Abstract
Heat shock protein 72 (Hsp72) is thought to protect cells against cellular stress. The protective role of Hsp72 was investigated by determining the effect of this protein on the stress-activated protein kinase signaling pathways. Prior exposure of NIH 3T3 cells to mild heat shock (43°C for 20 min) resulted in inhibition of H2O2-induced activation of apoptosis signal-regulating kinase 1 (ASK1). Overexpression of Hsp72 also inhibited H2O2-induced activation of ASK1 as well as that of downstream kinases in the p38 mitogen-activated protein kinase (MAPK) signaling cascade. Recombinant Hsp72 bound directly to ASK1 and inhibited ASK1 activity in vitro. Furthermore, coimmunoprecipitation analysis revealed a physical interaction between endogenous Hsp72 and ASK1 in NIH 3T3 cells exposed to mild heat shock. Hsp72 blocked both the homo-oligomerization of ASK1 and ASK1-dependent apoptosis. Hsp72 antisense oligonucleotides prevented the inhibitory effects of mild heat shock on H2O2-induced ASK1 activation and apoptosis. These observations suggest that Hsp72 functions as an endogenous inhibitor of ASK1.
Apoptosis signal-regulating kinase 1 (ASK1) is a widely expressed serine-threonine kinase that was initially discovered as a mitogen-activated protein kinase kinase kinase (MAPKKK) in the c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) and p38 MAPK signaling cascades (12, 13). ASK1 functions in apoptosis induced by diverse stimuli, including tumor necrosis factor alpha (TNF-α), Fas, and many apoptotic stresses (12, 13, 30). Overexpression of a dominant-negative mutant of ASK1 thus prevents apoptosis induced by withdrawal of growth factors, DNA-damaging agents, TNF-α, or agonistic antibodies to Fas (4, 13, 16, 30). Activation of ASK1 is reported to induce apoptotic cell death by triggering mitochondrial events that include the release of cytochrome c from mitochondria and the subsequent activation of caspase 9 and caspase 3 (11).
Many cellular stresses that stimulate the stress-activated MAPK pathways can also induce expression of heat shock proteins. Heat shock protein 72 (Hsp72) is the major inducible heat shock protein (35). It plays a role in many cellular activities including protein synthesis, folding, and translocation into organelles as well as the assembly of multiprotein complexes (2, 5, 24, 25, 33). Hsp72 contains two conserved domains, an ATP-binding domain (ABD) and a peptide-binding domain (PBD), that are important for its chaperon function (7, 21). Hsp72 also prevents cell death initiated by various apoptotic stresses including heat shock, ceramide, ionizing irradiation, TNF-α, and ischemia (8, 14, 20, 23, 32). Hsp72 suppresses several apoptotic signaling pathways, including caspase cascades and stress-activated MAPK pathways that include the JNK and p38 signaling cascades (3, 8, 9, 15, 17, 19, 22, 23, 29, 31). Furthermore, Hsp72 has been shown to physically interact with and inhibit Apaf-1 and apoptosis-inducing factor, resulting in suppression of caspase-dependent and -independent apoptosis, respectively (3, 28, 31). In addition, Hsp72 interacts through its peptide-binding domain with JNK, thereby inhibiting the JNK signaling pathway (27). The molecular mechanism by which Hsp72 inhibits the p38 MAPK pathway has remained unclear.
To better understand the mechanism by which Hsp72 modulates stress-activated signaling, we have now investigated the possible effects of Hsp72 on ASK1 and its downstream kinases in the p38 MAPK pathway. Our data show that Hsp72 inhibits both the stress-induced activation of ASK1 and ASK1-involved apoptosis. We also demonstrate that Hsp72 physically associates with ASK1, thereby inhibiting the homo-oligomerization of this kinase. Moreover, depletion of Hsp72 by the expression of specific antisense oligonucleotides indicates that endogenous Hsp72 is the major heat-inducible factor responsible for the inhibition of ASK1 activation by mild heat shock. Our results thus suggest that Hsp72 functions as an endogenous inhibitor of ASK1.
MATERIALS AND METHODS
Antibodies.
The mouse monoclonal anti-Flag M2 antibody was purchased from Sigma Chemical Co.; the mouse monoclonal anti-hemagglutinin (HA) antibody was obtained from Roche Molecular Biochemicals; the mouse monoclonal anti-Hsp72 antibody was obtained from Stressgen Biotechnologies; the rabbit polyclonal anti-ASK1 antibody was from Santa Cruz Biotechnology; and the mouse anti-thioredoxin antibody was from Pharmingen.
Cell culture and transfection.
NIH 3T3 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). NIH 3T3 cells were stably transfected with an empty pCMV vector or with pCMV-Hsp72 by the calcium phosphate method, and neomycin-resistant cells were selected in complete medium containing 500 μg of G418 (Gibco BRL)/ml (27). Heterogeneous populations of the stably transfected cells were used to avoid any possible clonal variations.
DNA constructs and production of fusion proteins.
ASK1-ΔN, which encodes amino acids 649 to 1375 of the ASK1 protein, was generated by PCR (6) and subcloned into pHM6 (Roche Molecular Biochemicals), which is a mammalian expression vector for HA-tagged proteins. The pcDNA3-HA-ASK1 and pcDNA3-HA-ASK1-K vectors were kindly provided by H. Ichijo (Tokyo Medical and Dental University, Tokyo, Japan). The cDNA for MKK6(K82A) was generated by use of a QuickChange site-directed mutagenesis kit (Stratagene). Construction of pCMV-Hsp72, pCMV-Hsp72ΔABD, pCMV-Hsp72ΔPBD, pCMV-Hsp72ΔN, pET30a-Hsp72, pET30a-Hsp72ΔABD, pET30a-Hsp72ΔPBD, and pET30a-Hsp72ΔN has been described previously (17, 27). Glutathione S-transferase (GST) fusion proteins and hexahistidine-tagged proteins were produced in Escherichia coli by use of pGEX-4T (Amersham Pharmacia Biotech) and pET30a (Novagen), respectively, and were purified with glutathione-Sepharose and Ni2+-nitrilotriacetic acid (NTA)-agarose beads, respectively.
Immunocomplex kinase assays.
Immunocomplex kinase assays were performed as described previously (6, 27). Cells were harvested and lysed with buffer A containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 2 μg of leupeptin/ml, 2 μg of aprotinin/ml, 25 mM glycerophosphate, 0.1 mM sodium orthovanadate, 1 mM sodium fluoride, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS). Cell lysates were subjected to centrifugation at 12,000 × g for 10 min at 4°C. The resulting supernatant was subjected to immunoprecipitation by incubation first for 2 h at 4°C with appropriate antibodies and then for 1 h at 4°C in the additional presence of protein G-Sepharose (Amersham Pharmacia Biotech). Immunoprecipitates were assayed for the indicated protein kinase activities by using GST fusion proteins as substrates. Reaction mixtures were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE), and the extent of substrate phosphorylation was analyzed with a Fuji BAS 2500 phosphorimager. GST-MKK6(K82A), GST-p38, and GST-ATF2(1-109) were used as substrates for ASK1, MKK3 or MKK6, and p38 MAPK, respectively. Protein concentration was determined by the Bradford assay (Bio-Rad).
