonstrate that drug-induced dimerization of FAK+ markedly and specifically stimulates its phosphorylation on Tyr-397.
Coumermycin-induced phosphorylation of Gyr-FAK+ on Tyr-397 is independent of cell attachment and of Src family kinases but requires the C-terminal region of the protein.
FAK phosphorylation is strongly dependent on cell attachment and, more precisely, on the integrin-mediated adhesion of cells to the extracellular matrix (50, 51). We examined the role of cell attachment in the dimerization-induced autophosphorylation of Gyr-FAK+. As described above, coumermycin induced a robust phosphorylation of Gyr-FAK+ on Tyr-397 in attached cells (Fig. 2D, lane 2). In suspended cells, although phosphorylation of endogenous FAK was undetectable (Fig. 2D, lanes 3 and 4), coumermycin stimulated phosphorylation of Gyr-FAK+ on Tyr-397 to the same levels as in attached cells (Fig. 2D, lane 4). These results demonstrate that drug-induced dimerization of Gyr-FAK+ increases its phosphorylation on Tyr-397 by a mechanism that bypasses the normal requirement for cell attachment.
Autophosphorylation of FAK on Tyr-397 allows the recruitment of Src family kinases and, thus, phosphorylation of several other tyrosine residues in the FAK sequence (4, 5, 47, 48). Src family kinases appear to be critical for full phosphorylation of Tyr-397 in response to integrin engagement, presumably through an amplification mechanism involving phosphorylation of the activation loop residues (42). We examined the role of Src family kinases in coumermycin-induced activation of Gyr-FAK+ with PP2, an inhibitor of these enzymes (25, 45).
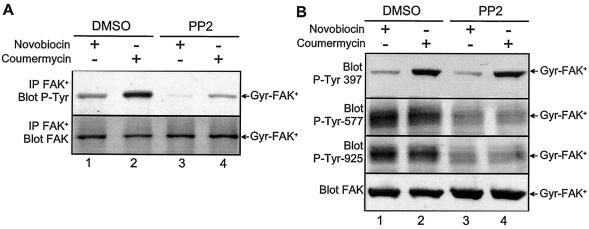
We immunoprecipitated Gyr-FAK+ with antibodies specific for the FAK+ isoform, which do not react with the FAK° isoform endogenously expressed in COS-7 cells (14), and measured its total tyrosine phosphorylation with anti-phospho-Tyr antibodies (Fig. 3A). The overall tyrosine phosphorylation of Gyr-FAK+ was increased by coumermycin compared to novobiocin (Fig. 3A, lanes 1, 2). Although PP2 markedly decreased the total tyrosine phosphorylation of Gyr-FAK+ in both novobiocin- and coumermycin-treated cells, a residual stimulatory effect of coumermycin was still present (Fig. 3A, lanes 3, 4).
FIG. 3.
Dimerization-induced phosphorylation of Tyr-397 in Gyr-FAK+ is independent of Src family kinases. COS-7 cells transfected with Gyr-FAK+ were treated for 15 min with 10 μM novobiocin or 10 μM coumermycin. A specific Src family kinase inhibitor, PP2 (10 μM), or vehicle (dimethyl sulfoxide, DMSO) was added 20 min prior to coumermycin or novobiocin. (A) Gyr-FAK+ was immunoprecipitated with antibodies specific for FAK+, and its total phosphorylation was assessed with anti-phospho-Tyr antibodies (IP-FAK+ blot P-Tyr). and with antibodies reacting with FAK independently of its state of phosphorylation (IP-FAK+ blot FAK). (B) Total lysates from the same cells were immunoblotted with antibodies specific for phospho-Tyr-397, phospho-Tyr-577, phospho-Tyr-925, or total FAK. Results are representative of at least three experiments.
To determine precisely the contribution of Src family kinases, we examined simultaneously the phosphorylation levels of Tyr-397, the autophosphorylation site, and of two residues phosphorylated by Src, Tyr-577 in the activation loop and Tyr-925 in the C-terminal focal adhesion targeting (FAT) domain (Fig. 3B). Although coumermycin markedly increased the phosphorylation of Tyr-397, it had no effect on phosphorylation of Tyr-577 and Tyr-925 (Fig. 3B, lanes 1, 2). In the presence of PP2, coumermycin-induced phosphorylation of Tyr-397 was unaltered, whereas phosphorylation of both Tyr-577 and Tyr-925 was dramatically decreased (Fig. 3B, lanes 3, 4). These results are important because they demonstrate that induced dimerization of Gyr-FAK+ promotes its phosphorylation on Tyr-397 independently of Src family kinases. In addition, they suggest that, although Gyr-FAK+ is phosphorylated by Src family kinases as well as endogenous FAK in the absence of coumermycin (data not shown), the formation of a stable Gyr-FAK+ dimer in the presence of coumermycin prevents access of phospho-Tyr-397 to Src family kinases.
The C-terminal region of FAK encompasses a focal adhesion targeting domain (FAT) that is necessary and sufficient for targeting the protein to focal adhesions (29). Because of the critical role of its C terminus in FAK activation (51), we examined the consequences of the 841 to 1054 deletion on coumermycin-induced phosphorylation of Gyr-FAK+ (Fig. 4). Since coumermycin-induced phosphorylation of Gyr-FAK+ occurred independently of cell adhesion, we expected that it would not be altered by the C-terminal deletion.
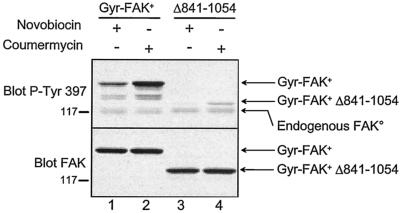
FIG. 4.
Dimerization-induced autophosphorylation of Gyr-FAK+ requires its C-terminal region. COS-7 cells were transfected with Gyr-FAK+ or Gyr-FAK+Δ841-1054 and incubated for 15 min in the presence of novobiocin or coumermycin. Cells were lysed, and phosphorylation of Tyr-397 and the total amount of FAK were determined by immunoblotting with specific antibodies. Note that endogenous FAK was not apparent in total FAK immunoblotting at the short exposure time used to allow comparison of Gyr-FAK+ and Gyr-FAK+Δ841-1054 levels. Results are representative of at least three experiments.
Contrary to that prediction, the effects of coumermycin on Gyr-FAK+Δ841-1054 were dramatically reduced (Fig. 4, lanes 3, 4) compared to full-length Gyr-FAK+ (Fig. 5, lanes 1, 2), although the two proteins were expressed at similar levels in transfected COS-7 cells. No further increase in Gyr-FAK+Δ841-1054 Tyr-397 phosphorylation was observed when coumermycin was applied for longer durations, up to 60 min (data not shown). The dramatic reduction of autophosphorylation of Gyr-FAK+Δ841-1054 was not due to a deficient kinase activity, since the full-length and truncated forms of Gyr-FAK+ had similar activities in vitro; the autophosphorylation activities of Gyr-FAK+ and Gyr-FAK+Δ841-1054 were, respectively, 205 ± 25 and 230 ± 20 (arbitrary units, mean ± standard error of the mean, n = 3). These observations reveal that the efficient phosphorylation of Gyr-FAK+ on Tyr-397 in response to induced dimerization in intact cells requires the presence of its C-terminal region.
