Abstract
In the murine model of infection, a Mycobacterium tuberculosis mce1 operon mutant elicits an aberrant granulomatous response, resulting in uncontrolled replication and failure to enter a persistent state. In this study, we demonstrate that the mce1 genes can be transcribed as a 13-gene polycistronic message encompassing Rv0166 to Rv0178. Quantitative reverse transcriptase PCR and immunoblot analyses revealed that the mce1 genes and proteins are expressed during in vitro growth but are significantly down-regulated in intracellular bacilli isolated from murine macrophages. A homologue of the FadR subfamily of GntR transcriptional regulators, Rv0165c (designated Mce1R), lies upstream and is divergently transcribed from the operon. To investigate whether this gene plays a role in regulation of mce1 expression, we created an M. tuberculosis mce1R deletion mutant. There was no difference in expression of mce1 operon genes in Δmce1R compared to expression in the wild type during logarithmic growth in vitro. However, in bacilli isolated from murine macrophages, expression of mce1 genes was significantly higher in Δmce1R. In addition, overexpression of mce1R resulted in repression of the mce1 genes. These data demonstrate that Mce1R is a negative regulator that acts intracellularly to repress expression of the mce1 operon. We propose that Mce1R facilitates balanced temporal expression of the mce1 products required for organized granuloma formation, which is both protective to the host and necessary for the persistence of M. tuberculosis.
Tuberculosis causes approximately two million deaths annually, and it has been estimated that two billion people are currently infected with the causative agent, Mycobacterium tuberculosis (10). Infection is initiated by inhalation of the bacilli into the lung, where they are ultimately ingested by alveolar macrophages. In the typical immunocompetent host, the cell-mediated immune response results in the formation of a granuloma, the pathological hallmark of tuberculosis. In the majority of cases, this response is sufficient to contain the infection and prevent disease; however, viable bacilli remain within granulomas and can cause active disease years later.
In the past decade, dramatic advances facilitating the molecular biological manipulation of mycobacteria have increased our knowledge of the mechanisms that tubercle bacilli use to survive within macrophages (33); however, little remains known about the bacterial determinants of infection outcome. Previous studies suggest that the M. tuberculosis mce1 operon is important in establishing a persistent infection in mice (32). It was shown that M. tuberculosis mce1 mutants failed to induce a characteristic proinflammatory response in ex vivo-infected murine macrophages. This phenotype was manifested in vivo by aberrant inflammatory cell migration and poor granuloma formation, leading to uncontrolled bacterial growth and rapid death of mutant-infected mice.
The six Mce1 proteins (Mce1A to Mce1F) contain putative signal sequences indicative of membrane localization (35). The surface exposure of Mce1A has been demonstrated by immunoelectron microscopy (4), and Mce1A to Mce1F were shown to localize to the cell wall fraction of M. tuberculosis (32). This localization supports the proposition that these proteins could interact with host components during infection and influence the immune response. The adjacent upstream genes, yrbE1A and yrbE1B, are predicted to encode integral membrane proteins, and, intriguingly, this set of eight genes (yrbE1A to mce1F) is repeated three more times elsewhere in the M. tuberculosis genome (5).
Given the proinflammatory effect of the mce1 products and the well-documented importance of correct regulation of virulence genes, we reasoned that the expression of the mce1 operon is tightly controlled. In this study, we examine the intracellular expression of the mce1 operon by quantitative methods and investigate the role of the proximal putative transcriptional regulator, Rv0165c.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. tuberculosis H37Rv (ATCC 25618) and Mycobacterium smegmatis mc2155 (ATCC 700084) were grown in Middlebrook 7H9 broth supplemented with albumin-dextrose-catalase (Becton Dickinson) and 0.05% Tween 80 or on Middlebrook 7H11 plates containing oleic acid-albumin-dextrose-catalase supplement (Becton Dickinson) and 0.5% glycerol. Hygromycin (50 μg/ml) or kanamycin (20 μg/ml) was included for maintenance of plasmids. Plasmid manipulation was carried out in the Escherichia coli host XL1-Blue (Stratagene), which was grown in Difco Luria-Bertani medium, supplemented with 200 μg/ml hygromycin or 50 μg/ml kanamycin where appropriate.
Isolation of intracellular M. tuberculosis.
RAW 264.7 cells (ATCC TIB-71) were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) containing 10% fetal bovine serum (Omega Scientific) at 37°C in a 5% CO2 humidified atmosphere. Prior to infection, 3 × 107 cells per sample were seeded into 175-cm2 triple-layer flasks (Nunc) and allowed to adhere overnight. In some experiments, 30 U/ml gamma interferon (IFN-γ) (R&D Systems) was included to activate macrophages. Mid-log-phase M. tuberculosis strains were harvested, resuspended in complete DMEM, and vortexed for 2 min with 3-mm glass beads to break up clumps. A sufficient number of bacilli were added to the RAW cells to achieve a multiplicity of infection of 10:1. After 4 h or 6 h, infected monolayers were washed three times to remove extracellular bacilli and fresh medium was added. At selected time points, intracellular bacteria were isolated by the differential lysis method described by Monahan et al. (22). Briefly, macrophages were lysed in 100 ml of a 4-M guanidinium isothiocyanate solution containing 0.5% sodium lauryl sarcosine, 25 mM trisodium citrate, and 0.1 M β-mercaptoethanol. Bacilli were harvested by centrifugation and rapidly washed in 1 ml 0.05% Tween 80 before RNA extraction or whole-cell lysate preparation.
Reverse transcriptase PCR (RT-PCR).
