Abstract
The RctB protein binds to the origin of replication of Vibrio cholerae chromosome II (chrII) and is required for oriCIIVc-based replication. Here, we found that RctB acts as an autorepressor, inhibiting rctB transcription. Integration host factor promotes rctB transcription, while Dam and DnaA, factors required for replication of both V. cholerae chromosomes, influence RctB autorepression. Thus, RctB appears to regulate chrII replication as both an initiator and a transcription repressor, and its synthesis is modulated by factors that govern replication of both chromosomes.
The genome of Vibrio cholerae, the causative agent of the severe diarrheal disease cholera, is divided unequally between two circular chromosomes. Most if not all of the many species that constitute the family Vibrionaceae have similarly divided genomes (12, 17-19). As more bacterial genomes have been investigated, it has become clear that multipartite genomes are not uncommon and are found among diverse prokaryotic phyla (2). Almost all studies of bacterial chromosome replication and segregation have utilized organisms with a single chromosome, yet the models derived from these studies may not fully apply to bacteria with multipartite genomes (2).
We previously constructed minichromosomes to identify the minimal replicons of the two V. cholerae chromosomes (4). We found that oriCIVc, the origin of replication of the larger chromosome, chromosome I (chrI), is similar in sequence to oriC, the well-characterized origin of replication of the Escherichia coli chromosome. Like oriC, oriCIVc includes five DnaA boxes (binding sites for the DnaA initiator protein), a putative binding site for integration host factor (IHF, a histone-like protein that bends DNA), several sites for methylation by DNA adenine methyltransferase (Dam, which is involved in regulating timing of replication in E. coli), and an AT-rich region (where strand opening is believed to originate).
The origin of replication of chrII, oriCIIVc, does not resemble oriCIVc in terms of sequence identity (4). However, it contains some features common to many bacterial origins of replication, such as one DnaA box, a binding site for IHF, several sites for Dam methylation, and an AT-rich region. In addition to these features, oriCIIVc contains a 12-base-pair repeat that is required for oriCIIVc-based replication. While the intergenic region containing oriCIVc can replicate autonomously in E. coli, oriCIIVc-based replication requires two novel V. cholerae genes which flank oriCIIVc (Fig. 1). One of these genes, rctB, encodes an origin-binding protein and is conserved among diverse genera of the family Vibrionaceae. The other gene, rctA, codes for an untranslated RNA and not a protein.
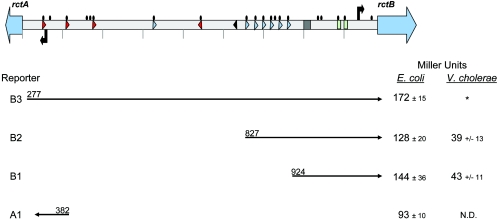
FIG. 1.
Reduced β-galactosidase activity of rctB::lacZ reporters in V. cholerae versus E. coli. (Top) Schematic representation of the ig2 region of V. cholerae chrII with the following features: the DnaA box is represented as a black triangle, the IHF site as a gray box, AT-rich regions as green boxes, Dam sites (GATC sequences) as black ovals, 12-mer repeats as blue triangles, and 11-mer repeats as red triangles. rctB and rctA are represented by the blue block arrows. The site of transcription initiation (+1) for rctB is represented by the black arrow upstream of rctB, and the presumed start site of transcription for rctA (based on the location of −10 and −35 sites) is represented by the black arrow upstream of rctA. Hatch marks reflect 100-bp intervals. (Bottom) β-Galactosidase activities (Miller units) in E. coli MC1061 and V. cholerae 2740-80 lacZ. The first three reporters are ig2 fragments B1 (bp 924 to 1265 according to TIGR annotation), B2 (bp 827 to 1265), and B3 (bp 277 to 1265) subcloned into the lacZ reporter vector pCB182N. pCB182N is a derivative of pCB182 (16) that contains an rRNA transcription terminator in the SmaI site. Reporter A1 contains a fragment from the left side of ig2 (bp 382 to 283) upstream of lacZ in pCB182. The asterisk indicates that we were unable to obtain a V. cholerae strain harboring reporter B3, and N.D. means not done. Each assay was conducted at least three times, and the means and standard deviations are presented.