FIG. 5.
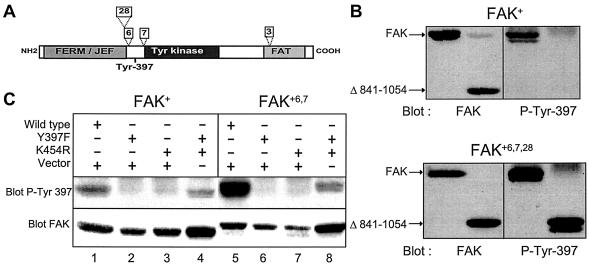
Differences in autophosphorylation between FAK splice isoforms in transfected cells. (A) The positions of the N-terminal FERM/JEF domain, the catalytic domain (Tyr kinase), the focal adhesion targeting (FAT) domain, and the peptides (boxes 28, 6, 7, and 3) coded by alternatively spliced exons are indicated. Tyr-397 is the autophosphorylation site. (B) Deletion of the C-terminal region (Δ841-1054) abolished autophosphorylation of FAK+ in transfected COS-7 cells, whereas it did not alter the autophosphorylation of FAK+6,7,28. The level of phosphorylation of Tyr-397 in intact cells was measured by immunoblotting with specific antibodies for phospho-Tyr-397 (blot P-Tyr-397). The total amount of FAK was measured with antibodies reacting with FAK independently of its state of phosphorylation (blot FAK). (C) Transphosphorylation in intact cells did not fully restore the increased autophosphorylation of FAK+6,7. COS-7 cells were transfected with 4 μg of plasmids coding for the wild-type, Y397F, or K454R forms of FAK+ or FAK+6,7, as indicated. Empty pBK-CMV2 vector was added when necessary to keep the total amount of transfected DNA constant. Cells were lysed on the culture dish. Immunoblotting was carried out with antibodies recognizing FAK phosphorylated on Tyr-397 (SL625857, blot P-Tyr 397) or independently of its phosphorylation state (A-17, blot FAK). Results are representative of three independent experiments which gave similar results.
Alternative splicing alters FAK autophosphorylation mechanism in transfected cells.
The neuronal splice variants of FAK that include boxes 6 and 7 (see Fig. 5A) have a dramatically increased autophosphorylation in immune precipitate kinase assays and in transfected cells (3, 55). Since the experiments with Gyr-FAK+ revealed the importance of the C-terminal region of FAK for dimerization-induced autophosphorylation, we compared the role of this region in the autophosphorylation of FAK+ and FAK+6,7,28 with truncated forms of either isoform (Fig. 5B). In transfected COS-7 cells, FAK+Δ841-1054 was not phosphorylated on Tyr-397 (Fig. 5B), confirming the importance of the C-terminal region of FAK+ for its autophosphorylation in intact cells. In contrast, the C-terminal truncation did not alter the phosphorylation of FAK+6,7,28 on Tyr-397 (Fig. 5B).
We verified that the C-terminal truncation did not alter the ability of FAK+ to autophosphorylate in immune precipitate kinase assays the autophosphorylation activity (arbitrary units, mean ± standard error of the mean, n = 4) was 363 ± 39 for FAK+ and 398 ± 52 for FAK+Δ841-1054. The truncation did not alter the ability of either of these kinases to phosphorylate an exogenous substrate, poly(Glu,Tyr), in immune precipitate kinase assays; the phosphorylation activity (arbitrary units, mean ± standard error of the mean, n = 3) was 1,665 ± 179 for FAK+ and 1,651 ± 204 for FAK+Δ841-1054. Thus, the different sensitivities of FAK isoforms to C-terminal truncation observed in transfected cells did not result from an alteration of the intrinsic kinase activity but more likely from a modification of the mechanism of autophosphorylation.
Since FAK+6,7 is the most abundant isoform expressed in neurons and has the same autophosphorylation characteristics as FAK+6,7,28 (3), further studies on the mechanism of autophosphorylation were carried out with FAK+6,7. To determine whether FAK+6,7 was able to undergo transautophosphorylation in intact cells, we used the approach described above for FAK+ (see Fig. 1). We transiently expressed wild-type and K454R and Y397F mutants of FAK+6,7, alone and in combination (Fig. 5C, lanes 5 to 8). For comparison, the same experiment was carried out in parallel with FAK+ (Fig. 5C, lanes 1 to 4). Although FAK+6,7 Y397F was able to transphosphorylate FAK+6,7 K454R (Fig. 5C, lane 8), this transphosphorylation did not reproduce the strong autophosphorylation observed with wild-type FAK+6,7 (Fig. 5C, compare lanes 5 and 8). These results suggest that although FAK+6,7 can undergo intermolecular phosphorylation on Tyr-397, this mechanism does not account for the increased level of autophosphorylation observed in intact cells.
Alternative splicing alters FAK autophosphorylation mechanism in vitro.
The experiments reported above provide strong evidence that FAK+ autophosphorylation is an intermolecular reaction in intact cells. Since the markedly increased autophosphorylation of FAK+6,7 appears to be independent of its C-terminal region, which is necessary for dimerization-induced autophosphorylation and is not reproduced by transphosphorylation in transfected cells (see above), we hypothesized that it might result from an intramolecular mechanism. To test this hypothesis, we investigated directly the inter- or intramolecular nature of the autophosphorylation reaction of FAK+ and FAK+6,7 in vitro.
Intermolecular autophosphorylation reactions are dependent on the kinase concentration, whereas intramolecular reactions are not. FAK autophosphorylation is usually measured in immune precipitate kinase assays, in which binding to antibodies may mimic the interaction of FAK with focal adhesion proteins. Since we have shown that FAK does not immunoprecipitate as oligomers (see Fig. 1C), it can be hypothesized that divalent antibodies artificially promote intermolecular phosphorylation. We designed simple experimental conditions to test this hypothesis and to study the mechanism of FAK autophosphorylation in immune precipitate kinase assays (referred to as CITIK assays).
