Abstract
A novel mechanism of Escherichia coli porin regulation was discovered from multicopy suppressors that permitted growth of cells expressing a mutant OmpC protein in the absence of DegP. Analyses of two suppressors showed that both substantially lowered OmpC expression. Suppression activities were confined to a short DNA sequence, which we designated ipeX for inhibition of porin expression, and to DNA containing a 3′-truncated ompR gene. The major effect of ipeX on ompC expression was exerted posttranscriptionally, whereas the truncated OmpR protein reduced ompC transcription. ipeX was localized within an untranslated region of 247 base pairs between the stop codon of nmpC—a remnant porin gene from the cryptic phage qsr′ (DLP12) genome—and its predicted Rho-independent transcriptional terminator. Interestingly, another prophage, PA-2, which encodes a porin similar to NmpC, known as Lc, has sequences downstream from lc identical to that of ipeX. PA-2 lysogenization leads to Lc expression and OmpC inhibition. Our data show that the synthesis of the lc transcript, whose 3′ end contains the corresponding ipeX sequence, inhibits OmpC expression. Overexpression of ipeX RNA inhibited both OmpC and OmpF expression but not that of OmpA. ompC-phoA chimeric gene constructs revealed a 248-bp untranslated region of ompC required for ipeX-mediated inhibition. However, no sequence complementarity was found between ipeX and this region of ompC, indicating that inhibition may not involve simple base pairing between the two RNA molecules. The effect of ipeX on ompC, but not on ompF, was independent of the RNA chaperone Hfq.
A special class of Escherichia coli outer membrane proteins (OMPs) called porins forms water-filled channels through which hydrophilic solutes gain access into the bacterial cell (27). Porins have led the way in providing insights on the structure, regulation, and assembly of membrane proteins. The two classical porins OmpC and OmpF consist of three 16-stranded β barrels, each of which forms a channel that is restricted in the middle due to the inward folding of a loop (8). By demanding bacterial growth on sugars too large to normally diffuse through porin channels, alterations in the channel loop resulting in functionally large channels were obtained (19). One such alteration in the OmpC porin was an R74C substitution (OmpCR74C, or OmpC1Cys). This mutant porin and its derivative, OmpCR74C,G154C, or OmpC2Cys (17), are the subjects of this work and are further discussed below.
The OmpC and OmpF porin genes are transcriptionally regulated by a classical two-component signal transduction regulatory system consisting of the OmpR and EnvZ proteins (12, 13). OmpC and OmpF are also subject to posttranscriptional regulation including the small regulatory RNA molecules micC (7) and micF (23), respectively. A characteristic of these regulatory RNA molecules is that they prevent translation by base pairing with their target mRNAs in the region encompassing the translation start site (31).
There also have been reports of some unusual and less well-understood mechanisms of porin regulation. For instances, it has been shown that the absence of an OMP, TolC, leads to lower OmpF levels (25). Although it is known that this effect on OmpF in tolC-null mutants involves micF up-regulation (22), the molecular mechanism behind this up-regulation is unknown. Another fascinating but even less understood example of porin regulation involves the lysogenization of E. coli K-12 cells by the PA-2 phage (28). Here, the lysogenization event leads to the expression of a phage-encoded porin, Lc, and the inhibition of the host's OmpC porin. The work conducted in this study will shed light on how phage porin expression regulates that of ompC.
Porins have also served as excellent models to study OMP assembly because they are abundant and a great deal is known about their genetics, biochemistry, and structures. A generally accepted view of the porin assembly pathway is that after the removal of the signal peptide from precursors, mature porin molecules transiently exist in the periplasm as soluble or peripherally membrane-associated, thermolabile intermediates. These intermediates interact with various soluble folding factors to gain assembly competence (9). The insertion of these intermediates into the outer membrane may be facilitated by YaeT (Omp85), an essential OMP (34, 35). Misfolded porins are degraded by DegP, a periplasmic protease (5, 20, 21).
The subject of this study is a mutant porin protein, OmpC2Cys, which contains two nonnative cysteine residues at positions 74 and 154 of the mature sequence (17). In the oxidizing environment of the periplasm, the two cysteine residues form disulfide bonds, causing OmpC2Cys misfolding and a partial loss of its cellular activities, including porin and phage receptor functions (17, 33, 34). These functions and normal folding of OmpC2Cys are restored in a background deficient in DsbA's periplasmic disulfide isomerase activity (17, 36). Expression of OmpC2Cys in a degP-null background is lethal at all growth temperatures unless dsbA is disrupted (5). Expressing a variant of DegP, DegPS210A (30), which lacks the proteolytic activity but maintains the normal polypeptide binding capacity (15), can reverse the OmpC2Cys-mediated lethality (5). This reversal is shown to be due to the capture of OmpC2Cys by DegPS210A, sequestering it away from the normal assembly pathway (5).
In this study we screened a plasmid library to identify cellular factors which, when overexpressed, can rescue OmpC2Cys-mediated lethality in a degP-null dsbA+ background. Two plasmid clones carrying different regions of the chromosome were characterized in detail. Although both plasmid clones inhibited OmpC2Cys expression, they achieved this feat by different mechanisms: one involved a well-known porin transcription factor, OmpR (12, 13), while the other involved a poorly understood strategy of porin regulation employed by certain temperate phages (28).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
Bacterial strains and plasmids used in this study are listed in Table 1. Luria broth (LB) and Luria agar (1.5% [wt/vol]) media were prepared according to the method of Silhavy et al. (29). When required, ampicillin (50 μg/ml), kanamycin (25 μg/ml), chloramphenicol (25 μg/ml), tetracycline (25 μg/ml), IPTG (isopropyl-β-d-thiogalactopyranoside) (0.4 mM), and l-arabinose (0.2%) were added to the growth medium.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain/plasmid | Relevant characteristics | Reference/source |
|---|---|---|
| Strains | ||
| MC4100 | F−araD139 Δ(argF-lac)U139 rpsL150 flbB5301 ptsF25 deoC1 thi-1 rbsR relA | 4 |
| PLB3260 | MC4100 ΔlamB106 ΦompF′::lacZ+ | S. Benson |
| PLB3261 | MC4100 ΔlamB106 ΦompC′::lacZ+ | S. Benson |
| RAM411 | PLB3261 ompC+zei::Tn10 (50% linkage to ompC by P1 transduction) | 17 |
| RAM412 | PLB3261 ompC234 (OmpC1Cys) zei::Tn10 | 17 |
| RAM415 | PLB3261 ompC402 (OmpC2Cys) zei::Tn10 | 17 |
| RAM1126 | RAM415 degP::Kmr pCS10 | 5 |
| RAM1304 | RAM412 hfq1::ΩKmr (hfq null) pTrc99A | This study; 32 |
| RAM1305 | RAM412 hfq2-ΩKmr (hfq+) pTrc99A | This study; 32 |
| RAM1306 | RAM412 hfq1::ΩKmr (hfq null) pTrc99-ipeX | This study; 32 |
| RAM1307 | RAM412 hfq2-ΩKmr (hfq+) pTrc-ipeX | This study; 32 |
| CS109 | W1485 F− | C. Schnaitman |
| CS137 | W1485 F− PA-2 lysogen | C. Schnaitman |
| Plasmids | ||
| pACYC184 | Cmr Tcr; p15A replicon | 6 |
| pC510 | pACYC-degPS210A | 30 |
| pBAD33 | Cmr; expression vector; p15A/M13 replicons | 11 |
| pBR322 | Apr Tcr; pMB1 replicon | 3 |
| pTrc99A | Apr; expression vector; pMB1 replicon | Pharmacia |
| pTrc-ybcQ | The ybcQ clone in pTrc99A encompassing ybcQ, ipeX and the 3′ end of nmpC | This study |
| pybcQ plus SL-1 through SL-5 | The ybcQ clone in pTrc99A encompassing ybcQ and all five stem-loops of ipeX | This study |
| pybcQ plus SL-2 through SL-5 | The ybcQ clone in pTrc99A encompassing ybcQ and stem-loops 2 to 5 of ipeX | This study |
| pybcQ plus SL-3 through SL-5 | The ybcQ clone in pTrc99A encompassing ybcQ and stem-loops 3 to 5 of ipeX | This study |
| pTrc-ipeX | The ipeX clone in pTrc99A containing all five stem loops of ipeX and the 3′ ends of ybcQ and nmpC | This study |
| pTrc-ipeX (SL-2*) | The ipeX clone in pTrc99A with a 3-base alteration in the stem-loop 2 of ipeX | This study |
| pRAM1006 | A 3.0-kb HindIII chromosomal fragment containing ompC was cloned into a pSC101-derived plasmid (Apr Kmr) | R. Misra |
| pTrc-ompC | A clone carrying the ompC coding region under the control of the pTrc99A promoter | This study |
| pompC-UTR[ss]-phoA | An ompC-phoA chimeric clone in pACYC184 from which PhoA, with OmpC's signal sequence, was synthesized under ompC's transcriptional and translational controls | This study |
| pompC-UTR-phoA | An ompC-phoA chimeric clone in pACYC184; PhoA, with its native signal sequence, was synthesized under ompC's transcriptional and translational controls | This study |
DNA methods.
