Abstract
The retinoblastoma (RB) gene product has been shown to restrict cell proliferation, promote cell differentiation, and inhibit apoptosis. Loss of RB function can induce both p53-dependent apoptosis and p53-independent apoptosis; little is known about the mechanisms of RB-regulated p53-independent apoptosis. Here we show that RB specifically activates transcription of the survival gene bcl-2 in epithelial cells but not in NIH 3T3 mesenchymal cells. This transcriptional activity is mediated by the transcription factor AP-2. By monitoring protein-DNA interactions in living cells using formaldehyde cross-linking and chromatin immunoprecipitation, we show that endogenous RB and AP-2 both bind to the same bcl-2 promoter sequence. In addition, we demonstrate that RB and AP-2 also bind to the E-cadherin gene promoter in vivo, consistent with regulation of this promoter by both AP-2 and RB in epithelial cells. This study provides evidence that RB activates bcl-2 and E-cadherin by binding directly to the respective promoter sequences and not indirectly by repressing an inhibitor. This recruitment is mediated by a transcription factor, in this case AP-2. For the first time, our results suggest a direct molecular mechanism by which RB might inhibit apoptosis independently of p53. The results are discussed in a context where RB and Bcl-2 contribute under nonpathological conditions to the maintenance of cell viability in association with a differentiated phenotype, contributing to the tumor suppressor function of RB and playing important roles in normal development.
The harmonious development of any organism requires a fine equilibrium between the processes of cell proliferation, differentiation, and apoptosis. These different functions are highly regulated and interdependent. Many of the genes involved in the regulation of these functions function as oncogenes or tumor suppressor genes.
The retinoblastoma (RB) gene is an important tumor suppressor, and its protein product has been shown to restrict cell proliferation, promote cell differentiation, and inhibit apoptosis (for reviews, see references 15, 28, and 53).
RB can act as either a negative or a positive regulator of transcription. In the context of cell proliferation, it acts as a negative regulator. The most widely accepted hypothesis proposes that RB represses transcription through the E2F family of transcription factors, partly through masking their activation domains and partly by recruiting a histone deacetylase to promoters that are repressed during the G1 phase of the cell cycle (for a review, see reference 37). It has also been suggested that repression and activation of E2F-responsive genes may occur through distinct E2F heterodimers (46). In contrast to cell proliferation, when RB promotes differentiation, it regulates the activity of several transcription factors in a positive manner (for a review, see reference 35). However, the precise molecular mechanism for this activity of RB has not yet been elucidated. Similarly, RB also inhibits apoptosis, but it is not yet known whether it acts positively or negatively on transcription in this context, nor are the relevant target genes known.
We have previously shown that RB plays a major role in the maintenance of the epithelial phenotype. In this regard, RB activates the master gene of epithelial differentiation, the E-cadherin gene, by directly binding to the transcription factor AP-2 in vivo and acting synergistically with it (1). In MDCK kidney epithelial cells, the inactivation of RB family proteins by simian virus 40 (SV40) large T antigen (LT) induces massive apoptosis and mesenchyme conversion, i.e., a loss of expression of all epithelial markers (30, 31). The stable reexpression of RB or of the antiapoptotic protein Bcl-2 allows a partial rescue of epithelial markers, including E-cadherin, and also restores cell viability, attesting that these two functions are linked and raising the possibility that bcl-2 might be directly regulated by RB.
The bcl-2 gene was originally identified by its involvement in the t(14:18) translocation that is associated with human follicular lymphoma. Its role in the pathogenesis of follicular lymphoma was attributed primarily to failure of programmed cell death rather than to rapid cell division (for a review, see reference 24).
In the adult, Bcl-2 is expressed by immature cell populations (bone marrow progenitors of cell lineages, epithelial progenitors in intestine and epidermis). Its expression is also especially prominent in the nervous system and during kidney development (52). Bcl-2−/− mice develop renal failure as a result of severe polycystic kidney decease (PKD). Interestingly, Bcl-2 expression levels do not mirror patterns of cell death in all tissues; and changes in its expression match cell differentiation more closely than patterns of death. In fact, developmental patterns of Bcl-2 expression suggest that Bcl-2 has a role beyond the regulation of cell death. Subsequent studies have confirmed this observation. Bcl-2 has been shown to have a role in hemopoietic differentiation (11), neuronal differentiation (5, 59) and epithelial differentiation (29; our unpublished results).
Whereas bcl-2 is regulated in both a tissue-specific and temporally specific manner, relatively little is known about the regulatory mechanism governing its transcription. Two promoter regions, P1 and P2, have been identified in the 5′-regulatory region. The major promoter, P1, located 1,386 to 1,423 bp upstream of the translation start site, is a TATA-less, GC-rich promoter containing multiple transcription initiation sites. The second promoter, P2, is located approximately 1.3 kb downstream of P1 and contains a canonical TATA element, an octamer element, and a CAAT box (36). This P2 promoter is responsible for a small percentage of the bcl-2 transcripts in most cell types (50).
The bcl-2 promoter was shown to be positively and negatively regulated, depending upon the transcription factor and the cell type. For example, bcl-2 was found to be negatively regulated by p53 in epithelial cells (34, 58) and positively regulated during B-cell activation through a cAMP-responsive element (57), by c-myb during hemopoiesis (47), by v-myb during differentiation of myoloblasts into macrophages (14), and by Brn-3a Pou during neuronal differentiation (44). WT1 was found to negatively regulate bcl-2 in HeLa cells and in t(14:18)-containing lymphoma cells (16, 18) while upregulating bcl-2 in rhabdoid tumor cells (32). However, still relatively little is understood about the mode of bcl-2 regulation in epithelial cells, where it is highly expressed both during embryogenesis and in the adult (52).
In this study, we demonstrate that RB participates in the positive regulation of the bcl-2 gene in epithelial cells. Interestingly, we found that this activation also requires interaction with the AP-2 transcription factor, as we previously demonstrated for the activation of the E-cadherin gene by RB in these same cells (1). Importantly, RB and AP-2 bind to both bcl-2 and E-cadherin gene promoters in epithelial cells, demonstrating that RB-mediated activation is direct.
Our findings also provide for the first time a direct putative mechanism by which RB affects cell survival, namely, by controlling expression of bcl-2.
MATERIALS AND METHODS
Cell culture and transfection.
MDCK cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum. NIH 3T3, HepG2, HaCat, MCF7, and C33 cell lines were grown in Dulbecco's modified Eagle's medium-10% fetal calf serum.
Dishes (diameter, 60 mm) containing 30 to 40% confluent cells fed with fresh medium 2 to 4 h earlier were transfected using the standard calcium phosphate precipitation technique. The amount of transfected DNA was normalized by adding pUC18 plasmid DNA. As an internal control for transfection efficiency, 1 μg of the pHβALacZ plasmid (Escherichia coli lacZ gene under the control of the β-actin promoter) was cotransfected with the other plasmids. CAT activity was measured by the method of Sleigh (43) and quantitated by liquid scintillation counting.
Plasmid constructs.
The Bcl-2P1P2CAT (MYH453-21), Bcl-2P1CAT(−4600) (TM438-2), and Bcl-2P1P2(Δ-755/-350)CAT (MYH453-17) constructs were previously described (34). The Bcl-2 CAT −1150, −1093, −946, −744, −670, −656, −648, −447, −231 and −134 constructs were constructed from Bcl-2P1P2CAT by unidirectional exonuclease III digestion of the bcl-2 promoter with the Erase-a-Base kit (Promega). Bcl-2(Δ −665/−640)CAT was constructed from Bcl-2(−744)CAT by site-directed deletion using the Quick-change site-directed mutagenesis kit (Stratagene). All plasmid sequences were confirmed by sequencing. The pSV-RB expression vector containing the human RB cDNA under SV40 promoter control was previously described (48). pSV-RBΔ was derived from pSV-RB by removing the EcoRI fragment carrying the RB cDNA nucleotides 900 to 4600 (1). The pPADH-AP2 expression vector containing the human AP-2 cDNA under alcohol dehydrogenase promoter control was previously described (56). Dominant-negative AP-2 Δ TA was obtained by deletion of the N-terminal domain of AP-2 (amino acids 51 to 138) (17).