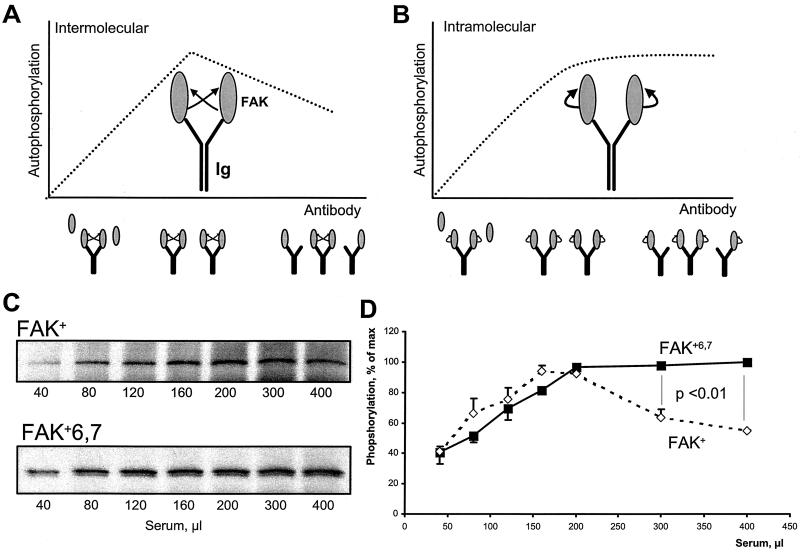
For CITIK assays, constant amounts of FAK were subjected to immunoprecipitation with increasing amounts of antiserum, with a fixed amount of protein A-Sepharose in a large excess in binding capacity, and an in vitro autophosphorylation reaction was carried out in the immune precipitate in the presence of [γ-32P]ATP. The principle of the assays is schematically illustrated in Fig. 6A and B. As the amount of serum is increased, two phases can be distinguished. With small amounts of serum, the available sites are saturated by the antigen and the amounts of precipitated FAK increase linearly with the amount of antibody. Above a certain threshold of serum, all the available antigen is precipitated, and further increasing the antibody level results in an excess of available binding sites for FAK, of which an increasing proportion are unoccupied. Study of the rate of phosphorylation in this second phase allows us to distinguish trans and cis mechanisms of autophosphorylation: intermolecular reactions, whose rate depends on the kinase concentration, will diminish when the amount of serum is increased above this threshold, since the probability that two molecules of FAK are close enough to transphosphorylate (i.e., presumably on the same immunoglobulin molecule) will decrease (Fig. 6A); in contrast, intramolecular reactions, whose rate is independent of the kinase concentration, will reach a plateau corresponding to the maximal amount of immunoprecipitated kinase (Fig. 6B). (In this model, the phosphorylation rate is proportional to the number of antibodies to which two molecules of FAK are bound. Assuming high affinities of antibodies for FAK, when the total number of antibodies [NAB] is greater than the number of antigens [NAG], i.e., FAK, the probability that the two sites of one antibody molecule are occupied varies as (NAG/NAB)2. Thus, when NAB > NAG, the phosphorylation rate varies as a function of 1/NAB2 when phosphorylation is intermolecular, whereas it is constant when it is intramolecular.)
FIG. 6.
Different mechanisms of autophosphorylation of FAK+ and FAK+6,7. (A and B) Principle of the CITIK assay. Equal amounts of lysates from COS-7 cells transfected with FAK are immunoprecipitated in the presence of increasing amounts of antiserum and constant amounts of protein A-Sepharose. Autophosphorylation assays are carried out in the immune precipitates, and the results are expected to be affected by the reaction mechanism as follows. For small amounts of antiserum, the quantity of specific antibodies against FAK is limiting, and all the available binding sites are expected to be occupied, as schematically indicated by two molecules of FAK per immunoglobulin (Ig). In these conditions, autophosphorylation increases with the amount of antiserum, reflecting the increasing amount of immunoprecipitated FAK, regardless of the cis or trans mechanism (ascending part of the curve). When the quantity of serum is further increased, the number of FAK-specific binding sites becomes greater than the number of FAK molecules, and an increasing proportion of immunoglobulin is expected to bind only one molecule of FAK or none. In these conditions, the trans and cis mechanisms of autophosphorylation can be clearly distinguished: intermolecular autophosphorylation will diminish with increasing the amount of serum, since the probability that two FAK molecules will be bound to the same immunoglobulin will decrease (A); intramolecular autophosphorylation is independent of immunoglobulin G-mediated interactions and will reach a plateau corresponding to the total amount of FAK (B). CITIK assays were carried out for FAK+ and FAK+6,7 as described in the text. Reaction products were separated by SDS-PAGE, transferred to nitrocellulose, and analyzed by immunoblotting (C), and incorporated 32P was quantified with an Instant Imager (D). The total amount of immunoprecipitated FAK, estimated by immunoblotting with FAK antibodies, was maximal with approximately 200 μl of antiserum and remained constant with higher amounts of antiserum (C). The amount of 32P incorporated was plotted as a function of the amount of antiserum for the two FAK isoforms (D). Results are expressed as percent of maximum to correct for the higher activity of FAK+6,7 compared to FAK+. Results are means ± standard error of the mean of three independent experiments. Statistical analysis was done with two-way analysis of variance. The results show a significant difference between FAK+6,7 and FAK+ for the relevant portion of the curve, supporting the hypothesis that phosphorylation is intramolecular in the case of FAK+6,7 and intermolecular in the case of FAK+.
We used CITIK assays to compare the mechanism of autophosphorylation of FAK+ and FAK+6,7. Immunoprecipitation was carried out with fixed amounts of COS-7 lysates and protein A-Sepharose and increasing amounts of an antiserum that specifically recognized FAK+ and FAK+6,7 but did not react with COS-7 cell endogenous FAK° (14). For each immunoprecipitation condition, a phosphorylation reaction was carried out in the presence of [γ-32P]ATP, and the phosphorylated proteins were separated by SDS-PAGE and transferred to nitrocellulose, allowing both immunoblotting to determine the total amount of FAK precipitated (Fig. 6C) and quantitative measurement of incorporated 32P with an Instant Imager (Fig. 6D).
As expected, the quantity of immunoprecipitated FAK increased progressively with the amount of antiserum and reached a plateau corresponding to complete immunoprecipitation at about 200 μl of antiserum (Fig. 6C). The differences between the phosphorylation of FAK+ and FAK+6,7 were readily apparent and statistically significant above this threshold of 200 μl (Fig. 6D): in the case of FAK+, the amount of 32P incorporated decreased progressively, as expected for an intermolecular autophosphorylation, whereas in the case of FAK+6,7, incorporation of 32P reached a plateau and did not decrease with increasing amounts of antibodies, showing that autophosphorylation was an intramolecular reaction.