RNA was extracted from M. tuberculosis essentially as previously described (9). Bacterial pellets, from macrophages or 2 ml log-phase culture, were resuspended in 1 ml Trizol (Invitrogen) and transferred to a 2-ml tube containing lysing matrix B (Qbiogene). Bacilli were disrupted by two 45-s pulses at 6,000 × g in a FastPrep machine (Qbiogene). After addition of 200 μl chloroform, the organic phase was separated by centrifugation. The aqueous phase was transferred to a clean tube, 500 μl of isopropanol was added, and RNA was precipitated at −80°C. RNA was recovered by centrifugation, washed in 70% ethyl alcohol, and resuspended in 100 μl water. DNA was digested with Ambion's DNA-free kit, and complete removal of DNA was confirmed by PCR. RNA was subsequently purified with QIAGEN RNeasy columns as described by the manufacturer.
RNA was quantified by a RiboGreen assay (Molecular Probes), and a 100- to 500-ng sample was reverse transcribed by Superscript II (Invitrogen) with random hexamer priming according to the manufacturer's instructions. For each sample, a reverse transcriptase negative reaction was also prepared. cDNA (1 to 5 μl) was used in standard PCRs with primers given in Table 1. Quantitative real-time RT-PCR (RT-qPCR) was performed with gene-specific TaqMan probes and Universal PCR master mix on an SDS 7700 apparatus (Applied Biosystems).
TABLE 1.
Oligonucleotide sequences
| Procedure | Gene | Forward primer | Reverse primer | TaqMan probe |
|---|---|---|---|---|
| RT-PCR | Rv0165c | CCACGTGAACGCACCTCTATC | AGCAGGAACCAGGCCAACTTG | |
| Rv0166 | TGCTGGAGGCAGAGAAGGTC | GGGTTGTTCCAGTAGCAGCTC | ||
| Rv0167 | ATGGTCTCGATACCGCTGACG | CACCTCCATCGCGTCGATTTC | ||
| Rv0168 | GCTTTGACCAGCATCGG | CACCGAGACCAACGAGAAACG | ||
| Rv0169 | CACCATCTCGGAGGTCACACG | CGCTCAGGGTCAGGTTCAGC | ||
| Rv0170 | AGTTCAGCAATGTCAGCGGG | GTCCAGCACGATGTTCAGGTT | ||
| Rv0171 | TCGCTGAATGTGTTGTCGGAG | CGGGTTGTCGTTGATAAGGTTTTG | ||
| Rv0172 | GTCGCGGAACATTCAGTTGG | GAATGCCTCGATGACTTCGC | ||
| Rv0173 | CTCTTGCTGAGTTCGTGCGG | GGTGGCATTTTTCGGTAGCG | ||
| Rv0174 | GCGCTGGACAATTCCAATCG | CGGAGTTCTCGATGATGTCG | ||
| Rv0175 | GGCTAGCATGCAGAAGATCATCG | CCTCATCCAGTGCCATCCG | ||
| Rv0176 | GCGCACTTGGTGGACACC | GATCGGTTTGCCATTCATTGACG | ||
| Rv0177 | CTGGCTCTGACCGCATCC | TTCCTGTGGTCGGTTCCACC | ||
| Rv0178 | GTTCGTGGGCTCGGCAG | GATCGGCCAGGGTGTCC | ||
| RT-qPCR | sigA | CCGATGACGACGAGGAGATC | GGCCTCCGACTCGTCTTCA | AAGGACAAGGCCTCCGGTGATTTCGa |
| fadD5 | CTTAACCGCACCGAGTTCGT | TGAGCCGGAAATTCAGTGGTA | TCGGTGCTGGCCGCCAACAb | |
| mce1A | TGCACGATCCGCAACTTCT | TCCTCAGCGAGTAGCCGTTAC | CGATGCCGATCCGCTCGCTAAAGb | |
| mce1F | CGTCGGTGACTTCAAAACCA | TTGACCTGGCTGTCCAAAATC | TTGGCGACGTCAACGACATCATCGb |
5′, 6-carboxyfluorescein; 3′, 6-carboxytetramethylrhodamine (TAMRA).
5′, VIC; 3′, TAMRA.
Immunoblot analysis.
Bacterial pellets, from macrophages or 2 ml log-phase culture, were resuspended in 100 μl 1% sodium dodecyl sulfate (SDS), transferred to a 2-ml tube containing 100 μl 0.1-mm zirconia beads (BioSpec Products), and lysed by two 45-s pulses at 6,000 × g in a FastPrep machine. The lysate was boiled in 1× SDS sample buffer (17), and insoluble material and beads were removed by centrifugation. Protein concentration was determined by amido black staining (14), and equal amounts of each sample were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (17). Proteins were transferred to a nitrocellulose membrane (Bio-Rad) by electroblotting using standard methods (2). Following blocking in 5% milk solution, the membrane was incubated with a 1:2,000 dilution of an anti-Mce1A or anti-Mce1F antibody raised in rabbits (4, 32). Subsequently, the membrane was washed and incubated with a 1:1,000 dilution of a horseradish peroxidase-conjugated anti-rabbit antibody (Amersham). Following further washing, immunoreactive proteins were detected with a luminol-based substrate (SuperSignal; Pierce).
β-Galactosidase assay.