Immunofluorescence.
MDCK cells were grown on coverslips. Fixation was with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7) for 15 min at 4°C. Fixed cells were incubated with the following antibodies: (i) the monoclonal antibody Bcl-2/B46620 (Transduction Laboratories), (ii) the polyclonal antibody RB/C15 (Santa Cruz), (iii) the fluorescein isothiocyanate-conjugated goat anti-mouse IgG, and (iv) the Texas Red dye-conjugated F(ab′)2 fragment of goat anti-rabbit IgG (Jackson Immunoresearch). All antibodies were diluted in blocking buffer (phosphate-buffered saline, 0.5% Triton X-100, 0.1% bovine serum albumin).
Electrophoretic mobility shift assay.
Nuclear extracts from subconfluent HaCat cells were prepared as previously described (9). HaCat nuclear extract (2 μg/reaction) or 1 μl of in vitro-transcribed and -translated hAP-2α from pGEM-hAP-2α (TnT T7/SP6; Promega) was incubated with 0.5 ng (70,000 to 80,000 cpm) of 32P-labeled probe and 1 μg of poly(dI-dC) in a 20-μl reaction volume (containing 20 mM HEPES [pH 7.9], 90 mM NaCl, 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl difluoride, 20% glycerol, and the competing oligonucleotides) for 30 min at 4°C. Supershift assays were performed by adding 5 μl of the polyclonal OB2 antibody or the monoclonal A6 antibody to the binding reaction for 2 h at 4°C. OB2 antibody is directed against AP-2α, AP-2β, and AP-2γ, and A6 antibody is directed against AP-2α and AP-2β (4). Samples were loaded onto 0.5× Tris-borate-EDTA-5% acrylamide gels and electrophoresed at 4°C.
The following oligonucleotides and their complements were synthesized, annealed, and purified on polyacrylamide gels: Bcl2wt, 5′-CTAATTTTTACTCCCTCTCCCCCCGACTCCTGA; SV40-AP2wt, 5′-GATCCAAAGTCCCCAGGCTCCCCAG; and SV40-AP2m, 5′-GATCCAAAGTCTCCGAATTCTCGAG. Double-stranded probes were end labeled by the T4 polynucleotide kinase process using [γ-32P]ATP.
Chromatin immunoprecipitation.
Chromatin immunoprecipitations were performed as described previously (12). Subconfluent MCF7 or C33 cells were treated with formaldehyde at a final concentration of 1% for 7 min at room temperature. Chemical cross-linking was terminated by addition of glycine to a final concentration of 0.125 M, followed by additional incubation for 5 min. After a wash with cold phosphate-buffered saline, cells were suspended in lysis buffer [5 mM piperazine N,N′-bis(2-ethanesulfonic acid) (pH 8.0), 85 mM KCl, 0.5% NP-40] and disrupted using a Dounce homogenizer. Nuclei were then pelleted and suspended in nuclear lysis buffer (50 mM Tris-HCl [pH 8.1], 10 mM EDTA, 1% sodium dodecyl sulfate [SDS]). Chromatin was sonicated with 16 10-s pulses (50 W; amplitude, 80%; Bioblock Vibra Cell 72434). After centrifugation, the supernatant was diluted 10-fold with TNE buffer (16.7 mM Tris-HCl [pH 8.1], 167 mM NaCl, 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA). Diluted chromatin was precleared with protein A/G agarose beads (Santa Cruz) saturated with bovine serum albumin and salmon sperm DNA and then incubated overnight at 4°C using anti-RB C-15, anti-AP-2 C-18, anti-acetylated H4 antibodies (Upstate Biotechnologies), or anti-mouse IgG (Santa Cruz) and immunoprecipitated with protein A/G agarose beads. The beads were extensively washed, and then chromatin was eluted from beads by incubation during vortexing in elution buffer (50 mM NaHCO3, 1% SDS). Cross-links were then reversed by overnight incubation at 65°C in elution buffer containing in addition 300 mM NaCl and 30 μg of RNase A/ml. An equivalent amount of diluted chromatin was similarly processed without immunoprecipitation and noted as “input” afterwards. DNA samples were then purified by phenol-chloroform extraction, ethanol precipitated, and further analyzed by real-time quantitative PCR (LightCycler; Roche Diagnostics) with monitoring of the accumulation of the amplified sequence in real time, using the FastStart DNA Master SYBR green I kit (Roche). Numbers of amplified copies of PCR product were estimated by reference to a linear standard curve obtained from PCRs run in parallel using known concentrations of a plasmid harboring the target sequence. Three dilutions of each sample were analyzed. Standardization of the chromatin inputs for immunoprecipitation was assessed in each experiment. The primers used were the following: Bcl-2 sequence, 5′-CGGTTGGGATTCCTGCGGATT and 5′-AATTGCATAAGGCAACGATCCC, E-cadherin sequence, 5′-TAGAGGGTCACCGCGTCTATG and 5′-GGGTGCGTGGCTGCAGCCAGG; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequence, 5′-GGACCTGACCTGCCGTCTAGAA and 5′-GGTGTCGCTGTTGAAGTCAGAG.
RESULTS
Endogenous Bcl-2 expression is activated by RB.
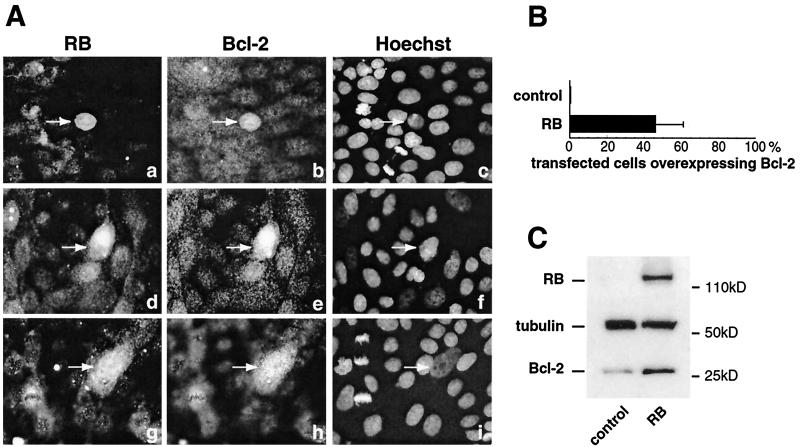
RB inactivation by SV40 LT specifically induces a mesenchyme-like conversion, i.e., a loss of expression of epithelial markers and induction of massive apoptosis, in epithelial MDCK cells (30, 31). Furthermore, the stable reexpression of RB or Bcl-2 in MDCK(LT) cells allows a partial restoration of the epithelial phenotype and a recovery of cell viability. We therefore sought to determine whether bcl-2 gene expression is regulated by RB. We overexpressed human RB in MDCK cells by transient transfection and determined the level of endogenous Bcl-2 expression in RB-transfected cells by immunofluorescence. We found that approximately half of RB-positive cells (Fig. 1A, panels a, d, and g) overexpressed Bcl-2 (Fig. 1A, panels b, e, and h; see Fig. 1B for quantification), indicating that RB can induce Bcl-2 expression. Cells transfected with pUC DNA or a control vector, pSVRBΔ (a vector containing a large deletion in the coding sequence), did not overexpress Bcl-2. The amount of RB expressed in transfected cells is variable, and this might explain why only about half of RB-transfected cells become Bcl-2 immunopositive. The cells were still growing and not confluent when they were fixed (36 h after transfection). Thus, this positive correlation between RB and Bcl-2 could not be due to growth arrest.
FIG. 1.