Immunoblot analysis.
Cells were lysed with buffer A, and lysates were subjected to centrifugation at 12,000 × g for 10 min at 4°C. The resulting supernatant was subjected to SDS-PAGE, and the separated proteins were transferred electrophoretically to a nitrocellulose membrane. The membrane was incubated with a Tris-buffered saline solution containing 5% nonfat milk and 0.1% Tween 20. The membrane was then incubated for 1 h at room temperature with the indicated antibodies in a Tris-buffered saline solution containing 0.1% Tween 20 and subsequently with appropriate secondary antibodies conjugated with horseradish peroxidase (Amersham Pharmacia Biotech). The immunoblots were visualized by an enhanced chemiluminescence method (Amersham Pharmacia Biotech).
Luciferase reporter assay for ATF2-dependent transcription.
The transcription-stimulating activity of ATF-2 was measured with a PathDetect luciferase reporter kit (Stratagene). NIH 3T3 cells were transfected by the calcium phosphate method for 32 h with pFR-Luc and pcDNA3-β-gal in the presence of the indicated combinations of pFA2-ATF2, pcDNA3-ASK1, pCMV-Hsp72, and pcDNA3-MKK6(K82A). Cell lysates were then prepared and subjected to centrifugation at 12,000 × g for 10 min at 4°C. The resulting supernatant was examined for luciferase activity with a luciferase assay kit (Promega). The luciferase reporter activity was normalized to the β-galactosidase activity of the same cells.
In vitro binding and coimmunoprecipitation analyses.
Recombinant hexahistidine-tagged Hsp72, Hsp72ΔABD, Hsp72ΔPBD, and Hsp72ΔN were expressed in E. coli and purified with Ni2+-NTA-agarose (Qiagen). In vitro-translated 35S-labeled protein kinases were incubated for 10 h at 4°C with full-length or mutant Hsp72 proteins immobilized on Ni2+-NTA-agarose beads in a solution containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1 mM phenylmethylsulfonyl fluoride, 2 μg of leupeptin/ml, 2 μg of aprotinin/ml, 25 mM glycerophosphate, 0.1 mM sodium orthovanadate, 1 mM sodium fluoride, 1% Nonidet P-40, and 10% glycerol. The beads were extensively washed with 50 mM Tris-HCl (pH 7.5), and bound proteins were then eluted from the beads and analyzed by SDS-PAGE and autoradiography. For coimmunoprecipitation experiments, cells were harvested and lysed with buffer B containing 50 mM Tris-HCl (pH 8.0), 120 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml, 2 mM sodium orthovanadate, 10 mM sodium fluoride, and 0.5% Nonidet P-40. Lysates were subjected to immunoprecipitation with appropriate antibodies. The resulting immunoprecipitates were washed three times with buffer B, subjected to SDS-PAGE, and then analyzed by immunoblotting with the indicated antibodies.
Antisense oligonucleotides of Hsp72.
NIH 3T3 cells were transiently transfected, by using GenePORTER 2 (Gene Therapy Systems), with an antisense oligonucleotide of the inducible hsp72 gene (5′-CACCTTGCCGTGCTGGAA-3′; nucleotides 61 to 78) at 10 μM or with the same concentration of a nonsense oligonucleotide (5′-TGGATCCGACATGTCAGA-3′) as described previously (27).
Apoptotic cell death.
NIH 3T3 cells were transiently transfected for 48 h with pEGFP and plasmid vectors encoding the indicated combinations of proteins by using GenePORTER 2. The cells were then fixed with 4% formaldehyde and stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI). The DAPI-stained nuclei of green fluorescent protein (GFP)-positive cells were analyzed for apoptotic morphology by fluorescence microscopy. The percentage of apoptotic cells was calculated as the number of GFP-positive cells with apoptotic nuclei divided by the total number of GFP-positive cells. Alternatively, for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate, and then incubated for 60 min at 37°C with the TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and fluorescein isothiocyanate-labeled dUTP by use of an in situ cell death detection kit (Roche Molecular Biochemicals). TUNEL-positive cells were detected by fluorescence microscopy.
RESULTS
Hsp72 inhibits ASK1 activation.
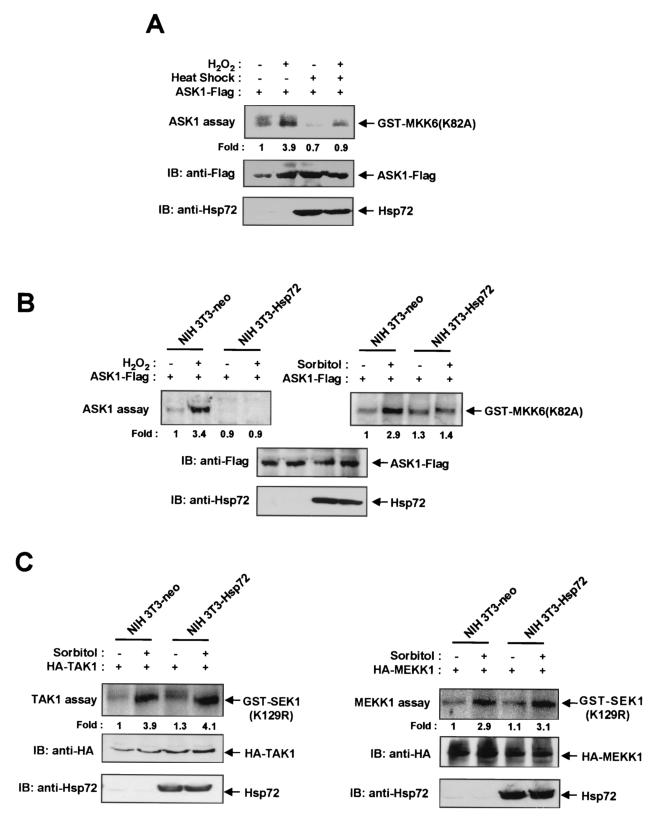
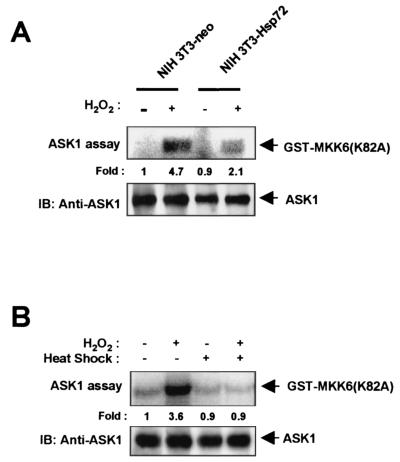