A previously constructed plasmid library was used to identify multicopy suppressor clones. The plasmid library was constructed by ligating Sau3A-digested chromosomal DNA fragments to BamHI-digested pBR322. Using this gene library, we have been able to isolate plasmids carrying a number of different genes, indicating that the library is highly representative. Smaller plasmid clones were constructed by cloning DNA fragments amplified by PCR using the original 12- and 76-minute suppressor plasmids as templates (see Table 1 for further description). Table 2 lists primers used for DNA amplifications. Some of these primers also introduced restrictions sites that were used for subsequent cloning into plasmid vectors. Site-directed mutagenesis was performed by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) per the manufacturer's instructions. Sequences of only forward primers used for mutagenesis are shown in Table 2. Clones and site-directed mutagenesis changes were confirmed by DNA sequence analysis.
TABLE 2.
Primers used in this study.
| Primer | Sequence (5′ to 3′) |
|---|---|
| Primers used for ybcQ and ipeX cloning and ipeX mutagenesis | |
| ybcQ | |
| Forward | TCTGGCTGGATccTGCTCAGGTCG (BamHI) |
| Reverse | GCACTGATGAatTCGTTGCTGTAGG (EcoRI) |
| ipeX | |
| Reverse | CCGGTTAGAGATGGATccCGTTG (BamHI) |
| Forward | GCACTGATGAatTCGTTGCTGTAGG (EcoRI) |
| SL-1 | CTAATCgaATTcCGAAAAAGATATGTTGCGGGAGGCG (EcoRI) |
| SL-2 | gGaattcGCCTCCCCAACATATAAGTGGC (EcoRI) |
| SL-3 | CCCTCAAGCgAaTTCCTTTAGAAGC (EcoRI) |
| ipeX SL-2 mutant | CCCCAACATATAAGTGGCTCaaaCAAGCCACTTCCTTTAGAAGC |
| Primers used to construct ompC and ompC-phoA clones | |
| ompC | |
| Forward | CTTGcATgcTTATTGCTTGATGTTAGGTGC (SphI) |
| Reverse | CTTtCATGATATTAACCCTCTGTTATATGCC (BspHI) |
| SS | AACTTcaTGAGCGTTTGCTGCGC (BspHI) |
| Whole | ATCCtCATGAGAACGGTCGCAAGAG (BspHI) |
| phoA | |
| Forward | |
| SS | AAATAtcaTGAAACAAAGCACTATTGCACTGGC (BspHI) |
| BspHI | acatcAtGaaCGGACACCAGAAATGCCTG (BspHI) |
| Reverse | CGAAgcTTCACTGCCGGG (HindIII) |
| Primers used in RT-PCR analysis | |
| ipeX | |
| Forward (RT) | CCCTCAAGCgAaTTCCTTTAGAAGC (EcoRI) |
| Reverse | CCGGTTAGAGATGGATccCGTTG (BamHI) |
| ipeX-SL-4 reverse | GGGTAATATATAACAGAAGGTTTATATAG |
| lc | |
| Forward | GGTCTGAACTTTGCTGCTCAGTACCAAGGC |
| Reverse | CTGGTAAACCAGACCTACAGCAAC |
| ompA | |
| Forward | GCTATCGCGATTGCAGTGGCAC |
| Reverse | CTGGAGCCGGAGCAACTACTG |
| ompC | |
| Forward | CGGTAAAGTAGACGGCCTGCAC |
| Reverse | CTGGTTGTCGTCCAGCAGGTTG |
Restriction sites are underlined and mutational sites are in lowercase letters.
RNA methods.
Total cellular RNA was purified using the TRIzol Max bacterial RNA isolation kit (Invitrogen). LB (4 ml) containing ampicillin, with and without IPTG, was inoculated with 1:50 dilution of overnight cultures. Cultures were grown at 37°C in a shaking water bath to an optical density at 600 nm (OD600) of 0.5. Cells from 3-ml cultures were pelleted in a microcentrifuge for 15 s at 15,000 × g. RNA was isolated from cell pellets after they had been resuspended in 200 μl of Max bacterial enhancement reagent preheated to 95°C.
RNA decay studies were conducted essentially as described by Gudapaty et al. (10). LB was inoculated with 1:50 dilution of an overnight culture, and cells were grown to OD600 of 0.5. At this point, rifampin (200 μg/ml final concentration) was added, and 2-ml samples were collected 0, 2, 8, and 16 min after the addition of rifampin. Withdrawn cells were centrifuged immediately at 15,000 × g for 15 s, and pellets were frozen in a dry-ice ethanol bath. RNA was then isolated, quantified, and stored at −80°C.
RT-PCR.
cDNA was obtained from total RNA by using the ProtoScript first-strand cDNA synthesis kit (New England BioLabs). PCR was performed using DynaZyme EXT DNA polymerase (Finnzymes) on serial dilutions of the cDNA template. Prior to the reverse transcription (RT) reaction, 1 μg RNA was treated with amplification grade DNase I (Invitrogen) to remove any traces of genomic DNA. RT reactions were performed using each of the 120 ng/μl ompC and ompA reverse primers (Table 2), after which RNA templates were removed by incubation with RNase H (2 U/μl). Undiluted and diluted cDNA (1:5, 1:25, and 1:125) from RT reactions were used as templates for PCR. Amplification reactions rendered ompC and ompA DNA fragments of approximately 900 and 600 nucleotides, respectively. For the ompC RNA decay study, 1:10 dilution cDNA template was used in the PCR. RT-PCR was performed to detect ipeX RNA in cells. All DNA products were separated in 0.8% agarose gels except for the small ipeX PCR product, which was separated in a 2% agarose gel. DNA bands were stained with ethidium bromide and visualized in a Bio-Rad Fluor-S imager.
β-Galactosidase assay.
ompC and ompF transcriptional activities were determined by measuring β-galactosidase activities of ompC′::lacZ+ and ompF′::lacZ+ operon fusions. Cells were grown to an OD600 of 0.5 in LB supplemented with or without IPTG to induce ipeX expression from a plasmid clone. Assays were carried out in duplicate as described by Miller (16).
Protein analysis.