Endogenous Bcl-2 expression is activated by RB. (A) MDCK cells were transfected by an RB expression vector (a, d, and g) and tested 36 h after transfection for endogenous Bcl-2 (b, e, and h) expression. RB and Bcl-2 expression was detected by immunofluorescence using specific antibodies against RB and Bcl-2. Cells were also stained with Hoechst as a control (c, f, and i). (B) Quantification of panel A showing the percentage of RB-transfected cells overexpressing Bcl-2. (C) MDCK cells were transfected with both RB and IL-2 receptor-expressing vectors. Transfected cells were selected as described in the text. Total protein samples were extracted, and Bcl-2, tubulin, and RB protein expression was analyzed by immunoblotting on SDS-15% polyacrylamide gels.
To confirm this result, MDCK cells were cotransfected with RB and an interleukin 2 (IL-2) receptor-expressing plasmid. The transfected cells were selected with magnetic beads bearing an anti-IL-2 receptor antibody. Then, the amount of endogenous Bcl-2 in these cells was analyzed by immunoblotting. As shown in Fig. 1C, an increase of Bcl-2 protein levels was observed when cells were transfected with RB, whereas tubulin expression did not change, serving as a control.
RB transactivates the bcl-2 promoter in a cell-type-specific manner.
Having established a correlation between RB and Bcl-2 expression, we next explored whether RB can regulate bcl-2 transcription. To this end, we performed transient transfection assays and examined the ability of human RB to transactivate reporter plasmids containing the bcl-2 promoter (Fig. 2). MDCK epithelial cells were cotransfected with the Bcl-2 P1P2CAT construct, containing 1,644 bp upstream of the translation start site, including the two promoters P1 and P2, along with the pSVRB expression plasmid or the control vector pSVRBΔ and a vector containing only the SV40 early promoter (data not shown). Figure 2A shows that the Bcl-2 P1P2CAT construct was strongly activated by pSVRB, up to 13-fold. In contrast, the Bcl-2 P1CAT construct (−4644), lacking the P2 promoter, was very weakly transactivated by pSVRB, revealing that the region located between −1644 and the transcription initiation site +1 was important for RB transactivation. However, we cannot formally exclude the possibility that a sequence located between −4600 and −1644 might also play a role in the RB-mediated effect. Nevertheless, we decided to first analyze the region located between −1644 and +1. The specificity of RB activation was demonstrated by the observation that RB activated the bcl-2 promoter in a concentration-dependent fashion (Fig. 2B). Moreover, RB had no effect on the Bax promoter, another Bcl-2 protein family member (data not shown). Cotransfection experiments in mesenchymal NIH 3T3 cells of Bcl2 P1P2CAT using increasing amounts of pSVRB did not result in significant activation of the bcl-2 promoter. We also analyzed ectopic Bcl-2 regulation in MDCK cells transformed by either wild-type SV40 LT, i.e., MDCK(1-6) cells, or by a K1 LT mutant, unable to bind RB family proteins but still capable of inactivating p53, i.e., MDCK(2a5) cells (1). The MDCK(1-6) cells have lost the expression of all epithelial markers and are fibroblast-like cells, whereas the MDCK(2a5) cells retain an epithelial phenotype. In addition, cotransfection experiments were performed with another epithelial cell line, MCF7 cells. Figure 2C shows that the RB-mediated activation of the bcl-2 promoter was similar in epithelial MDCK, MDCK(2a5), and MCF7 cells, whereas it was abolished in fibroblast-like MDCK(1-6) cells, thus mimicking the results obtained with NIH 3T3 fibroblasts. Taken together, these results indicate that RB transactivates the bcl-2 promoter in a cell-type-specific manner, i.e., in MDCK and MCF7 epithelial cells but not in fibroblasts. Several possibilities could explain these results. Very low levels of AP-2 proteins and activity were detected in fibroblasts (data not shown). A cofactor and/or posttranslational modification may be missing in fibroblasts, or alternatively an inhibitor may be present.
FIG. 2.
Cell-type-specific activation of bcl-2 promoter by RB. (A) Effects of RB on the activity of Bcl-2 P1P2 and Bcl-2 P1 promoters in MDCK epithelial cells. One microgram of each Bcl-2 CAT construct was transfected with 5 μg of pSV-RB expression vector (black bars) or the pSV-RBΔ vector as a control (white bars). The results shown are averages of values obtained in three independent experiments expressed as fold activation of CAT activity relative to the basal promoter activity, which is assigned a value of 1. Error bars indicate standard deviation. (B) Dose-dependent effect of RB on transcriptional activity of the Bcl-2 P1P2 promoter in MDCK epithelial cells and in NIH 3T3 fibroblasts. One microgram of Bcl-2 P1P2 CAT construct was cotransfected with increasing amounts of pSV-RB expression vector (solid lines, filled circles) or the pSV-RBΔ vector as a control (stippled lines, triangles). Each value is expressed as fold activation of CAT activity relative to the baseline value obtained by cotransfecting the Bcl-2 P1P2 CAT vector with the empty expression vector. The results shown are the averages of values obtained in three independent experiments performed in duplicate. Error bars indicate standard deviations. (C) Effects of RB on the activity of Bcl-2 P2 (−1093) promoter in MDCK epithelial cells, in two stably transformed cell lines: fibroblast-like MDCK(1-6) transformed by wild-type LT and epithelial-cell-like MDCK(2a5) transformed by mutated LT(K1) and in MCF7 cells. Cells were transfected with 1 μg of the Bcl-2 P2 (−1093) CAT construct and 5 μg of the pSV-RB expression vector (black bars) or the empty expression vector (white bars). The results are averages of values obtained in three independent experiments performed in duplicate and are expressed as in panel A.
Characterization of the RB-responsive element.
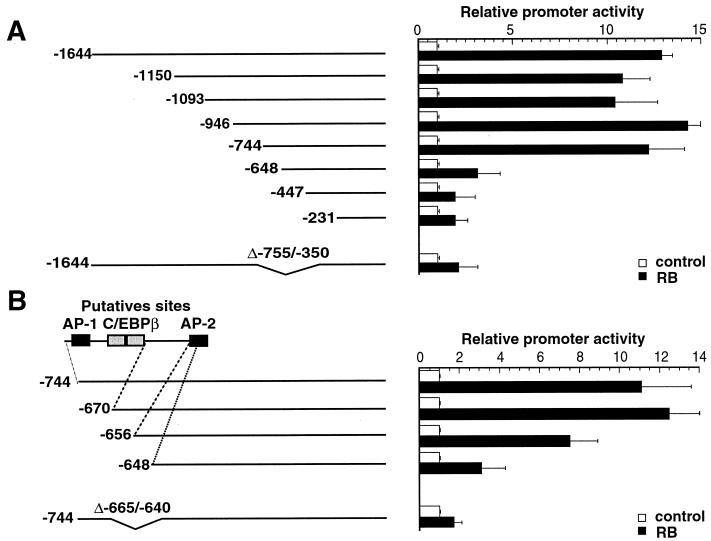
To further explore the molecular basis for this RB-mediated activation, a series of regularly spaced processive 5′ deletions of the bcl-2 promoter were created. When the panel of deletion mutants was assayed, the region of the promoter responsible for transactivation by RB was found to be located between −744 and −648, in a 96-bp region upstream from the transcriptional initiation site (Fig. 3A). Analysis of this region revealed putative binding sites for the transcription factors AP-1, C/EBPβ, and AP-2. To more precisely define this region, further 5′ promoter deletion mutants were constructed and analyzed. The results shown in Fig. 3B indicate that the sequence located between −656 and −648 contains the target site for RB transactivation, which might be an AP-2 binding site based on its DNA sequence.
FIG. 3.
Identification of the region of the bcl-2 promoter that responds to RB protein in MDCK epithelial cells. (A) MDCK cells were transfected with a series of bcl-2 promoter deletion constructs (1 μg of each) and either 5 μg of pSV-RB expression vector (black bars) or the empty expression vector (white bars). The results are averages of values obtained in three independent experiments and are expressed as promoter activity relative to the basal promoter activity, which is assigned a value of 1. Error bars indicate standard deviations. bcl-2 constructs are schematically represented on the left. Numbering of the bcl-2 sequence is relative to the translation start site. (B) Fine mapping of the RB response element. One microgram of each bcl-2 construct (with the 5′ extremity deleted as indicated) was transfected with 5 μg of pSV-RB expression vector (black bars) or the empty expression vector (white bars). Experiments were performed and results were expressed as in panel A. (C) MCF7 cells were transfected as in panel A with three bcl-2 promoter constructs.