To determine whether Hsp72 is able to modulate ASK1-mediated signaling, we subjected NIH 3T3 cells to transient transfection with an expression plasmid encoding Flag epitope-tagged ASK1 (ASK1-Flag) and then examined the effect of a mild heat shock (43°C for 20 min) on the H2O2-induced activation of the recombinant ASK1 in the transfected cells (Fig. 1A). Prior treatment of the transfected cells with the mild heat shock resulted in induction of Hsp72 expression. Whereas H2O2 induced ASK1 activation in control NIH 3T3 cells, this effect was markedly inhibited in the heat-treated cells. We next examined H2O2-induced ASK1 activation in NIH 3T3 cells stably transfected with an expression vector for human Hsp72 (NIH 3T3-Hsp72 cells) or with the corresponding empty vector (NIH 3T3-neo cells) as previously described (27). The abundance of ectopic Hsp72 in NIH 3T3-Hsp72 cells is similar to that of endogenous Hsp72 in NIH 3T3 cells exposed to a mild heat shock (27) (see also Fig. 5B). Both NIH 3T3-neo and NIH 3T3-Hsp72 cells were transiently transfected with the ASK1-Flag construct and then treated with 2 mM H2O2 for 20 min. The H2O2-induced activation of ectopic ASK1 apparent in the control NIH 3T3-neo cells was abolished in NIH 3T3-Hsp72 cells (Fig. 1B). The ASK1 activation induced by other stimuli, including sorbitol and UV light, was also inhibited in NIH 3T3-Hsp72 cells (Fig. 1B; also data not shown). In comparison, overexpression of ectopic Hsp72 failed to inhibit the sorbitol-induced stimulation of either TAK1 or MEKK1 activities in NIH 3T3-Hsp72 cells (Fig. 1C). We also examined the H2O2-induced stimulation of endogenous ASK1 activity in NIH 3T3-neo and NIH 3T3-Hsp72 cells (Fig. 2). The increase in the activity of endogenous ASK1 induced by H2O2 was much smaller in NIH 3T3-Hsp72 cells than in NIH 3T3-neo cells (Fig. 2A). Furthermore, prior exposure of NIH 3T3 cells to a mild heat shock inhibited the H2O2-induced activation of endogenous ASK1 (Fig. 2B). Together, these results suggest that Hsp72 inhibits ASK1 activation in intact cells.
FIG. 1.
Hsp72 inhibits H2O2-induced ASK1 activation in NIH 3T3 cells. (A) NIH 3T3 cells were transiently transfected for 30 h with the pcDNA3 vector encoding Flag-tagged ASK1. The cells were then either left untreated or exposed to a mild heat shock (43°C for 20 min), incubated for an additional 16 h at 37°C, and either left untreated or treated with 2 mM H2O2 for 20 min. Cell lysates were subjected to immunoprecipitation with an anti-Flag antibody, and the resulting precipitates were examined for ASK1 activity by an immunocomplex kinase assay with GST-MKK6(K82A) as a substrate (top panel). The abundances of ASK1-Flag and Hsp72 in cell lysates were also examined by immunoblot analysis (IB) with mouse monoclonal antibodies to the Flag epitope (middle panel) and to Hsp72 (bottom panel). (B) NIH 3T3-neo or NIH 3T3-Hsp72 cells were transiently transfected for 48 h with the ASK1-Flag vector and then incubated in the absence or presence of 2 mM H2O2 for 20 min or of 600 mM sorbitol for 30 min. Cell lysates were assayed for ASK1 activity by an immunocomplex kinase assay and subjected to immunoblot analysis as described for panel A. (C) NIH 3T3-neo or NIH 3T3-Hsp72 cells were transiently transfected with an expression vector encoding HA-tagged TAK1 or HA-tagged MEKK1, respectively. After 48 h of transfection, cells were incubated in the absence or presence of 600 mM sorbitol for 30 min. Cell lysates were subjected to immunoprecipitation with an anti-HA antibody, and the resulting precipitates were assayed for TAK1 and MEKK1 activities.
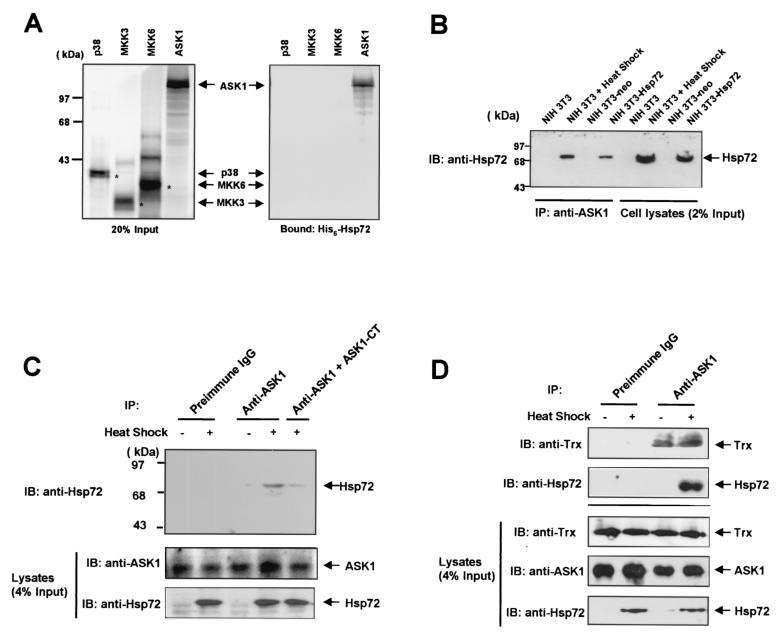
FIG. 5.
Hsp72 physically interacts with ASK1. (A) Hexahistidine-tagged recombinant Hsp72 was immobilized on Ni2+-NTA-agarose beads and incubated with in vitro-translated 35S-labeled p38, MKK3, MKK6, or ASK1. The beads were then washed extensively, after which bound proteins were eluted and analyzed by SDS-PAGE (on an 8% polyacrylamide gel) and autoradiography. A portion of the 35S-labeled proteins corresponding to 20% of the input for the binding reaction is also shown. (B) Cell lysates prepared from either untreated NIH 3T3 cells, NIH 3T3 cells treated with a mild heat shock (43°C for 20 min), NIH 3T3-neo cells, or NIH 3T3-Hsp72 cells were subjected to immunoprecipitation (IP) with an anti-ASK1 antibody. The resulting immunoprecipitates were then subjected to SDS-PAGE on a 10% polyacrylamide gel, followed by immunoblot (IB) analysis with a mouse monoclonal anti-Hsp72 antibody. A portion of the cell lysates corresponding to 2% of the input for immunoprecipitation was also subjected to immunoblot analysis with the anti-Hsp72 antibody. (C and D) NIH 3T3 cells were either left untreated or treated with a mild heat shock (43°C for 20 min), and cell lysates were subjected to immunoprecipitation with rabbit preimmune immunoglobulin G or an anti-ASK1 antibody. Where indicated in panel C, immunoprecipitation was carried out in the presence of 10 μg of a carboxy-terminal fragment (amino acids 1014 to 1376) of ASK1 (ASK1-CT). The resulting immunoprecipitates were analyzed by immunoblotting using an antibody against Hsp72 or thioredoxin (Trx) as for panel B.