Several control experiments were carried out to validate this conclusion. First, in both cases, the results obtained by immunoblotting for phospho-Tyr-397 were similar to those obtained by measurement of 32P incorporation, although they could not be quantified as accurately (data not shown). Second, we verified that the apparent intramolecular phosphorylation of FAK+6,7 was not due to its ability to form stable multiprotein complexes containing several molecules of FAK+6,7. To do so, experiments similar to those described for FAK+ in Fig. 1C were carried out with FAK+6,7, with identical results (data not shown). Finally, we used a completely different approach, in which constant amounts of either FAK+ or FAK+6,7 were immunoprecipitated with fixed amounts of antiserum but in the presence of increasing amounts of the FAK+ Y397F/K454R double mutant, resulting in dilution of the active kinase in the immune precipitate. These experiments also supported the contribution of an intramolecular mechanism of autophosphorylation for FAK+6,7 and not for FAK+ (data not shown).
Altogether, our results provide strong evidence that although FAK+6,7, like FAK+, is able to undergo intermolecular autophosphorylation, it possess a unique ability to undergo intramolecular phosphorylation. This distinctive capacity of FAK+6,7 is likely to account for its increased autophosphorylation in cells and in vitro.
Box 7 is sufficient to allow intramolecular autophosphorylation of FAK.
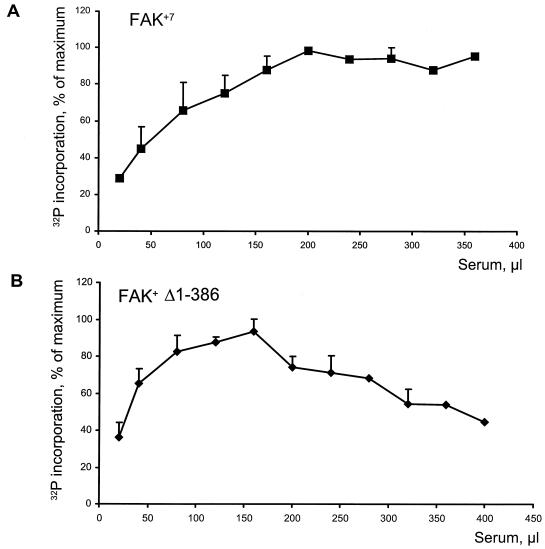
One simple hypothesis to account for the ability of the FAK neuronal isoform to undergo intramolecular phosphorylation is that the extra length of peptide chain generated by alternative splicing allows Tyr-397 to reach and/or to be correctly positioned in the active site of the same molecule. This hypothesis predicts a critical role for the 7-residue peptide (box 7) which is inserted at position 412, between Tyr-397 and the catalytic domain that starts around residue 422. Accordingly, we have shown previously that the presence of box 7 alone is sufficient to markedly increase the autophosphorylation of FAK (55). To test whether the presence of this peptide was also sufficient to alter the mechanism of autophosphorylation, we carried out a CITIK assay with FAK+7, an isoform of FAK which contains only box 7 (Fig. 7A). The results were similar to those obtained with FAK+6,7, demonstrating that FAK+7 could undergo intramolecular phosphorylation (Fig. 7A).
FIG. 7.
Autophosphorylation mechanism of FAK is altered by the presence of box 7 but not by N-terminal truncation. (A) The presence of box 7 is sufficient for intramolecular phosphorylation of Tyr-397. The CITIK assay was carried out with FAK+7 as described in the legend to Fig. 6. The results indicate that phosphorylation was intramolecular. (B) Deletion of the N-terminal FERM/JEF domain does not alter the autophosphorylation mechanism of FAK+. The CITIK assay was carried out with a truncated form of FAK+ (Δ1-386). The results indicate that autophosphorylation is intermolecular. In both cases, data are means ± standard error of the mean of three independent experiments.
Alternative splicing alleviates the inhibitory effects of the FAK N-terminal domain on autophosphorylation.
The N-terminal domain of FAK displays significant sequence similarity with the band 4.1 domain (21, 22), now referred to as the 4.1 ezrin, radixin, moesin (FERM) domain (10) or JAK, ERM, FAK (JEF) domain (21). Deletion of this domain or part of it has been reported to increase tyrosine phosphorylation and autophosphorylation activity (7, 49), indicating that the N-terminal FERM/JEF domain of FAK may have an inhibitory effect on FAK activity.
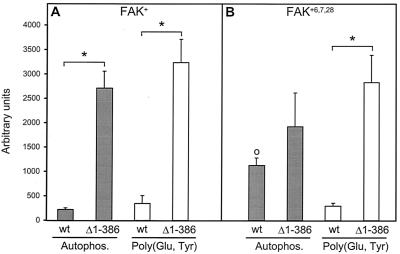
We examined the effects of removal of the N-terminal domain (1 to 386) on the autophosphorylation of FAK+ in immune precipitates and its ability to phosphorylate an exogenous substrate, poly(Glu, Tyr). The N-terminal truncation dramatically increased both the autophosphorylation of FAK+ and its kinase activity towards poly(Glu, Tyr) (Fig. 8A). However, in spite of its very strong effect on autophosphorylation, the truncation did not allow intramolecular phosphorylation, as judged by the CITIK assay (Fig. 7B). This demonstrates that the N-terminal domain exerts its inhibitory effect on intermolecular phosphorylation of Tyr-397 and is not responsible for the inability of FAK to undergo intramolecular phosphorylation.
FIG. 8.
Effects of deletion of the N-terminal FERM/JEF domain on autophosphorylation of FAK isoforms and their ability to phosphorylate an exogenous substrate. Wild-type (wt) FAK+, its N-terminally truncated form (FAK+Δ1-386) (left panel), and wild-type (wt) FAK+6,7,28 and its truncated form (FAK+6,7,28Δ1-386) (right panel) were expressed in COS-7 cells, dephosphorylated in vitro, and immunoprecipitated, and their autophosphorylation was assayed in the presence of [γ-32P]ATP (Autophos). In the same conditions, the ability of each immunoprecipitated isoform of FAK to phosphorylate an exogenous substrate, poly(Glu, Tyr), was examined. The amount of radioactivity incorporated into FAK or poly(Glu, Tyr) was measured with an Instant Imager after SDS-PAGE, and the counts per minute were normalized to the total amount of immunoprecipitated FAK, measured by immunoblotting. The autophosphorylation of FAK+6,7,28 was higher than that of FAK+ (○; P < 0.01, two-tailed Student's t test). N-terminal truncation increased the ability of FAK+ to autophosphorylate (∗; P < 0.005, two-tailed Student's t test), whereas it did not significantly alter the autophosphorylation of FAK+6,7,28 (left panel). In contrast, N-terminal truncation dramatically increased the ability of both isoforms to phosphorylate poly(Glu, Tyr) (∗, P < 0.05, two-tailed Student's t test). Values are means ± standard error of the mean for four (autophosphorylation) or three [phosphorylation of poly(Glu, Tyr)] experiments.