An E. coli mycobacterium shuttle vector for promoter analysis was constructed by cloning the promoterless lacZ gene from pSV-β-galactosidase (Promega) into pMS2 (15), creating pIGn. DNA fragments containing the putative promoter and first three codons of mce1R were cloned into pIGn to create LacZ translational fusions (see Fig. 3A). Plasmids were electroporated into M. smegmatis (23). Colonies were picked and transferred directly into 2-ml tubes containing 100 μl phosphate-buffered saline, pH 7.4 (Sigma), with 1× Complete protease inhibitor (Roche) and 100 μl 0.1-mm zirconia beads. Bacilli were lysed in a FastPrep machine by two 45-s pulses at 6,000 × g. Insoluble material and beads were removed by centrifugation. The Bio-Rad DC protein assay was used to determine the concentration of proteins in the lysates. β-Galactosidase activity was assayed by the method of Miller (20), using the substrate o-nitrophenyl-β-d-galactopyranoside (Sigma), which is hydrolyzed by the enzyme to the chromogenic product, o-nitrophenol (ONP). Activity is expressed as nmol of ONP produced per milligram of lysate per minute.
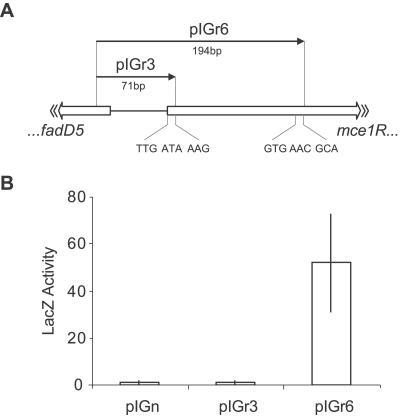
FIG. 3.
Activity of mce1R′-′lacZ translational fusions. (A) Schematic representation of the genomic regions cloned into pIGn to create the mce1R′-′lacZ translational fusions, pIGr3 and pIGr6. (B) β-Galactosidase activity in cell lysates of M. smegmatis transformed with the promoter constructs. The vector pIGn contains no promoter. Values represent the mean activities of three colonies (nmol ONP mg−1 min−1), and error bars show 1 standard deviation.
Rv0165c gene disruption and overexpression.
An Rv0165c (mce1R) knockout was constructed by the two-step counterselection strategy described by Parish and Stoker (24). The suicide delivery vector, pCER6, contained a 2.5-kb genomic region in which the central region of the target gene (corresponding to nucleotides 194284 to 194791 [TubercuList]) was deleted and replaced by a hygromycin resistance gene. The vector included a selection cassette encoding SacB and LacZ, as well as a kanamycin resistance gene. The delivery vector was electroporated into M. tuberculosis (23), and single-crossover colonies were selected on medium containing hygromycin, kanamycin, and 50 μg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). An antibiotic-resistant blue colony was picked and incubated for 7 to 10 days in medium containing hygromycin to allow the second crossover to occur. Serial dilutions were plated onto hygromycin, X-Gal, and 2% sucrose to select for double crossovers. DNA was isolated from white colonies (29) and analyzed by Southern blotting (27) to confirm the correct genotype.
Vectors for the overexpression of Mce1R were constructed in the phagemid pYUB178 (25) by fusing the mce1R coding sequence to the M. tuberculosis glnA1 promoter (12, 40). The vector pCER8 contains the 264-amino-acid Mce1R sequence (nucleotides 194938 to 194144 [TubercuList]), and pCER17 contains the coding sequence from the downstream GTG start (223 amino acids; nucleotides 194815 to 194144).
Sequence analysis.
M. tuberculosis sequences were acquired from the TubercuList web server (http://genolist.pasteur.fr/TubercuList), and other mycobacterial genome sequences were accessed via the Comprehensive Microbial Resource server (http://www.tigr.org). Sequences of GntR family regulators (26) were retrieved from the NCBI sequence database (http://www.ncbi.nlm.nih.gov). Multiple alignments were performed with CLUSTAL_X (36), and phylogenetic trees were generated by the PHYLIP/MOLPHY interface, PIE, using the maximum likelihood method with default parameters. Secondary structures were computed by the JPred consensus secondary structure prediction server (6).
RESULTS
Demarcation of the mce1 operon.
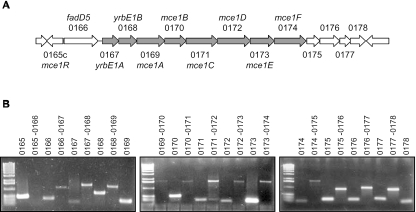
The Mce1 proteins are encoded in a region that contains 13 genes transcribed in the same direction (Fig. 1A). The first gene in this cluster, Rv0166, encodes a putative fatty acyl coenzyme A (CoA) synthetase, designated FadD5 (39). The following eight genes (Rv0167 to Rv0174) comprise the “core operon” that is repeated three more times in the M. tuberculosis H37Rv genome (5). The predicted start codon of each of these eight gene products either overlaps with the upstream gene or is preceded by a gap of less than 3 codons, suggesting that their expression is transcriptionally and perhaps translationally coupled. Paralogues of the adjacent genes, Rv0175 to Rv0178, are located downstream of the mce3 and mce4 gene clusters. We sought to define the boundaries of the mce1 operon by using RT-PCR. Amplification with intragenic primer pairs for the genes Rv0165c to Rv0178 demonstrated that each of these genes was expressed during in vitro growth. The same primers were used in pairs that spanned each intergenic region. PCR products were obtained for each region from Rv0166 to Rv0178 (Fig. 1B). These data indicate that the mce1 operon can be transcribed as a 13-gene polycistronic message encompassing Rv0166 to Rv0178.
FIG. 1.
RT-PCR analysis of the M. tuberculosis mce1 region. (A) Schematic representation of the genes predicted within the mce1 genomic region. The core operon is shaded. Numbers correspond to TubercuList Rv designations. (B) RT-PCR amplification of intragenic and intergenic segments within the mce1 region with primer pairs listed in Table 1. Reverse transcriptase negative reactions showed no amplification products with any pair of primers.