To confirm that these sequences were required for RB transactivation, we also created internal deletions of two different 5′-end deletion mutants. Results obtained with these mutants clearly demonstrate that the RB target sequences are located between −665 and −640 bp upstream of the transcriptional start site (Fig. 3A and B).
RB transcriptional activation is mediated by the transcription factor AP-2.
It was previously shown that RB activates the E-cadherin gene in epithelial cells through functional and physical interactions with the transcription factor AP-2 (1). Since a putative AP-2 binding site was identified in the RB-responsive sequence of the bcl-2 promoter, we first tested whether the RB-mediated activation required AP-2. A dominant-negative mutant of AP-2 (AP-2 ΔTA) (17) which lacks the transactivation domain was used in a cotransfection experiment. AP-2 ΔTA was found to inhibit RB-mediated activation of the −670 Bcl-2 reporter in a dose-dependent manner. Basal expression levels of a reporter construct with a deletion of the AP-2 binding site (−134 bcl-2 reporter) were not affected (Table 1). We next performed cotransfection assays with an AP-2 expression vector, pPADH-AP2. To avoid self-interference phenomena which can occur between endogenous and exogenous AP-2 (23), we performed these assays with HepG2 cells, which lack endogenous AP-2 activity (21). Whereas RB and pPADH-AP2 alone did not stimulate bcl-2 promoter activity, transcriptional activity was enhanced fourfold when both RB and pPADH-AP2 were cotransfected (Fig. 4).
TABLE 1.
Suppression of RB-mediated activation of the bcl-2 promoter by dominant-negative AP-2
| Activation by: | Amt of dominant-negative AP-2 (μg) | Promoter activity
|
|
|---|---|---|---|
| −670 Bcl-2a | −134 Bcl-2b | ||
| RB | 0 | 100.0 ± 0.5 | |
| 1 | 20.8 ± 2.8 | ||
| 2 | 6.4 ± 3.6 | ||
| Basal level | 0 | 100.0 ± 0.5 | |
| 1 | 99.5 ± 0.2 | ||
| 2 | 89.5 ± 6.3 | ||
One-half microgram of −670 Bcl-2 CAT was transfected into MDCK cells either with 2.5 μg of pUC DNA or with pSV-RB expression vector, as indicated, plus increasing amounts of dominant-negative AP-2 in which the N-terminal region has been deleted. The activity of the −670 Bcl-2 CAT reporter in the presence of the control vector without the AP-2 sequence was set at 100%.
The negative control −134 Bcl-2 CAT vector, which does not contain the AP-2 binding site, was used. Its basal level was determined in a cotransfection assay with increasing amounts of dominent-negative AP-2.
FIG. 4.
Transcriptional activation of the bcl-2 promoter in HepG2 cells. Cells were transfected with 1 μg of −670 Bcl-2 CAT construct in the presence of 4 μg of the pSV-RB expression vector (cross-hatched and black bars) or the empty expression vector (white and stippled bars) and 4 μg of the pPADH-AP2 expression vector (stippled and black bars) or the corresponding empty expression vector (white and cross-hatched bars). Values were obtained from two independent experiments, each analyzed in duplicate. The results are averages expressed as fold activation of CAT activity relative to the basal promoter activity, which is assigned a value of 1. Error bars indicate standard deviations.
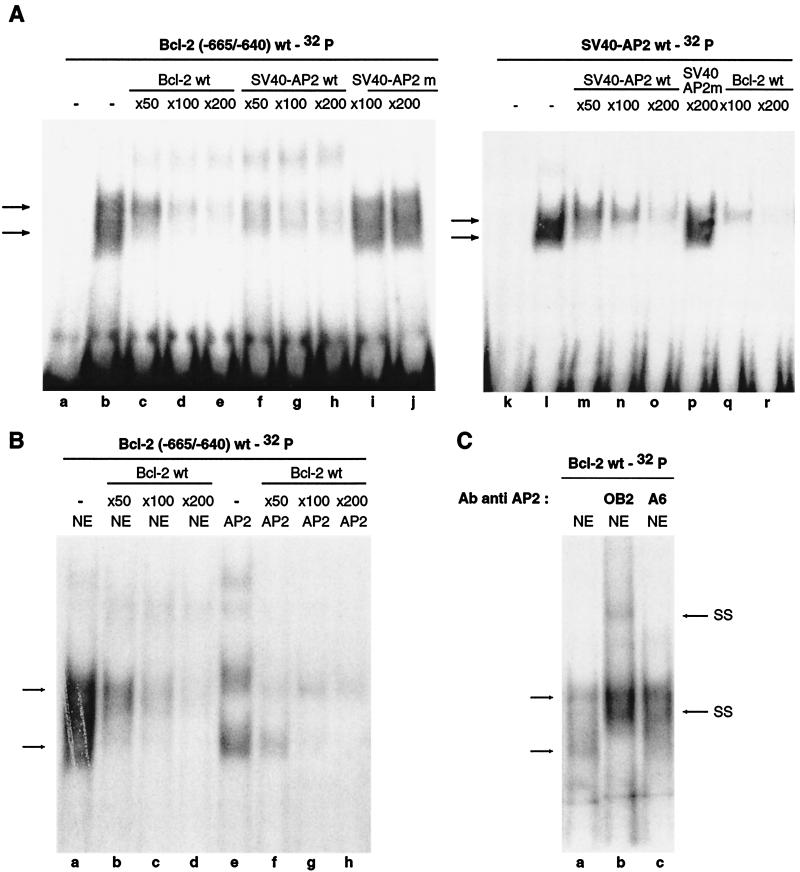
To determine whether the AP-2 protein interacts with the sequences located in the −665/−640 region, electrophoretic mobility shift assays (EMSA) were performed using this sequence and nuclear extracts prepared from human epithelial HaCat cells. Gel shift assays performed with these extracts and 32P-end-labeled −665/−640 Bcl-2 probe revealed two major specific complexes of retarded mobility (Fig. 5A). This binding could be competed away by an excess of unlabeled probe (Fig. 5A). Interestingly, these bands could also be competed away by oligonucleotides containing the AP-2 binding site of the SV40 enhancer but not by oligonucleotides containing a mutated SV40 AP-2 binding site. Importantly, use of the 32P-end-labeled wild-type SV40 AP-2 binding site resulted in detection of the same two retarded bands, which were competed away by an excess of either SV40 or Bcl-2 unlabeled, wild-type oligonucleotides (Fig. 5A). EMSA were next performed with HaCat nuclear extracts and human recombinant AP-2 that was translated in vitro, using the Bcl-2 probe. The AP-2 recombinant protein gave rise to the same retarded bands as the HaCat nuclear extract, and these were competed away by an excess of unlabeled Bcl-2 oligonucleotide (Fig. 5B). As a control, experiments were also performed with the SV40-AP-2 probe, and similar results were obtained (data not shown). Finally, to confirm the binding of the AP-2 protein to the RB target site of the bcl-2 promoter, gel shift assays were performed in the presence of two specific anti-AP-2 antibodies (4). These studies revealed that the two retarded bands can be supershifted with the anti-AP-2 antibodies tested (Fig. 5C); the polyclonal OB2 antibody supershifted the retarded bands more efficiently than the monoclonal A6 antibody. The presence of RB was also investigated by using two different RB antibodies, but we did not observe supershifted bands (data not shown), as previously reported by other groups in similar cases (6, 8, 38, 49). Together these results demonstrate that the RB-mediated activation of the bcl-2 promoter requires the binding of transcription factor AP-2 on the sequences located between −665 and −640 bp upstream of the transcriptional start site. Moreover, they are consistent with results of the transfection assays, showing that when the AP-2 binding site is deleted, the bcl-2 promoter construct (−648) is no longer activated by RB (Fig. 3).
