Abstract
The stress-activated protein kinase p38 stabilizes a number of mRNAs encoding inflammatory mediators, such as cyclooxygenase 2 (Cox-2). In HeLa cells the anti-inflammatory glucocorticoid dexamethasone destabilizes Cox-2 mRNA by inhibiting p38 function. Here we demonstrate that this effect is phosphatase dependent. Furthermore, in HeLa cells dexamethasone induced the sustained expression of mitogen-activated protein kinase phosphatase 1 (MKP-1), a potent inhibitor of p38 function. The inhibition of p38 and the induction of MKP-1 by dexamethasone occurred with similar dose dependence and kinetics. No other known p38 phosphatases were induced by dexamethasone, and other cell types which failed to express MKP-1 also failed to inhibit p38 in response to dexamethasone. The proinflammatory cytokine interleukin 1 (IL-1) induced MKP-1 expression in a p38-dependent manner and acted synergistically with dexamethasone to induce MKP-1 expression. In HeLa cells treated with IL-1 or IL-1 and dexamethasone, the dynamics of p38 activation mirrored the expression of MKP-1. These observations suggest that MKP-1 participates in a negative-feedback loop which regulates p38 function and that dexamethasone may inhibit proinflammatory gene expression in part by inducing MKP-1 expression.
Members of the three mitogen-activated protein kinase (MAPK) families mediate transcriptional and posttranscriptional changes in gene expression in response to proinflammatory stimuli (reviewed in references 15, 25, and 33). In addition to its effects on transcription (69), the MAPK p38 pathway positively regulates the stability of several proinflammatory mRNAs, including tumor necrosis factor alpha, vascular endothelial growth factor, interleukin 6 (IL-6), IL-8, and cyclooxygenase 2 (Cox-2) (8, 19, 41, 46, 49, 54, 74, 76). Glucocorticoids are widely used in the treatment of inflammation because of their ability to inhibit proinflammatory gene expression. This inhibitory effect involves direct interactions of the glucocorticoid receptor with transcription factors such as NF-κB and AP-1, resulting in the inhibition of their function (reviewed in references 1 and 47). However, glucocorticoids also posttranscriptionally repress a number of proinflammatory genes, several of which are known targets of the p38 pathway (3, 26, 48, 60, 67). As glucocorticoids have been shown to inhibit other members of the MAPK family (10, 27, 30, 31, 35, 73), we hypothesized that posttranscriptional effects of dexamethasone involve the inhibition of p38 function. The synthetic glucocorticoid dexamethasone was demonstrated to inhibit p38 activity in a manner requiring ongoing, glucocorticoid receptor-mediated gene expression (40). Here the link between dexamethasone, p38 activity, and proinflammatory gene expression is investigated in further detail.
Activation of MAPKs requires phosphorylation of both threonine and tyrosine residues within a Thr-Xxx-Tyr activation motif, where the central residue is glutamic acid in the case of the extracellular-signal-regulated kinase (ERK) family, proline in the case of the JNK family, and glycine in the case of the p38 family (15, 25, 33). Cellular function is profoundly affected by both strength and duration of MAPK activation, which must therefore be strictly controlled (45, 68). In part this control is mediated by a family of about 12 dual-specificity phosphatases (DUSPs) or MAPK phosphatases (MKPs), which inactivate MAPKs by dephosphorylation of both threonine and tyrosine residues within the activation motif (reviewed in references 11 and 36). These phosphatases differ in their target specificities, subcellular localizations, and patterns of expression. In many cases their expression or function is regulated by MAPKs, and they may also tightly associate with their substrates in vivo. The participation of MKPs in feedback regulation of MAPK activity has been described in Saccharomyces cerevisiae, Drosophila melanogaster, and mammals and is thought to be critical to the dynamic regulation of MAPK responses. MKP-1 (otherwise known as CL-100, hVH1, Erp, or DUSP1) is the archetypal member of the MAPK phosphatase family and is expressed as an immediate-early gene in response to serum, growth factors, or cellular stresses (16, 37, 59, 78). Although MKP-1 is able to dephosphorylate ERKs (2, 59), it appears to act preferentially against the stress-activated protein kinases (21, 22, 44) and forms a stable association with p38 in vivo or in vitro (32).
The actions of glucocorticoids are mediated by a 777-amino-acid receptor (glucocorticoid receptor [GR]), which migrates from the cytoplasm to the nucleus upon binding of ligand and in the nucleus exerts both positive and negative effects upon transcription (reviewed in reference 47). Classically, positive regulation is mediated by the binding of GR dimers to palindromic glucocorticoid response elements (GREs). The negative regulatory interaction of GR with NF-κB and AP-1, known as transrepression, is independent of GR dimerization (28, 29). Although the absence of GR is lethal (18), mice expressing only a dimerization-deficient version of GR are viable (51) and show normal glucocorticoid-mediated down-regulation of proinflammatory genes in a phorbol myristate acetate-induced skin inflammation model (34, 51, 52). A degree of functional uncoupling can also be achieved using “dissociated” glucocorticoid analogues, which are selectively impaired in transactivating or transrepressing activity. Anti-inflammatory effects are displayed by glucocorticoid analogues which are relatively poor at transactivation of GRE-dependent reporters (5, 28, 71, 72). These observations suggest that critical anti-inflammatory actions of glucocorticoids are independent of gene induction. However, some glucocorticoid-induced genes do not possess GREs (13, 14, 20), and their activation may or may not require GR dimerization. It remains possible that induction of gene expression by glucocorticoids plays a significant role in the inhibition of inflammatory responses (4, 50, 77).
Here we report that the inhibition of p38 function by dexamethasone is phosphatase mediated. Circumstantial evidence implicates MKP-1 in the suppression of p38 function and Cox-2 gene expression by dexamethasone. Expression of MKP-1 is induced by proinflammatory stimuli in a p38-dependent manner, suggesting that MKP-1 participates in feedback regulation of p38 function. The rapid, sustained, and p38-independent induction of MKP-1 gene expression by dexamethasone may provide a novel mechanism for the posttranscriptional suppression of proinflammatory gene expression.
MATERIALS AND METHODS
Materials.
Dexamethasone and RU486 were from Sigma-Aldrich. SB203580, GF109203X, and protein kinase A inhibitor 14-22 amide were from Calbiochem-Novabiochem. U-0126 was from Promega. The rabbit antiserum to the C-terminal peptide of p38α MAPK used for both immunoprecipitation and Western blotting has been described previously (55). The rabbit anti-human antibodies against the phosphorylated form of p38 MAPK (catalog no. 9211L), the phosphorylated form of ERK (catalog no. 9101S), and total ERK (catalog no. 9122) were from New England Biolabs. Rabbit antiserum to DUSP-1 (M-18; catalog no. sc-1102) was from Santa Cruz Biotech. Monoclonal antibody HA.11 was from BAbCO. Rabbit antiserum to hsp27 was previously described (24). IL-1 was purified from lysates of overexpressing bacteria (75) by serial ion-exchange chromatography and dialyzed against phosphate-buffered saline. Epidermal growth factor was from BD Bioscience.
Plasmids.