Whole cell envelopes were extracted by the French press cell lysis method as described previously (18). Membrane pellets were resuspended in 20 mM Tris-HCl, pH 7.5, and stored frozen at −20°C. Membrane proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide (11%) gel electrophoresis. To better resolve OmpC and OmpF, 4 M urea was added to the separation gel solution. For Western blot analysis, proteins from SDS-polyacrylamide gel electrophoresis gels were transferred onto Immobilon-P polyvinylidene difluoride transfer membranes (Millipore) by using a Mini Transblot electrophoretic transfer cell (Bio-Rad). Membrane blots were blocked overnight in 5% (wt/vol) nondairy cream. The next day, they were incubated for 1.5 h with primary rabbit antibodies against OmpC (1:10,000 dilution), followed by 1-h incubation with goat anti-rabbit immunoglobulin G secondary antibodies (Sigma). Detection was carried out using ECF substrate (Pierce) as per manufacturer's instructions. Bands were visualized using a Molecular Dynamics Storm imager.
RESULTS
Multicopy suppressors of OmpC2Cys.
We sought plasmid clones that could overcome the lethal effect of OmpC2Cys in a degP-null background. The viability of the ompC2Cys degP::Kmr strain depends on the expression of protease-deficient DegPS210A by an IPTG-inducible plasmid promoter. That is, the ompC2Cys degP::Kmr/pdegPS210A strain (RAM1126) grows on media supplemented with IPTG but not on media lacking IPTG. A random chromosomal gene library, generated on a plasmid with a replicon (ColE1) and an antibiotic resistance gene (Apr) compatible with the resident pdegPS210A plasmid (p15A; Cmr), was transformed into RAM1126, and transformants were selected at 30°C on antibiotic plates lacking IPTG. Several hundred transformants were obtained, and due to this large number, they were pooled and the plasmids extracted from this pool were retransformed into RAM1126 to ensure that plasmid-encoded functions rescued the OmpC2Cys lethality. When MC4100-competent cells were transformed with the same amount of the same gene library DNA, approximately 100,000 transformants were typically obtained. Thus, about 0.1% of transformants contained plasmid genes that could reverse the conditional lethal phenotype of RAM1126.
EcoRI restriction analysis of plasmids from fifty random colonies obtained through the second round of transformation revealed five distinct groups of plasmid clones. DNA sequence analysis from one member of each group revealed three subgroups that encompassed DNA from the 12-, 56-, and 76-minute region of the chromosome. Plasmids from these subgroups were tested to see whether they could support the viability of the ompC2Cys degP::Kmr strain in the absence of pdegPS210A. Since the ompC2Cys degP::Kmr strain could not be constructed in the absence of pdegPS210A, the three suppressor plasmids and the vector plasmid were first introduced into the ompC2Cys degP+ strain, followed by transduction of the degP::Kmr-null allele by the P1 phage. The fact that stable Kmr (degP::Kmr) transductants were obtained only when the recipient ompC2Cys degP+ cells carrying the suppressor plasmids (but not the vector plasmid) were used showed that the three suppressor plasmids are capable of supporting growth without the pdegPS210A plasmid. We have been unable to resolve the mechanism of suppression by the plasmid encompassing the 56-minute chromosomal region. Results for the other two suppressor plasmids are presented here.
Suppression of OmpC2Cys-mediated lethality by a plasmid clone encompassing the 12-minute chromosomal region.
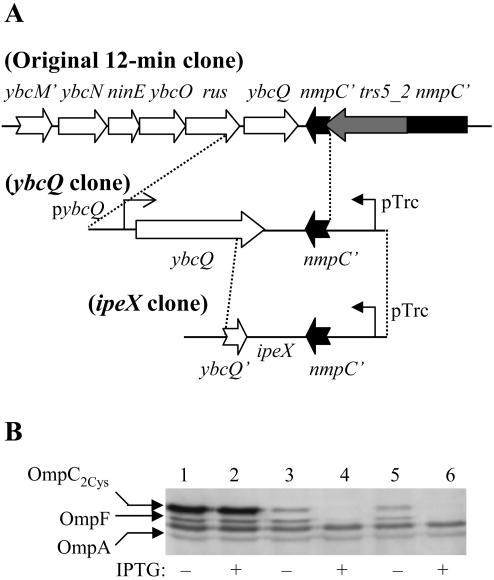
A suppressor plasmid that reversed the lethal phenotype of OmpC2Cys in a DegP− background carried a chromosomal insert of 4,212 bp and contained six complete genes (ybcN, ninE, ybcO, rus, ybcQ, and trs5-2) flanked by two truncated genes (ybcM′ and nmpC′) at both ends (Fig. 1A). All of these genes are from the genome of a cryptic lambdoid phage qsr′ (DLP12) integrated at the 12-minute region of several E. coli strains, including MC4100. Of the six complete genes, the functions of only rus and trs5-2 are known, and they are involved in recombination events. The trs5-2 gene is a part of the IS5 element that disrupts the phage-encoded nmpC porin gene in most laboratory E. coli K-12 strains (2, 28). In the absence of any obvious candidate for the suppression, subcloning experiments were carried out to narrow down the responsible gene. Initially, two subclones were constructed, one carrying ybcN to rus and the other carrying ybcQ to the truncated nmpC gene. The subclone carrying ybcN to rus could not suppress OmpC2Cys-mediated lethality but the ybcQ to nmpC′ clone could. Moreover, an even smaller subclone (referred to as the ybcQ clone in Fig. 1A) containing the ybcQ gene, and 318 bp upstream and 230 bp downstream from the ybcQ open reading frame (ORF) was found to suppress OmpC2Cys-mediated lethality. Western blot analysis of envelopes revealed that the presence of the ybcQ clone substantially reduced OmpC2Cys levels (Fig. 1B, lanes 1 and 3), suggesting that the plasmid clone most likely achieved suppression by lowering the mutant OmpC protein level.
FIG. 1.
(A) Schematic diagram of the plasmid clones that suppress OmpC2Cys-mediated lethality. As described in the text, the ybcQ and the ipeX clones, both of which lower OmpC2Cys and OmpF expression, were subcloned from the original 12-minute clone. (B) OmpC, OmpF, and OmpA from RAM415 were detected in whole cell envelopes by Western blot analyses using OmpC polyclonal antibodies that also recognize OmpF and OmpA. Envelope samples were obtained from cells containing pTrc99A vector plasmid (lanes 1 and 2), the ybcQ clone (lanes 3 and 4) and the ipeX clone (lanes 5 and 6). The presence (+) or absence (−) of IPTG in bacterial cultures is indicated.
The ybcQ ORF within the 922-nucleotide-long ybcQ clone is oriented in the opposite direction of the IPTG-inducible promoter of pTrc99A (Fig. 1A). We noted that this clone produced a dramatically greater inhibitory effect on ompC expression when IPTG was present in the growth medium than when it was absent (Fig. 1B, lanes 3 and 4). This indicated that expression of a product in the direction of the pTrc99A promoter is possibly involved in porin inhibition (see below). The effect of the ybcQ clone was not specific to OmpC2Cys, as the levels of OmpC1Cys and wild-type OmpC were also significantly reduced (data not shown). Surprisingly, when IPTG was present, the ybcQ clone also exerted a strong inhibitory effect on OmpF but not on OmpA (Fig. 1B). Lastly, the inhibitory effect of the ybcQ clone on OmpC and OmpF was not influenced by the presence or absence of DegP, even though the original plasmid clone was isolated in a genetic background lacking DegP (data not shown).
Identification of the porin inhibitor sequence.