As reported previously (3, 55), the presence of peptide boxes coded by alternative exons around Tyr-397 increased its autophosphorylation, whereas it had little effect on the ability of FAK to phosphorylate poly(Glu, Tyr) (Fig. 8). Remarkably, however, the presence of these peptides altered the consequences of the removal of the FERM/JEF N-terminal domain: truncation of the FERM domain of FAK+6,7,28 did not significantly enhance its autophosphorylation (Fig. 8B). In contrast, the same deletion increased its ability to phosphorylate poly(Glu, Tyr) to the same extent as truncated FAK+ (Fig. 8B). These results indicate that the presence of additional peptides around Tyr-397 markedly diminishes the inhibitory effects of the N-terminal domain on FAK autophosphorylation but does not allow access to exogenous substrates. Thus, our results show that alternative splicing dramatically alters the ability of FAK to undergo autophosphorylation by two distinct mechanisms: by allowing intramolecular autophosphorylation and by alleviating the inhibitory effect of the N-terminal FERM/JEF domain.
DISCUSSION
Autophosphorylation of FAK on Tyr-397 plays a critical role in its functional activation in response to various stimuli (see references 20 and 50 for reviews). In contrast to many other tyrosine kinases (32), autophosphorylation of FAK takes place not in the A-loop, but on a linker region between the N-terminal FERM/JEF domain and the central kinase domain.
The present study clarifies the mechanism of FAK autophosphorylation and demonstrates that this mechanism differs markedly between alternatively spliced isoforms. Our results with Tyr-397→Phe and kinase-dead mutants show that FAK+ undergoes intermolecular phosphorylation in intact cells. Since FAK+ and FAK° (the isoform without additional alternatively spliced exons) have similar autophosphorylation properties (3), the results reported here with FAK+ can be generalized to the FAK° isoform. The observation that increasing amounts of FAK+ double mutant (Y397F, K454R) decreased the phosphorylation on Tyr-397 of cotransfected FAK+ strongly suggests that intermolecular phosphorylation is the major mechanism of autophosphorylation in intact cells. However, we were unable to isolate protein complexes containing several molecules of FAK, indicating that, in spite of its ability to bind various proteins of focal adhesions and signaling molecules, FAK does not form stable complexes with itself.
Interestingly, it has been reported that PYK2, a tyrosine kinase related to FAK (≈45% overall sequence identity), was able to transphosphorylate FAK on Tyr-397 in transfected cells, although the converse reaction did not occur (36). In that study, FAK and PYK2 did not coimmunoprecipitate (36). The fact that FAK molecules do not form stable homo-oligomers may have functional importance, allowing separation of the polypeptide chains once they are autophosphorylated and their action on other FAK molecules, a condition that seems to be a prerequisite for amplification by Src family kinases (see below).
Since autophosphorylation of FAK appears to be intermolecular in intact cells, we examined whether bringing two molecules of FAK into close proximity was sufficient to induce its autophosphorylation. To do so, we used fusion proteins of FAK+ with gyrase B, which dimerizes in the presence of coumermycin (17). This system has been used successfully to induce the dimerization of protein kinases (17, 35, 39) and other types of proteins (34, 43) in intact cells. Treatment of transfected cells with coumermycin was sufficient to increase tyrosine phosphorylation of Gyr-FAK+. Tyrosine phosphorylation occurred on Tyr-397, as demonstrated by immunoblotting with a specific antibody. Importantly, PP2, a Src family kinase inhibitor (25), had no effect on phosphorylation of Tyr-397 in response to coumermycin, demonstrating that dimerization-induced autophosphorylation of FAK is independent of these kinases.
Coumermycin-induced dimerization of Gyr-FAK+ did not trigger the phosphorylation of Tyr-577 or Tyr-925, presumably because Src family kinases could not access the autophosphorylated Tyr-397 in stable Gyr-FAK+ dimers. This observation underscores the importance of the reversibility of the interactions between FAK molecules, discussed above. Dimerization-induced autophosphorylation of Gyr-FAK+ was independent of cell adhesion, showing that dimerization of FAK induces its autophosphorylation independently of any other stimulus. However, we found that deletion of the C-terminal region of Gyr-FAK+ (residues 841 to 1054) dramatically decreased the level of coumermycin-induced autophosphorylation. This unexpected finding was not accounted for by an alteration of intrinsic kinase activity. It also seems unlikely that the phosphorylation deficit was due to an increased sensitivity to tyrosine phosphatases, since C-terminal deletions have been reported to provide resistance to dephosphorylation (51).
The C-terminal region of FAK contains binding sites for talin (9), paxillin (30, 54), Graf (31), and Hic-5 (18). It also encompasses the FAT sequence (29), which mediates targeting of FAK to focal adhesions, at least in part through its interaction with the focal adhesion proteins talin and paxillin (12, 30). One possible explanation for the effects of the C-terminal deletion on coumermycin's ability to induce Gyr-FAK+ autophosphorylation is the loss of such protein-protein interactions. These interactions could facilitate the effects of coumermycin by locally enriching the concentration of Gyr-FAK+, for example, in the vicinity of the membrane and/or cytoskeletal elements. Additional experiments will be required to determine whether any of these proteins exerts a more specific effect on FAK activation in intact cells.
When we examined the role of the C-terminal region in the phosphorylation of Tyr-397 of wild-type FAK in intact cells, we disclosed a dramatic difference between splicing isoforms, although this deletion had no effect on the intrinsic kinase activity of FAK in immune precipitate kinase assays. In transfected COS-7 cells, the C-terminal deletion prevented Tyr-397 phosphorylation in FAK+, whereas it had no effect on FAK+6,7,28. The lack of effect of the C-terminal deletion on isoforms with boxes 6 and 7 strongly suggested that the presence of additional peptide sequences surrounding Tyr-397 altered the mechanism of autophosphorylation. In addition, although FAK+6,7 was able to undergo intermolecular phosphorylation in intact cells, the cotransfection of Y397F and kinase-dead mutants of FAK+6,7 did not restore the high level of autophosphorylation of wild-type FAK+6,7. This observation also pointed at a possible difference in the autophosphorylation mechanism.
To address the intra- or intermolecular mechanism of autophosphorylation of FAK isoforms, we used a modified version of the immune precipitate kinase assays, referred to as the CITIK assay. This procedure has the advantage that it mimics to some extent the conditions in which FAK undergoes autophosphorylation in intact cells, since binding to antibodies is likely to mimic binding to cytoskeletal and/or membrane proteins. Moreover, CITIK assays allow us to address the mechanism of the reaction in the conditions of the widely used immune precipitate kinase assays. The results indicated that autophosphorylation of FAK+ was predominantly intermolecular, whereas autophosphorylation of FAK+6,7 was intramolecular. It should be emphasized that FAK+6,7 is able to undergo intermolecular phosphorylation on Tyr-397, as shown by transphosphorylation of Y397F and kinase-dead mutants in transfected cells (see above), as well as in vitro (data not shown). However, this isoform has the additional ability to undergo intramolecular phosphorylation, a process that seems predominant. The ability to undergo intramolecular phosphorylation accounts for the lack of sensitivity of FAK+6,7 to C-terminal truncation, which was shown to dramatically reduce dimerization-induced autophosphorylation.