The p38 Asp-316-Asn mutant construct (HA-p38D316N) and wild-type p38 construct HA-p38WT (32) were kindly supplied by Yusen Liu (Baltimore, Md.). Expressed sequence tag (EST) clones (42) encoding DUSPs were identified and obtained from the United Kingdom Human Genome Mapping Project Resource Centre as follows: MKP-1, IMAGE4819422; DUSP2, IMAGE4385332; DUSP3, IMAGE3831894; DUSP4, IMAGE3920222; DUSP5, IMAGE1723172; DUSP6, IMAGE2960126; DUSP7, IMAGE4398897; DUSP8, IMAGE2675291; DUSP9, IMAGE2356893; MKP-10, IMAGE2310556; MKP-11, IMAGE3914630; MKP-12, IMAGE3958403; and MKP7, IMAGE2358910. The coding sequence of MKP-1 was amplified from the IMAGE4819422 clone using primers D51 (5′-GCGAATTCGGTCATGGAAGTGGGCACCCTG-3′) and D31 (5′-GCGAATTCTCAGCAGCTGGGAGAGGTCG-3′). The PCR product was cut with EcoRI and inserted in frame at the EcoRI site of pFlagCMV2 (Sigma-Aldrich). To generate the catalytically inactive Cys-258-Ser MKP-1 mutant, coding fragments were amplified from the IMAGE4819422 clone using primers D51 and D32 (5′-GCCTGCCTGGGAGTGCACAAACACCCTTCC-3′) or primers D31 and D52 (5′-GGAAGGGTGTTTGTGCACTCCCAGGCAGGC-3′). The amino-terminal and carboxy-terminal coding fragments were cut with EcoRI and ApaLI, ligated together, and inserted at the EcoRI site of pFlagCMV2. Both constructs were checked by sequencing.
Cell culture and transfection.
HeLa-TO cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum. Cells were seeded in six-well plates, and the following day the cells were transfected using Superfect (Qiagen). After 24 h, cells were incubated with dexamethasone and then irradiated in a Stratalinker (40 J/m2; UV-C) (Stratagene). After the period indicated in each figure, the cells were harvested and processed for Western blotting. HeLa (American Type Culture Collection), human skin fibroblast, and RAW264.7 cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum. Human peripheral blood T cells were prepared from the buffy coat fraction of a unit of blood from a single donor. T cells were isolated and maintained in RPMI 1640 medium as described previously (40).
Immunoprecipitation and assay of p38 MAPK.
HeLa cells were incubated in the absence or presence of IL-1 and dexamethasone as described in the figure legends. After the period indicated in each figure, the cells were lysed and the p38 MAPK was immunoprecipitated as previously described (40). p38 kinase assays were performed as described previously (40), using MAPKAPK-2 as the substrate.
Immunoprecipitation of MKP-1.
HeLa cells were incubated as described in the figure legends and then harvested in lysis buffer (20 mM HEPES [pH 7.4], 50 mM sodium β-glycerophosphate, 2 mM EGTA, 1% Triton X-100, 10% glycerol, 150 mM NaCl, 10 mM NaF, 1 mM sodium orthovanadate, 2 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride, 3 μg of aprotinin per ml, 10 μM E64). The lysates were clarified by centrifugation at 13,000 × g for 10 min at 4°C and incubated for 1 h at 4°C with a rabbit antiserum to hsp27 previously linked to protein A-Sepharose beads. The supernatants were then incubated for 2 h at 4°C with an anti-MKP-1 antibody linked to protein A-Sepharose beads. The beads were washed, resuspended in sample buffer, and processed for Western blotting.
Western blotting.
HeLa cells were incubated as described in the figure legends and then harvested in lysis buffer as described above, separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and electrophoretically transferred to nitrocellulose membranes (Sartorius). The membranes were probed with primary antibodies as indicated and then with a peroxidase-coupled second antibody (Dako). Proteins were detected using the enhanced chemiluminescence system (Amersham).
Northern blotting.
Total RNA was isolated using the RNeasy Kit from Qiagen, and 10-μg RNA samples were electrophoresed on denaturing formaldehyde-agarose gels. Gels were stained with SYBR green II RNA gel stain (Molecular Probes) and visualized using a phosphorimager (Fuji FLA-2000). RNA was then transferred to a Hybond N membrane by capillary transfer and fixed by UV cross-linking. cDNA probes for the different DUSPs were prepared by appropriate restriction digestion of EST clones as described above and labeled with 50 μCi of [α-32P]dCTP using the Ready-to-go kit (Amersham). Prehybridization (2 h) and hybridization (overnight) were performed at 42°C in Ultrahyb solution (Ambion). Blots were washed three times for 30 min each time at 42°C with the following three solutions: (i) 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% SDS, (ii) 1× SSC and 0.1% SDS, and (iii) 0.1× SSC and 0.1% SDS. Signals were quantified by a phosphorimager (Fuji FLA2000).
Microarray analysis.
HeLa-TO cells were incubated in the absence or presence of dexamethasone for 2 h, and total RNA was isolated using the RNeasy kit (Qiagen). Subsequent steps were performed according to protocols provided by Affymetrix. First-strand cDNA synthesis was done at 42°C in 20-μl reaction mixture volumes containing 5 μM T7(T)24 primer (Helena Biosciences), 500 μM (each) deoxynucleoside triphosphates, and 400 U of Superscript II reverse transcriptase (Life Technologies). Second-strand cDNA synthesis was performed at 16°C in 150-μl reaction mixture volumes containing 200 μM (each) deoxynucleoside triphosphates, 10 U of DNA ligase, 40 U of DNA polymerase I, and 2 U of RNase H (Life Technologies). Double-stranded cDNA was purified by phenol extraction and ethanol precipitation. cRNA was synthesized using the Enzo RNA transcript labeling kit (Affymetrix) according to the manufacturer's instructions and purified using the RNeasy mini kit (Qiagen). Application of cRNA to the U95Av2 chip, amplification of signal, and collection of data were performed using an Affymetrix fluidics station and chip reader according to the manufacturer's instructions.
RESULTS
Inhibition of stress-activated protein kinases by dexamethasone is phosphatase mediated.
Dexamethasone suppressed the activity and decreased the phosphorylation of p38 in UV-stimulated HeLa cells but did not affect the phosphorylation of the p38 activator MKK6 (40). To investigate whether the inhibition of p38 could be mediated by a phosphatase, we tested the effects of sodium orthovanadate, an efficient inhibitor of tyrosine phosphatases and DUSPs (37, 64, 65). Sodium orthovanadate did not increase basal p38 phosphorylation in unstimulated cells (Fig. 1A). In UV-stimulated cells sodium orthovanadate very slightly increased p38 phosphorylation, probably due to the inhibition of UV-induced phosphatase activity (see below). The phosphatase inhibitor significantly blocked the dexamethasone-dependent inhibition of p38 activation. Sodium orthovanadate was not toxic within the range of concentrations used (data not shown). Okadaic acid, a serine/threonine phosphatase inhibitor, was unable to block the effect of dexamethasone upon p38 activation (data not shown).
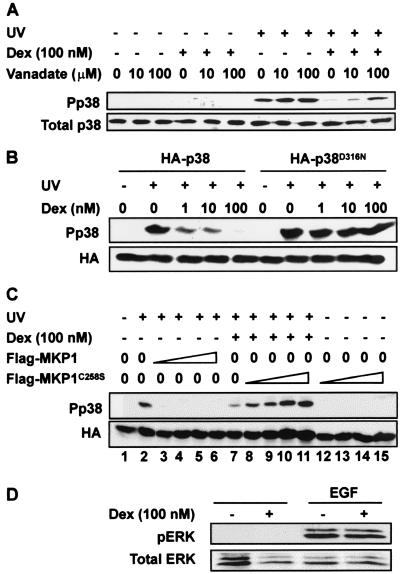
FIG. 1.