FIG. 2.
Hsp72 inhibits H2O2-induced activation of endogenous ASK1 in NIH 3T3 cells. (A) NIH 3T3-neo or NIH 3T3-Hsp72 cells were incubated in the absence or presence of 2 mM H2O2 for 20 min, after which cell lysates were subjected to immunoprecipitation with an anti-ASK1 antibody and the resulting precipitates were examined for ASK1 activity by an immunocomplex kinase assay. IB, immunoblot. (B) NIH 3T3 cells were either left untreated or treated with a mild heat shock (43°C for 20 min), incubated for 16 h at 37°C, and then incubated in the absence or presence of 2 mM H2O2 for 20 min. Cell lysates were processed for the assay of ASK1 activity as described for panel A.
Hsp72 inhibits signaling downstream of ASK1.
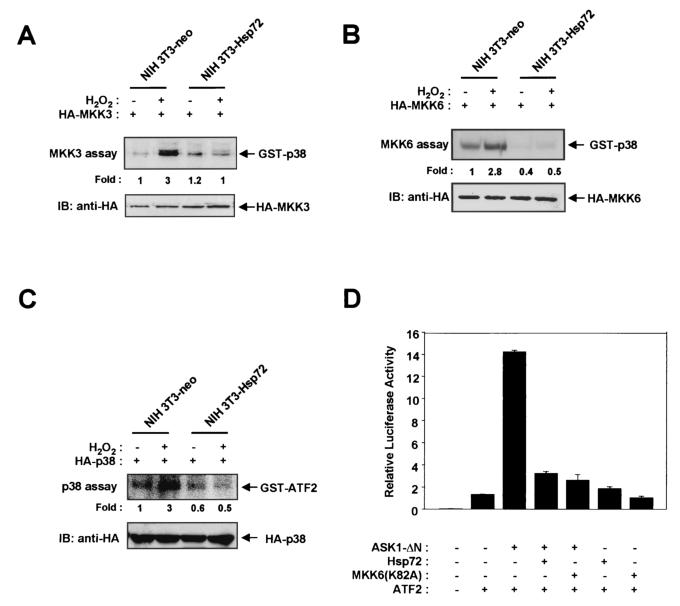
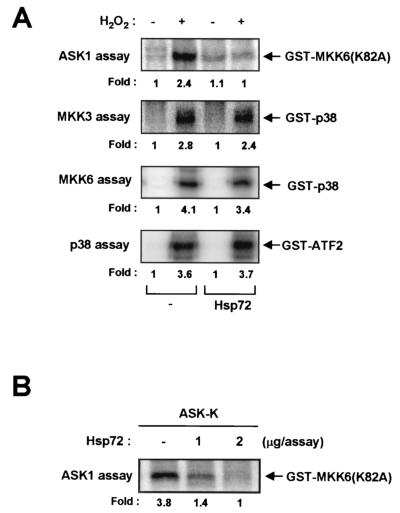
We next examined whether Hsp72 affects signaling downstream of ASK1, which includes the MKK3-p38 (or MKK6-p38) pathway (13). NIH 3T3-neo and NIH 3T3-Hsp72 cells were transiently transfected with vectors expressing HA epitope-tagged MKK3, HA-MKK6, or HA-p38 and were then exposed to 2 mM H2O2 for 20 min. Whereas H2O2 markedly stimulated the activities of MKK3 (Fig. 3A), MKK6 (Fig. 3B), and p38 (Fig. 3C) in NIH 3T3-neo cells, these effects were greatly reduced in NIH 3T3-Hsp72 cells. The transcription factor ATF2 is a physiological target of p38 MAPK. We therefore determined whether Hsp72 affects the ASK1-dependent increase in the trans-activation activity of ATF2 by use of a luciferase reporter gene assay (Fig. 3D). Overexpression in NIH 3T3 cells of ASK1-ΔN, which contains amino acids 649 to 1375 of the ASK1 protein and functions as a constitutively active form of the enzyme, induced a marked increase in ATF2-dependent luciferase activity, and this effect of ASK1 was inhibited by MKK6(K82A), a kinase-inactive mutant of MKK6. These data indicate that ASK1 stimulates ATF2 through the p38 signaling pathway. Overexpression of Hsp72 also blocked the ASK1-induced increase in the transcription-stimulating activity of ATF2.
FIG. 3.
Hsp72 inhibits signaling downstream of ASK1 in NIH 3T3 cells. (A through C) NIH 3T3-neo or NIH 3T3-Hsp72 cells were transiently transfected for 48 h with plasmid vectors encoding HA-tagged MKK3 (A), HA-tagged MKK6 (B), or HA-tagged p38 MAPK (C). The cells were then incubated in the absence or presence of 2 mM H2O2 for 20 min. Cell lysates were subjected to immunoprecipitation with a mouse monoclonal anti-HA antibody, and the resulting precipitates were examined for MKK3 (A), MKK6 (B), or p38 MAPK (C) activities by immunocomplex kinase assays with GST-p38 (A and B) or GST-ATF2(1-109) (C) as the substrate. Expression of HA-MKK3 (A), HA-MKK6 (B), and HA-p38 MAPK (C) was also examined by immunoblot (IB) analysis of cell lysates with an anti-HA antibody. (D) NIH 3T3 cells were transiently transfected with pcDNA3-β-gal and a luciferase reporter plasmid, pFR-Luc, in the presence of vectors for the indicated combinations of ATF2, ASK1-ΔN, Hsp72, and MKK6(K82A). After 36 h of transfection, cell lysates were assayed for luciferase and β-galactosidase activities. The luciferase activity in each sample was normalized to the β-galactosidase activity of the same sample. Data are means ± standard deviations of triplicates from one of two representative experiments.
Hsp72 inhibits ASK1 activity in vitro.
The effect of Hsp72 on ASK1 activity in vitro was examined in order to determine whether Hsp72 targets ASK1 directly (Fig. 4). NIH 3T3 cells were transiently transfected with Flag-tagged ASK1, incubated for 20 min in the absence or presence of 2 mM H2O2, and then subjected to immunoprecipitation with an anti-Flag antibody. The resulting Flag immmunoprecipitates were assayed for ASK1 activity in the absence or presence of purified recombinant Hsp72. Hsp72 inhibited ASK1 activity in vitro (Fig. 4A). In contrast, Hsp72 did not inhibit the kinase activities of MKK3, MKK6, or p38 MAPK in vitro. These results suggest that Hsp72 suppresses ASK1-mediated signaling through direct inhibition of ASK1. In a separate in vitro kinase assay, Hsp72 also inhibited an enzymatic activity of ASK1-K, corresponding to amino acids 667 to 936 of ASK1, which include the kinase domain of the enzyme (Fig. 4B). Moreover, an in vitro binding study using purified Hsp72 protein and in vitro-translated 35S-labeled ASK1-K revealed that Hsp72 bound ASK1-K (data not shown). Hsp72 was not phosphorylated by ASK1 in vitro (data not shown).