Intracellular expression of the mce1 operon.
Intracellular expression of the mce1 operon was investigated by infection of RAW murine macrophages with M. tuberculosis at a multiplicity of infection of 10:1. Macrophages were washed to remove extracellular bacteria after 4 h, and incubation continued up to 48 h. Microscopic analysis indicated that the macrophage monolayers remained intact during this time period. At selected time points, monolayers were lysed with a guanidine isothiocyanate solution, preventing further transcriptional changes and allowing the separation of intracellular bacilli from macrophage components (22).
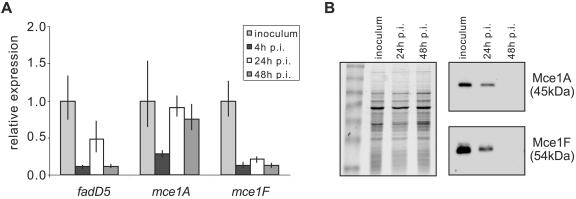
Extracted M. tuberculosis RNA was analyzed by RT-qPCR to assess expression of selected mce1 operon genes: fadD5, mce1A, and mce1F. The expression of each gene was normalized to sigA, which has been shown to be constitutively expressed (8, 18). This analysis revealed that expression of the mce1 operon genes was down-regulated within macrophages compared to expression in bacilli growing in standard 7H9 growth medium (Fig. 2A). Repression of mRNA synthesis occurred rapidly, and the level of transcript was reduced approximately 10-fold by 4 h postinfection. At 24 h and 48 h postinfection, the expression level of mce1A appeared to increase; this difference may reflect the enhanced relative stability of the mce1A transcript compared to that of the others.
FIG. 2.
Expression of the mce1 operon in RAW macrophages. (A) RT-qPCR assessment of expression of selected mce1 operon genes. Results are presented as the ratio of the amount of target gene at each time point postinfection (p.i.) to that in 7H9 medium (inoculum) for each gene. Values shown are means of triplicate PCRs, and error bars represent 1 standard deviation. (B) Immunoblot analyses of Mce protein expression in M. tuberculosis isolated from RAW cells 24 h or 48 h postinfection compared to expression in bacilli grown in vitro (inoculum). Equal amounts of bacterial lysate were separated by SDS-PAGE (left panel), transferred to a nitrocellulose membrane, and hybridized to polyclonal antibodies raised against recombinant Mce1A or Mce1F (right two panels).
To confirm that the mce1 operon was repressed intracellularly, we assessed the expression levels of the corresponding Mce1 proteins. Whole-cell lysates were prepared from intracellular bacilli isolated using the method described above (22). Identically prepared uninfected RAW cells did not contain detectable levels of protein (data not shown), demonstrating that the lysates did not contain macrophage proteins and permitting semiquantitative immunoblot analysis. Equal amounts of M. tuberculosis proteins were separated by SDS-PAGE and analyzed by Western blotting. Antibodies raised against Mce1A and Mce1F bound to proteins of the expected sizes (48 kDa and 54 kDa, respectively) in lysates prepared from in vitro-grown bacilli. Comparison to lysates from intracellular bacilli revealed that the levels of these proteins decreased by 24 h postinfection and were undetectable by 48 h postinfection (Fig. 2B). These results confirm that the mce1 operon is repressed in the macrophage environment compared to standard growth media.
Rv0165c is a GntR family transcriptional regulator.
The gene upstream and divergently transcribed from the mce1 operon is predicted to encode a GntR family transcriptional regulator (TubercuList). Since prokaryotic regulators are frequently located adjacent to genes under their control, we hypothesized that this gene encoded a regulator of the mce1 operon, and it was designated mce1R.
Sequence alignment of Mce1R with homologous GntR family regulators revealed that the predicted 264-amino-acid open reading frame extended much further upstream than other members of the family. A possible alternative start codon (GTG; genome position 194815) in a more homologous position was identified. The use of this start codon in vivo was confirmed by employing a β-galactosidase reporter gene assay (Fig. 3). Thus, we propose that mce1R encodes a 223-amino-acid protein.
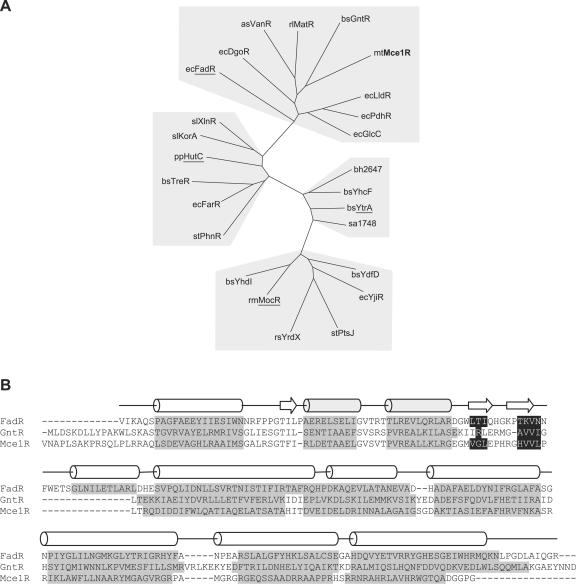
The GntR family is defined by a 64-amino-acid N-terminal DNA-binding motif (13). Rigali et al. (26) showed that the family could be divided into four major subfamilies based on homology within the C-terminal effector-binding domain and that clustering predicted functional as well as structural relatedness. Sequence comparisons of Mce1R with 24 GntR family regulators showed that Mce1R was a member of the FadR subfamily and was most closely related to the archetypal family member, the Bacillus subtilis gluconate operon regulator, GntR (37% similarity) (Fig. 4A).