Dexamethasone-dependent inhibition of p38 is mediated by a stress-activated protein kinase (SAPK)-specific phosphatase(s). (A) HeLa-TO cells were incubated (+) for 2 h with 100 nM dexamethasone (Dex), and the cells were treated (+) with UV C and sodium orthovanadate was added to the medium at the indicated concentrations. After 30 min, total cell lysates were prepared and analyzed by Western blotting with anti-phospho-p38 antibody or total p38 antiserum. (B) HeLa-TO cells were transiently transfected with 500 ng of either pSR-HA-p38 or pSR-HA-p38N316. After 24 h, cells were incubated for 2 h with dexamethasone at the indicated concentrations and then treated with UV C (+) or left untreated (−). After 30 min, total cell lysates were prepared and analyzed by Western blotting with anti-phospho-p38 antibody or HA antibody as a control for transfection. (C) HeLa cells were transiently transfected with 500 ng of pSR-HA-p38 and cotransfected with 20 ng (lane 3), 50 ng (lane 4), 75 ng (lane 5), or 150 ng (lane 6) of pFlagCMV-MKP-1 or 20 ng (lanes 8 and 12), 50 ng (lanes 9 and 13), 75 ng (lanes 10 and 14), or 150 ng (lanes 11 and 15) of pFlagCMV-MKP-1C258S. The empty expression vector pFlagCMV was added as appropriate to bring the total quantity of DNA up to 1 μg. After 24 h, cells were treated as indicated with vehicle (methanol) or 100 nM dexamethasone. After a further 2 h, cells were left untreated or stimulated with UV C, and lysates were prepared 30 min later and subjected to Western blotting using anti-phospho-p38 or anti-HA epitope antibodies. (D) HeLa cells were incubated for 2 h with 100 nM dexamethasone and then stimulated with 10 ng of epidermal growth factor (EGF) per ml for 10 min. Cells were harvested, and total cell lysates were analyzed by Western blotting with anti-phospho-ERK or total ERK antibodies.
As an alternative approach, an epitope-tagged sevenmaker mutant of p38 was expressed in HeLa cells. The sevenmaker mutation, first described in a Drosophila MAPK homologue (9), is a single amino acid substitution within an acidic patch that is conserved between members of the MAPK superfamily and is involved in the interaction between DUSPs and MAPKs (63). This mutation inhibits the kinase-phosphatase interaction and renders the kinase resistant to dephosphorylation. Analogous mutations in mammalian ERKs or p38 result in a similar gain of function, demonstrated by reduced DUSP-mediated dephosphorylation in vitro or in vivo, and extended or enhanced activation in vivo (6, 12, 17, 32, 57). Wild-type and sevenmaker epitope-tagged p38 were expressed at similar levels and were both phosphorylated in response to UV light (Fig. 1B). The activation of endogenous p38, with a mobility slightly different from those of the epitope-tagged versions, could be detected in longer exposures of the Western blot shown in Fig. 1B. The phosphorylation or activation of wild-type p38 was inhibited by dexamethasone in a dose-dependent manner, as previously shown (40); however, the sevenmaker mutant was resistant to the effects of dexamethasone.
Catalytically inactive mutants of MAPK phosphatases interact strongly with their substrates and protect them from dephosphorylation by endogenous phosphatases, a process known as substrate trapping (11, 32, 36, 57, 59). A catalytically inactive MKP-1 mutant was constructed and coexpressed with hemagglutinin (HA)-tagged p38 (Fig. 1C). UV treatment induced phosphorylation of p38 (lanes 1 and 2), which was inhibited by 100 nM dexamethasone (lane 7) or by coexpression of wild-type MKP-1 (lanes 3 to 6). Complete inhibition of p38 phosphorylation was observed with as little as 20 ng of the vector pCMV-Flag-MKP-1 (lane 3); however, the epitope-tagged phosphatase was detectable only with the highest quantities of the expression vector (data not shown). The inactive mutant MKP-1 reversed the inhibition of p38 phosphorylation in dexamethasone-treated cells in a dose-dependent manner (lanes 8 to 11). In the absence of any stimulus, expression of the mutant MKP-1 did not cause accumulation of phosphorylated p38 (lanes 12 to 15) or phosphorylated ERK (not shown). Hence, the inactive phosphatase does not activate p38 but prevents its inactivation in response to dexamethasone.
Dexamethasone treatment of HeLa cells inhibits the function of both JNK and p38 (40). The function of ERK was investigated in HeLa cells pretreated with 100 nM dexamethasone for 2 h and then stimulated with epidermal growth factor (Fig. 1D). Phosphorylation or activation of ERK was strongly induced but was not sensitive to dexamethasone under these conditions. These observations suggest that the inhibition of p38 function by dexamethasone is mediated by a tyrosine-specific or dual-specificity phosphatase (or phosphatases) selective for the stress-activated protein kinases.
Expression of MKP-1 is induced by dexamethasone.
The inactivation of p38 may be mediated by tyrosine-specific phosphatases such as HePTP (56) or by serine/threonine-specific phosphatases of the protein phosphatase 2 (PP2) class, PP2Cα and Wip1 (61, 62). The former are restricted in expression, whereas the latter probably do not play a role in the inhibition of p38 by dexamethasone, since this effect is sensitive to sodium orthovanadate but not okadaic acid. The principal candidates are therefore members of the large family of DUSPs (11, 36). Several of these have been shown to inactivate p38 in vivo or in vitro, notably DUSP1/MKP-1 (22), DUSP2/PAC1 (17), DUSP10/MKP-5 (64, 66), and the recently described MKP-7 (65).
The inhibition of p38 enzyme activity by dexamethasone was prevented by RU486 or actinomycin D, and therefore depends upon GR-mediated transcription (40). Treatment of HeLa cells with dexamethasone for 2 h was sufficient for the inhibition of p38 function and the destabilization of p38 sensitive reporter mRNAs (40). To search for candidate dexamethasone-induced genes which might be involved in the negative regulation of p38, an oligonucleotide microarray experiment was performed. RNA was isolated from HeLa cells which had been treated for 2 h with vehicle (methanol), 100 nM or 1 μM dexamethasone, and analyzed using Affymetrix U95Av2 chips. A gene was considered to be induced by dexamethasone if its expression level was at least 200 U and was increased at least 50% by both 100 nM and 1 μM dexamethasone treatments. A total of 172 genes fitted these criteria, including known glucocorticoid-induced genes metallothionein 1A (MT1A) (53) and the inhibitor of nuclear factor NF-κB (IκBα) (4) (Table 1). Basal expression of the glucocorticoid-repressed IL-8 gene (67) was completely eliminated (Table 1). Basal expression of Cox-2 was close to the detection limit but was also eliminated by dexamethasone (data not shown). Levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin mRNAs were not affected by dexamethasone. HePTP and PP2Cα mRNAs were not detected, while Wip1 mRNA was constitutively expressed at a low level and not induced by dexamethasone. Most members of the DUSP family were not expressed at the mRNA level or were not significantly induced by dexamethasone. Exceptions were DUSP2 (PAC-1), which was weakly expressed but apparently up-regulated by dexamethasone, and DUSP1 (MKP-1), which was expressed moderately strongly and induced almost twofold by 100 nM or 1 μM dexamethasone. The pattern of expression of MKP-1 was similar to that of MT1A in this experiment. No other dexamethasone-induced potential inhibitors of the p38 pathway were identified in this screen.
TABLE 1.
Regulation of various genes by dexamethasone
| Gene | Synonym(s) | Expression level of gene (arbitrary units)a
|
||
|---|---|---|---|---|
| Control | 100 nM Dex | 1 μM Dex | ||
| GAPDH | 18,911 | 20,491 | 19,537 | |
| β-Actin | 18,544 | 19,673 | 18,732 | |
| MT-1A | 3,883 | 6,710 | 8,508 | |
| IκBα | 1,726 | 3,206 | 3,109 | |
| IL-8 | 527 | ND | ND | |
| DUSP1 | MKP-1, CL-100, Erp | 3,387 | 6,436 | 5,951 |
| DUSP2 | PAC-1 | 343 | 903 | 879 |
| DUSP4 | MKP-2, hVH2 | 230 | 389 | 339 |
| DUSP5 | hVH3 | 324 | ND | ND |
| DUSP10 | MKP-5 | 288 | 305 | 356 |
| DUSP11 | PIR1 | 1,960 | 1,576 | 1,508 |
| Wip1 | 685 | 573 | 506 | |
HeLa-TO cells were treated for 2 h with vehicle (methanol) (control) or with 100 nM or 1 μM dexamethasone (Dex). Total RNA was prepared and analyzed using Affymetrix U95Av2 arrays as described in Materials and Methods. Raw data sets from the chip reader were normalized against one another using the mean expression level of all detected transcripts under each condition. Correction factors applied to the 100 nM and 1,000 nM dexamethasone data sets were 1.156 and 0.99, respectively. Normalization using the GAPDH or β-actin signals gave almost identical results. Normalization using internal control poly(A) mRNAs (added at the cRNA synthesis step) suggested slightly stronger up-regulation of MKP-1 gene expression by 100 and 1,000 nM dexamethasone (2.4- and 2.6-fold rather than 1.7-and 1.5-fold as shown above). An arbitrary cutoff of 200 expression units was employed, and genes expressed below this level were considered not detected (ND).