FIG. 5.
Binding of AP-2 to the bcl-2 promoter. (A) EMSA analysis of nuclear extracts from Hacat cells with probes representing the −665/−640 sequence of the bcl-2 promoter (lanes a to j) and the AP-2 binding site of the SV40 enhancer (lanes k to r). Lanes a and k, absence of nuclear proteins; lanes b to j and l to r, presence of nuclear proteins. Arrows indicate the specific complexes. Competitions were carried out with a 50- to 200-fold molar excess of the unlabeled oligonucleotides Bcl-2wt (lanes c to e and q to r), SV40-AP2wt (lanes f to h and m to o), and SV40-AP2m (lanes i to j and lane p). SV40-AP2wt and SV40-AP2m correspond, respectively, to the wild-type or mutated AP-2 binding site of the SV40 enhancer. (B) Comparison of retarded bands obtained with Hacat nuclear extract (lanes a to d) and in vitro-transcribed and -translated AP-2 protein (lanes e to h), using the −665/−640 sequence of the bcl-2 promoter as a probe. Competitions were carried out with a 50- to 200-fold molar excess of the unlabeled Bcl-2wt oligonucleotide (lanes b to d and f to h). (C) EMSA was performed as for panel A with the Bcl-2wt oligonucleotide as a probe in the presence of antibodies directed against known AP-2 family members: polyclonal OB2 (lane b) or monoclonal A6 (lane c) antibodies. The arrows SS indicate the supershifted complexes.
RB and AP2 specifically associate on bcl-2 and E-cadherin gene promoters in vivo.
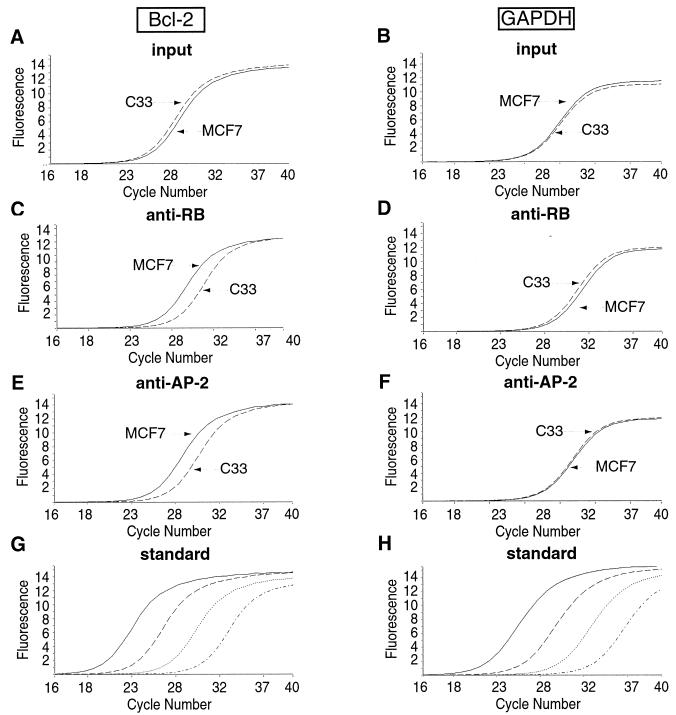
The RB-mediated activation of Bcl-2 as well as of E-cadherin requires the binding of AP-2 on the RB target sequences. Because endogenous RB and AP-2 interact in vivo in epithelial cells (1), we sought to determine whether a complex containing these molecules exists on the endogenous bcl-2 and E-cadherin gene promoter sequences. We assessed the association of these proteins with DNA in vivo by chromatin immunoprecipitation (ChIP) (13, 25) of endogenous RB and AP-2 from human MCF7 epithelial cells. We chose these cells because they express a large amount of both Bcl-2 and E-cadherin proteins. Moreover, we showed that RB activates the bcl-2 promoter in these cells (Fig. 2C). The RB target sequences of the bcl-2 promoter (−737/−566) and of the E-cadherin gene promoter (−171/−6) both contain AP-2 binding sites. A sequence of the GAPDH gene, which is not regulated by either RB or AP-2, was used as control. None of these sequences contain recognizable E2F-binding sites, excluding indirect effects of RB due to its interaction with E2F family transcription factors. Histone H4 acetylation was also followed (Fig. 7). Representative quantitative PCR amplification experiments are shown in Fig. 6. Quantitative analyses of all results are shown in Fig. 7 and 8.
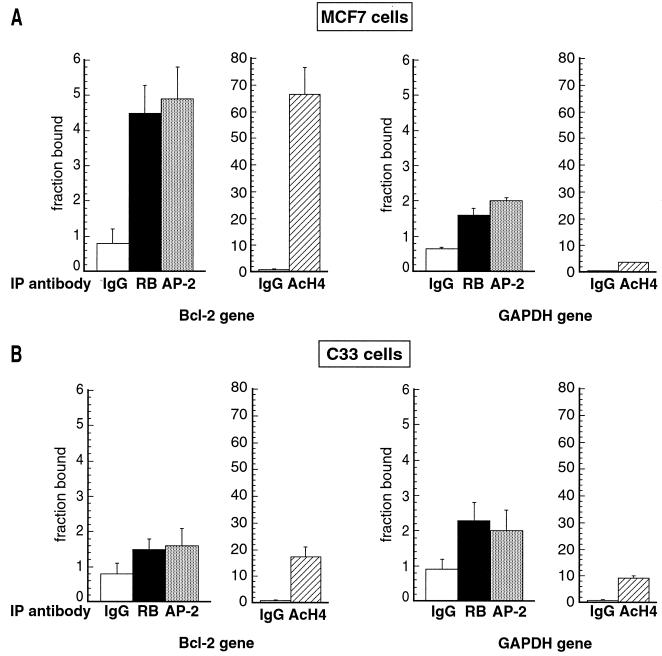
FIG. 7.
In vivo recruitment of RB and AP-2 proteins and histone acetylation on the bcl-2 promoter in MCF7 and C33 cells. Graphic representation of results obtained by real-time PCR as described in the legend to Fig. 6. The values are expressed as a fraction of the total number of copies (input) detected as antibody-bound material (percentage of input multiplied by 100). Averaged values were obtained from three independent experiments, each analyzed in triplicate. Error bars indicate standard deviations of the means. Equal amounts of chromatin extracted from MCF7 (A) and C33 (B) cells were subjected to immunoprecipitation using anti-RB (black bar), anti-AP-2 (grey bar), or anti-AcH4 (cross-hatched bar) antibodies. bcl-2 sequence (Bcl-2 gene) was detected by real-time quantitative PCR. A sample immunoprecipitated with an anti-mouse IgG antibody (IgG) (white bars) was used as a negative control and revealed no significant association with the bcl-2 promoter. The GAPDH sequence (GAPDH gene) was also used as a negative control.
FIG. 6.
Quantification of chromatin immunoprecipitation by real-time PCR. Chromatin from cross-linked subconfluent MCF7 or C33 cells was immunoprecipitated with antibodies specific for RB (C and D) and AP-2 (E and F). bcl-2 or GAPDH sequences were detected by PCR analysis of eluted DNA using a LightCycler (Roche). A known amount of chromatin from each sample was removed before immunoprecipitation and PCR amplified like the immunoprecipitates (input, panels A and B). The curves show the accumulation of PCR products plotted against the number of cycles (bcl-2: panels A, C, and E; GAPDH: panels B, D, and F). The results are shown for only one dilution of the immunoprecipitates, but three dilutions were analyzed for each sample. Copy numbers were estimated by reference to a plasmid DNA containing the bcl-2 or GAPDH sequence using the second derivative maximum method. Curves obtained with reference plasmid DNA for bcl-2 (G) or GAPDH (H), using (from right to left in each case) 1, 10, 100, and 1,000 fg of plasmid, are shown (standard).