FIG. 4.
Sequence analysis of Mce1R. (A) Unrooted phylogenetic tree of GntR family regulators, showing division of the four subfamilies, FadR, YtrA, MocR, and HutC (underlined). Organism abbreviations are as follows: as, Acinetobacter sp. strain ADP1; bh, Bacillus halodurans; bs, Bacillus subtilis; ec, E. coli; mt, M. tuberculosis; pp, Pseudomonas putida; rl, Rhizobium leguminosarum; rm, Rhizobium meliloti; rs, Rhodobacter sphaeroides; sa, Staphylococcus aureus; sl, Streptomyces lividans; and st, Salmonella enterica serovar Typhimurium. (B) Secondary-structure-based alignment of Mce1R with GntR and FadR. Predicted α-helical regions are highlighted in gray and β-strands in black. The structural elements of FadR determined from its crystal structure are shown above the alignment. The helix-turn-helix DNA-binding motif is shaded.
The DNA-binding domains of GntR regulators have a winged helix-turn-helix structure, and the C-terminal domains of FadR-like regulators all form an α-helical bundle (26, 41). Comparison of the predicted secondary structure of Mce1R with crystallographic data from E. coli FadR showed that Mce1R contains the expected characteristic structural elements, supporting the primary sequence analysis (Fig. 4B).
Construction of an mce1R mutant of M. tuberculosis.
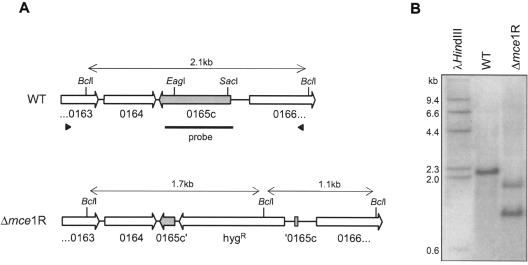
In order to investigate the role of Mce1R in regulation of the mce1 operon, we constructed an mce1R mutant by homologous recombination using the two-step counterselection strategy described by Parish and Stoker (24). In this mutant, 505 bp of the central portion of the target gene is deleted and replaced by a hygromycin resistance cassette (Fig. 5A). The genotype of the mce1R mutant was confirmed by Southern blotting and hybridization (Fig. 5B). The in vitro growth kinetics of the mce1R mutant was indistinguishable from that of the parental H37Rv strain (data not shown).
FIG. 5.
Disruption of the mce1R gene in M. tuberculosis. (A) Genomic organizations of the WT and mutant strains. Black arrowheads indicate the region cloned into the knockout vector. The region between the EagI and SacII sites was deleted and replaced by a hygromycin resistance cassette to create the mutant strain. The black bar corresponds to the probe used for hybridization. (B) Southern blot analysis of genomic DNA from WT and mutant strains digested with BclI.
Expression of the mce1 operon in Δmce1R.
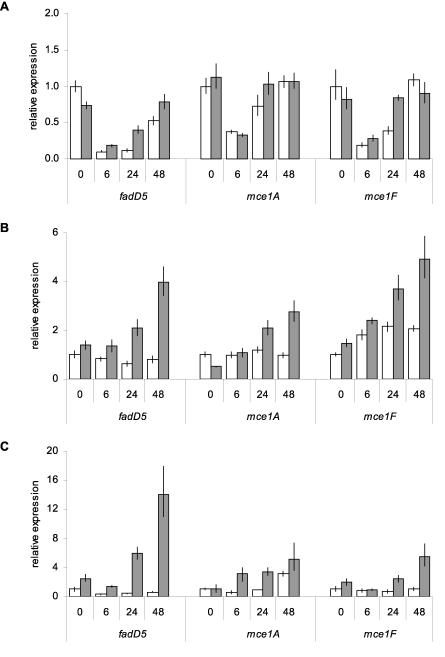
The expression levels of mce1 operon genes in wild-type (WT) M. tuberculosis and the mce1R mutant were assessed by RT-qPCR. This comparison revealed that the absence of mce1R had no significant effect on expression of mce1 genes during log-phase growth in vitro in standard media (Fig. 6A, 0-h time point). Differences in intracellular expression of the mce1 operon between the WT and Δmce1R strains were investigated by infection of RAW murine macrophages. There was no difference in growth of WT and mutant strains in RAW cells during the time period of infection (data not shown). In a preliminary experiment to determine the effect of transferring bacilli from standard 7H9 growth medium into complete DMEM, the medium used for RAW cell culture, we found that after 6 h of incubation, this change promoted a 10-fold down-regulation of the mce1 genes in both WT and Δmce1R strains. After 48 h, expression of the mce1 operon in both strains returned to levels observed with 7H9 medium (Fig. 6A). Relative to bacilli incubated in cell culture medium, expression of fadD5 and mce1A remained unchanged in intracellular WT bacilli, and expression of mce1F increased less than twofold during the 48-h infection period. However, with the mce1R mutant, expression of all three genes increased in a coordinated time-dependent fashion in intracellular bacilli compared to that in cell culture media (Fig. 6B). The expression of each gene was higher at all time points in the mce1R mutant than in the WT, and this difference increased as infection progressed.
FIG. 6.