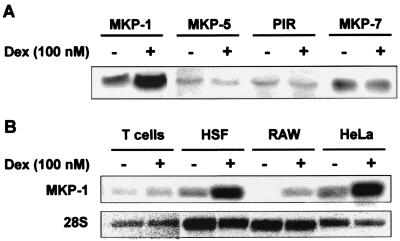
To confirm these observations and to study the expression of MKP-7 (which was not represented on the Affymetrix U95Av2 chip), mRNA from dexamethasone-treated or control HeLa cells was subjected to Northern blotting using probes against all of the known DUSPs. Some representative results are illustrated in Fig. 2A. The up-regulation of DUSP1 (MKP-1) mRNA was similar to that observed in the gene expression array experiment. In more than eight independent experiments MKP-1 mRNA was induced two- to fivefold by dexamethasone, while in two experiments basal expression was lower and apparent gene induction was greater than 20-fold (data not shown). PAC1, VHR, MKP-2, and hVH3 mRNAs were not detected by Northern blotting (data not shown). MKP-5 and MKP-7 mRNAs were expressed at relatively low levels and did not respond to dexamethasone.
FIG. 2.
Dexamethasone induces the expression of MAPK phosphatase 1. (A) HeLa cells were treated for 2 h with vehicle (methanol) (−) or 100 nM dexamethasone (Dex) (+). Total RNA was prepared and subjected to Northern blotting using probes against MKP-1 (DUSP1), MKP-5 (DUSP10), and MKP-7 and PIR (DUSP11). Exposures of the different Northern blots are not identical, and relative expression levels cannot be inferred. (B) HeLa cells, RAW264.7 (RAW) cells, human skin fibroblasts (HSF), and human peripheral blood T cells were treated for 2 h with vehicle (methanol) or 100 nM dexamethasone. Total RNA was prepared and subjected to Northern blotting using a probe against MKP-1.
We reported previously that dexamethasone inhibits p38 function in HeLa cells, mouse macrophage-like RAW264.7 cells, and human skin fibroblasts, although not in primary human T cells (40). The induction of MKP-1 mRNA by dexamethasone in the same cells was assessed by Northern blotting (Fig. 2B). In HeLa, RAW264.7, and human skin fibroblast cells, MKP-1 mRNA was induced by treatment with 100 nM dexamethasone for 2 h. In contrast, MKP-1 mRNA was constitutively expressed at a low level and not induced by dexamethasone in human peripheral blood T cells. Dexamethasone also failed to inhibit p38 or to induce MKP-1 expression in mouse embryonic fibroblasts (data not shown). Thus, the induction of MKP-1 correlates with the ability of dexamethasone to inhibit p38 activity within the cell types that we have studied.
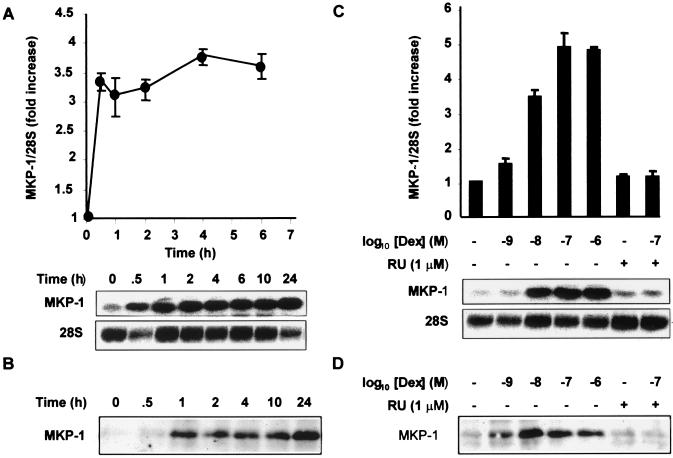
The kinetics of MKP-1 mRNA and protein expression were examined following stimulation of HeLa cells with 100 nM dexamethasone (Fig. 3A and B). The expression of MKP-1 mRNA was induced rapidly, within half an hour, and was sustained for at least 24 h. The protein was virtually undetectable in untreated cells, and its induction by dexamethasone appeared much stronger than at the mRNA level. Expression of protein reached a peak roughly 1 h after dexamethasone treatment and was sustained for at least 24 h (Fig. 3B). The dexamethasone dose dependence of MKP-1 mRNA and protein induction were also examined by Northern and Western blotting (Fig. 3C and D). Both mRNA and protein were induced by dexamethasone with an apparent 50% inhibitory concentration (IC50) between 1 and 10 nM, and this induction was almost completely blocked by the glucocorticoid antagonist RU486. These observations are consistent with a role of MKP-1 in the down-regulation of p38 function by dexamethasone. MAPK p38 enzyme activity was inhibited by dexamethasone with an IC50 between 1 and 10 nM, in an RU486-sensitive manner. Inhibition was dependent on time and appeared to reach a maximum half an hour to an hour after the addition of dexamethasone (40).
FIG. 3.
Dexamethasone induces MKP-1 mRNA and protein in a time- and dose-dependent manner. (A) HeLa-TO cells were incubated with 100 nM dexamethasone for the times shown, and then MKP-1 mRNA was analyzed by Northern blotting. The 28S rRNA is shown as a loading control. Mean MKP-1/28S rRNA ratios from four experiments are shown. (B) HeLa-TO cells were incubated as described above for panel A, and then MKP-1 protein was immunoprecipitated and detected by Western blotting. (C) HeLa-TO cells were incubated with different doses of dexamethasone (Dex) alone or dexamethasone plus RU486 (RU) (1 μM) for 6 h. MKP-1 mRNA was analyzed by Northern blotting. Mean MKP-1/28S rRNA ratios from three independent experiments are shown. (D) HeLa-TO cells were incubated as described above for panel C, and MKP-1 protein was immunoprecipitated and detected by Western blotting.
Proinflammatory stimuli cooperate with dexamethasone to induce MKP-1 gene expression.
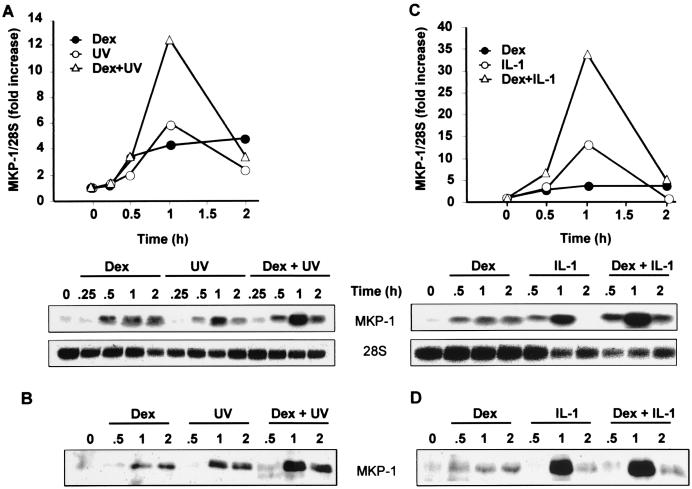
The effects of dexamethasone upon the p38 pathway following proinflammatory stimuli were measured (40); however, in the experiments described above, MKP-1 gene expression was investigated in the presence of dexamethasone only. Therefore, MKP-1 expression was examined following treatment of HeLa cells with a proinflammatory stimulus (IL-1 or UV), with dexamethasone alone, or with dexamethasone and proinflammatory stimulus (Fig. 4). As before, dexamethasone induced MKP-1 mRNA three- to fourfold and in a sustained manner. Induction by UV or IL-1 was stronger but more transient, rising to sixfold (UV) or 13-fold (IL-1) within 1 h but then returning close to basal levels by the 2-h time point. The presence of both dexamethasone and proinflammatory stimulus resulted in a cooperative stimulation of MKP-1 expression at the mRNA level (Fig. 4A and C). The effects of dexamethasone and UV were approximately additive, while those of dexamethasone and IL-1 were synergistic. Cooperativity between dexamethasone and UV or IL-1 also occurred at the protein level, as confirmed by the results of Western blotting (Fig. 4B and D).