The negative effects on OmpC and OmpF described above most likely resulted from the expression of some insert DNA under the control of the IPTG-regulated plasmid promoter of the ybcQ clone. The ybcQ ORF is oriented opposite to the IPTG-regulated plasmid promoter, which suggested that ybcQ might not be involved in inhibiting porin expression. We proceeded to test this by both disrupting the ybcQ reading frame and constructing subclones that lacked the majority of the ybcQ gene. The ybcQ gene encodes a 127-residue-long protein that is homologous to the lambda antitermination protein Q. We introduced a premature stop codon by altering the 40th codon of the ybcQ ORF. The plasmid clone capable of expressing only the truncated YbcQ protein suppressed lethality and reduced OmpC and OmpF levels as effectively as the parental plasmid containing the intact ybcQ ORF (data not shown). Thus, it is unlikely that the ybcQ ORF mediates suppression.
Next, we built several smaller subclones to further pinpoint the region responsible for ompC and ompF inhibition. The porin inhibition activity was narrowed down to a 247-bp sequence that excluded all but 17 nucleotides from the 3′ end of the ybcQ ORF (Fig. 1A). We call this 247-bp sequence ipeX, for inhibitor of porin expression. The ipeX clone was as effective in reducing OmpC and OmpF levels (Fig. 1B, lanes 5 and 6) as the ybcQ clone (Fig. 1B, lanes 3 and 4). A hypothetical ORF of 78 nucleotides can be identified within the 247-bp DNA insert. But clones lacking the potential Shine-Dalgarno (SD) sequence and start codon of this hypothetical ORF retained the inhibitory effect on porins (data not shown), thus eliminating the possibility that a small peptide from the ipeX sequence mediated the inhibition of porin expression. Taken together, these results suggested that an RNA molecule made from the ORF-free DNA sequence is the inhibitor.
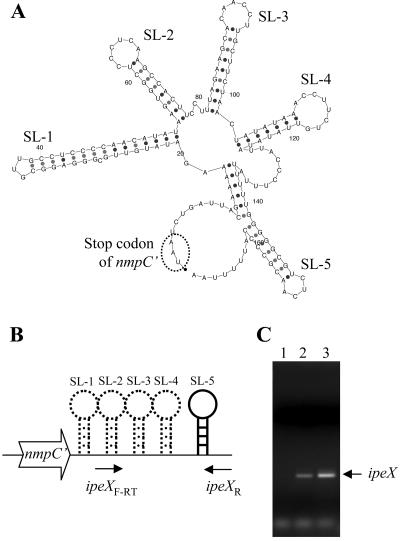
Characteristics of ipeX.
MFOLD software (http://bioweb.pasteur.fr/seqanal/interfaces/mfold-simple.html), which predicts RNA secondary structures, suggested multiple secondary structures within the ipeX sequence. Most notably, it predicted five adjacent stem-loop structures of 34, 22, 24, 22, and 18 nucleotides (SL-1 to SL-5) (Fig. 2A and 3A). SL-5, which is followed by a string of five consecutive U's, resembles a conventional Rho-independent transcription terminator (Fig. 2A). Synthesis of a small RNA corresponding to the ipeX DNA on a plasmid clone was confirmed by RT-PCR analysis (Fig. 2B and C). A greater amount of ipeX RNA was present when IPTG was added in the culture, showing both leaky (without IPTG) and induced expression of ipeX from the pTrc promoter of the ipeX clone (Fig. 2C, lanes 2 and 3). These results are consistent with the notion that the elevated ipeX RNA level in the presence of IPTG produces a greater inhibitory effect on porin expression. ipeX RNA could not be detected in bacterial cells devoid of the ipeX plasmid clone (Fig. 2C, lane 1), suggesting that ipeX is not normally expressed from the MC4100 chromosome.
FIG. 2.
(A) The 167-bp-long portion of the ipeX RNA sequence was analyzed using MFOLD software, which predicted the RNA secondary structure. There are four stem-loop structures (SL-1 to SL-4) and a presumed Rho-independent transcription terminator (SL-5). The stop codon of the truncated nmpC′ gene is circled. (B) A cartoon showing the ipeX region and locations of ipeX primers used in the RT-PCR analysis. (C) ipeX RNA was analyzed by RT-PCR from cells containing just the vector plasmid (lane 1) and the ipeX clone (lanes 2 and 3). RNA was isolated from cells grown in the absence (lane 2) and presence (lane 3) of IPTG to induce ipeX expression from the pTrc99A promoter.
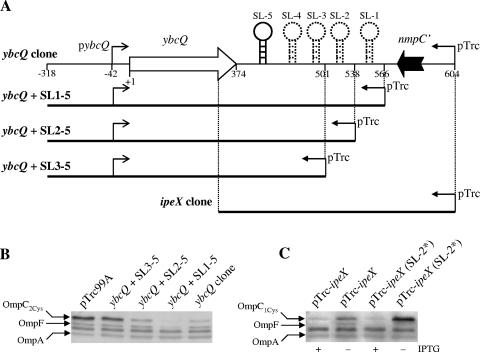
FIG. 3.
(A) Schematic diagrams of the ybcQ clone and its derivatives lacking one or two predicted stem-loops (SL) or almost the entire ybcQ gene (ipeX clone). Small leftward arrows indicate the location of IPTG-inducible pTrc99A promoter while small rightward arrows show the location of the predicted ybcQ transcription start site. (B) OmpC, OmpF, and OmpA were detected in whole cell envelopes by Western blot analyses with OmpC polyclonal antibodies that also recognize OmpF and OmpA. Shown are the names of the plasmids that were transformed into RAM415 (OmpC2Cys). ipeX expression was not induced by IPTG; instead, it occurred from the leaky pTrc99A promoter. (C) OMPs were detected from whole cell envelopes. Shown are the names of plasmids transformed into RAM412 (OmpC1Cys). pTrc-ipeX (SL-2*) refers to a mutant plasmid derived from pTrc-ipeX in which three residues of ipeX SL-2, which are complementary to the ompC and ompF SD sequences, have been altered by site-directed mutagenesis. ipeX expression was induced by IPTG.
The involvement of ipeX stem-loops on porin expression was investigated by constructing various plasmids lacking one or more predicted stem-loop structures (Fig. 3A). The data showed that the presence of all five stem-loops imposed the strongest inhibitory effect on ompC expression, a modest effect in the presence of stem-loops 2 to 5, and no inhibition when only stem-loops 3 to 5 were present (Fig. 3B). Thus, the region encompassing stem-loops 1 and 2 is mandatory for porin inhibition. However, we cannot completely rule out the possibility that a lack of the inhibitory effect of smaller clones is due to the instability of the truncated ipeX transcript. It is unclear why the ybcQ clone with all five stem-loops but lacking 38-bp from the 3′ end of nmpC′ imposes a greater inhibitory effect on porin expression than a similar clone containing the 38-bp 3′ nmpC DNA (Fig. 3B, lanes 4 and 5).
Previously two regulatory RNA molecules that negatively regulate ompC and ompF expression have been identified, micC (7) and micF (23), respectively. Both of these RNA molecules have extended sequence complementarities to the 5′ end of their target mRNAs and exert their inhibitory effects on porin expression by preventing translation (7, 23). No such extended complementarity between ipeX and ompC or ompF was found. Interestingly, however, bases present in the loop region of stem-loop 2 LSL-2 of ipeX are complementary to the SD region of ompF (6 of 6 nucleotides), ompC (5 of 6 nucleotides), and ompA (4 of 6 nucleotides). Although all 6 bases of LSL-2 are complementary to the ompF SD region, the effect of ipeX on ompC is more severe than on ompF, suggesting that a sequence other than that in LSL-2 plays a greater role in porin regulation. To assess the involvement of the LSL-2 sequence in ompC regulation, we altered 3 of its 6 nucleotides (CCCTCA to CAAACA) by site-directed mutagenesis and found only a modest reduction (without overexpression) in the ability of the mutant ipeX clone to reduce OmpC levels (Fig. 3C). These results affirmed that although the LSL-2 sequence plays a role in regulation, additional ipeX sequences must also participate in porin regulation and inhibition specificity.