The distinct replication requirements for the two V. cholerae chromosomes suggest that initiation of chrII replication may, at least in part, be controlled independently from chrI. However, our studies of the kinetics of V. cholerae chromosome replication revealed that the two chromosomes initiate replication in a coordinated, synchronous manner (3). We found that oriCIVc- and oriCIIVc-based replication share requirements for certain factors that have been implicated in the regulation of bacterial chromosome replication in other organisms, such as DnaA and Dam methyltransferase (4). These shared replication factors may help mediate coordinated replication of the chromosomes. However, our understanding of the interplay of the various V. cholerae replication factors and how they act to coordinate chromosome replication is limited.
Since the V. cholerae RctB protein binds specifically to oriCIIVc and is required only for oriCIIVc-based replication, RctB appears to be a chrII-specific replication initiator. Here, we identified the rctB promoter and investigated its regulation. We found that RctB is autoregulatory and represses its own transcription. IHF enhances rctB transcription, and Dam and DnaA influence RctB-mediated repression of rctB transcription. In addition to its own promoter, RctB also regulates the promoter for rctA.
To identify the rctB transcriptional start site, we isolated RNA from V. cholerae strain N16961 and used 5′ rapid amplification of cDNA ends (Invitrogen). This analysis revealed a single putative transcriptional start site 40 bp upstream of the rctB start codon (Fig. 1; see also Fig. S1 in the supplemental material). A 209-bp fragment spanning this site could drive transcription from a promoterless lacZ gene (see below), suggesting that this region harbors the rctB promoter, PrctB. BPROM, a computer program designed to identify sigma 70-dependent promoters for bacterial genes (SoftBerry, Mount Kisco, NY), predicted −10 and −35 sites upstream of the rctB transcriptional start site (see Fig. S1 in the supplemental material). Experimental verification that the predicted −10 and −35 sequences are required for activity of PrctB has recently been provided by another lab (14).
We explored the influence of sequences upstream of PrctB on rctB expression by studying the activity of PrctB using a set of rctB transcriptional reporter plasmids. DNA fragments of various lengths from the intergenic region (ig2) upstream of the rctB open reading frame were introduced upstream of a promoterless lacZ gene. The smallest fragment, found in the B1 reporter, started at base pair 924 (numbers are according to the TIGR annotation of the N16961 genome) and included 209 bp upstream of the rctB start codon; the largest fragment, in the B3 reporter, included the entire ig2 intergenic region between rctB and the divergently transcribed rctA. Each of these reporters exhibited significant β-galactosidase activity in E. coli, indicating that there are no V. cholerae-specific factors required for PrctB function. Also, the amounts of β-galactosidase activity of all three reporters were very similar (Fig. 1), suggesting that there is only one rightward-facing promoter within ig2 and that there are no key cis-acting sites in ig2 upstream of base pair 924 that control PrctB expression. In V. cholerae, the level of β-galactosidase activity generated by these reporters was ∼3-fold lower than in E. coli, suggesting that there is a V. cholerae-specific factor(s) that represses PrctB activity (Fig. 1). As in E. coli, the activities of the B1 and B2 reporters in V. cholerae were similar, providing further support for the idea that sequences upstream of base pair 924 do not control PrctB expression. The B3 reporter contains inc sequences that negatively regulate chrII replication (4), and as expected, we were unable to introduce this reporter into V. cholerae.
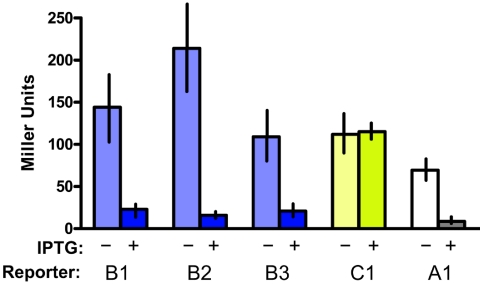
We speculated that RctB autorepression might account for the reduced activity of PrctB in V. cholerae compared to E. coli for two reasons. First, we previously showed that RctB binds to oriCIIVc in the region now known to contain PrctB (4), and second, initiator proteins are commonly autoregulated (1, 10). This hypothesis proved correct; the expression of all three PrctB reporters was repressed by RctB more than fivefold when RctB was overproduced from a plasmid in E. coli (Fig. 2). RctB binds to multiple sites in ig2 (4); however, there was no obvious correlation between the degree of RctB-mediated repression of the three rctB reporters and the number of RctB binding sites in the individual reporters, suggesting that potential interactions between different RctB molecules bound throughout ig2 do not influence PrctB activity. As in E. coli, when rctB was overexpressed in V. cholerae, PrctB activity was also reduced (data not shown). Overexpression of RctB did not repress the activity of a control reporter, indicating that RctB repression of PrctB is specific (Fig. 2). Thus, like both DnaA and Rep proteins of iteron plasmids, RctB appears to be an autoregulatory initiator protein that represses its own synthesis.