FIG. 4.
Proinflammatory stimuli and dexamethasone cooperatively increase MKP-1 mRNA and protein expression. (A) HeLa-TO cells were stimulated with UV C and simultaneously incubated with dexamethasone (Dex) (100 nM) or vehicle (methanol). After the indicated time period, cells were harvested and MKP-1 mRNA was analyzed by Northern blotting. The 28S rRNA is shown as a loading control. (B) HeLa-TO cells were incubated as described above for panel A, and then MKP-1 protein was immunoprecipitated and detected by Western blotting. (C) HeLa cells were incubated in the absence or presence of IL-1 (20 ng/ml) with or without dexamethasone (100 nM). After the indicated time period, cells were collected, and MKP-1 mRNA was then analyzed by Northern blotting. (D) HeLa cells were incubated as described above for panel C, and MKP-1 protein was immunoprecipitated and detected by Western blotting.
MAPK p38 is required for IL-1-induced expression of MKP-1.
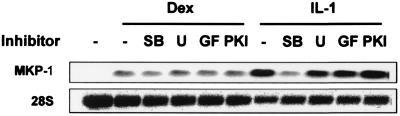
In different cellular contexts, the expression of MKP-1 may be regulated by several signal transduction pathways, including ERK, p38, protein kinase C (PKC), PKA, and calcineurin (38, 43, 44, 58, 70). To investigate the roles of different signaling pathways in the control of MKP-1 gene expression in HeLa cells, MKP-1 mRNA induction was stimulated with dexamethasone or IL-1 in the absence or presence of various inhibitors. Gene induction by dexamethasone was not sensitive to any of the inhibitors tested (Fig. 5), including the calcineurin inhibitor cyclosporine A (not shown). Induction by IL-1 (Fig. 5) or UV (data not shown) was strongly inhibited by 1 μM SB203580 and therefore requires p38 activity. ERK, PKC, PKA, and calcineurin are dispensable for induction of MKP-1 gene expression in response to UV or IL-1.
FIG. 5.
MKP-1 mRNA induction by proinflammatory stimuli is dependent upon p38. HeLa cells were incubated for 10 min with different inhibitors as follows: no inhibitor (control) (−), 1 μM SB203580 (SB), 10 μM U0126 (U), 1 μM GF109203X (GF), and 0.5 μM protein kinase A inhibitor (PKI). Some cells were then incubated for 1 h in the presence of 100 nM dexamethasone (Dex lanes). Some cells were then stimulated with 20 ng of IL-1 per ml for 1 h (IL-1 lanes). MKP-1 mRNA was analyzed by Northern blotting. The 28S rRNA is shown as a loading control.
The activity of p38 mirrors the expression of MKP-1.
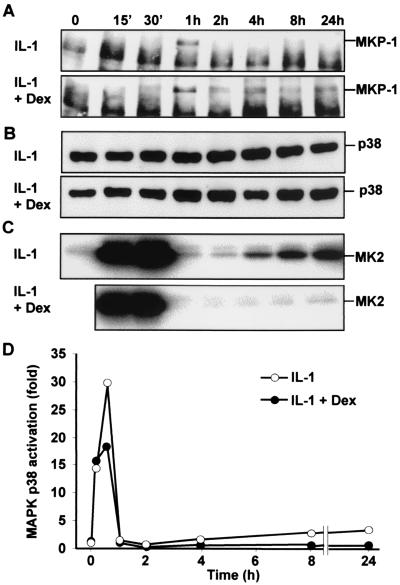
The relationship between MKP-1 expression and p38 activity was investigated further. HeLa cells were treated with IL-1 alone or with IL-1 and dexamethasone. Over the next 24 h, expression of MKP-1 was assessed by immunoprecipitation and Western blotting, total p38 was quantified by Western blotting, and its activity was measured by in vitro kinase assay. IL-1 induced transient expression of MKP-1, with no protein detected beyond 2 h after the stimulus (Fig. 6A). Under these conditions, the activation of p38 was biphasic (Fig. 6C and D). A rapid, transient peak of strong activity was followed by a return to basal levels at the 2-h time point and then by a second phase of increasing activity. Maximum p38 activity during this second phase was approximately 10% of that in the initial peak (Fig. 6D). No significant variations in the expression of p38 were observed (Fig. 6B), confirming the equal loading of protein for measurement of MKP-1 expression and p38 activity. In the presence of IL-1 and dexamethasone, the expression of MKP-1 showed a transient peak but did not return to zero, instead remaining at a sustained, moderate level for the duration of the time course. Under these conditions, the initial peak of p38 activity was inhibited by approximately 40%; however, the second phase of activity was almost completely inhibited.
FIG. 6.
Time courses of p38 activation and MKP-1 expression in the absence or presence of dexamethasone. HeLa cells were left untreated or treated with IL-1 (20 ng/ml) alone or with IL-1 (20 ng/ml) and dexamethasone (Dex) (1 μM), and lysates were prepared after the times indicated (15 min [15′]). (A) MKP-1 was immunoprecipitated from lysate (1.5 mg) and subjected to Western blotting. The band below MKP-1 is the light chain of the immunoprecipitating antibody. (B) Lysate (50 μg) was subjected to Western blotting using an antibody against p38α. (C) MAPK p38 was immunoprecipitated from lysate (500 μg) and used in immune complex kinase assays with recombinant MAPKAPK2 (MK2) as the substrate. (D) Quantitation of the data represented in panel C. The results shown are representative of at least two independent experiments.
Sustained p38 activity is required for the expression of Cox-2 in response to IL-1.
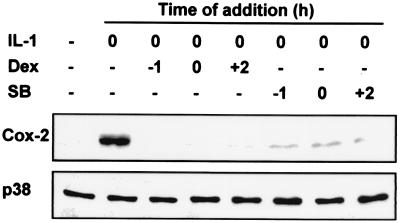
Under identical conditions, dexamethasone completely blocked the induction of Cox-2 gene expression by IL-1 (Fig. 7 and data not shown). Because dexamethasone modestly affected the first phase of p38 activity but profoundly inhibited the second phase, we hypothesized that the second phase was critical for the expression of Cox-2. To test this hypothesis, HeLa cells were treated with IL-1, and the expression of Cox-2 protein was examined 24 h later (Fig. 7). Dexamethasone or SB203580 was added 1 h before the stimulus, at the same time as the stimulus, or 2 h later (after the transient peak of p38 activation). Both dexamethasone and SB203580 efficiently blocked the IL-1-induced expression of Cox-2 regardless of time of addition. Hence, the initial transient phase of p38 activity is not sufficient for Cox-2 expression, but the sustained second phase is necessary.
FIG. 7.
Sustained MAPK p38 activity is necessary for the expression of Cox-2 in response to IL-1. HeLa cells were either not treated (−) or treated by stimulation with 20 ng of IL-1 per ml at time zero. Lysates were prepared 24 h later, and Western blotting was performed to assess the expression of Cox-2 or p38. Dexamethasone (Dex) (1 μM) or SB203580 (SB) (1 μM) was added to the cells 1 h before the IL-1 stimulus (−1), at the same time as the IL-1 stimulus (0), or 2 h after the IL-1 stimulus (+2).