FIG. 4.
Hsp72 inhibits ASK1 activity, but not MKK3, MKK6, or p38 activity, in vitro. (A) NIH 3T3 cells were transfected for 48 h with expression vectors encoding Flag-tagged ASK1, HA-tagged MKK3, HA-tagged MKK6, or HA-tagged p38, as indicated. The cells were incubated in the absence or presence of 2 mM H2O2 for 20 min, and then cell lysates were subjected to immunoprecipitation using a mouse monoclonal anti-Flag or anti-HA antibody. The Flag or HA immunoprecipitates were incubated for 1 h at room temperature in 50 μl of HEPES buffer (pH 7.4) in the absence or presence of 2 μg of purified human recombinant Hsp72 protein, washed twice with the HEPES buffer, and then assayed for the indicated kinase activities. (B) NIH 3T3 cells were transiently transfected for 48 h with a pcDNA3 vector encoding HA-tagged ASK1-K. Cell lysates were subjected to immunoprecipitation with an anti-HA antibody. The immunoprecipitates were incubated for 1 h at room temperature with the indicated amount of Hsp72 protein in 50 μl of HEPES buffer (pH 7.4), washed twice with the HEPES buffer, and assayed for ASK1 activity by using GST-MKK6(K82A) as a substrate.
Hsp72 physically interacts with ASK1.
We next examined whether Hsp72 interacts with ASK1 in vitro. 35S-labeled ASK1, MKK3, MKK6, and p38 were produced by in vitro translation and incubated individually with purified hexahistidine-tagged Hsp72 that had been immobilized on Ni2+-NTA-agarose beads (Fig. 5A). In vitro binding data revealed that Hsp72 protein bound to 35S-labeled ASK1 but not to 35S-labeled p38, MKK3, or MKK6. The possible interaction of Hsp72 with ASK1 in intact cells was also examined (Fig. 5B). Immunoblot analysis with an anti-Hsp72 antibody of ASK1 immunoprecipitates prepared from NIH 3T3-Hsp72 cells demonstrated that ectopic Hsp72 physically interacted with endogenous ASK1 in these cells. Furthermore, exposure of NIH 3T3 cells to a mild heat shock induced a physical interaction between the two endogenous Hsp72 and ASK1 proteins in intact cells (Fig. 5B). We tested the specificity of the anti-ASK1 antibody used in this coimmunoprecipitation by using a carboxy-terminal fragment (amino acids 1014 to 1376) of ASK1 (Fig. 5C). Addition of the carboxy-terminal fragment of ASK1 in the immunoprecipitation using the anti-ASK1 antibody mitigated the coimmunoprecipitation of ASK1 with Hsp72.
ASK1 has been shown to form an inactive complex with thioredoxin (30). Therefore, we investigated whether mild heat shock could modulate the interaction between ASK1 and thioredoxin in NIH 3T3 cells. NIH 3T3 cells were either left untreated or treated with a mild heat shock (43°C for 20 min), and the cell lysates were subjected to immunoprecipitation by use of rabbit preimmune immunoglobulin G or an anti-ASK1 antibody. Immunoblot analysis of the resulting immunoprecipitates by use of an anti-thioredoxin antibody revealed that ASK1 physically interacted with thioredoxin in NIH 3T3 cells (Fig. 5D). The mild heat shock did not affect the interaction between endogenous ASK1 and thioredoxin proteins, while it induced the interaction between ASK1 and Hsp72 in NIH 3T3 cells.
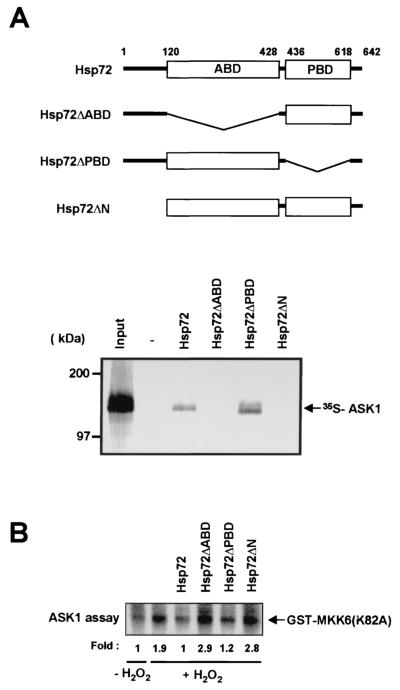
We next examined which domain of Hsp72 was required for its association with ASK1. Hsp72 contains the ABD and the PBD (7, 21). In vitro binding data demonstrated that 35S-labeled full-length ASK1 bound to an Hsp72 mutant that lacks the PBD (Hsp72ΔPBD) but not to mutants that lack either the ABD (Hsp72ΔABD) or the NH2-terminal region (Hsp72ΔN) (Fig. 6A). These data suggest that both the NH2-terminal region and the ABD of Hsp72 are important for the interaction of this protein with ASK1. We also investigated the effects of the Hsp72 variants on ASK1 activity in vitro (Fig. 6B). ASK1 activity was inhibited by Hsp72 and by Hsp72ΔPBD but not by Hsp72ΔABD or Hsp72ΔN. Thus, the in vitro kinase activity results were consistent with the in vitro binding data shown in Fig. 6A.
FIG. 6.
Interaction of ASK1 with the NH2-terminal region and the ABD of Hsp72. (A) In vitro-translated 35S-labeled full-length ASK1 was applied to hexahistidine-tagged Hsp72 variants that had been immobilized to Ni2+-NTA-agarose beads. Bead-bound proteins were subsequently eluted and analyzed by SDS-PAGE and autoradiography as for Fig. 5A. The input 35S-labeled ASK1 (20%) is also shown. A schematic diagram of Hsp72 and its mutants is shown above the gel. (B) NIH 3T3 cells were transiently transfected for 48 h with pcDNA3-Flag-ASK1 and were then incubated for 20 min at 37°C in the absence or presence of 2 mM H2O2. Cell lysates were subjected to immunoprecipitation with an anti-Flag antibody. The resulting precipitates were incubated for 1 h at room temperature with 2 μg of purified wild-type Hsp72, Hsp72ΔABD, Hsp72ΔPBD, or Hsp72ΔN in 50 μl of HEPES buffer (pH 7.4), washed twice with the HEPES buffer, and then assayed for ASK1 activity with GST-MKK6(K82A) as the substrate.
Hsp72 interferes with the homo-oligomerization of ASK1 and with the interaction between ASK1 and MKK3.