Intracellular expression of mce1 genes in Δmce1R. RT-qPCR comparison of expression levels of the mce1 operon in WT (white bars) and Δmce1R (gray bars) strains. (A) Expression of mce1 genes in bacilli incubated in complete DMEM medium for 6 h, 24 h, and 48 h, presented as the ratio of the amount of target gene at each time point to that of the WT strain in 7H9 medium (0 h) for each gene. Expression of mce1 genes in bacilli isolated from naïve (B) or IFN-γ-stimulated (C) RAW macrophages at 6 h, 24 h, and 48 h postinfection. The 0-h time point represents bacilli incubated in complete DMEM for 6 h. Results are presented as the ratio of the amount of target gene at each time point postinfection to the amount of target in the WT strain at 0 h for each gene. Values shown are means of triplicate PCRs, and error bars represent 1 standard deviation.
To further characterize this phenomenon, we compared mce1 expression levels in WT and Δmce1R isolates from IFN-γ-activated RAW cells. In this case, we observed a slight decrease in expression of fadD5, with no change in mce1A or mce1F, in WT intracellular bacilli relative to the control. In the mce1R mutant, all three genes again showed an increase in expression over time (Fig. 6C). The difference between the WT and the mutant strain was more pronounced than in bacilli isolated from naïve macrophages. These observations demonstrate that Mce1R can negatively affect the intracellular transcription of the mce1 operon.
Expression of the mce1 operon in M. tuberculosis overproducing Mce1R.
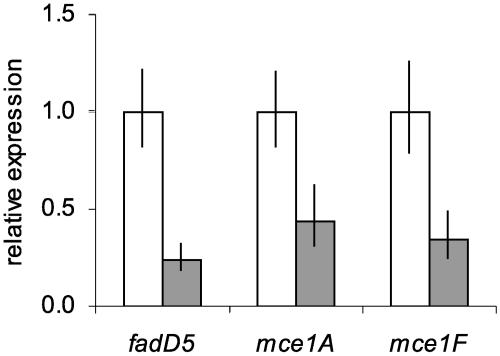
In many cases, overproduction of a transcription regulator results in operator binding even in the absence of the proper environmental conditions. Since Mce1R appeared to be inactive during in vitro growth, we reasoned that its overexpression in this condition might be sufficient to promote DNA binding and repress transcription of the mce1 operon. We used an integrating vector carrying the glutamine synthetase (glnA1) promoter, which has been shown to promote high-level expression of heterologous proteins (40), to create translational fusions with the mce1R gene. Two vectors were constructed: one carried the glnA1 promoter and cognate start codon fused to the sequence downstream of the published mce1R TTG start (pCER8), and the second used the sequence downstream of our predicted GTG start (pCER17) (Fig. 3A). RT-qPCR analysis showed that pCER8 had no effect on expression of the mce1 operon in transformed M. tuberculosis (data not shown). However, in a recombinant M. tuberculosis strain carrying pCER17 the mce1 genes were down-regulated approximately threefold compared to levels for the WT (Fig. 7). These data confirm that Mce1R is translated from the GTG start codon and provide further evidence that Mce1R is a negative regulator of the mce1 operon.
FIG. 7.
Effect of overexpression of Mce1R on transcription of mce1 genes. RT-qPCR analysis of mce1 gene expression in WT (white bars) and attB::glnA1p′-mce1R (pCER17; gray bars) in 7H9 medium. Results are presented as the ratio of the amount of target gene in the recombinant strain to the amount in the WT strain for each gene. Values shown are means of triplicate PCRs, and error bars represent 1 standard deviation.
DISCUSSION
Shimono et al. (32) showed that an M. tuberculosis mce1 mutant exhibited uncontrolled growth in mouse lungs, suggesting that the Mce1 proteins are required for the establishment of persistent infection. M. tuberculosis strains that contained a deletion of either yrbE1B or mce1A exhibited indistinguishable phenotypes, suggesting that the mutations were polar. Immunoblot analysis revealed that neither mutant expressed the proteins Mce1A to Mce1F, and thus they were considered to be mce1 operon knockouts (32). Here we show that the mce1 operon actually consists of 13 genes: Rv0166 to Rv0178, including fadD5, yrbE1A, yrbE1B, mce1A to mce1F, and the four downstream open reading frames. This result is substantiated by a statistical analysis of transcriptome data which revealed that these 13 genes formed a significant cluster of similarly regulated genes, suggesting they are cotranscribed (30).
We have shown that, compared to expression in M. tuberculosis growing in vitro, expression of the mce1 operon is repressed within a murine macrophage cell line at both the RNA and protein levels. This result is corroborated by reported microarray analyses demonstrating that the 13 M. tuberculosis mce1 genes are also significantly down-regulated in bone marrow-derived murine macrophages (30).
Rv0165c, the gene encoded upstream and divergently transcribed from the mce1 operon, belongs to the GntR family of transcriptional regulators. Typically, members of this family negatively regulate nearby genes. Thus, we hypothesized that Rv0165c, which we have designated Mce1R, is responsible for the intracellular repression of the mce1 operon. Quantitative comparison of mce1 expression in WT and mce1R mutant M. tuberculosis isolated from macrophages demonstrated that this was indeed the case. In addition, overexpression of Mce1R was sufficient to repress expression of the mce1 genes in M. tuberculosis grown in vitro, confirming the role of Mce1R as a negative regulator of the mce1 operon.
GntR family regulators repress transcription by binding to operator sequences within the promoters of target genes. DNA-binding activity is modulated by an inducer molecule that binds to the C-terminal domain of the regulator. In general, when the inducer is present, DNA binding is inhibited and repression of target genes is relieved (26). Mce1R belongs to the FadR subfamily, whose members are typically induced by oxidized metabolites that are substrates of the target enzymes. For example, GntR binds gluconate, thereby relieving repression of the gluconate utilization operon (21), and FadR binds fatty acyl CoA, resulting in derepression of genes involved in fatty acid degradation (7). Our data show that Mce1R represses transcription of the mce1 operon in the intracellular environment but not during in vitro growth. Thus, modeling the activity of Mce1R on the GntR paradigm suggests that the hypothetical inducer of Mce1R is present in 7H9 growth medium but not within macrophages.