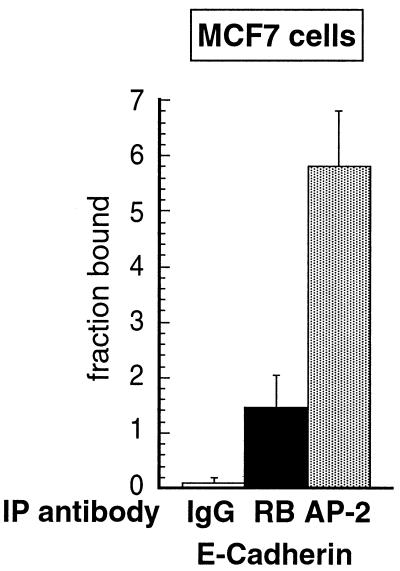
FIG. 8.
In vivo recruitment of RB and AP-2 proteins on the E-cadherin promoter in MCF7 cells. Equal amounts of chromatin extracted from MCF7 cells were subjected to immunoprecipitation using anti-RB (black bar), anti-AP-2 (grey bar), or anti-mouse IgG (white bar) antibodies. E-cadherin sequence was detected by real-time quantitative PCR. The values are expressed as a fraction of the total number of copies (input) detected as antibody-bound material (percentage of input multiplied by 100). Averaged values were obtained from three independent experiments, each analyzed in triplicate. Error bars indicate standard deviations of the means.
A significant and similar fraction of the bcl-2 promoter sequence was found in association with the anti-RB and anti-AP-2 immunoprecipitates in chromatin extracted from MCF7 cells, representing approximately 0.05% of the total input chromatin (Fig. 7A). Consistently, the RB target sequences were found to be highly acetylated (Fig. 7A). Irrelevant anti-mouse IgG antibody failed to immunoprecipitate significant levels of any of the sequences tested, and neither RB nor AP-2 was significantly associated with the GAPDH sequence. Nevertheless, to confirm the specificity of our results, we performed another negative control, immunoprecipitating chromatin from C33 cells, which have nonfunctional RB and AP-2 proteins. No significant amounts of bcl-2 promoter were detected (Fig. 7B). In addition, the amount of the negative control, GAPDH sequence, is the same in both cell types and also comparable to bcl-2 sequence immunoprecipitated in C33 cells. Interestingly, in C33 cells the bcl-2 RB target sequences were not acetylated (Fig. 7B).
Figure 8 shows the results obtained with the E-cadherin gene promoter. AP-2 and RB coprecipitated with significant amounts of E-cadherin gene promoter. However, anti-RB immunoprecipitated less E-cadherin DNA than did anti-AP-2 but still 16-fold more than the irrelevant antibody. This difference between bcl-2 and E-cadherin gene promoters might be explained by the fact that the bcl-2 sequence contains only one AP-2 binding site, whereas the E-cadherin gene promoter has four AP-2 binding sites (2). The binding of RB in vivo might be more specific for one of these sites or, alternatively, RB could bind to any one of these sites but not to all of them simultaneously. We favor the first possibility, since it has been shown that the E-Pal sequence (AP-2 binding site) is responsible for the cell-type-specific expression of E-cadherin (2) and that the effect of RB on E-cadherin is cell type specific (1). Globally, the amounts of binding we detect are consistent with results of a previous study using ChIP to examine the binding of E2F family proteins and RB on E2F target genes during the cell cycle (55). This study found for all the samples examined a maximum binding of 0.03% of the total chromatin input.
Together with our previous data (1), the results presented in this study strongly indicate that RB is recruited to the native bcl-2 and E-cadherin gene promoters by AP-2. More importantly, they show that the molecular mechanism used by RB, when acting as a positive regulator, can involve direct activation of the target gene, rather than the down-regulation of a repressor. In addition, the RB-mediated activation is correlated with increased acetylation of the activated gene.
DISCUSSION
In recent years, several in vivo and ex vivo studies of RB function have clearly demonstrated a link between the maintenance of a differentiated state (i.e., induction of tissue-specific genes) and cell viability (27; see reference 28 for a review). In RB knockout mice, aberrant differentiation of erythrocytes and neurons occurs with apoptotic death (7, 22, 26, 27). Specific inactivation of RB by the viral oncoprotein E7 targeted to the retina of transgenic mice prevented terminal differentiation, and these cells instead underwent apoptosis (19, 39). A similar process occurs in mouse embryonic carcinoma cells, in which the absence of functional RB during differentiation into neuroectoderm results in apoptosis (42). In most of the above-mentioned studies, ectopic mitotic figures are also visible. Their presence is in keeping with the role of RB as a cell cycle regulator. In addition, our previous studies showed a link between the maintenance of the epithelial state and cell viability (30, 31), with loss of the former resulting in loss of the latter. In all these cases, the absence of a functional RB and/or RB-related protein was shown to interfere with a differentiation program and result in apoptosis. Thus, these two RB functions, differentiation and promotion of cell survival, have been clearly demonstrated to be linked. However, it is not yet known how RB acts as a survival gene and what its target genes are.
Importantly, both during development and in adults, the expression patterns of RB and Bcl-2 have several features in common. Both are specifically and highly expressed in epithelial tissues, among others. Thus, developmental patterns of Bcl-2 expression suggested that Bcl-2 might have a role beyond the regulation of cell death (52). In fact, Bcl-2 was shown to have an important function in several cellular models of differentiation: in neuronal, hematopoietic and epithelial cell lines (5, 11, 29, 59), similar to RB. Moreover, Bcl-2 was also shown to negatively regulate cell growth in certain cell lines and even to inhibit tumor cell growth (3, 20, 33, 40, 51), a property that further reinforces the similarity between Bcl-2 and RB activities.
We demonstrate here that RB participates in the positive regulation of the bcl-2 gene in epithelial cells.
Interestingly, we found that regulation of bcl-2 by RB occurs through the AP-2 transcription factor, which is specific for epithelial marker genes, as previously shown for the E-cadherin gene (1), and is independent of p53. Importantly, ChIP experiments showed that RB and AP-2, which we previously found to be associated in vivo (1), in fact bound to the same bcl-2 promoter sequence, strongly suggesting that a protein complex containing these proteins forms on DNA in vivo. Most importantly, we also show here, by ChIP, that RB and AP-2 bind in vivo to the same E-cadherin gene promoter sequence, suggesting that a DNA-protein complex (RB/AP-2) can also form on this gene involved in apoptosis regulation.
The RB-mediated activation of bcl-2 is independent of p53, as is the apoptosis mediated by RB inactivation (30). The RB-binding site on the bcl-2 promoter was found to differ from the p53 target sequences. Furthermore, RB-mediated activation of bcl-2 occurred in MDCK(2a5) cells, in which only p53 is inactivated, but not in MDCK(1-6) cells, where both RB and p53 were inactivated (see Fig. 2). In addition, in these MDCK epithelial cells, the RB-mediated activation of bcl-2 and consequently its effect on cell survival is unlikely to be dependent on E2F for at least two reasons. One is that no E2F-binding site is found in the bcl-2 promoter. The second is that RB mutants such RBΔ22 and RBC706F, which affect E2F-binding, do not interfere with AP-2 (1).
Intriguingly, a correlation between AP-2β, Bcl-2 expression, apoptosis, and the maintenance of differentiated renal epithelia was previously described for AP-2β null mice (36). These mice display massive apoptotic cell death during kidney development, with down-regulation of Bcl-2. Furthermore, they develop renal cysts resembling PKD. The resemblance to the Bcl-2 null mouse phenotype is striking. Bcl-2 null mice also develop PKD (52), with altered expression of epithelial markers (45). Thus, studies using knockout mice indicate that the absence of AP-2β is associated with down-regulation of Bcl-2 expression and loss of epithelial differentiation, as expected from our findings that AP-2 regulates Bcl-2 expression.