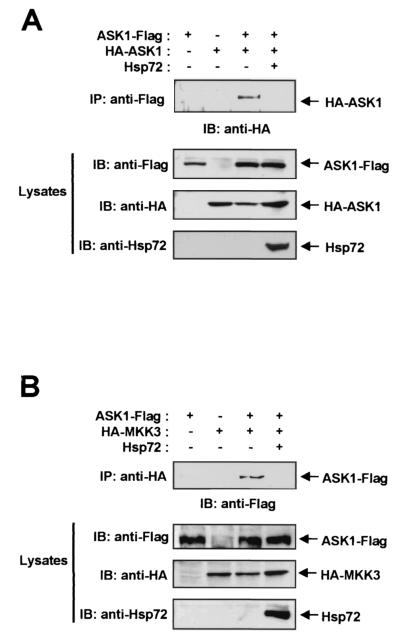
Given that Hsp72 physically associates with ASK1, we investigated whether Hsp72 prevents the homo-oligomerization of ASK1, which is thought to contribute to the activation of this kinase (10, 18). NIH 3T3 cells were transfected with expression vectors encoding Flag-tagged ASK1 and HA-tagged ASK1 in the absence or presence of an Hsp72 construct (Fig. 7A). Cell lysates were then subjected to immunoprecipitation with an anti-Flag antibody, and the resulting immunoprecipitates were subjected to immunoblot analysis with an anti-HA antibody. The immunoblot data showed that HA-tagged ASK1 associated with Flag-tagged ASK1 and that ASK1 homo-oligomerization was inhibited by coexpression of Hsp72. These results thus suggest that inhibition of ASK1 homo-oligomerization may contribute to the inhibition of ASK1 activation by Hsp72.
FIG. 7.
Hsp72 inhibits ASK1 homo-oligomerization and the interaction between ASK1 and MKK3. (A) NIH 3T3 cells were transfected for 48 h with the indicated combinations of ASK1-Flag, HA-ASK1, and Hsp72 constructs. Cell lysates were then subjected to immunoprecipitation (IP) with a mouse monoclonal anti-Flag antibody, and the resulting precipitates were subjected to immunoblot (IB) analysis with a mouse monoclonal anti-HA antibody (upper panel). Cell lysates were also directly subjected to immunoblot analysis with an antibody to Flag, to HA, or to Hsp72 (lower panels). (B) NIH 3T3 cells were transfected for 48 h with the indicated combinations of vectors encoding ASK1-Flag, HA-MKK3, and Hsp72. Cell lysates were then subjected to immunoprecipitation with an anti-HA antibody, and the resulting precipitates were subjected to immunoblot analysis with an anti-Flag antibody. Cell lysates were also directly subjected to immunoblot analysis as described for panel A.
We next examined whether Hsp72 interferes with the interaction between ASK1 and its substrate MKK3. NIH 3T3 cells were transfected with vectors expressing Flag-tagged ASK1 and HA-tagged MKK3 in the absence or presence of an Hsp72 construct (Fig. 7B). Immunoprecipitation and immunoblot analysis demonstrated that Flag-tagged ASK1 interacted with HA-tagged MKK3 in cotransfected cells and that this interaction was blocked by Hsp72. In a similar experiment, Hsp72 also inhibited the interaction of ASK1 with MKK6 (data not shown).
Hsp72 prevents ASK1-mediated apoptosis.
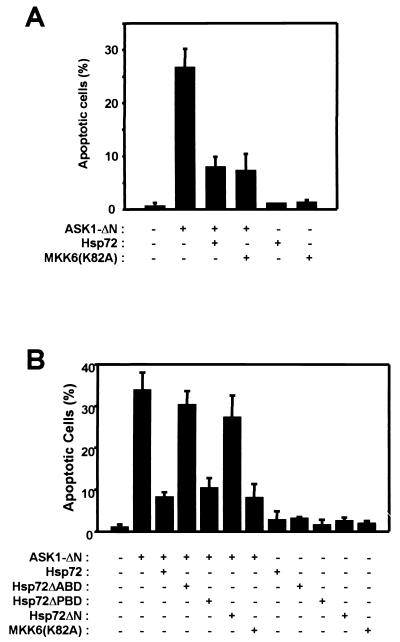
Activation of ASK1 induces apoptotic cell death under various conditions (4, 12, 13, 16, 30). We therefore investigated whether Hsp72 prevents the induction of apoptosis by ASK1-ΔN, a constitutively active form of ASK1 (Fig. 8). Expression of ASK1-ΔN in NIH 3T3 cells resulted in an increase in the percentage of apoptotic cells, as determined by staining with DAPI (Fig. 8A) or by TUNEL staining (data not shown). This effect of ASK1-ΔN was inhibited by coexpression of MKK6(K82A), a kinase-inactive mutant of MKK6. Hsp72 also inhibited ASK1-ΔN-induced apoptosis. In a separate experiment, ASK1-ΔN-induced apoptosis was inhibited by full-length Hsp72 and Hsp72ΔPBD but not by Hsp72ΔABD or Hsp72ΔN (Fig. 8B). These results are in good agreement with the in vitro binding and kinase data shown in Fig. 6.
FIG. 8.
Hsp72 inhibits ASK1-dependent cell death. NIH 3T3 cells were transfected for 48 h with pEGFP and the indicated combinations of expression vectors encoding ASK-ΔN, Hsp72, Hsp72ΔABD, Hsp72ΔPBD, Hsp72ΔN, and MKK6(K82A). Cells were then fixed and stained with DAPI. DAPI-stained nuclei of GFP-positive cells were examined for apoptotic morphology by fluorescence microscopy, and the percentages of apoptotic cells in five randomly chosen fields were determined. Data are means ± standard deviations of triplicates from one experiment representative of three independent experiments.
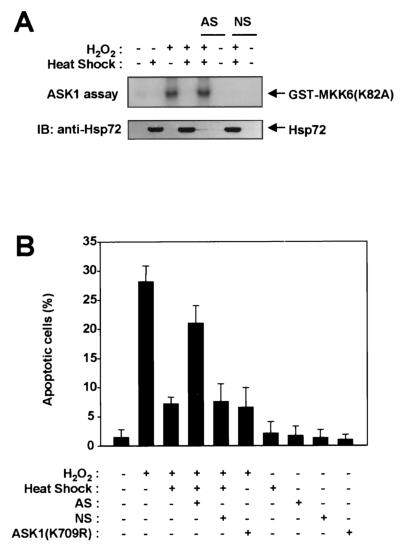
Hsp72 antisense oligonucleotides prevent the inhibitory effects of mild heat shock on H2O2-induced ASK1 activation and apoptosis.