Given the degree of homology between Mce1R and FadR (33% similarity), it is intriguing that the first gene in the mce1 operon encodes the fatty acyl CoA synthetase, FadD5, predicted to catalyze the first step in fatty acid degradation (39). However, Mce1R is no more homologous to FadR than to other subfamily members and the amino acid residues shown to be important for fatty acyl CoA binding are not well conserved (41).
Genes that are cotranscribed generally encode proteins with related or dependent functions. Comparison of the mce1 regions in Mycobacterium bovis, Mycobacterium avium, Mycobacterium marinum, and M. smegmatis revealed that the orthologue of FadD5 was encoded in the same position, upstream of the core mce1 operon, in each of these genomes (data not shown). Conservation of genomic organization also suggests functional association. Thus, inclusion of fadD5 in the mce1 operon raises the hypothesis that the role of the other proteins may be related to fatty acid uptake.
A review of published microarray data indicates that the mce1 operon is down-regulated as a result of exposure to a variety of stimuli, such as oxygen deprivation, nutrient starvation, and SDS, which are proposed to simulate aspects of the intraphagosomal environment (3, 19, 31). We found that the mce1 operon can be repressed in an Mce1R-independent manner by incubation in DMEM cell culture medium. Together, these results suggest that the operon is under the control of either a global stress regulator or multiple negative regulators. Further comparison of microarray transcriptome data reveals that the four M. tuberculosis mce operons are not coregulated (3, 19, 30, 31). This is not surprising in view of the fact that a TetR family transcriptional regulator divergently transcribed from the mce3 operon (Mce3R, Rv1963) has been shown to negatively affect regulation of this operon (28) and that a third probable transcriptional regulator (Rv0586) is associated with the mce2 operon (5). The differential expression levels of these operons may reflect their different roles.
Although the mce1 operon is down-regulated in macrophages, several lines of evidence suggest that the Mce1 proteins are expressed in vivo. Expression of the mce1 operon has been detected in bacilli isolated from both mouse and rabbit lungs (16, 34). In addition, sera from tuberculosis patients were shown to react with recombinant Mce1A and Mce1E (1). Given the importance of the mce1 operon on disease progression and outcome in mice (32), this suggests that the operon products function either extracellularly or within nonmacrophage cells. Alternatively, the in vivo intramacrophage environment may not be effectively mimicked by in vitro-infected cells. Indeed, it seems likely that there are profound differences in the environmental signals experienced by bacilli in short-term in vitro-infected macrophages and those experienced by persistent bacilli residing within granulomas in vivo.
Numerous techniques, such as those employing promoter probes (37), promoter traps (in vivo expression technology [8]), or subtractive hybridization (selective capture of transcribed sequences [11]), are directed at identifying genes that are up-regulated within macrophages or in vivo. Although these screens have been instrumental in identifying genes that are required for survival in the host and whose disruption results in an attenuated phenotype, such genes commonly encode metabolic rather than virulence functions (33, 38). In striking contrast, the mce1 operon is intracellularly down-regulated and its disruption leads to uncontrolled growth of M. tuberculosis in mouse lungs, demonstrating that important virulence genes may not necessarily be those that are highly expressed in macrophages.
Expression of the M. tuberculosis mce1 operon is necessary to elicit the host proinflammatory response that is critical for the establishment of a persistent infection (32). We propose that Mce1R is responsible for the correct temporal and spatial expression of the mce1 operon products required for organized granuloma formation, which is both protective to the host and necessary for the persistence of M. tuberculosis.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIAID, grant RO1AI53266) and the Ellison Medical Foundation Senior Investigator Award in Global Infectious Diseases.
REFERENCES
- 1.Ahmad, S., P. K. Akbar, H. G. Wiker, M. Harboe, and A. S. Mustafa. 1999. Cloning, expression and immunological reactivity of two mammalian cell entry proteins encoded by the mce1 operon of Mycobacterium tuberculosis. Scand. J. Immunol. 50:510-518. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, Indianapolis, Ind.
- 3.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 4.Chitale, S., S. Ehrt, I. Kawamura, T. Fujimura, N. Shimono, N. Anand, S. Lu, L. Cohen-Gould, and L. W. Riley. 2001. Recombinant Mycobacterium tuberculosis protein associated with mammalian cell entry. Cell. Microbiol. 3:247-254. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Cuff, J. A., M. E. Clamp, A. S. Siddiqui, M. Finlay, and G. J. Barton. 1998. JPred: a consensus secondary structure prediction server. Bioinformatics 14:892-893. [DOI] [PubMed] [Google Scholar]
- 7.DiRusso, C. C., and P. N. Black. 2004. Bacterial long-chain fatty acid transport: gateway to a fatty acid-responsive signaling system. J. Biol. Chem. 279:49563-49566. [DOI] [PubMed] [Google Scholar]
- 8.Dubnau, E., P. Fontan, R. Manganelli, S. Soares-Appel, and I. Smith. 2002. Mycobacterium tuberculosis genes induced during infection of human macrophages. Infect. Immun. 70:2787-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrt, S., M. I. Voskuil, G. Schoolnik, and D. Schnappinger. 2002. Genome-wide expression profiling of intracellular bacteria: the interaction of Mycobacterium tuberculosis with macrophages, p. 169-180. In S. H. E. Kaufmann and D. Kabelitz (ed.), Immunology of infection. Academic Press, London, United Kingdom.