DISCUSSION
In several cell types, dexamethasone inhibited the function but not the expression of p38 (40). This effect was dependent upon GR-mediated transcription and was accompanied by a decrease in p38 phosphorylation, but no effect on signaling events upstream of p38 could be shown. These observations are consistent with phosphatase-mediated reversal of p38 activation or with prevention of phosphorylation of p38 by active MKK6, for example due to the sequestration of one or both of these proteins. Here we provide three lines of evidence that the inhibition of p38 function by dexamethasone is phosphatase mediated. (i) Inhibition of p38 is blocked by sodium orthovanadate, an efficient inhibitor of tyrosine-specific and dual-specificity phosphatases (11, 32, 36). (ii) A sevenmaker mutation of p38, a single amino acid substitution previously shown to confer resistance to phosphatases (32), also conferred resistance to the inhibitory effects of dexamethasone. (iii) Inhibition of p38 was blocked by a catalytically inactive mutant of the DUSP MKP-1, which was previously shown to bind strongly to p38, and protect it from dephosphorylation (32).
A gene expression array approach was used to search for phosphatases which could be involved in the negative regulation of p38 by dexamethasone. Of the candidate phosphatase genes, only MKP-1 was expressed at significant levels and induced by dexamethasone treatment of HeLa cells. This finding was confirmed by Northern blotting using probes against all of the known DUSPs. For example, the expression array data suggested that both DUSP2 (PAC1) and DUSP4 (MKP-2) mRNAs were expressed at low levels and responded to dexamethasone, but neither transcript could be detected by Northern blotting. The stress-activated protein kinase phosphatase MKP7 (65), not represented on the Affymetrix U95Av2 chip, was additionally shown by Northern blotting not to be regulated by dexamethasone.
In HeLa cells, the induction of MKP-1 mRNA was consistently in the three- to fivefold range, but the protein was virtually undetectable in untreated cells and appeared to be induced much more strongly than the mRNA. The time course and dose dependence of MKP-1 gene induction by dexamethasone were consistent with a role for this phosphatase in the inactivation of p38. In human peripheral blood lymphocytes, as well as in mouse embryonic fibroblasts, dexamethasone failed to inhibit p38 function and failed to induce the expression of MKP-1. The induction of MKP-1 in HeLa cells was accompanied by inhibition of both p38 and JNK but not ERK, consistent with evidence that MKP-1 preferentially targets the stress-activated protein kinases (21, 22). However, the possibility that p38 inhibition by dexamethasone involves a phosphatase or phosphatases other than MKP-1 remains. We have attempted to address this possibility by using two different antisense oligonucleotides and four different short interfering RNA duplexes to inhibit MKP-1 gene expression but were unable to block the induction of MKP-1 gene expression by dexamethasone. Therefore, the role of MKP-1 in the inhibition of stress-activated protein kinases by glucocorticoids will be investigated using an MKP-1 knockout mouse strain. The absence of functional MKP-1 in this strain does not result in dysregulation of ERK or abnormalities of cell division, but the stress-activated protein kinase pathways were not investigated (21).
Inhibition of MAPK function by glucocorticoids has been described elsewhere (10, 27, 30, 31, 40, 73). In a mast cell line, dexamethasone induced the expression of MKP-1 within 5 h, but inhibition of ERK function was observed only at much later time points (35). The acquisition of ERK-inactivating function required a relatively slow change in the sensitivity of MKP-1 to proteasome-mediated degradation (7, 35). Hence, there appear to be two distinct mechanisms for the modulation of MKP-1 function by glucocorticoids, and their relative contributions may differ according to cell type. In HeLa cells, the expression of MKP-1 and the inhibition of p38 function both occurred rapidly after dexamethasone treatment; therefore, the modulation of MKP-1 protein stability may not play a significant role. We have not determined whether prolonged treatment of HeLa cells with dexamethasone results in the inhibition of ERK function.
Dexamethasone and IL-1 cooperate to induce MKP-1 gene expression, a highly unusual synergy between pro- and anti-inflammatory stimuli, which merits further investigation. The expression of MKP-1 in response to dexamethasone is sustained, in contrast to the transient induction by growth factors and cellular stresses demonstrated elsewhere (16, 37, 39, 59, 70, 78), or the induction by IL-1 shown here. IL-1-induced MKP-1 expression is dependent on p38, MKP-1 may be catalytically activated by p38 (32), and MKP-1 efficiently inactivates p38 (22, 23, 32, 44, 57). These observations suggest the participation of MKP-1 in a complex feedback loop which modulates p38 function. This is consistent with the dynamics of p38 activation and MKP-1 expression in HeLa cells (although a causal relationship between these phenomena remains to be established). The activation of p38 by IL-1 is biphasic, with a strong transient peak followed by a sustained phase of increasing activity. The down-regulation of the initial peak coincides with the up-regulation of MKP-1 expression, and the second phase of p38 activation occurs in the absence of detectable MKP-1 expression. In the presence of both IL-1 and dexamethasone, the initial peak of MKP-1 expression is augmented, and sustained expression is also observed. This is accompanied by a significant blunting of the initial peak of p38 activity and a complete ablation of the second, sustained phase of p38 activity.
Addition of SB203580 during this second phase causes a rapid destabilization of Cox-2 mRNA (54). The addition of SB203580 or dexamethasone 2 h after IL-1 stimulation of HeLa cells completely blocks IL-1-induced Cox-2 expression; therefore, the prolonged late phase of p38 activity appears to play a critical role in Cox-2 gene expression by maintaining the stability of the transcript. A similar inhibition of IL-1-induced Cox-2 gene expression by late addition of dexamethasone has been described in a pulmonary epithelial cell line (48). This underscores a potential difference between transrepressive and posttranscriptional mechanisms of inhibition of gene expression by glucocorticoids. The former can function only if the glucocorticoids are present during the phase of active transcription, which may be relatively brief in the case of proinflammatory genes. In contrast, the inhibition of p38 and the destabilization of mRNA by glucocorticoids may result in the rapid shutoff of gene expression even when the glucocorticoids are present long after the initial proinflammatory stimulus. This may be relevant to the role of endogenous corticosteroids in negative-feedback control and the resolution of inflammatory responses. Future experiments using the MKP-1 knockout mouse strain will address the physiological relevance of this phosphatase in the control of inflammation.
Acknowledgments
We are grateful to Yusen Liu for provision of reagents, to Helen Causton and Peter Broderick of the CSC/IC Microarray Centre for expert help with Affymetrix microarray experiments, and to Jon Dean for constructive criticism of the manuscript.
This work was supported in part by Programme Grant G8623776 from the Medical Research Council of the United Kingdom.