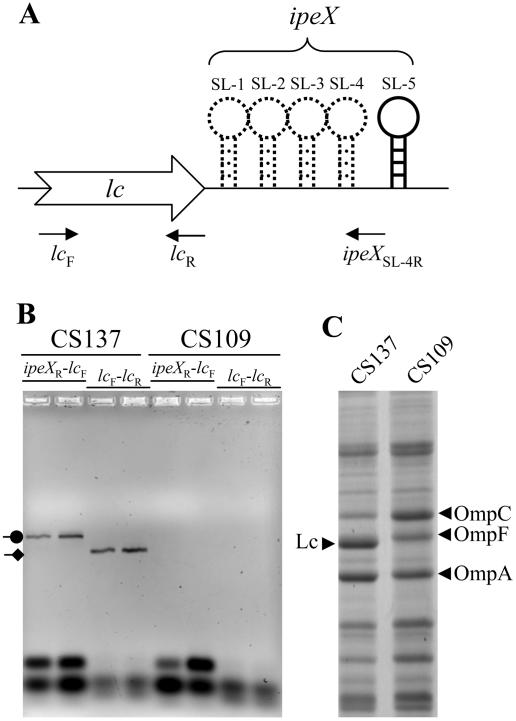
In PA-2 lysogens, ipeX is transcribed in cis with the phage porin gene.
The ipeX sequence located downstream of the nmpC porin gene of the cryptic phage qsr′ is identical to the sequence present downstream of the lc gene of the PA-2 phage. We asked whether ipeX RNA is made in PA-2 lysogens and, if so, whether it is made as a part of the lc transcript. These questions were addressed by conducting RT-PCR analysis of RNA obtained from the PA-2 lysogen (CS137) and its parental strain (CS109) (Fig. 4). In the PCR step, we carried out two separate amplifications using the same forward primer, which was specific to the lc gene but different reverse primers, one specific to the lc gene while the other specific to the ipeX sequence (Fig. 4A). Transcription of the lc gene from PA-2 lysogens should give a RT-PCR product using lc-specific forward (lcF) and reverse (lcR) primers. If ipeX is transcribed as part of lc, we should detect a RT-PCR product by using lcF and ipeXR primers. The data presented in Fig. 4B showed a RT-PCR product of the expected size from PA-2 lysogens, using lcF and ipeXR primers, thus showing that ipeX RNA is synthesized attached to the 3′ end of the lc RNA.
FIG. 4.
RT-PCR and envelope protein analyses from the parental (CS109) and PA-2 lysogen (CS137) strains. (A) A cartoon showing the lc-ipeX region of the PA-2 phage genome. Locations of various stem-loops (SL-1 to SL-5) and primers used in the RT-PCR analysis are shown. (B) After RT reactions using the ipeX reverse primer (ipeXR), a subsequent PCR step was carried out using primers to amplify the lc region (lcF and lcR) and the lc-ipeX region (lcF and ipeXR). PCR-amplified products were analyzed on an agarose gel, and bands were visualized after ethidium bromide staining. PCR-amplified products corresponding to lc (diamond arrowhead) and lc-ipeX (circle arrowhead) RNA were obtained only from the PA-2 lysogen strain. (C) Envelopes isolated from CS109 and CS137 were analyzed by SDS-polyacrylamide gel electrophoresis. Proteins bands were visualized after Coomassie brilliant blue staining. Positions of Lc, OmpC, OmpF, and OmpA are shown.
ipeX exerts its negative effect by destabilizing the target mRNA.
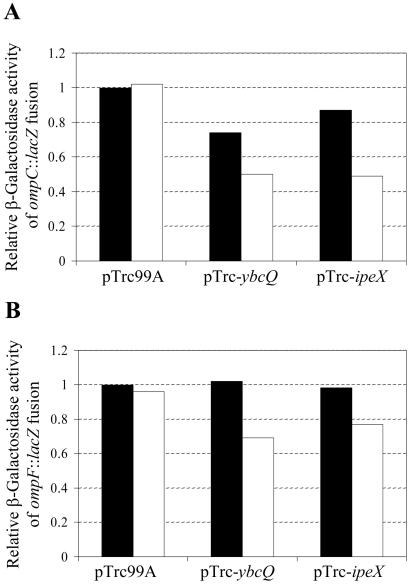
The IPTG-induced expression of ipeX from the plasmid clone only moderately reduced ompC (50%) and ompF (20%) transcription from the ompC′::lacZ+ and ompF′::lacZ+ operon fusion constructs, respectively (Fig. 5). This effect of ipeX on ompC and ompF transcription was significantly less than that on OmpC and OmpF protein levels (Fig. 1B), suggesting that the bulk of the inhibitory effect must be exerted posttranscriptionally. Since pulse-chase experiments failed to show any OmpC protein when cells expressing high levels of ipeX were labeled (data not shown), indicating an inhibition on OmpC protein synthesis, we focused on RNA analysis.
FIG. 5.
Transcriptional activities of ompC (A) and ompF (B) as analyzed through assaying β-galactosidase activities of the ompC′::lacZ+ and ompF′::lacZ+ operon fusion constructs, respectively. Cultures were grown in the absence (solid bars) or presence (empty bars) of IPTG. Enzymatic activities are relative to those obtained from pTrc99A-containing cells grown without IPTG. Names of plasmids present in the bacterial strains are shown.
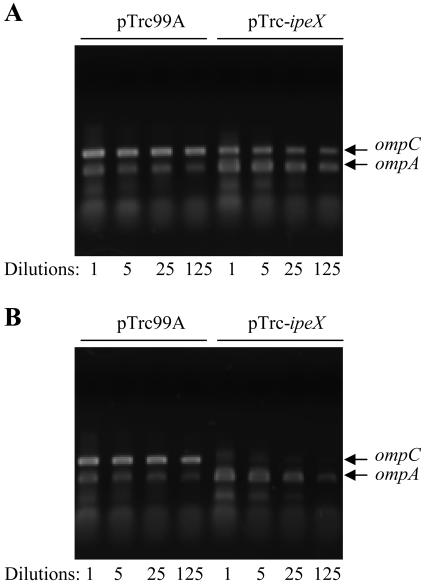
Direct evidence for the posttranscriptional effect of ipeX on ompC and ompF expression came from RT-PCR analysis of RNA isolated from cells expressing ipeX. ompA mRNA was analyzed as a control, since OmpA levels remained unaffected when ipeX was overexpressed (Fig. 1B). When cells were grown in the absence of IPTG so that only leaky expression of ipeX could occur from the plasmid promoter, a modest reduction in ompC mRNA level was observed (Fig. 6A). In contrast, a dramatic reduction in ompC mRNA resulted when ipeX expression was fully induced by the addition of IPTG to the growth medium, while ompA mRNA levels remained unaffected (Fig. 6B). ompF transcripts were also reduced by ipeX overexpression but to a lesser extent than that of ompC (data not shown). These results showed that porin mRNA production and/or stability is lowered when ipeX is overexpressed.
FIG. 6.
Semiquantitative analysis of OmpC and OmpA RNAs through RT-PCR. RNA was isolated from cultures grown in the absence (A) or presence (B) of IPTG. Undiluted (1-fold) and 5-, 25-, and 125-fold diluted cDNAs were amplified by PCR using ompC- and ompA-specific primers. Both ompC and ompA PCRs were carried out in the same tube. Amplified DNAs were analyzed on agarose gels.