Our results demonstrate that exposure of NIH 3T3 cells to a mild heat shock inhibits ASK1 activation (Fig. 1 and 2). Heat shock can increase the expression of various stress-inducible proteins, including heat shock proteins. We therefore transfected NIH 3T3 cells with Hsp72 antisense oligonucleotides in order to determine whether induction of Hsp72 mediates the inhibition of ASK1 activation by heat shock (Fig. 9A). Immunoblot analysis with an anti-Hsp72 antibody revealed that mild heat shock increased the abundance of Hsp72 in NIH 3T3 cells and that this accumulation of Hsp72 was prevented by prior transfection of the cells with the Hsp72 antisense oligonucleotides (Fig. 9A). In contrast, transfection of cells with nonsense oligonucleotides had no effect on the heat shock-induced accumulation of Hsp72. Transfection of cells with the Hsp72 antisense oligonucleotides, but not transfection with the nonsense oligonucleotides, also blocked the inhibitory effect of mild heat shock on H2O2-induced ASK1 activation. Finally, we examined the role of Hsp72 in the heat shock-induced inhibition of apoptosis triggered by H2O2 (Fig. 9B). Treatment of NIH 3T3 cells with 200 μM H2O2 for 12 h resulted in an increase in the percentage of apoptotic cells, and this effect was inhibited by expression of ASK1(K709R), a kinase-inactive mutant of ASK1. These data suggest that ASK1 mediates apoptosis initiated by H2O2. Prior exposure of cells to a mild heat shock inhibited H2O2-induced apoptosis in control NIH 3T3 cells and in those transfected with the nonsense oligonucleotides, but it did not do so in cells transfected with the Hsp72 antisense oligonucleotides. Collectively, these data indicate that Hsp72 mediates the mild-heat-shock-induced inhibition of both ASK1 activation and apoptosis induced by H2O2.
FIG. 9.
Hsp72 antisense oligonucleotides prevent the inhibitory effects of mild heat shock on H2O2-induced ASK1 activation and apoptosis. (A) NIH 3T3 cells were transiently transfected for 30 h with Hsp72 antisense (AS) or nonsense (NS) oligonucleotides, where indicated, by using GenePORTER 2. Cells were then either left untreated or exposed to a mild heat shock (43°C for 20 min), incubated for an additional 16 h at 37°C, and either left untreated or treated with 2 mM H2O2 for 20 min. Cell lysates were subjected to immunoprecipitation with an anti-ASK1 antibody, and the resulting precipitates were examined for ASK1 activity by an immunocomplex kinase assay (upper panel). Cell lysates were also subjected to immunoblot (IB) analysis with an anti-Hsp72 antibody (lower panel). (B) NIH 3T3 cells were transfected for 30 h with pEGFP and the indicated combinations of pcDNA3-HA-ASK1(K709R) and Hsp72 antisense (AS) or nonsense (NS) oligonucleotides. Cells were then either left untreated or exposed to a mild heat shock (43°C for 20 min), incubated further for 16 h at 37°C, and either left untreated or treated with 200 μM H2O2 for 12 h. They were subsequently fixed and stained with DAPI. GFP-positive cells were examined for apoptotic nuclei with a fluorescence microscope, and the percentages of apoptotic cells in five randomly chosen fields were determined. Data are means ± standard deviations of triplicates from a representative experiment performed twice.
DISCUSSION
We have shown that Hsp72 physically interacts with ASK1 and inhibits the activation of ASK1, resulting in suppression of ASK1-mediated signaling. Furthermore, Hsp72 was shown to prevent ASK1-dependent apoptotic cell death. These observations suggest that inhibition of ASK1 is an important mechanism by which Hsp72 functions as a negative regulator of SAPK signaling cascades.
Hsp72, when induced in the cellular response to cytotoxic stress, prevents cell death triggered by a variety of apoptotic stimuli, including heat shock, ionizing radiation, TNF-α, and ischemia (8, 14, 20, 23, 32). Hsp72 modulates several signaling processes that are associated with the regulation of cell death. It thus down-regulates the caspase, JNK, and p38 MAPK signaling cascades (3, 8, 9, 15, 17, 19, 22, 23, 29, 31). Moreover, Hsp72 physically interacts with Apaf-1, thereby blocking Apaf-1/cytochrome c-mediated caspase activation (3, 31). Hsp72 also binds to and antagonizes apoptosis-inducing factor, thereby inhibiting caspase-independent apoptosis (28). Several mechanisms have been proposed for the down-regulation of the JNK signaling pathway by Hsp72. Hsp72 directly interacts with and thereby inhibits the activation of JNK, resulting in suppression of JNK-mediated apoptosis (27). Other studies have proposed that Hsp72 inhibits JNK activation by promoting JNK dephosphorylation catalyzed by a JNK phosphatase (19). Our data now reveal a new mechanism: Hsp72 suppresses the JNK pathway by means of direct inhibition of ASK1. Given that ASK1 is a MAP3K that contributes to both the JNK and p38 MAPK signaling cascades, inhibition of ASK1 activation by Hsp72 may also be an important mechanism through which Hsp72 inhibits the p38 MAPK signaling pathway.
ASK1 plays a role in the mechanisms of apoptosis, cell growth, and cell differentiation (1, 4, 11, 12, 13, 30, 34). The biological function of ASK1 is modulated by ASK1-interacting proteins, which include thioredoxin and other proteins (1, 4, 6, 18, 26, 30, 36). TRAF2 and Daxx have been shown to mediate ASK1 activation by TNF-α and Fas, respectively (4, 26), whereas thioredoxin, p21, 14-3-3, and GST μ function as cellular inhibitors of ASK1 (1, 6, 30, 36). Our data in this study demonstrate that Hsp72 also functions as a negative regulator of ASK1. Hsp72, by binding to ASK1, prevents ASK1 homo-oligomerization, which is thought to be a mechanism of ASK1 activation (6, 10, 18). Furthermore, Hsp72 inhibits the binding of ASK1 to substrates such as MKK3 and MKK6 in intact cells. On the basis of these data, it may be proposed that Hsp72 inhibits ASK1-mediated signaling by at least two distinct mechanisms: (i) inhibition of an ASK1-activating process and (ii) inhibition of ASK1-mediated activation of its substrate proteins.
Exposure of cells to a variety of cellular stresses results in stimulation of stress-activated MAPKs, including JNK and p38. The JNK and p38 signaling cascades are thought to mediate intracellular signaling initiated by cellular stress. Sustained activation of these signaling cascades by cellular stress may result in irreversible cellular damage including cell death. Given that ASK1 functions as a MAP3K in both the JNK and p38 signaling pathways, our results suggest that Hsp72, by antagonizing ASK1, suppresses stress-activated MAPK signaling with high efficiency. In addition, Hsp72 can tightly regulate the JNK signaling pathway by targeting at least two components of this pathway, JNK (27) and ASK1. Our present observations thus suggest that ASK1 is an intracellular target of Hsp72 and that inhibition of ASK1 activation is an important component of the mechanism by which Hsp72 modulates stress-activated signaling.
Acknowledgments
We thank H. Ichijo, J. Han, K. Matsumoto, R. J. Ulevitch, G. L. Johnson, and R. J. Davis for providing ASK1, MKK6, TAK1, p38, MEKK1, and MKK3 cDNA clones, respectively. We also thank W. A. Toscano, Jr., for critical reading of the manuscript.
This work was supported by grants to E.-J.C and S.K. from the Creative Research Initiatives Program of the Korean Ministry of Science and Technology and by a grant to Y.-G.K. from the Korean Ministry of Public Health and Welfare.
