Abstract
The plasmids R1162 and pSC101 have origins of conjugative transfer (oriTs) and corresponding relaxases that are closely related. The oriTs are made up of a highly conserved core, where DNA is cleaved by the relaxase prior to transfer, and an inverted repeat that differs in size and sequence. We show that in each case the seven base pairs adjacent to the core and within one arm of the inverted repeat are sufficient to determine specificity. Within this DNA there are three AT base pairs located 4 bp from the core. Mutations in the AT base pairs suggest that the relaxase makes essential contacts at these locations to the minor groove of the DNA. The remaining four bases are different for each oriT and are both necessary and sufficient for stringent recognition of oriT by the pSC101 mobilization proteins. In contrast, the R1162 mobilization proteins have a much more relaxed requirement for the base sequence of this specificity region. As a result, the R1162 mobilization proteins can initiate transfer from a variety of sites, including those derived from the chromosome. The R1162 mobilization proteins could therefore contribute to the horizontal gene transfer of DNA from diverse sources.
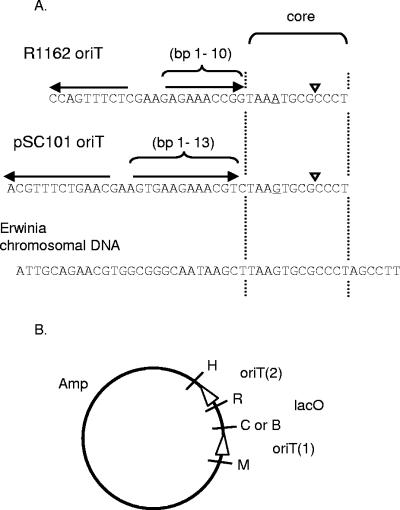
The plasmids R1162 and pSC101 have closely related systems for conjugative mobilization, although they are otherwise unrelated. These mobilization (Mob) systems belong to a large family, with examples found in plasmids from both gram-negative and gram-positive bacteria (12). Within this family, the general structures of the origins of conjugative transfer (oriTs) are similar and are characterized by a highly conserved region called the core and an adjacent, inverted repeat that varies in both size and sequence (3). These features are shown for the R1162 and pSC101 oriTs in Fig. 1A. We recently suggested that the oriTs of this family were generated by the duplication and inversion of the core and adjacent DNA on several separate occasions (3). The resulting second copy of the core was then lost by deletion, although remnants have remained in several cases, including the oriT of pSC101. The properties of the relaxases indicated that this duplication and inversion was necessary in the evolution of a system for the efficient transfer of circular DNA. The cognate relaxase binds double-stranded DNA, initiates strand separation within the core, and cleaves one of these strands at the nick site. The cleavage takes place by a transesterification that results in transient covalent linkage of the protein to the 5′ end of the DNA strand (8, 22, 23). The relaxase and the attached strand, unwound from its complement, are then transferred into a recipient cell by type IV secretion (11). Duplication and inversion of DNA next to the core generates an inverted repeat, which re-creates the binding site for the attached relaxase. This allows the subsequent reversal of the transesterification to regenerate a circular molecule of DNA. The outer arm of the inverted repeat is not required for relaxase-induced nicking of supercoiled DNA at the beginning of a round of transfer (16), in agreement with this model for the evolution of oriT.
FIG. 1.
(A) Minimal oriTs of R1162 and pSC101. The inverted repeats in each case are indicated by the horizontal arrows, and the single base difference in the core is underlined. The base pairs making up the inner arms of the R1162 and pSC101 inverted repeats and mutated in this study are numbered for identification. Below is the Erwinia carotovora chromosomal DNA cloned to test for initiation of transfer. Inverted triangles indicate the cleavage site for the plasmid relaxase. (B) General structure of plasmids used to determine the efficiency of initiation of transfer at a mutated oriT. oriT(1) contains the mutations; the direction of transfer (16) from each oriT is indicated by the arrowheads. Restriction sites are BamHI (B), ClaI (C), HindIII (H), MfeI (M), and EcoRI (R). Amp, gene encoding ampicillin resistance.
The R1162 relaxase is active on the pSC101 oriT, but the reciprocal combination, the R1162 oriT and the pSC101 relaxase, is inactive for both initiation and termination of transfer (3, 19). In fact, core DNA can be nicked by the R1162 relaxase when the adjacent DNA, corresponding to the inner arm of an oriT, has been highly mutated (3). We show here that such an oriT is functional for initiation of transfer by the R1162 Mob proteins, sometimes at frequencies similar to that of the normal oriT. In contrast, the pSC101 relaxase has a more stringent requirement for the base sequence of the inner arm, and we have mapped this requirement to the DNA just adjacent to the core. We demonstrate that the relaxed specificity of the R1162 system means that it can act in trans to enable the horizontal transfer of DNA of chromosomal origin.
MATERIALS AND METHODS
Strains and bacterial mating.
The Escherichia coli K-12 strains used for cloning were the C600 derivative MV10 (13), DH5α, and TOP10 (Invitrogen). Conjugative mating with E. coli donors and recipients was done on semisolid agar medium as described previously (9). The donor was MV10, containing the mobilizing plasmid R751 (26) and a second plasmid acting as a source of either the R1162 or the pSC101 Mob proteins. The recipient was C600 NalR. Transconjugants were selected on medium containing Turboamp (100 μg/ml; Stratagene), 25 μg/ml nalidixic acid, and 0.8% X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Donors were enumerated on medium containing ampicillin (100 μg/ml). For matings with donor strains containing pUT1883 or pUT1884, the procedure was scaled up: 0.1 to 1.0 ml of a mid-log-phase culture of donor cells and 1.0 ml recipient cells were drawn onto a 0.22-μm filter by gentle vacuum and deposited cell-side up on a broth plate. After incubation for about 2 h, the cells were resuspended by vortexing and plated for donors and transconjugants.