FIG. 2.
RctB repression of rctB and rctA transcription. RctB was produced from pRctB-119, which is a derivative of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible expression vector pGZ119 (6). C1, a grpE::lacZ fusion in pCB182N, served as a control. The Miller units presented are the means of at least three independent experiments, and vertical lines represent standard deviations.
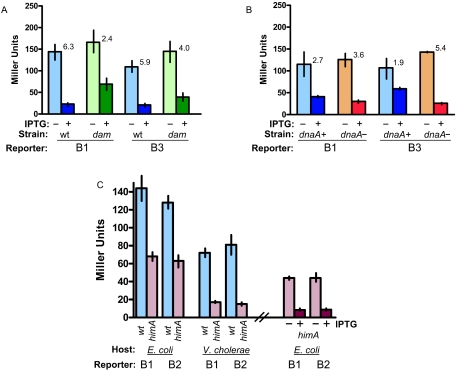
To study whether rctB transcription is influenced by other factors that are required for chrII replication, such as Dam methyltransferase and DnaA, we measured PrctB activity from the B1 and B3 rctB reporters in E. coli strains harboring mutations in dam or dnaA. Dam methylation is known to regulate gene expression (7), and there are several potential sites for methylation by Dam methyltransferase in the vicinity of PrctB (Fig. 1). The β-galactosidase activities of the two rctB reporters were similar in wild-type and dam E. coli strains, indicating that Dam methylation does not significantly influence PrctB activity (Fig. 3A). However, Dam methylation appears to enhance RctB autorepression. In dam E. coli, overexpression of rctB resulted in somewhat less repression of the rctB reporters than was observed in wild-type E. coli; this effect was strongest for reporter B1 (Fig. 3A). These findings may suggest that methylation increases the affinity of RctB for its promoter.
FIG. 3.
Host factors influence the activity and regulation of PrctB. In all cases, RctB was produced from the IPTG-inducible expression plasmid pRctB-119, which is a derivative of pGZ119 (6). (A) The wild-type strain was E. coli MC1061, and the dam strain was E. coli KO1607 (MC1061 dam13::Tn9, gift of A. Wright). (B) The wild-type strain was E. coli EH 3896, and the isogenic dnaA-independent strain was EH 3894 (CM1565 zig::pKN500 ΔdnaA mad-1 tnaA::Tn10 [5]). (C) The wild-type strain was E. coli MC1061, and the himA strain was an isogenic derivative of MC1061 with a transposon-linked C-terminal deletion of himA. This deletion originated from strain RJ3138 (gift of R. Johnson). The V. cholerae wild-type strain was 2740-80, and V. cholerae himA was an in-frame deletion of himA (gift of S. McLeod). All assays were done at least three times, and the means and standard errors of the mean are presented. The repression (n-fold) in the presence of overproduced RctB is shown for each reporter.
DnaA is known to act as a transcription regulator as well as an initiator of DNA replication (11). Since PrctB is close to the single DnaA box in oriCIIVc (Fig. 1), we tested whether DnaA influences PrctB activity by comparing the levels of lacZ expression from the B3 and B1 rctB reporters, one of which (B3) includes the DnaA box and the other of which does not (Fig. 1). The β-galactosidase activities of these two rctB reporters were similar (Fig. 1), suggesting that DnaA binding to ig2 does not influence PrctB promoter activity. Consistent with this idea, we found that the β-galactosidase activities of these two reporters were similar in a dnaA deletion strain (with an integrated R1 ori [5]) and isogenic wild-type E. coli (Fig. 3B). However, the degrees of RctB autorepression were different in these strains (Fig. 3B). Although in both backgrounds RctB repressed its own synthesis, in the absence of DnaA, RctB-mediated repression was enhanced 2.8-fold for reporter B3 and less so for B1. This may suggest that DnaA antagonizes RctB binding to PrctB.