REFERENCES
- 1.Adcock, I. M., and G. Caramori. 2001. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell. Biol. 79:376-384. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., C. Smythe, and S. M. Keyse. 1993. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene 8:2015-2020. [PubMed] [Google Scholar]
- 3.Amano, Y., S. W. Lee, and A. C. Allison. 1993. Inhibition by glucocorticoids of the formation of interleukin-1 alpha, interleukin-1 beta, and interleukin-6: mediation by decreased mRNA stability. Mol. Pharmacol. 43:176-182. [PubMed] [Google Scholar]
- 4.Auphan, N., J. A. DiDonato, C. Rosette, A. Helmberg, and M. Karin. 1995. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science 270:286-290. [DOI] [PubMed] [Google Scholar]
- 5.Belvisi, M. G., S. L. Wicks, C. H. Battram, S. E. Bottoms, J. E. Redford, P. Woodman, T. J. Brown, S. E. Webber, and M. L. Foster. 2001. Therapeutic benefit of a dissociated glucocorticoid and the relevance of in vitro separation of transrepression from transactivation activity. J. Immunol. 166:1975-1982. [DOI] [PubMed] [Google Scholar]
- 6.Bott, C. M., S. G. Thorneycroft, and C. J. Marshall. 1994. The sevenmaker gain-of-function mutation in p42 MAP kinase leads to enhanced signalling and reduced sensitivity to dual specificity phosphatase action. FEBS Lett. 352:201-205. [DOI] [PubMed] [Google Scholar]
- 7.Brondello, J. M., J. Pouyssegur, and F. R. McKenzie. 1999. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286:2514-2517. [DOI] [PubMed] [Google Scholar]
- 8.Brook, M., G. Sully, A. R. Clark, and J. Saklatvala. 2000. Regulation of tumour necrosis factor alpha mRNA stability by the mitogen-activated protein kinase p38 signalling cascade. FEBS Lett. 483:57-61. [DOI] [PubMed] [Google Scholar]
- 9.Brunner, D., N. Oellers, J. Szabad, W. H. Biggs III, S. L. Zipursky, and E. Hafen. 1994. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76:875-888. [DOI] [PubMed] [Google Scholar]
- 10.Caelles, C., J. M. Gonzalez-Sancho, and A. Munoz. 1997. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 11:3351-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camps, M., A. Nichols, and S. Arkinstall. 2000. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 14:6-16. [PubMed] [Google Scholar]
- 12.Camps, M., A. Nichols, C. Gillieron, B. Antonsson, M. Muda, C. Chabert, U. Boschert, and S. Arkinstall. 1998. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280:1262-1265. [DOI] [PubMed] [Google Scholar]
- 13.Cella, N., B. Groner, and N. E. Hynes. 1998. Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocorticoid receptor in mammary cells. Mol. Cell. Biol. 18:1783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cha, H. H., E. J. Cram, E. C. Wang, A. J. Huang, H. G. Kasler, and G. L. Firestone. 1998. Glucocorticoids stimulate p21 gene expression by targeting multiple transcriptional elements within a steroid responsive region of the p21waf1/cip1 promoter in rat hepatoma cells. J. Biol. Chem. 273:1998-2007. [DOI] [PubMed] [Google Scholar]
- 15.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 16.Charles, C. H., H. Sun, L. F. Lau, and N. K. Tonks. 1993. The growth factor-inducible immediate-early gene 3CH134 encodes a protein-tyrosine-phosphatase. Proc. Natl. Acad. Sci. USA 90:5292-5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu, Y., P. A. Solski, R. Khosravi-Far, C. J. Der, and K. Kelly. 1996. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J. Biol. Chem. 271:6497-6501. [DOI] [PubMed] [Google Scholar]
- 18.Cole, T. J., J. A. Blendy, A. P. Monaghan, K. Krieglstein, W. Schmid, A. Aguzzi, G. Fantuzzi, E. Hummler, K. Unsicker, and G. Schutz. 1995. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 9:1608-1621. [DOI] [PubMed] [Google Scholar]
- 19.Dean, J. L., M. Brook, A. R. Clark, and J. Saklatvala. 1999. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J. Biol. Chem. 274:264-269. [DOI] [PubMed] [Google Scholar]
- 20.Diamond, M. I., J. N. Miner, S. K. Yoshinaga, and K. R. Yamamoto. 1990. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science 249:1266-1272. [DOI] [PubMed] [Google Scholar]
- 21.Dorfman, K., D. Carrasco, M. Gruda, C. Ryan, S. A. Lira, and R. Bravo. 1996. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene 13:925-931. [PubMed] [Google Scholar]
- 22.Franklin, C. C., and A. S. Kraft. 1997. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J. Biol. Chem. 272:16917-16923. [DOI] [PubMed] [Google Scholar]
- 23.Franklin, C. C., S. Srikanth, and A. S. Kraft. 1998. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc. Natl. Acad. Sci. USA 95:3014-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freshney, N. W., L. Rawlinson, F. Guesdon, E. Jones, S. Cowley, J. Hsuan, and J. Saklatvala. 1994. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 78:1039-1049. [DOI] [PubMed] [Google Scholar]
- 25.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 26.Gille, J., K. Reisinger, B. Westphal-Varghese, and R. Kaufmann. 2001. Decreased mRNA stability as a mechanism of glucocorticoid-mediated inhibition of vascular endothelial growth factor gene expression by cultured keratinocytes. J. Investig. Dermatol. 117:1581-1587. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez, M. V., B. Jimenez, M. T. Berciano, J. M. Gonzalez-Sancho, C. Caelles, M. Lafarga, and A. Munoz. 2000. Glucocorticoids antagonize AP-1 by inhibiting the activation/phosphorylation of JNK without affecting its subcellular distribution. J. Cell Biol. 150:1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heck, S., K. Bender, M. Kullmann, M. Gottlicher, P. Herrlich, and A. C. Cato. 1997. IκBα-independent downregulation of NF-κB activity by glucocorticoid receptor. EMBO J. 16:4698-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heck, S., M. Kullmann, A. Gast, H. Ponta, H. J. Rahmsdorf, P. Herrlich, and A. C. Cato. 1994. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 13:4087-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirasawa, N., Y. Sato, Y. Fujita, S. Mue, and K. Ohuchi. 1998. Inhibition by dexamethasone of antigen-induced c-Jun N-terminal kinase activation in rat basophilic leukemia cells. J. Immunol. 161:4939-4943. [PubMed] [Google Scholar]
- 31.Hulley, P. A., F. Gordon, and F. S. Hough. 1998. Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cell line (MBA 15.4) by dexamethasone: role of protein phosphatases. Endocrinology 139:2423-2431. [DOI] [PubMed] [Google Scholar]
- 32.Hutter, D., P. Chen, J. Barnes, and Y. Liu. 2000. Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: critical role of the p38 C-terminal domain in its negative regulation. Biochem. J. 352:155-163. [PMC free article] [PubMed] [Google Scholar]
- 33.Ip, Y. T., and R. J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr. Opin. Cell Biol. 10:205-219. [DOI] [PubMed] [Google Scholar]
- 34.Karin, M. 1998. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell 93:487-490. [DOI] [PubMed] [Google Scholar]
- 35.Kassel, O., A. Sancono, J. Kratzschmar, B. Kreft, M. Stassen, and A. C. Cato. 2001. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 20:7108-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keyse, S. M. 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 12:186-192. [DOI] [PubMed] [Google Scholar]
- 37.Keyse, S. M., and E. A. Emslie. 1992. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature 359:644-647. [DOI] [PubMed] [Google Scholar]
- 38.Kim, F., and M. A. Corson. 2000. Adhesion to fibronectin enhances MKP-1 activation in human endothelial cells. Biochem. Biophys. Res. Commun. 273:539-545. [DOI] [PubMed] [Google Scholar]
- 39.Laderoute, K. R., H. L. Mendonca, J. M. Calaoagan, A. M. Knapp, A. J. Giaccia, and P. J. Stork. 1999. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. J. Biol. Chem. 274:12890-12897. [DOI] [PubMed] [Google Scholar]