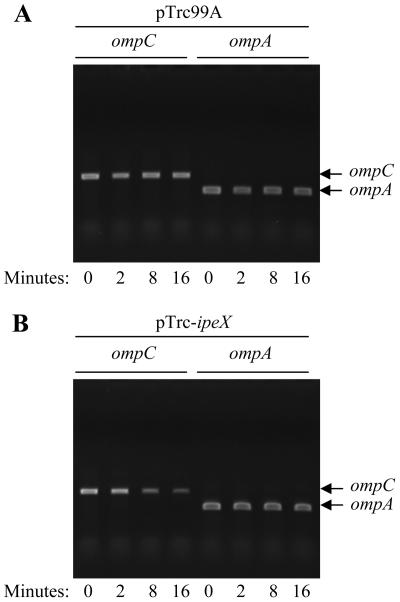
We determined the half-life of ompC mRNA to gain a better insight into the ipeX-mediated inhibition mechanism. Since the overexpression of ipeX severely lowered ompC mRNA levels, making it difficult to evaluate half-lives, experiments were performed under conditions of modest ipeX expression (without IPTG induction) that still produced a negative effect on ompC mRNA levels (Fig. 6A). Cultures were grown to mid-log phase, and rifampin was added to prevent new rounds of transcription initiation. Cells were collected immediately after the addition of rifampin (time zero) and 2, 4, 8, and 16 min thereafter. Cells withdrawn at various times were instantly frozen to minimize mRNA decay. ompC and ompA (control) mRNAs were analyzed by RT-PCR (Fig. 7). The results clearly show that only when cells are expressing ipeX do we see a significant reduction in the half-life of ompC mRNA (Fig. 7B). Consistent with the protein data, the half-life of ompA mRNA was unaffected by ipeX (Fig. 7B).
FIG. 7.
Half-life determination of ompC and ompA RNA by RT-PCR. RNA was isolated from bacterial cultures treated with rifampin for various time durations without (pTrc99A) (A) or with (pTrc-ipeX) (B) ipeX expression. One-in-ten diluted cDNA products were used in separate PCRs using ompC- or ompA-specific primers. Amplified DNAs were analyzed on agarose gels.
Regulatory regions of ompC required for ipeX-mediated inhibition.
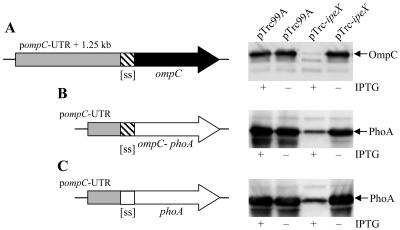
Overexpression of ipeX from an IPTG-inducible promoter effectively inhibited OmpC when it was produced from a compatible plasmid (pSC101 replicon) containing the entire ompC coding region under the control of ompC's native promoter and the untranslated region (UTR) between the promoter and initiation codon (Fig. 8A). On other hand, when the ompC coding region was placed under the transcriptional and translational control sequences of the pTrc99A vector plasmid, OmpC synthesis was unaffected when ipeX was expressed from the arabinose-inducible promoter of pBAD33 (data not shown). These results suggested that ipeX-mediated inhibition requires the noncoding region of the ompC gene. To narrow down this region, we constructed two ompC-phoA chimeras. In one chimera, PhoA was synthesized under the transcriptional and translational controls of ompC and used OmpC's signal sequence (Fig. 8B). The other chimera had the ompC promoter and UTR of ompC but incorporated PhoA's native signal sequence instead of OmpC's (Fig. 8C). Both chimeras synthesized PhoA that could be readily detected by Western blot analyses using PhoA antibodies (Fig. 8B and C). However, PhoA synthesis was inhibited when ipeX was overproduced (Fig. 8B and C). These results showed that the 248-bp sequence encompassing the promoter and UTR of ompC is sufficient to observe the ipeX-mediated inhibition of ompC expression.
FIG. 8.
Effects of ipeX overexpression on OmpC and PhoA synthesis from various plasmid constructs. ipeX was expressed from an IPTG-inducible promoter of pTrc99A. ompC was expressed from a 1.5-kb noncoding region of ompC which contains its native 248-bp promoter and UTR (gray box) (A). This ompC clone was present on a plasmid replicon (pSC101) compatible with the ColE1 replicon of pTrc99A. phoA clones were present on pACYC184, which is also compatible with pTrc99A. PhoA synthesis was placed under the control of the 248-bp ompC promoter and UTR sequences (gray box) (B and C). Filled and open arrows show mature protein-coding regions of ompC and phoA, respectively. phoA constructs used either ompC's (striped box) (B) or phoA's (open box) (C) signal sequence-coding region (ss). Plus and minus signs refer to cultures with or without the IPTG inducer, respectively. OmpC and PhoA proteins were visualized from whole cells by Western blot analyses with OmpC (A) and PhoA (B and C) antibodies.
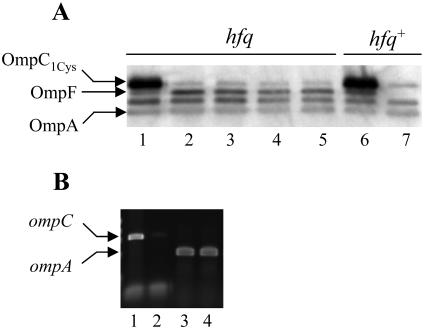
hfq is not required for the ipeX-mediated inhibition of ompC expression.
It is well documented that several small regulatory RNAs that act by base pairing to their target mRNA require Hfq, a RNA chaperone (24, 37). Both micC and micF RNAs bind to Hfq (7, 37) and micC is shown to require Hfq for its action (7). We asked whether Hfq is also required for the ipeX-mediated regulation of ompC and ompF expression. Our results showed no change in the ipeX-mediated inhibition of OmpC expression when Hfq was absent from the cell (Fig. 9A). However, ipeX could not inhibit OmpF expression in cells lacking Hfq (Fig. 9A). It should be noted that we introduced the hfq-null allele into the ompC1Cys strain because ompC2Cys hfq-null cells produced extremely small and heterogeneous colonies. Lastly, even though elevated expression of ipeX in an hfq-null background severely reduced ompC mRNA levels, ompA mRNA levels remained unaffected (Fig. 9B).
FIG. 9.
Effects of Hfq on ipeX-mediated inhibition of OmpC, OmpF, and OmpA expression. (A) OmpC, OmpF, and OmpA were analyzed from envelopes of hfq-null or hfq wild-type strains carrying either the pTrc99A vector (lanes 1 and 6) or the ipeX clone (lanes 2 to 5 and 7). OMPs were detected by Western blot analyses using OmpC polyclonal antibodies that also recognize OmpF and OmpA. Due to an apparent growth defect of the strain carrying the hfq-null allele, multiple (four) independent cultures (lane 2 to 5) were analyzed to assess the effect of ipeX on porin expression. (B) ompC and ompA transcripts were analyzed by RT-PCR from hfq-null strains carrying the vector plasmid (lanes 1 and 3) or the ipeX clone (lanes 2 and 4). ipeX expression from the resident plasmid was induced by IPTG.
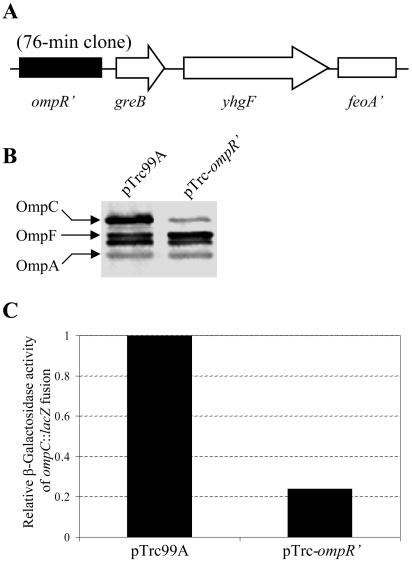
Suppression by the plasmid clone containing the 76-minute chromosomal region.