Taken together with our previous results, the study presented here demonstrates that RB directly activates Bcl-2 and E-cadherin by being recruited to DNA by a transcription factor, in this case by AP-2, and not indirectly by repressing an inhibitor. Furthermore, this mechanism may in fact be general, since in another model, it has been shown by ChIP that during osteogenic differentiation RB is recruited by CBFA1 to specific differentiation-associated promoters (49).
Thus, we are faced with two different molecular mechanisms for RB, depending on whether it acts as a negative transcriptional regulator, when inhibiting cell growth, versus as a positive transcriptional regulator, when promoting cell differentiation and cell survival. In the first case, it is accepted that RB represses E2F family proteins and might recruit a histone deacetylase (HDAC). In the second case, RB is recruited to differentiation and survival gene promoters by specific transcription factors, enhancing their transcriptional activity. It is likely that other associated factors are also part of this protein complex, such histone acetyltransferases (HAT), since the bcl-2 and E-cadherin gene promoters were found to be specifically acetylated when RB and AP-2 bound to them (Fig. 7) (see model in Fig. 9). In addition, consistent with this hypothesis, we found that AP-2 associates in vitro and in vivo with the coactivator p300 (unpublished results), which exhibits histone acetyltransferase activity. These observations are compatible with the idea that RB functions as a molecular matchmaker, assembling different protein complexes in different cells (54).
FIG. 9.
Model for RB function.
However, the largely accepted model of RB as being an E2F-dependent transcriptional corepressor of S phase genes and so accounting for its clinical role as a tumor suppressor has recently been challenged by several studies (10, 37, 41, 46, 55). Takahashi et al., using ChIP, could not find RB binding to transcriptionally repressed genes in living cells in G0. Wells et al. demonstrated that the standard model for E2F-mediated regulation does not reflect the E2F-pocket protein binding pattern for the majority of E2F target genes. Most of the promoters of E2F target genes were bound by E2F pocket protein complexes during mid-S phase. Among the hypotheses discussed, these authors surmised that the pocket proteins may not always serve as transcriptional repressors but sometimes may serve as activators, even when bound on these E2F target promoters.
In addition, cancer-associated mutations that have been identified to date were located in RB-1 and its upstream regulators but not in downstream components, such as E2F (41). Moreover, the majority of tumor-derived RB mutants described are defective for all of RB's biochemical activities, making it difficult to discern the relative contribution of these activities to RB-mediated tumor suppression. These and other observations (41) thus possibly suggest that in vivo all these activities of RB are linked.
Thus, we speculate that bcl-2 is, through AP-2, one of the targets of RB in vivo, participating in the maintenance of a differentiated state, cell survival, and tissue architecture (29) and thereby helping to prevent cancer and tumor progression. Clearly, bcl-2 is not the only RB target of relevance to epithelial cells. E-cadherin and p21 genes, other targets of RB through its interactions with AP-2 and SP-1, respectively, would also participate in this homeostasis (1, 8). Together, the various target genes of RB thus contribute to cell quiescence, allowing survival of terminally differentiated epithelial cells.
Acknowledgments
We are very grateful to L. Pritchard, H. Lehrman, and D. Thomas for critical reading of the manuscript. We thank R. Ferreira for helpful discussions and F. Petit for help in the beginning of this work.
This work was supported by grants from La Ligue contre le Cancer, the CNRS, and NIH (gramt GM-60554). S.D. was supported by a postdoctoral fellowship from ARC, and J.D. was supported by predoctoral fellowships from the Ministère de la Recherche.
REFERENCES
- 1.Batsche, E., C. Muchardt, J. Behrens, H. C. Hurst, and C. Cremisi. 1998. RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol. Cell. Biol. 18:3647-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens, J., O. Lowrick, L. Klein-Hitpass, and W. Birchmeier. 1991. The E-cadherin promoter: functional analysis of a G·C-rich region and an epithelial cell-specific palindromic regulatory element. Proc. Natl. Acad. Sci. USA 88:11495-11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borner, C. 1996. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J. Biol. Chem. 271:12695-12698. [DOI] [PubMed] [Google Scholar]
- 4.Bosher, J. M., T. Williams, and H. C. Hurst. 1995. The developmentally regulated transcription factor AP-2 is involved in c-erbB-2 overexpression in human mammary carcinoma. Proc. Natl. Acad. Sci. USA 92:744-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D. F., G. E. Schneider, J. C. Martinou, and S. Tonegawa. 1997. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature 385:434-439. [DOI] [PubMed] [Google Scholar]
- 6.Chen, P. L., D. J. Riley, Y. Chen, and W. H. Lee. 1996. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 10:2794-2804. [DOI] [PubMed] [Google Scholar]
- 7.Clarke, A. R., E. R. Maandag, M. van Roon, N. M. van der Lugt, M. van der Valk, M. L. Hooper, A. Berns, and H. te Riele. 1992. Requirement for a functional Rb-1 gene in murine development. Nature 359:328-330. [DOI] [PubMed] [Google Scholar]
- 8.Decesse, J. T., S. Medjkane, M. B. Datto, and C. E. Cremisi. 2001. RB regulates transcription of the p21/WAF1/CIP1 gene. Oncogene 20:962-971. [DOI] [PubMed] [Google Scholar]
- 9.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 11.Fairbairn, L. J., G. J. Cowling, B. M. Reipert, and T. M. Dexter. 1993. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell 74:823-832. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira, R., I. Naguibneva, M. Mathieu, S. Ait-Si-Ali, P. Robin, L. L. Pritchard, and A. Harel-Bellan. 2001. Cell cycle-dependent recruitment of HDAC-1 correlates with deacetylation of histone H4 on an Rb-E2F target promoter. EMBO Rep. 2:794-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrester, W. C., L. A. Fernandez, and R. Grosschedl. 1999. Nuclear matrix attachment regions antagonize methylation-dependent repression of long-range enhancer-promoter interactions. Genes Dev. 13:3003-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frampton, J., T. Ramqvist, and T. Graf. 1996. v-Myb of E26 leukemia virus up-regulates bcl-2 and suppresses apoptosis in myeloid cells. Genes Dev. 10:2720-2731. [DOI] [PubMed] [Google Scholar]
- 15.Harbour, J. W., and D. C. Dean. 2000. Rb function in cell-cycle regulation and apoptosis. Nat. Cell Biol. 2:E65-E67. [DOI] [PubMed] [Google Scholar]
- 16.Heckman, C., E. Mochon, M. Arcinas, and L. M. Boxer. 1997. The WT1 protein is a negative regulator of the normal bcl-2 allele in t(14;18) lymphomas. J. Biol. Chem. 272:19609-19614. [DOI] [PubMed] [Google Scholar]
- 17.Hennig, G., O. Lowrick, W. Birchmeier, and J. Behrens. 1996. Mechanisms identified in the transcriptional control of epithelial gene expression. J. Biol. Chem. 271:595-602. [DOI] [PubMed] [Google Scholar]
- 18.Hewitt, S. M., S. Hamada, T. J. McDonnell, F. J. Rauscher III, and G. F. Saunders. 1995. Regulation of the proto-oncogenes bcl-2 and c-myc by the Wilms' tumor suppressor gene WT1. Cancer Res. 55:5386-5389. [PubMed] [Google Scholar]
- 19.Howes, K. A., N. Ransom, D. S. Papermaster, J. G. Lasudry, D. M. Albert, and J. J. Windle. 1994. Apoptosis or retinoblastoma: alternative fates of photoreceptors expressing the HPV-16 E7 gene in the presence or absence of p53. Genes Dev. 8:1300-1310. [DOI] [PubMed] [Google Scholar]