The nucleoid-associated factor IHF is a heterodimer consisting of HimA and HimB and is known to modulate transcription of some bacterial genes (9). We investigated whether IHF influences PrctB activity. oriCIIVc contains a DNA sequence that is similar to the E. coli IHF consensus binding site (4), and we have found that the E. coli IHF protein binds to the region of oriCIIVc containing this site (our unpublished observations). IHF appears to activate transcription from PrctB, as transcription from PrctB was reduced ∼2-fold in an E. coli himA mutant strain compared with wild-type E. coli (Fig. 3C). The similar reductions in the β-galactosidase activities of the B1 and B2 reporters in himA versus wild-type E. coli suggest that the activating effect of IHF requires only, at the most, the sequence present in the small B1 reporter. The activity of a control reporter was not reduced in himA E. coli, indicating that the effect of the himA deletion is not due to a global defect in transcription levels. In V. cholerae, IHF activation of transcription from PrctB was even more pronounced, as the β-galactosidase activities of the B1 and B2 reporters were four to five times lower in himA than in wild-type V. cholerae. These observations suggest that IHF contributes to efficient PrctB activity, as is the case for several other promoters where IHF promotes architectural changes in the DNA. Alternatively, IHF could also activate transcription by directly contacting RNA polymerase (9). IHF does not appear to influence the binding of RctB to PrctB, since the reporters were repressed to similar extents by RctB in himA and wild-type E. coli (Fig. 3C).
Since RctB binds to the left side of ig2 just upstream of rctA (4), we tested whether RctB regulates rctA transcription. Using BPROM, we identified a putative rctA promoter (PrctA) approximately 80 bp upstream of the annotated gene (Fig. 1). This region of DNA can drive lacZ transcription, since a PrctA::lacZ reporter (A1) yielded ∼93 Miller units of β-galactosidase activity in E. coli (Fig. 1). Introduction of a 3-bp substitution mutation into the putative −10 sequence of PrctA in A1 decreased β-galactosidase activity ∼10-fold, indicating that PrctA is indeed a true promoter. RctB overproduction reduced the β-galactosidase activity from A1 more than eightfold, indicating that PrctA is repressed by RctB (Fig. 2). Thus, RctB represses at least two genes, rctA and rctB, required for chrII replication.
Our findings reveal that RctB is a multifunctional protein. Previously, we found that RctB is required for oriCIIVc-based replication and that RctB binds to several sites in ig2, suggesting that RctB functions as a chrII-specific replication initiator. This idea is supported by the recent observation that RctB levels determine the copy number of an oriCIIVc-based minichromosome in E. coli (14). Besides acting as a chrII-specific initiator, RctB also functions as a transcriptional repressor, inhibiting transcription from PrctB and from PrctA. Thus, RctB controls oriCIIVc-based replication on at least three levels: as an initiator of replication, as an autorepressor, and as a repressor of rctA.
RctB binds in the vicinity of PrctB, and therefore it is likely that RctB directly represses its own promoter. RctB autorepression, which may be a critical determinant of RctB levels and chrII replication, appears to be influenced by DnaA and Dam, two host factors that are essential for replication of both V. cholerae chromosomes (4). Dam modestly potentiates RctB autorepression, probably by altering RctB binding to sites that influence PrctB activity. DnaA modestly inhibits RctB autorepression, perhaps by interacting with RctB and inhibiting its binding to PrctB. Interactions between DnaA and plasmid Rep proteins have been described previously (8). Thus, there appears to be a complex interplay of factors essential for chrI and chrII replication that, along with RctB, govern rctB transcription. Understanding the molecular details of RctB-DNA and RctB-protein interactions will enhance our understanding of how the two chromosomes initiate replication in synchrony.
IHF promotes transcription from PrctB but does not influence RctB binding to this region. The putative IHF binding site is 129 bp from the start site of rctB transcription, and the region upstream of the IHF binding site is relatively AT rich. This arrangement of an AT-rich region and IHF site upstream of a promoter is similar to that of the ilvPG promoter in E. coli, which is also activated by IHF (13). IHF activates ilvPG by translocating superhelical energy; binding to its cognate site prevents destabilization of the upstream AT-rich region while promoting duplex destabilization of the −10 region of the promoter (15). Destabilization is correlated with open complex formation as well as increased transcriptional activity of ilvPG. It is tempting to speculate that IHF activates PrctB by a similar mechanism.