Western analysis of envelope proteins revealed that the presence of the suppressor plasmid significantly reduced OmpC2Cys levels compared to that observed in an ompC2Cys degP+ strain (data not shown). As with the ipeX clone, the effect of this suppressor was not specific to OmpC2Cys, as the levels of OmpC1Cys and wild-type OmpC were also reduced by the plasmid. Nucleotide sequence analysis revealed that the insert DNA contained a contiguous chromosomal region encompassing the 3′-truncated ompR, greB, and yhgF and 5′-truncated feoA genes (Fig. 10A). We suspected that the expression of the 3′-truncated ompR, lacking the last 75 nucleotides of the 717-bp ompR ORF, is likely responsible for the reduced OmpC level. This assertion was based on the previous characterization of a specific class of ompR mutants, referred to as ompR2 (12, 13), which displays an OmpF+ OmpC− phenotype and produces either C terminus-truncated OmpR (1) or full-length OmpR with alterations at the C terminus (26). To test this, a plasmid clone containing all but the last 75 nucleotides (i.e., the last 25 codons) of the ompR gene was created and transformed into strains expressing various ompC alleles. The presence of the ompR′ plasmid substantially reduced OmpC levels (Fig. 10B), thus confirming that the expression of C terminus-truncated OmpR from the suppressor plasmid is responsible for the reduced OmpC expression. These results also showed that the plasmid-encoded truncated OmpR mutant is dominant to the chromosomally encoded wild-type protein. Finally, since the effect of the OmpR2 mutants is exerted at the transcription level, we examined the effect of the ompR′ clone on ompC transcription by assaying β-galactosidase activities from an ompC′::lacZ+ operon fusion. Compared to the control strain containing just the vector plasmid, the presence of the ompR′ clone inhibited the β-galactosidase activity by almost 80% (Fig. 10C), thus demonstrating that the inhibitory effect of the plasmid clone on ompC is primarily exerted at the transcription level. Taken together, these data showed that the expression of the C terminus-truncated OmpR protein from the suppressor plasmid reduces ompC2Cys transcription, thus eliminating the toxic effect of OmpC2Cys in a degP-null background.
FIG. 10.
(A) A schematic diagram of the 76-min clone that suppresses OmpC2Cys-mediated lethality. The ompR and feoA genes are truncated at their 3′ and 5′ ends, respectively. (B) Western blot analysis of OmpC, OmpF, and OmpA from envelopes of strains carrying the plasmid vector or the truncated ompR clone lacking the last 25 codons of the ompR gene. The orientation of the ompR′ gene in the pTrc99A clone is opposite to that of the pTrc99A promoter, thus ompR′ was expressed from its indigenous promoter. OMPs were detected by Western blot analyses using OmpC polyclonal antibodies that also recognize OmpF and OmpA. (C) Effect of the truncated ompR clone on ompC transcription relative to the pTrc99A vector. OmpC expression was measured by assaying β-galactosidase activities of an ompC′::lacZ+ operon fusion construct.
DISCUSSION
The search for multicopy suppressors of the assembly-defective and lethal OmpC2Cys mutant protein yielded two plasmid clones that rescued lethality by reducing OmpC expression. One plasmid produced a C terminus-truncated OmpR protein, which exerted its effect by lowering ompC transcription, resulting in an OmpF+ OmpC− phenotype typical of previously characterized OmpR2 mutants (1, 12, 13, 26). The other plasmid clone also reduced OmpC expression but exerted its effect mainly at the posttranscription level. Further analysis of this latter plasmid led to the discovery of a regulatory strategy, possibly employed by certain temperate phages, by which OmpC expression is down-regulated concomitant to the synthesis of a phage-encoded porin.
Over 30 years ago, Schnaitman's lab reported that the lysogenization of E. coli strains by a lambdoid phage, PA-2, resulted in the production of a phage-encoded membrane protein called Lc and the inhibition of synthesis of OmpC, which had been shown to serve as the cell surface receptor for PA-2 (28). Subsequently, it was determined that Lc is highly homologous to the classical porins, OmpC and OmpF (2). Although the exact reason for the Lc expression-mediated inhibition of OmpC was unknown, it was speculated that reduced levels of OmpC on the cell surface either render cells ineffective in neutralizing the phage progeny generated when the lysogen is induced or prevent superinfection of lysogens (2, 28).
The data presented in this work showed that transcription from a 247-bp-long clone containing the 3′ end of the phage-encoded porin nmpC′ gene produced the inhibitory effect on OmpC expression. The E. coli K-12 strains used in our laboratory are not PA-2 lysogens but contain a remnant of the cryptic lambdoid phage, qsr′ (DLP12), genome incorporated at the 12-min region of the chromosome (14). The qsr′ phage genome contains a segment encompassing the NmpC porin gene that is identical to the PA-2 phage genome (14). The NmpC porin from the qsr′ phage is not expressed due to the presence of an insertion element (IS5) separating the last 19 codons of nmpC from the rest of its reading frame (2). We have determined that the 247-bp DNA located at the 3′ end of the nmpC gene contains an inhibitor of porin expression, ipeX, which is transcriptionally inactive in our E. coli strains. Expression of ipeX from the original suppressor plasmid occurred presumably from the vector plasmid promoter; ipeX from subsequent smaller clones was expressed exclusively from an IPTG-inducible pTrc99A promoter. Thus, ipeX was discovered serendipitously through our efforts to seek multicopy suppressors of the toxic OmpC2Cys protein.
The 247-bp qsr′ phage sequence encompassing ipeX is identical to the DNA sequence present downstream from the PA-2 phage's lc gene. Therefore, it is reasonable to assume that in PA-2 lysogens, transcription of the lc gene from its promoter also transcribes the immediately downstream region corresponding to ipeX, which in turn inhibits ompC expression. The first stem-loop of ipeX commences only 16 nucleotides downstream from the translation stop codon of the lc and nmpC genes and all four ipeX stem loops are sandwiched between the stop codon and the predicted Rho-independent transcription terminator (referred to as SL-5) of these genes (Fig. 2A). We suspect that the location of the ipeX stem-loops at the end of the lc/nmpC transcript ensures that the expression of the host ompC porin gene is inhibited only when the phage porin gene is completely transcribed in lysogens.
Data presented here suggest that the ipeX sequence does not code for a protein; rather, it is transcribed only into a RNA molecule. Since ipeX and the targeted porin genes do not share significant sequence complementarities, it is likely that the ipeX-mediated regulation is different from that mediated by micC and micF. There is a region of short complementarity between the loop 2 sequence of ipeX and the SD sequence of ompC and ompF, but this region is not critical for overall inhibition or specificity. This is because the inhibitory effect of ipeX on ompC, with five nucleotides complementary to its SD region, is significantly greater than that on ompF, in which all 6 nucleotides of loop 2 are complementary to the ompF SD region. Moreover, elimination of complementary nucleotides between the loop 2 of ipeX and ompC SD region only slightly weakened the mutant ipeX's ability to inhibit ompC expression. Thus, unlike with micC and micF, extended base pairing between ipeX and the porin RNA molecule does not appear to be involved in, or critical for, inhibition.
It is interesting that whereas the inhibitory actions of ipeX and micF on OmpF and micC on OmpC are dependent on an RNA chaperone, Hfq, the ipeX-mediated inhibition of ompC is independent of Hfq. The reason for this anomaly is unclear but may possibly reflect different mechanisms or strengths of RNA-RNA interactions. A reduced half-life of ompC mRNA by ipeX fits well with the ompC-lacZ fusion data and together they show that the inhibitory action of ipeX on ompC expression is largely posttranscriptional. Our data also narrowed down where ipeX acts to the 248-bp promoter and UTR of ompC. However, since there is no obvious base pairing involved between ipeX and ompC UTRs, we are unable to pinpoint a precise site of action. Additional work is needed to find this and the underlying molecular mechanism by which ipeX inhibits porin gene expression.