- 40.Lasa, M., M. Brook, J. Saklatvala, and A. R. Clark. 2001. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol. Cell. Biol. 21:771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasa, M., K. R. Mahtani, A. Finch, G. Brewer, J. Saklatvala, and A. R. Clark. 2000. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol. Cell. Biol. 20:4265-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lennon, G., C. Auffray, M. Polymeropoulos, and M. B. Soares. 1996. The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics 33:151-152. [DOI] [PubMed] [Google Scholar]
- 43.Li, J., M. Gorospe, D. Hutter, J. Barnes, S. M. Keyse, and Y. Liu. 2001. Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol. Cell. Biol. 21:8213-8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim, H. W., L. New, J. Han, and J. D. Molkentin. 2001. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J. Biol. Chem. 276:15913-15919. [DOI] [PubMed] [Google Scholar]
- 45.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 46.Miyazawa, K., A. Mori, H. Miyata, M. Akahane, Y. Ajisawa, and H. Okudaira. 1998. Regulation of interleukin-1β-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J. Biol. Chem. 273:24832-24838. [DOI] [PubMed] [Google Scholar]
- 47.Newton, R. 2000. Molecular mechanisms of glucocorticoid action: what is important? Thorax 55:603-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton, R., J. Seybold, L. M. Kuitert, M. Bergmann, and P. J. Barnes. 1998. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J. Biol. Chem. 273:32312-32321. [DOI] [PubMed] [Google Scholar]
- 49.Pages, G., E. Berra, J. Milanini, A. P. Levy, and J. Pouyssegur. 2000. Stress-activated protein kinases (JNK and p38/HOG) are essential for vascular endothelial growth factor mRNA stability. J. Biol. Chem. 275:26484-26491. [DOI] [PubMed] [Google Scholar]
- 50.Re, F., M. Muzio, M. De Rossi, N. Polentarutti, J. G. Giri, A. Mantovani, and F. Colotta. 1994. The type II “receptor” as a decoy target for interleukin 1 in polymorphonuclear leukocytes: characterization of induction by dexamethasone and ligand binding properties of the released decoy receptor. J. Exp. Med. 179:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reichardt, H. M., K. H. Kaestner, J. Tuckermann, O. Kretz, O. Wessely, R. Bock, P. Gass, W. Schmid, P. Herrlich, P. Angel, and G. Schutz. 1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93:531-541. [DOI] [PubMed] [Google Scholar]
- 52.Reichardt, H. M., J. P. Tuckermann, M. Gottlicher, M. Vujic, F. Weih, P. Angel, P. Herrlich, and G. Schutz. 2001. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 20:7168-7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards, R. I., A. Heguy, and M. Karin. 1984. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell 37:263-272. [DOI] [PubMed] [Google Scholar]
- 54.Ridley, S. H., J. L. Dean, S. J. Sarsfield, M. Brook, A. R. Clark, and J. Saklatvala. 1998. A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 439:75-80. [DOI] [PubMed] [Google Scholar]
- 55.Saklatvala, J., L. Rawlinson, R. J. Waller, S. Sarsfield, J. C. Lee, L. F. Morton, M. J. Barnes, and R. W. Farndale. 1996. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J. Biol. Chem. 271:6586-6589. [DOI] [PubMed] [Google Scholar]
- 56.Saxena, M., S. Williams, J. Brockdorff, J. Gilman, and T. Mustelin. 1999. Inhibition of T cell signaling by mitogen-activated protein kinase-targeted hematopoietic tyrosine phosphatase (HePTP). J. Biol. Chem. 274:11693-11700. [DOI] [PubMed] [Google Scholar]
- 57.Slack, D. N., O. M. Seternes, M. Gabrielsen, and S. M. Keyse. 2001. Distinct binding determinants for ERK2/p38α and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J. Biol. Chem. 276:16491-16500. [DOI] [PubMed] [Google Scholar]
- 58.Sommer, A., H. Burkhardt, S. M. Keyse, and B. Luscher. 2000. Synergistic activation of the mkp-1 gene by protein kinase A signaling and USF, but not c-Myc. FEBS Lett. 474:146-150. [DOI] [PubMed] [Google Scholar]
- 59.Sun, H., C. H. Charles, L. F. Lau, and N. K. Tonks. 1993. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75:487-493. [DOI] [PubMed] [Google Scholar]
- 60.Swantek, J. L., M. H. Cobb, and T. D. Geppert. 1997. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol. 17:6274-6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takekawa, M., M. Adachi, A. Nakahata, I. Nakayama, F. Itoh, H. Tsukuda, Y. Taya, and K. Imai. 2000. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 19:6517-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takekawa, M., T. Maeda, and H. Saito. 1998. Protein phosphatase 2Cα inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 17:4744-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanoue, T., M. Adachi, T. Moriguchi, and E. Nishida. 2000. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2:110-116. [DOI] [PubMed] [Google Scholar]
- 64.Tanoue, T., T. Moriguchi, and E. Nishida. 1999. Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J. Biol. Chem. 274:19949-19956. [DOI] [PubMed] [Google Scholar]
- 65.Tanoue, T., T. Yamamoto, R. Maeda, and E. Nishida. 2001. A novel MAPK phosphatase MKP-7 acts preferentially on JNK/SAPK and p38 alpha and beta MAPKs. J. Biol. Chem. 276:26629-26639. [DOI] [PubMed] [Google Scholar]
- 66.Theodosiou, A., A. Smith, C. Gillieron, S. Arkinstall, and A. Ashworth. 1999. MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene 18:6981-6988. [DOI] [PubMed] [Google Scholar]
- 67.Tobler, A., R. Meier, M. Seitz, B. Dewald, M. Baggiolini, and M. F. Fey. 1992. Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8, and IL-6, but not of M-CSF in human fibroblasts. Blood 79:45-51. [PubMed] [Google Scholar]
- 68.Tombes, R. M., K. L. Auer, R. Mikkelsen, K. Valerie, M. P. Wymann, C. J. Marshall, M. McMahon, and P. Dent. 1998. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem. J. 330:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Treisman, R. 1996. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8:205-215. [DOI] [PubMed] [Google Scholar]
- 70.Valledor, A. F., J. Xaus, M. Comalada, C. Soler, and A. Celada. 2000. Protein kinase C epsilon is required for the induction of mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J. Immunol. 164:29-37. [DOI] [PubMed] [Google Scholar]
- 71.Vanden Berghe, W., E. Francesconi, K. De Bosscher, M. Resche-Rigon, and G. Haegeman. 1999. Dissociated glucocorticoids with anti-inflammatory potential repress interleukin-6 gene expression by a nuclear factor-κB-dependent mechanism. Mol. Pharmacol. 56:797-806. [PubMed] [Google Scholar]
- 72.Vayssiere, B. M., S. Dupont, A. Choquart, F. Petit, T. Garcia, C. Marchandeau, H. Gronemeyer, and M. Resche-Rigon. 1997. Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit antiinflammatory activity in vivo. Mol. Endocrinol. 11:1245-1255. [DOI] [PubMed] [Google Scholar]
- 73.Ventura, J. J., C. Roncero, I. Fabregat, and M. Benito. 1999. Glucocorticoid receptor down-regulates c-Jun amino terminal kinases induced by tumor necrosis factor alpha in fetal rat hepatocyte primary cultures. Hepatology 29:849-857. [DOI] [PubMed] [Google Scholar]
- 74.Wang, S. W., J. Pawlowski, S. T. Wathen, S. D. Kinney, H. S. Lichenstein, and C. L. Manthey. 1999. Cytokine mRNA decay is accelerated by an inhibitor of p38-mitogen-activated protein kinase. Inflamm. Res. 48:533-538. [DOI] [PubMed] [Google Scholar]
- 75.Wingfield, P., M. Payton, J. Tavernier, M. Barnes, A. Shaw, K. Rose, M. G. Simona, S. Demczuk, K. Williamson, and J. M. Dayer. 1986. Purification and characterization of human interleukin-1 beta expressed in recombinant Escherichia coli. Eur. J. Biochem. 160:491-497. [DOI] [PubMed] [Google Scholar]
- 76.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C. Y. Chen, A. B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao, X. L., M. J. Cowan, M. T. Gladwin, M. M. Lawrence, C. W. Angus, and J. H. Shelhamer. 1999. Dexamethasone alters arachidonate release from human epithelial cells by induction of p11 protein synthesis and inhibition of phospholipase A2 activity. J. Biol. Chem. 274:17202-17208. [DOI] [PubMed] [Google Scholar]
- 78.Zheng, C. F., and K. L. Guan. 1993. Dephosphorylation and inactivation of the mitogen-activated protein kinase by a mitogen-induced Thr/Tyr protein phosphatase. J. Biol. Chem. 268:16116-16119. [PubMed] [Google Scholar]