Our results provide important clues about the possible mechanisms of FAK autophosphorylation. A simple hypothesis explaining the ability of FAK+6,7 to undergo cis autophosphorylation is that the presence of additional residues on either sides of Tyr-397 provides additional length and flexibility to the peptide chain, allowing it access to the catalytic site. This hypothesis would predict that the peptide (box 7) located between Tyr-397 and the catalytic site plays a major role in allowing intramolecular autophosphorylation. Interestingly, the presence of box 7 alone has a greater effect on autophosphorylation than box 6 alone (located between Tyr-397 and the N-terminal side of FAK) (55). The CITIK assay results showing that the presence of box 7 is sufficient to allow intramolecular phosphorylation support this hypothesis.
Other mechanisms are also likely to be involved in the control of FAK autophosphorylation. The present study, confirming previous suggestions (7, 49), demonstrates that the N-terminal FERM/JEF domain of FAK inhibits its ability to autophosphorylate as well as its ability to phosphorylate an exogenous substrate. Since deletion of the FERM/JEF domain of FAK+ did not alter the mechanism of autophosphorylation itself, which was still intermolecular, it can be concluded that the N-terminal domain hinders intermolecular autophosphorylation. The N-terminal domain may prevent the correct positioning of the two molecules and/or the access of Tyr-397 from one peptide chain to the active site of the other peptide chain. The presence of alternatively spliced peptides flanking Tyr-397 alleviated the inhibitory effects of the FERM/JEF domain on autophosphorylation, whereas it did not prevent the inhibition exerted by this domain on the phosphorylation of poly(Glu, Tyr). A possible explanation of these effects (diagrammed in Fig. 9) is that alternative splicing allows the positioning of Tyr-397 in the active site of the same molecule, in a position that is insensitive to the effects of the N-terminal domain.
FIG. 9.
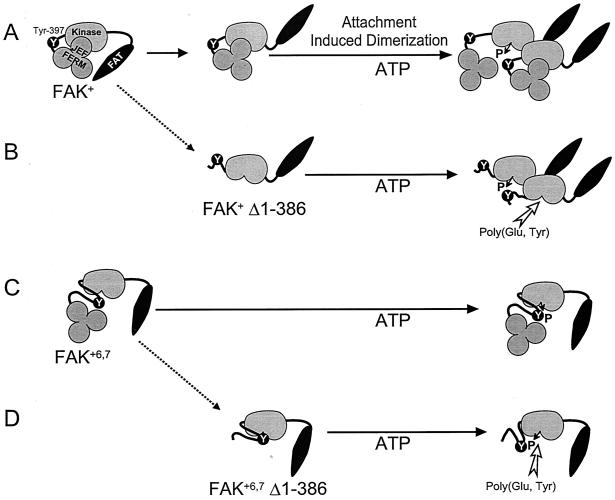
Schematic representation of FAK autophosphorylation mechanisms and differences between isoforms, based on the results of the present study. (A) FAK+ (as well as FAK°, which has the same low autophosphorylation) is in a poorly active state in basal conditions, presumably due to inhibition by the N-terminal domain. The FERM/JEF domain is indicated here as a trilobate structure, on the basis of the recently determined structure of several ERM domains (24, 41). The C-terminal region, including the FAT domain, is necessary for phosphorylation in intact cells. Cell attachment or artificial dimerization (as shown in the present study) promotes intermolecular phosphorylation of Tyr-397. (B) Deletion of the FERM/JEF domain of FAK+ increases intermolecular autophosphorylation in vitro by removing the inhibitory effect of the N-terminal FERM/JEF and facilitating the access of substrates to the active site [arrow, poly(Glu, Tyr)]. (C) The neuronal isoform, FAK+6,7 (as well as FAK+6,7,28, which has the same high autophosphorylation), has an increased autophosphorylation because of its ability to undergo intramolecular phosphorylation. The presence of box 7 is sufficient to allow intramolecular phosphorylation, presumably by allowing access of Tyr-397 to the active site located on the same peptide chain. However, in FAK+6,7, the N-terminal FERM/JEF domain still hampers the access of external substrates. (D) The deletion of the FERM/JEF domain has little effect on FAK+6,7 autophosphorylation but increases the accessibility of substrates to the active site [arrow, poly(Glu, Tyr)]. Whereas the C-terminal FAT domain is essential for the attachment- or artificial dimerization-induced autophosphorylation of FAK+, this does not appear to be the case for FAK+6,7. This is symbolized by the ordered open position of the C terminus in A and B and its loosely closed position in C and D.
Previous observations and the results of the present study support a model for FAK activation in which recruitment of this kinase to focal adhesions and its interaction with other proteins at these sites induce its transautophosphorylation when integrins are engaged. This clustering step, dependent on cell adhesion, is likely to be the one mimicked by coumermycin-induced dimerization of Gyr-FAK+ molecules. Thus, transphosphorylation of FAK may result from a mere increase in its local concentration or involve more directed interactions with specific proteins. It does not implicate stable dimers or oligomers of FAK, but rather transient interactions between multiple FAK molecules.
This model accounts well for the role of Src family kinases in FAK activation (40). Phosphorylation of FAK by Src is greatly enhanced by its binding to phospho-Tyr-397 (47). In turn, phosphorylation of A-loop residues Tyr-576 and Tyr-577 by Src increases FAK kinase activity (4) and may thus enhance further phosphorylation of Tyr-397 (40). Our data support a model in which autophosphorylation of Tyr-397 would be the starting point resulting from a transient intermolecular interaction between FAK molecules. Src could then be involved in a positive feed-back loop, as suggested previously (40), in which autophosphorylated FAK recruits Src, which phosphorylates Tyr-576 and -577 in the A-loop, increasing FAK catalytic activity and its ability to transphosphorylate additional molecules of FAK. It is noteworthy that such a model implies that interactions between FAK molecules are dynamic and reversible, in keeping with the lack of stable complexes found in the present study. Interestingly, the Src inhibitor PP2 had no effect on coumermycin-induced phosphorylation of Tyr-397 in Gyr-FAK+. This is consistent with the fact that coumermycin-bound dimers of Gyr-FAK+ are stable and do not allow the amplification mechanism mediated by Src.
In contrast to FAK° or FAK+, the major neuronal isoform FAK+6,7 is able to undergo intramolecular phosphorylation of Tyr-397, a property that accounts to a large extent for its increased autophosphorylation. The specific features of FAK+6,7 suggest that its regulation in neurons involves mechanisms different from integrin-mediated clustering, which may include relief of inhibitory influences on the cis-autophosphorylation reaction and/or control of protein tyrosine phosphatases. Thus, our results illustrate how alternative RNA splicing, a common process particularly frequent in neurons (23), has dramatic consequences on the properties of a ubiquitous and important protein kinase: the presence of short peptides on either side of the autophosphorylated tyrosine enhances the rate of autophosphorylation by switching it from an intermolecular to an intramolecular reaction.