- 10.Frieden, T. R., T. R. Sterling, S. S. Munsiff, C. J. Watt, and C. Dye. 2003. Tuberculosis. Lancet 362:887-899. [DOI] [PubMed] [Google Scholar]
- 11.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harth, G., and M. A. Horwitz. 1997. Expression and efficient export of enzymatically active Mycobacterium tuberculosis glutamine synthetase in Mycobacterium smegmatis and evidence that the information for export is contained within the protein. J. Biol. Chem. 272:22728-22735. [DOI] [PubMed] [Google Scholar]
- 13.Haydon, D. J., and J. R. Guest. 1991. A new family of bacterial regulatory proteins. FEMS Microbiol. Lett. 63:291-295. [DOI] [PubMed] [Google Scholar]
- 14.Henkel, A. W., and S. C. Bieger. 1994. Quantification of proteins dissolved in an electrophoresis sample buffer. Anal. Biochem. 223:329-331. [DOI] [PubMed] [Google Scholar]
- 15.Kaps, I., S. Ehrt, S. Seeber, D. Schnappinger, C. Martin, L. W. Riley, and M. Niederweis. 2001. Energy transfer between fluorescent proteins using a co-expression system in Mycobacterium smegmatis. Gene 278:115-124. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, A., M. Bose, and V. Brahmachari. 2003. Analysis of expression profile of mammalian cell entry (mce) operons of Mycobacterium tuberculosis. Infect. Immun. 71:6083-6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Manganelli, R., E. Dubnau, S. Tyagi, F. R. Kramer, and I. Smith. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31:715-724. [DOI] [PubMed] [Google Scholar]
- 19.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor σE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423-437. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Miwa, Y., and Y. Fujita. 1988. Purification and characterization of a repressor for the Bacillus subtilis gnt operon. J. Biol. Chem. 263:13252-13257. [PubMed] [Google Scholar]
- 22.Monahan, I. M., J. A. Mangan, and P. D. Butcher. 2001. Extraction of RNA from intracellular Mycobacterium tuberculosis. Methods Mol. Med. 54:31-42. [DOI] [PubMed] [Google Scholar]
- 23.Parish, T., and N. G. Stoker. 1998. Electroporation of mycobacteria. Methods Mol. Biol. 101:129-144. [DOI] [PubMed] [Google Scholar]
- 24.Parish, T., and N. G. Stoker. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146:1969-1975. [DOI] [PubMed] [Google Scholar]
- 25.Pascopella, L., F. M. Collins, J. M. Martin, M. H. Lee, G. F. Hatfull, C. K. Stover, B. R. Bloom, and W. R. Jacobs, Jr. 1994. Use of in vivo complementation in Mycobacterium tuberculosis to identify a genomic fragment associated with virulence. Infect. Immun. 62:1313-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigali, S., A. Derouaux, F. Giannotta, J. Dusart, D. J. Haydon, and J. R. Guest. 2002. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J. Biol. Chem. 277:12507-12515. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Santangelo, M. P., J. Goldstein, A. Alito, A. Gioffre, K. Caimi, O. Zabal, M. Zumarraga, M. I. Romano, A. A. Cataldi, and F. Bigi. 2002. Negative transcriptional regulation of the mce3 operon in Mycobacterium tuberculosis. Microbiology 148:2997-3006. [DOI] [PubMed] [Google Scholar]
- 29.Santos, A. R., A. B. de Miranda, L. M. Lima, P. N. Suffys, and W. M. Degrave. 1992. Method for high yield preparation in large and small scale of nucleic acids from mycobacteria. J. Microbiol. Methods 15:83-94. [Google Scholar]
- 30.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimono, N., L. Morici, N. Casali, S. Cantrell, B. Sidders, S. Ehrt, and L. W. Riley. 2003. Hypervirulent mutant of Mycobacterium tuberculosis resulting from disruption of the mce1 operon. Proc. Natl. Acad. Sci. USA 100:15918-15923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, I. 2003. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 16:463-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talaat, A. M., R. Lyons, S. T. Howard, and S. A. Johnston. 2004. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 101:4602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tekaia, F., S. V. Gordon, T. Garnier, R. Brosch, B. G. Barrell, and S. T. Cole. 1999. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 79:329-342. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triccas, J. A., F. X. Berthet, V. Pelicic, and B. Gicquel. 1999. Use of fluorescence induction and sucrose counterselection to identify Mycobacterium tuberculosis genes expressed within host cells. Microbiology 145:2923-2930. [DOI] [PubMed] [Google Scholar]
- 38.Triccas, J. A., and B. Gicquel. 2000. Life on the inside: probing Mycobacterium tuberculosis gene expression during infection. Immunol. Cell Biol. 78:311-317. [DOI] [PubMed] [Google Scholar]
- 39.Trivedi, O. A., P. Arora, V. Sridharan, R. Tickoo, D. Mohanty, and R. S. Gokhale. 2004. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature 428:441-445. [DOI] [PubMed] [Google Scholar]
- 40.Tullius, M. V., G. Harth, and M. A. Horwitz. 2001. High extracellular levels of Mycobacterium tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism. Infect. Immun. 69:6348-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Aalten, D. M., C. C. DiRusso, J. Knudsen, and R. K. Wierenga. 2000. Crystal structure of FadR, a fatty acid-responsive transcription factor with a novel acyl coenzyme A-binding fold. EMBO J. 19:5167-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]