- 20.Huang, D. C., L. A. O'Reilly, A. Strasser, and S. Cory. 1997. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 16:4628-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imagawa, M., R. Chiu, and M. Karin. 1987. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell 51:251-260. [DOI] [PubMed] [Google Scholar]
- 22.Jacks, T., A. Fazeli, E. M. Schmitt, R. T. Bronson, M. A. Goodell, and R. A. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359:295-300. [DOI] [PubMed] [Google Scholar]
- 23.Kannan, P., R. Buettner, P. J. Chiao, S. O. Yim, M. Sarkiss, and M. A. Tainsky. 1994. N-ras oncogene causes AP-2 transcriptional self-interference, which leads to transformation. Genes Dev. 8:1258-1269. [DOI] [PubMed] [Google Scholar]
- 24.Korsmeyer, S. J. 1992. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood 80:879-886. [PubMed] [Google Scholar]
- 25.Kuo, M. H., and C. D. Allis. 1999. In vivo cross-linking and immunoprecipitation for studying dynamic protein:DNA associations in a chromatin environment. Methods 19:425-433. [DOI] [PubMed] [Google Scholar]
- 26.Lee, E. Y., C. Y. Chang, N. Hu, Y. C. Wang, C. C. Lai, K. Herrup, W. H. Lee, and A. Bradley. 1992. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359:288-294. [DOI] [PubMed] [Google Scholar]
- 27.Lee, E. Y., N. Hu, S. S. Yuan, L. A. Cox, A. Bradley, W. H. Lee, and K. Herrup. 1994. Dual roles of the retinoblastoma protein in cell cycle regulation and neuron differentiation. Genes Dev. 8:2008-2021. [DOI] [PubMed] [Google Scholar]
- 28.Lipinski, M. M., and T. Jacks. 1999. The retinoblastoma gene family in differentiation and development. Oncogene 18:7873-7882. [DOI] [PubMed] [Google Scholar]
- 29.Lu, P. J., Q. L. Lu, A. Rughetti, and J. Taylor-Papadimitriou. 1995. bcl-2 overexpression inhibits cell death and promotes the morphogenesis, but not tumorigenesis, of human mammary epithelial cells. J. Cell Biol. 129:1363-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martel, C., E. Batsche, and C. Cremisi. 1996. Inactivation of the retinoblastoma gene product or RB-related protein by SV40 T antigen in MDCK epithelial cells results in massive apoptosis. Cell Death Differ. 3:61-74. [PubMed] [Google Scholar]
- 31.Martel, C., F. Harper, S. Cereghini, V. Noe, M. Mareel, and C. Cremisi. 1997. Inactivation of retinoblastoma family proteins by SV40 T antigen results in creation of a hepatocyte growth factor/scatter factor autocrine loop associated with an epithelial-fibroblastoid conversion and invasiveness. Cell Growth Differ. 8:165-178. [PubMed] [Google Scholar]
- 32.Mayo, M. W., C. Y. Wang, S. S. Drouin, L. V. Madrid, A. F. Marshall, J. C. Reed, B. E. Weissman, and A. S. Baldwin. 1999. WT1 modulates apoptosis by transcriptionally upregulating the bcl-2 proto-oncogene. EMBO J. 18:3990-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazel, S., D. Burtrum, and H. T. Petrie. 1996. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J. Exp. Med. 183:2219-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyashita, T., M. Harigai, M. Hanada, and J. C. Reed. 1994. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 54:3131-3135. [PubMed] [Google Scholar]
- 35.Morris, E. J., and N. J. Dyson. 2001. Retinoblastoma protein partners. Adv. Cancer Res. 82:1-54. [DOI] [PubMed] [Google Scholar]
- 36.Moser, M., A. Pscherer, C. Roth, J. Becker, G. Mucher, K. Zerres, C. Dixkens, J. Weis, L. Guay-Woodford, R. Buettner, and R. Fassler. 1997. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev. 11:1938-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller, H., and K. Helin. 2000. The E2F transcription factors: key regulators of cell proliferation. Biochim. Biophys. Acta 1470:M1-M12. [DOI] [PubMed] [Google Scholar]
- 38.Nead, M. A., L. A. Baglia, M. J. Antinore, J. W. Ludlow, and D. J. McCance. 1998. Rb binds c-Jun and activates transcription. EMBO J. 17:2342-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan, H., and A. E. Griep. 1994. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 8:1285-1299. [DOI] [PubMed] [Google Scholar]
- 40.Pietenpol, J. A., N. Papadopoulos, S. Markowitz, J. K. Willson, K. W. Kinzler, and B. Vogelstein. 1994. Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res. 54:3714-3717. [PubMed] [Google Scholar]
- 41.Sellers, W. R., B. G. Novitch, S. Miyake, A. Heith, G. A. Otterson, F. J. Kaye, A. B. Lassar, and W. G. Kaelin, Jr. 1998. Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 12:95-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slack, R. S., I. S. Skerjanc, B. Lach, J. Craig, K. Jardine, and M. W. McBurney. 1995. Cells differentiating into neuroectoderm undergo apoptosis in the absence of functional retinoblastoma family proteins. J. Cell Biol. 129:779-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sleigh, M. J. 1986. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal. Biochem. 156:251-256. [DOI] [PubMed] [Google Scholar]
- 44.Smith, M. D., E. A. Ensor, R. S. Coffin, L. M. Boxer, and D. S. Latchman. 1998. Bcl-2 transcription from the proximal P2 promoter is activated in neuronal cells by the Brn-3a POU family transcription factor. J. Biol. Chem. 273:16715-16722. [DOI] [PubMed] [Google Scholar]
- 45.Sorenson, C. M. 1999. Nuclear localization of beta-catenin and loss of apical brush border actin in cystic tubules of bcl-2 −/− mice. Am. J. Physiol. 276:F210-F217. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi, Y., J. B. Rayman, and B. D. Dynlacht. 2000. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14:804-816. [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor, D., P. Badiani, and K. Weston. 1996. A dominant interfering Myb mutant causes apoptosis in T cells. Genes Dev. 10:2732-2744. [DOI] [PubMed] [Google Scholar]
- 48.Templeton, D. J., S. H. Park, L. Lanier, and R. A. Weinberg. 1991. Nonfunctional mutants of the retinoblastoma protein are characterized by defects in phosphorylation, viral oncoprotein association, and nuclear tethering. Proc. Natl. Acad. Sci. USA 88:3033-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas, D. M., S. A. Carty, D. M. Piscopo, J. S. Lee, W. F. Wang, W. C. Forrester, and P. W. Hinds. 2001. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol. Cell 8:303-316. [DOI] [PubMed] [Google Scholar]
- 50.Tsujimoto, Y., and C. M. Croce. 1986. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc. Natl. Acad. Sci. USA 83:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vairo, G., K. M. Innes, and J. M. Adams. 1996. Bcl-2 has a cell cycle inhibitory function separable from its enhancement of cell survival. Oncogene 13:1511-1519. [PubMed] [Google Scholar]
- 52.Veis, D. J., C. M. Sorenson, J. R. Shutter, and S. J. Korsmeyer. 1993. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229-240. [DOI] [PubMed] [Google Scholar]
- 53.Wang, J. Y. 1997. Retinoblastoma protein in growth suppression and death protection. Curr. Opin. Genet. Dev. 7:39-45. [DOI] [PubMed] [Google Scholar]
- 54.Wang, J. Y., E. S. Knudsen, and P. J. Welch. 1994. The retinoblastoma tumor suppressor protein. Adv. Cancer Res. 64:25-85. [DOI] [PubMed] [Google Scholar]
- 55.Wells, J., K. E. Boyd, C. J. Fry, S. M. Bartley, and P. J. Farnham. 2000. Target gene specificity of E2F and pocket protein family members in living cells. Mol. Cell. Biol. 20:5797-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams, T., A. Admon, B. Luscher, and R. Tjian. 1988. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 2:1557-1569. [DOI] [PubMed] [Google Scholar]
- 57.Wilson, B. E., E. Mochon, and L. M. Boxer. 1996. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol. Cell. Biol. 16:5546-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, Y., J. W. Mehew, C. A. Heckman, M. Arcinas, and L. M. Boxer. 2001. Negative regulation of bcl-2 expression by p53 in hematopoietic cells. Oncogene 20:240-251. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, K. Z., J. A. Westberg, E. Holtta, and L. C. Andersson. 1996. BCL2 regulates neural differentiation. Proc. Natl. Acad. Sci. USA 93:4504-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]