Acknowledgments
We thank Pascal Ezan and Sylvie Clain for valuable assistance and Hervé Enslen for critical reading of the manuscript. Michael Ferrar and Leon Perlmutter are gratefully acknowledged for providing the gyrase B construct, and Janine Ragab is gratefully acknowledged for providing RPTP-β.
A.C. was the recipient of a fellowship from the French Ministry of Foreign Affairs and the French Embassy in Uruguay. The work was supported in part by grants from the European Community (Bio4 CT98 0526), the Human Frontier Science Program, the Fondation pour la Recherche Medicale, and the Fondation Schlumberger pour l'Enseignement et la Recherche (Dotation Annette Gruner Schlumberger) to J.A.G.
The first two authors contributed equally to this work.
REFERENCES
- 1.Baron, V., V. Calléja, P. Ferrari, F. Alengrin, and E. Van Obberghen. 1998. p125Fak focal adhesion kinase is a substrate for the insulin and insulin-like growth factor-I tyrosine kinase receptors. J. Biol. Chem. 273:7162-7168. [DOI] [PubMed] [Google Scholar]
- 2.Burgaya, F., and J. A. Girault. 1996. Cloning of focal adhesion kinase from rat stiatum reveals multiple transcripts. Mol. Brain Res. 37:63-73. [DOI] [PubMed] [Google Scholar]
- 3.Burgaya, F., M. Toutant, J. M. Studler, A. Costa, M. Le Bert, M. Gelman, and J. A. Girault. 1997. Alternatively spliced focal adhesion kinase in rat brain with increased autophosphorylation activity. J. Biol. Chem. 272:28720-28725. [DOI] [PubMed] [Google Scholar]
- 4.Calalb, M. B., T. R. Polte, and S. K. Hanks. 1995. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: A role for Src family kinases. Mol. Cell. Biol. 15:954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calalb, M. B., X. E. Zhang, T. R. Polte, and S. K. Hanks. 1996. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem. Biophys. Res. Commun. 228:662-668. [DOI] [PubMed] [Google Scholar]
- 6.Cary, L. A., R. A. Klinghoffer, C. Sachsenmaier, and J. A. Cooper. 2002. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22:2427-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, P.-Y., S. B. Kanner, G. Whitney, and A. Aruffo. 1994. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK. J. Biol. Chem. 269:20567-20574. [PubMed] [Google Scholar]
- 8.Chen, H. C., P. A. Appeddu, H. Isoda, and J. L. Guan. 1996. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 271:26329-26334. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H.-C., P. A. Appeddu, J. T. Parsons, J. D. Hildebrand, M. D. Schaller, and J.-L. Guan. 1995. Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 270:16995-16999. [DOI] [PubMed] [Google Scholar]
- 10.Chishti, A. H., A. C. Kim, S. M. Marfatia, M. Lutchman, M. Hanspal, H. Jindal, S. C. Liu, P. S. Low, G. A. Rouleau, et al. 1998. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem. Sci. 23:281-282. [DOI] [PubMed] [Google Scholar]
- 11.Cobb, B. S., M. D. Schaller, T.-H. Leu, and J. T. Parsons. 1994. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol. Cell. Biol. 14:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooley, M. A., J. M. Broome, C. Ohngemach, L. H. Romer, and M. D. Schaller. 2000. Paxillin binding is not the sole determinant of focal adhesion localization or dominant-negative activity of focal adhesion kinase/focal adhesion kinase-related nonkinase. Mol. Biol. Cell 11:3247-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derkinderen, P., J. Siciliano, M. Toutant, and J. A. Girault. 1998. Differential regulation of FAK+ and PYK2/Cakβ, two related tyrosine kinases, in rat hippocampal slices: effects of LPA, carbachol, depolarization and hyperosmolarity. Eur. J. Neurosci. 10:1667-1675. [DOI] [PubMed] [Google Scholar]
- 14.Derkinderen, P., M. Toutant, F. Burgaya, M. Le Bert, J. C. Siciliano, V. De Franciscis, M. Gelman, and J. A. Girault. 1996. Regulation of a neuronal form of focal adhesion kinase by anandamide. Science 273:1719-1722. [DOI] [PubMed] [Google Scholar]
- 15.Derkinderen, P., M. Toutant, G. Kadaré, C. Ledent, M. Parmentier, and J. A. Girault. 2001. Dual role of Fyn in the regulation of FAK+6,7 by cannabinoids in hippocampus. J. Biol. Chem. 276:38289-38296. [DOI] [PubMed] [Google Scholar]
- 16.Eide, B. L., C. W. Turck, and J. A. Escobedo. 1995. Identification of Tyr-397 as the primary site of tyrosine phosphorylation and pp60src association in the focal adhesion kinase, pp125FAK. Mol. Cell. Biol. 15:2819-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrar, M. A., J. Alberola-Ila, and R. M. Perlmutter. 1996. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature 383:178-181. [DOI] [PubMed] [Google Scholar]
- 18.Fujita, H., K. Kamiguchi, D. Cho, M. Shibanuma, C. Morimoto, and K. Tachibana. 1998. Interaction of Hic-5, a senescence-related protein, with focal adhesion kinase. J. Biol. Chem. 273:26516-26521. [DOI] [PubMed] [Google Scholar]
- 19.Gabarra-Niecko, V., P. J. Keely, and M. D. Schaller. 2002. Characterization of an activated mutant of focal adhesion kinase: superFAK. Biochem. J. 365:591-603. [DOI] [PMC free article] [PubMed]
- 20.Girault, J. A., A. Costa, P. Derkinderen, J. M. Studler, and M. Toutant. 1999. FAK and PYK2/CAK in the nervous system, a link between neuronal activity, plasticity and survival? Trends Neurosci. 22:257-263. [DOI] [PubMed] [Google Scholar]
- 21.Girault, J. A., G. Labesse, J.-P. Mornon, and I. Callebaut. 1998. The FAKs and JAKs play in the 4.1 band: a superfamily of band 4.1 domains important for cell structure and signal transduction. Mol. Medicine. 4:751-769. [PMC free article] [PubMed] [Google Scholar]
- 22.Girault, J. A., G. Labesse, J.-P. Mornon, and I. Callebaut. 1999. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem. Sci. 24:54-57. [DOI] [PubMed] [Google Scholar]
- 23.Grabowski, P. J., and D. L. Black. 2001. Alternative RNA splicing in the nervous system. Prog. Neurobiol. 65:289-308. [DOI] [PubMed] [Google Scholar]
- 24.Hamada, K., T. Shimizu, T. Matsui, S. Tsukita, and T. Hakoshima. 2000. Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 19:4449-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanke, J. H., J. P. Gardner, R. L. Dow, P. S. Changelian, W. H. Brissette, E. J. Weringer, K. Pollok, and P. A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor — study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 26.Hanks, S. K., M. B. Calalb, M. C. Harper, and S. K. Patel. 1992. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. USA 89:8487-8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harte, M. T., J. D. Hildebrand, M. R. Burnham, A. H. Bouton, and J. T. Parsons. 1996. p130Cas, a substrate associated with v-Src and v-Crk, localizes tofocal adhesions and binds to focal adhesion kinase. J. Biol. Chem. 271:13649-13655. [DOI] [PubMed] [Google Scholar]