REFERENCES
- 1.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, K. Fukumuro, and S. Mizutani. 1999. Apoptosis inhibitory activity of cytoplasmic p21 (Cip1/WAF1) in monocytic differentiation. EMBO J. 18:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckmann, R. P., L. E. Mizzen, and W. J. Welch. 1990. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science 248:850-854. [DOI] [PubMed] [Google Scholar]
- 3.Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 4.Chang, H. Y., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281:1860-1863. [DOI] [PubMed] [Google Scholar]
- 5.Chirico, W. J., M. G. Waters, and G. Blobel. 1988. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature 332:805-810. [DOI] [PubMed] [Google Scholar]
- 6.Cho, S. G., Y. H. Lee, H. S. Park, K. Ryoo, K. W. Kang, J. Park, S. J. Eom, M. J. Kim, T. S. Chang, S. Y. Choi, J. Shim, Y. Kim, M. S. Dong, M. J. Lee, S. G. Kim, H. Ichijo, and E.-J. Choi. 2001. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J. Biol. Chem. 276:12749-12755. [DOI] [PubMed] [Google Scholar]
- 7.Freeman, B. C., M. P. Myers, R. Schumacher, and R. I. Morimoto. 1995. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 14:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabai, V. L., A. B. Meriin, D. D. Mosser, A. W. Caron, S. Rits, V. I. Shifrin, and M. Y. Sherman. 1997. Hsp70 prevents activation of stress kinases: a novel pathway of cellular thermotolerance. J. Biol. Chem. 272:18033-18037. [DOI] [PubMed] [Google Scholar]
- 9.Gabai, V. L., J. A. Yaglom, V. Volloch, A. B. Meriin, T. Force, M. Koutroumanis, B. Massie, D. D. Mosser, and M. Y. Sherman. 2000. Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol. Cell. Biol. 20:6826-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotoh, Y., and J. A. Cooper. 1998. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J. Biol. Chem. 273:17477-17482. [DOI] [PubMed] [Google Scholar]
- 11.Hatai, T., A. Matsuzawa, S. Inoshita, Y. Mochida, T. Kuroda, K. Sakamaki, K. Kuida, S. Yonehara, H. Ichijo, and K. Takeda. 2000. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J. Biol. Chem. 275:26576-26581. [DOI] [PubMed] [Google Scholar]
- 12.Ichijo, H. 1999. From receptors to stress-activated MAP kinases. Oncogene 18:6087-6093. [DOI] [PubMed] [Google Scholar]
- 13.Ichijo, H., E. Nishida, K. Irie, P. ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90-94. [DOI] [PubMed] [Google Scholar]
- 14.Jaattela, M. 1993. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J. Immunol. 151:4286-4294. [PubMed] [Google Scholar]
- 15.Jaattela, M., D. Wissing, K. Kokholm, T. Kallunki, and M. Egeblad. 1998. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17:6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanamoto, T., M. Mota, K. Takeda, L. L. Rubin, K. Miyazono, H. Ichijo, and C. E. Bazenet. 2000. Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons. Mol. Cell. Biol. 20:196-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, C. Y., J. S. Lee, Y. G. Ko, J. I. Kim, and J. S. Seo. 2000. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J. Biol. Chem. 275:25665-25671. [DOI] [PubMed] [Google Scholar]
- 18.Liu, H., H. Nishitoh, H. Ichijo, and J. M. Kyriakis. 2000. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol. 20:2198-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meriin, A. B., J. A. Yaglom, V. L. Gabai, D. D. Mosser, L. Zon, and M. Y. Sherman. 1999. Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol. Cell. Biol. 19:2547-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meriin, A. B., V. L. Gabai, J. Yaglom, V. I. Shifrin, and M. Y. Sherman. 1998. Proteasome inhibitors activate stress kinases and induce Hsp72. Diverse effects on apoptosis. J. Biol. Chem. 273:6373-6379. [DOI] [PubMed] [Google Scholar]
- 21.Milarski, K. L., and R. I. Morimoto. 1989. Mutational analysis of the human HSP70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J. Cell Biol. 109:1947-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosser, D. D., A. W. Caron, L. Bourget, A. B. Meriin, M. Y. Sherman, R. I. Morimoto, and B. Massie. 2000. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 20:7146-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosser, D. D., A. W. Caron, L. Bourget, C. Denis-Larose, and B. Massie. 1997. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17:5317-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami, H., D. Pain, and G. Blobel. 1988. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J. Cell Biol. 107:2051-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson, R. J., T. Ziegelhoffer, C. Nicolet, M. Werner-Washburne, and E. A. Craig. 1992. The translation machinery and 70 kD heat shock protein cooperate in protein synthesis. Cell 71:97-105. [DOI] [PubMed] [Google Scholar]
- 26.Nishitoh, H., M. Saitoh, Y. Mochida, K. Takeda, H. Nakano, M. Rothe, K. Miyazono, and H. Ichijo. 1998. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell 2:389-395. [DOI] [PubMed] [Google Scholar]
- 27.Park, H. S., J. S. Lee, S. H. Huh, J. S. Seo, and E.-J. Choi. 2001. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 20:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravagnan, L., S. Gurbuxani, S. A. Susin, C. Maisse, E. Daugas, N. Zamzami, T. Mak, M. Jaattela, J. M. Penninger, C. Garrido, and G. Kroemer. 2001. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell Biol. 3:839-843. [DOI] [PubMed] [Google Scholar]
- 29.Robertson, J. D., K. Datta, S. S. Biswal, and J. P. Kehrer. 1999. Heat-shock protein 70 antisense oligomers enhance proteasome inhibitor-induced apoptosis. Biochem. J. 344:477-485. [PMC free article] [PubMed] [Google Scholar]
- 30.Saitoh, M., H. Nishitoh, M. Fujii, K. Takeda, K. Tobiume, Y. Sawada, M. Kawabata, K. Miyazono, and H. Ichijo. 1998. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17:2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saleh, A., S. M. Srinivasula, L. Balkir, P. D. Robbins, and E. S. Alnemri. 2000. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2:476-483. [DOI] [PubMed] [Google Scholar]
- 32.Sharp, F. R., S. M. Massa, and R. A. Swanson. 1999. Heat-shock protein protection. Trends Neurosci. 22:97-99. [DOI] [PubMed] [Google Scholar]
- 33.Shi, Y., and J. O. Thomas. 1992. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol. Cell. Biol. 12:2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda, K., T. Hatai, T. S. Hamazaki, H. Nishitoh, M. Saitoh, and H. Ichijo. 2000. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem. 275:9805-9813. [DOI] [PubMed] [Google Scholar]
- 35.Wu, B., C. Hunt, and R. Morimoto. 1985. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol. Cell. Biol. 5:330-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, L., J. Chen, and H. Fu. 1999. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc. Natl. Acad. Sci. USA 96:8511-8515. [DOI] [PMC free article] [PubMed] [Google Scholar]