We find it interesting that ipeX, micC, and micF sequences are located adjacent to a porin gene. Close proximity is presumably important in the coordinated regulation of porin genes. A fascinating aspect of the ipeX location is that unlike micC and micF, which are synthesized as independent units from their own promoters, we have shown that ipeX is synthesized from the phage's porin gene promoter as part of the phage porin transcript at the 3′ terminus. Our experiments also demonstrated that the inhibitory action of ipeX does not require that it be physically attached to the porin gene transcript. Thus, it is possible that the ipeX portion of the lc-ipeX transcript is cleaved after synthesis. We hypothesize that the coupling of the porin gene and ipeX to the same transcript ensures that the ipeX-mediated inhibition of ompC expression occurs only when the phage-encoded porin gene is expressed in lysogens.
Acknowledgments
We thank Leanne Misra for critically reading the manuscript and N. Ruiz and Joe Fralick for providing bacterial strains.
This work was supported by a grant from the National Institute of General Medical Sciences (R01 GM48167).
REFERENCES
- 1.Berman, M. E., and D. E. Jackson. 1984. Selection of lac gene fusions in vivo: ompR-lacZ fusions that define a functional domain of the ompR gene product. J. Bacteriol. 159:750-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasband, A. J., W. R. Marcotte, Jr., and C. A. Schnaitman. 1986. Structure of the lc and nmpC outer membrane protein genes of lambdoid bacteriophages. J. Biol. Chem. 261:12723-12732. [PubMed] [Google Scholar]
- 3.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 4.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 141:541-555. [DOI] [PubMed] [Google Scholar]
- 5.Castillo-Keller, M., and R. Misra. 2003. Protease-deficient DegP suppresses lethal effects of a mutant OmpC protein by its capture. J. Bacteriol. 185:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S., A. Zhang, L. B. Lawrence, and G. Storz. 2004. MicC, a second small-RNA regulator of Omp protein expression in Escherichia coli. J. Bacteriol. 186:6689-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowen, S. W., T. Schirmer, G. Rummel, M. Steiert, R. Ghose, R. A. Pauptit, J. N. Jansonius, and J. P. Rosenbusch. 1992. Crystal structures explain functional properties of two E. coli porins. Nature 358:727-733. [DOI] [PubMed] [Google Scholar]
- 9.Danese, P. N., and T. J. Silhavy. 1999. Targeting and assembly of periplasmic and outer membrane proteins in Escherichia coli. Annu. Rev. Genet. 32:59-94. [DOI] [PubMed] [Google Scholar]
- 10.Gudapaty, S., K. Suzuki, X. Wang, P. Babitzke, and T. Romeo. 2001. Regulatory interactions of Csr components: the RNA binding protein CsrA activates csrB transcription in Escherichia coli. J. Bacteriol. 183:6017-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall, M. N., and T. J. Silhavy. 1981. The ompB locus and the regulation of the major outer membrane protein of Escherichia coli K-12. J. Mol. Biol. 146:23-43. [DOI] [PubMed] [Google Scholar]
- 13.Hall, M. N., and T. J. Silhavy. 1981. Genetic analysis of the ompB locus of Escherichia coli K-12. J. Mol. Biol. 151:1-15. [DOI] [PubMed] [Google Scholar]
- 14.Highton, P. J., Y. Chang, W. R. Marcotte, Jr., and C. A. Schnaitman. 1985. Evidence that the outer membrane protein gene nmpC of Escherichia coli K-12 lies within the defective prophage. J. Bacteriol. 162:256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krojer, T., M. Garrido-Franco, R. Huber, M. Ehrmann, and T. Clausen. 2002. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature 416:455-459. [DOI] [PubMed] [Google Scholar]
- 16.Miller, J. H. 1971. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Misra, R. 1993. A novel ompC mutation of Escherichia coli K-12 that reduces OmpC and OmpF levels in the outer membrane. Mol. Microbiol. 10:1029-1035. [DOI] [PubMed] [Google Scholar]
- 18.Misra, R. 1993. OmpF assembly mutants of Escherichia coli K-12: isolation, characterization, and suppressor analysis. J. Bacteriol. 175:5049-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra, R., and S. A. Benson. 1988. Genetic identification of the pore domain of the OmpC porin of Escherichia coli K-12. J. Bacteriol. 170:3611-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra, R., M. Castillo-Keller, and M. Deng. 2000. Overexpression of protease deficient DegPS210A rescues the lethal phenotype of Escherichia coli OmpF assembly mutants in a degP background. J. Bacteriol. 182:4882-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra, R., A. Peterson, T. Ferenci, and T. J. Silhavy. 1991. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli. J. Biol. Chem. 266:13592-13597. [PubMed] [Google Scholar]
- 22.Misra, R., and P. R. Reeves. 1987. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J. Bacteriol. 169:4722-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuno, T., M. Y. Chou, and M. Inouye. 1984. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc. Natl. Sci. Acad. USA 81:1966-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moll, I., D. Leitsch, T. Steinhauser, and U. Blasi. 2003. RNA chaperone activity of the sm-like Hfq protein. EMBO Rep. 4:284-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morona, R., and P. Reeves. 1982. The tolC locus of Escherichia coli affects the expression of three major outer membrane proteins. J. Bacteriol. 150:1016-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nara, F. S. Matsuyama, T. Mizuno, and S. Mizushima. 1986. Molecular analysis of mutant ompR genes exhibiting different phenotypes as to osmoregulation of the ompF and ompC genes of Escherichia coli. Mol. Gen. Genet. 202:194-199. [DOI] [PubMed] [Google Scholar]
- 27.Nikaido, H. 1994. Porins and specific diffusion channels in bacterial membranes. J. Biol. Chem. 269:3905-3908. [PubMed] [Google Scholar]
- 28.Schnaitman, C. A., D. Smith, and M. F. de Salas. 1975. Temperate bacteriophage which causes the production of a new major outer membrane protein by Escherichia coli. J. Virol. 15:1121-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Spiess, C., A. Bell, and M. Ehrmann. 1999. A temperature-dependent switch from chaperones to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 31.Storz, G., J. A. Opdyke, and A. Zhang. 2004. Controlling mRNA stability and translation with small, noncoding RNAs. Curr. Opin. Microbiol. 7:140-144. [DOI] [PubMed] [Google Scholar]
- 32.Tsui, H. C., H. C. Leung, and M. E. Winkler. 1994. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol. Microbiol. 13:35-49. [DOI] [PubMed] [Google Scholar]
- 33.Vakharia, H., and R. Misra. 1996. A genetic approach for analyzing surface-exposed regions of the OmpC protein of Escherichia coli K-12. Mol. Microbiol. 19:881-889. [DOI] [PubMed] [Google Scholar]
- 34.Werner, J., and R. Misra. 2005. YaeT (Omp85) affects the assembly of lipid-dependent and lipid-independent outer membrane proteins of Escherichia coli. Mol. Microbiol. 57:1450-1459. [DOI] [PubMed]
- 35.Wu, T., J. Malinverni, N., Ruiz, S. Kim, T. J. Silhavy, and D. Kahne. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235-245. [DOI] [PubMed] [Google Scholar]
- 36.Xiong, X., J. N. Deeter, and R. Misra. 1996. Assembly-defective OmpC mutants of Escherichia coli K-12. J. Bacteriol. 178:1213-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, A., K. M. Wassarman, C. Rosenow, B. C. Tjaden, G. Storz, and S. Gottesman. 2003. Global analysis of small, R. N. A., and mRNA targets of Hfq. Mol. Microbiol. 50:1111-1124. [DOI] [PubMed] [Google Scholar]