- 28.Hens, M. D., and D. W. DeSimone. 1995. Molecular analysis and developmental expression of the focal adhesion kinase pp125FAK in Xenopus laevis. Dev. Biol. 170:274-288. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrand, J. D., M. D. Schaller, and J. T. Parsons. 1993. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J. Cell Biol. 123:993-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebrand, J. D., M. D. Schaller, and J. T. Parsons. 1995. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol. Biol. Cell 6:637-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildebrand, J. D., J. M. Taylor, and J. T. Parsons. 1996. An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol. Cell. Biol. 16:3169-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubbard, S. R., M. Mohammadi, and J. Schlessinger. 1998. Autoregulatory mechanisms in protein-tyrosine kinases. J. Biol. Chem. 273:11987-11990. [DOI] [PubMed] [Google Scholar]
- 33.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, T. Yamamoto, and S. Aizawa. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- 34.Inouye, K., S. Mizutani, H. Koide, and Y. Kaziro. 2000. Formation of the Ras dimer is essential for Raf-1 activation. J. Biol. Chem. 275:3737-3740. [DOI] [PubMed] [Google Scholar]
- 35.Knight, E. L., A. J. Warner, A. Maxwell, and S. A. Prigent. 2000. Chimeric VEGFRs are activated by a small-molecule dimerizer and mediate downstream signalling cascades in endothelial cells. Oncogene 19:5398-5405. [DOI] [PubMed] [Google Scholar]
- 36.Li, X., R. C. Dy, W. G. Cance, L. M. Graves, and H. S. Earp. 1999. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J. Biol. Chem. 274:8917-8924. [DOI] [PubMed] [Google Scholar]
- 37.Menegon, A., F. Burgaya, P. Baudot, D. D. Dunlap, J. A. Girault, and F. Valtorta. 1999. FAK+ and PYK2/CAKβ, two related tyrosine kinases highly expressed in the central nervous system: Similarities and differences in the expression pattern. Eur. J. Neurosci. 11:3777-3788. [DOI] [PubMed] [Google Scholar]
- 38.Menegoz, M., P. Gaspar, M. Le Bert, T. Galvez, F. Burgaya, C. Palfrey, P. Ezan, F. Amos, and J. A. Girault. 1997. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron 19:319-331. [DOI] [PubMed] [Google Scholar]
- 39.Mohi, M. G., K. Arai, and S. Watanabe. 1998. Activation and functional analysis of Janus kinase 2 in BA/F3 cells with the coumermycin/gyrase B system. Mol. Biol. Cell 9:3299-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen, J. D., P. J. Ruest, D. W. Fry, and S. K. Hanks. 1999. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhance cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 19:4806-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson, M. A., D. Reczek, A. Bretscher, and P. A. Karplus. 2000. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101:259-270. [DOI] [PubMed] [Google Scholar]
- 42.Perez Salazar, E., and E. Rozengurt. 2001. Src family kinases are required for integrin-mediated but not for G protein-coupled receptor stimulation of FAK autophosphorylation at Tyr-397. J. Biol. Chem. 276:17788-17795. [DOI] [PubMed] [Google Scholar]
- 43.Prodromou, C., B. Panaretou, S. Chohan, G. Siligardi, R. O'Brien, J. E. Ladbury, S. M. Roe, P. W. Piper, and L. H. Pearl. 2000. The ATPase cycle of Hsp90 drives a molecular "clamp' via transient dimerization of the N-terminal domains. EMBO J. 19:4383-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruest, P. J., S. Roy, E. Shi, R. L. Mernaugh, and S. K. Hanks. 2000. Phosphospecific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site. Cell Growth Differ. 11:41-48. [PubMed] [Google Scholar]
- 45.Salazar, E. P., and E. Rozengurt. 1999. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J. Biol. Chem. 274:28371-28378. [DOI] [PubMed] [Google Scholar]
- 46.Schaller, M. D., C. A. Borgman, B. S. Cobb, R. R. Vines, A. B. Reynolds, and J. T. Parsons. 1992. pp125FAK, A structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA 89:5192-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaller, M. D., J. D. Hildebrand, J. D. Shannon, J. W. Fox, R. R. Vines, and J. T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlaepfer, D. D., S. K. Hanks, T. Hunter, and P. Van der Geer. 1994. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372:786-791. [DOI] [PubMed] [Google Scholar]
- 49.Schlaepfer, D. D., and T. Hunter. 1996. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src family protein-tyrosine kinases. Mol. Cell. Biol. 16:5623-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlaepfer, D. D., and T. Hunter. 1998. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 8:151-157. [DOI] [PubMed] [Google Scholar]
- 51.Shen, Y., and M. D. Schaller. 1999. Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol. Biol. Cell 10:2507-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siciliano, J. C., M. Toutant, P. Derkinderen, T. Sasaki, and J. A. Girault. 1996. Differential regulation of proline-rich tyrosine kinase 2 cell adhesion kinase β (PYK2/CAKβ) and pp125FAK by glutamate and depolarization in rat hippocampus. J. Biol. Chem. 271:28942-28946. [DOI] [PubMed] [Google Scholar]
- 53.Sonoda, Y., Y. Matsumoto, M. Funakoshi, D. Yamamoto, S. K. Hanks, and T. Kasahara. 2000. Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J. Biol. Chem. 275:16309-16315. [DOI] [PubMed] [Google Scholar]
- 54.Tachibana, K., T. Sato, N. D'Avirro, and C. Morimoto. 1995. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J. Exp. Med. 182:1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toutant, M., J. M. Studler, F. Burgaya, A. Costa, P. Ezan, M. Gelman, and J. A. Girault. 2000. Molecular characterization of FAK neuronal isoforms. Biochem. J. 348:119-128. [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss, A., and J. Schlessinger. 1998. Switching signals on or off by receptor dimerization. Cell 94:277-280. [DOI] [PubMed] [Google Scholar]
- 57.Zachary, I., and E. Rozengurt. 1992. Focal adhesion kinase (p125FAK): A point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell 71:891-894. [DOI] [PubMed] [Google Scholar]