Supplementary Material
Acknowledgments
We are grateful to Sarah McLeod for E. coli and V. cholerae himA strains, helpful discussions, and critical reading of the manuscript; to Dhruba Chattoraj for sharing unpublished data and for strains EH 3896 and EH 3894; to Harvey Kimsey for plasmid pCB182N; to Reid C. Johnson for E. coli himA strain RJ3138; and to Andrew Wright for the dam strain KO1607.
This work was supported by grants from NIH, HHMI, INRA, and the NEMC GRASP Center.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.del Solar, G., R. Giraldo, M. J. Ruiz-Echevarria, M. Espinosa, and R. Diaz-Orejas. 1998. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 62:434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan, E. S., M. A. Fogel, and M. K. Waldor. 2005. Divided genomes: negotiating the cell cycle in prokaryotes with multiple chromosomes. Mol. Microbiol. 56:1129-1138. [DOI] [PubMed] [Google Scholar]
- 3.Egan, E. S., A. Lobner-Olesen, and M. K. Waldor. 2004. Synchronous replication initiation of the two Vibrio cholerae chromosomes. Curr. Biol. 14:R501-R502. [DOI] [PubMed] [Google Scholar]
- 4.Egan, E. S., and M. K. Waldor. 2003. Distinct replication requirements for the two Vibrio cholerae chromosomes. Cell 114:521-530. [DOI] [PubMed] [Google Scholar]
- 5.Hansen, E. B., and M. B. Yarmolinsky. 1986. Host participation in plasmid maintenance: dependence upon dnaA of replicons derived from P1 and F. Proc. Natl. Acad. Sci. USA 83:4423-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessl, M., D. Balzer, R. Lurz, V. L. Waters, D. G. Guiney, and E. Lanka. 1992. Dissection of IncP conjugative plasmid transfer: definition of the transfer region Tra2 by mobilization of the Tra1 region in trans. J. Bacteriol. 174:2493-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobner-Olesen, A., O. Skovgaard, and M. G. Marinus. 2005. Dam methylation: coordinating cellular processes. Curr. Opin. Microbiol. 8:154-160. [DOI] [PubMed] [Google Scholar]
- 8.Lu, Y. B., H. J. Datta, and D. Bastia. 1998. Mechanistic studies of initiator-initiator interaction and replication initiation. EMBO J. 17:5192-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLeod, S. M., and R. C. Johnson. 2001. Control of transcription by nucleoid proteins. Curr. Opin. Microbiol. 4:152-159. [DOI] [PubMed] [Google Scholar]
- 10.Messer, W. 2002. The bacterial replication initiator DnaA. DnaA and oriC, the bacterial mode to initiate DNA replication. FEMS Microbiol. Rev. 26:355-374. [DOI] [PubMed] [Google Scholar]
- 11.Messer, W., and C. Weigel. 1997. DnaA initiator—also a transcription factor. Mol. Microbiol. 24:1-6. [DOI] [PubMed] [Google Scholar]
- 12.Okada, K., T. Iida, K. Kita-Tsukamoto, and T. Honda. 2005. Vibrios commonly possess two chromosomes. J. Bacteriol. 187:752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagel, J. M., and G. W. Hatfield. 1991. Integration host factor-mediated expression of the ilvGMEDA operon of Escherichia coli. J. Biol. Chem. 266:1985-1996. [PubMed] [Google Scholar]
- 14.Pal, D., T. Venkova-Canova, P. Srivasta, and D. K. Chattoraj. 2005. Multipartite regulation of rctB, the replication initiator gene of Vibrio cholerae chromosome II. J. Bacteriol. 187:7167-7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parekh, B. S., and G. W. Hatfield. 1996. Transcriptional activation by protein-induced DNA bending: evidence for a DNA structural transmission model. Proc. Natl. Acad. Sci. USA 93:1173-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider, K., and C. F. Beck. 1986. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene 42:37-48. [DOI] [PubMed] [Google Scholar]
- 17.Thompson, F. L., T. Iida, and J. Swings. 2004. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 68:403-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trucksis, M., J. Michalski, Y. K. Deng, and J. B. Kaper. 1998. The Vibrio cholerae genome contains two unique circular chromosomes. Proc. Natl. Acad. Sci. USA 95:14464-14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaichi, Y., T. Iida, K. S. Park, K. Yamamoto, and T. Honda. 1999. Physical and genetic map of the genome of Vibrio parahaemolyticus: presence of two chromosomes in Vibrio species. Mol. Microbiol. 31:1513-1521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.