Plasmids. (i) Plasmids used for the oriT libraries.
The plasmid pUT1616 is a pBR322 derivative containing lacO (see Fig. 4 in reference 2). The R1162 Mob proteins were provided by pUT1309, which is R1162 ΔoriT (20), or pUT1841, which consists of the R1162 Mob genes cloned into the vector pACYC184 (10) between the HindIII and BamHI sites. The R1162 DNA in the plasmid consists of a ScaI-EcoRV fragment containing the oriT deletion from pUT1309. The plasmid pUT1705 was used as a source of the pSC101 Mob proteins. This plasmid consists of a pSC101 DNA fragment (bp 2269 to 4069 [6]) that includes the mob genes but lacks oriT. The fragment was cloned between the EcoRV and BamHI sites of pACYC184.
FIG. 4.
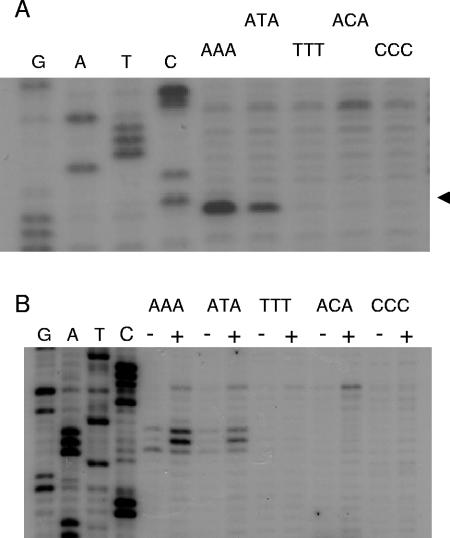
(A) Nicking at R1162 oriTs (core plus inner arm) mutated within the conserved sequence AAA in the inner arm. The nick is shown by the arrowhead. Nicking was assayed by primer extension. The unmutated, conserved DNA is lane AAA, adjacent to the sequencing ladder. The other lanes contain conserved DNA with the sequence shown at the top of the figure. (B) Sensitivity of mutated oriT DNA to oxidation by permanganate. DNA was assayed by primer extension as in panel A, except that the complement to the nicked strand was used as the template. Plasmid DNAs were extracted from cleared lysates treated (+) or untreated (−) with permanganate.
(ii) Plasmids for testing initiation from a highly mutated R1162 oriT.
The plasmids have the general structure shown in Fig. 1B and are derived from pUT1616. There are unique ClaI/MfeI and HindIII/EcoRI cloning sites for inserting an oriT at positions 1 and 2, respectively. The R1162 oriT at position 2 is within an 81-bp, HindIII-EcoRI fragment (18). Selected, tailed oriTs from the libraries, cloned into pUC19, were amplified with the primers TACGCCAAGCTTATCGATCC and CAGAATTCCCCCCCCAGGGCGCACTTA. An effect of the amplification is to restore the nick site in the oriT. The PCR product was digested with ClaI and EcoRI and cloned between the ClaI and MfeI sites.
(iii) Plasmids containing mutations in AAA within the inner arm of the R1162 oriT.
A set of R1162 derivatives containing mutations at positions 4, 5, and 6 in the inner arm were constructed using complementary oligonucleotides degenerate in sequence at these positions. Mutations were introduced into R1162 by the PCR method of Horton et al. (14), and the base changes for each recombinant plasmid were determined by sequencing. DNA containing the oriT inner arm and core was then cloned to test for initiation of transfer. The oriT DNA was amplified by PCR with the primers CGTTAGGCCAGTTCTTCGAAG and CCGAATTCAGGGCGCATTTACCG. The product was digested with BstBI and EcoRI and cloned between the ClaI and MfeI sites at position 1 of the test plasmid (Fig. 1B).
(iv) Plasmids containing mutations in the inner arm of the pSC101 oriT.
We used a plasmid similar to pUT1705 but containing an intact oriT. Mutations in the specificity region (CGTC) in the inner arm were introduced by two-step PCR (14). We also constructed hybrid oriTs, consisting of inner arm and core with elements from both R1162 and pSC101, and tested these for initiation of transfer by using plasmids with the structure shown in Fig. 1B. In these plasmids, position 2 contained pSC101 oriT DNA (bp 4070 to 4129 [6]). The oligonucleotide GCCCCAAGCTTATGGATCCGAAGAGAAACGTCTAAGTGCGCCCTCCCTTTTGGCAATTGGGCCC and the same oligonucleotide except with a T at position 27 were amplified with the primers GCCCAAGCTTATGGATC and GGGCCCAATTGGGCCC, and the product was cloned between the BamHI and MfeI sites at position 1, as shown in Fig. 1B. A plasmid essentially similar but containing the normal inner arm plus core of pSC101 at position 1 was also constructed by amplifying the analogous oligonucleotide.
(v) Plasmids containing Erwinia carotovora subsp. atroseptica DNA.
The oligonucleotide ATTGCAGAACGTGGCGGGCAATAAGCTTAAGTGCGCCCTAGCCTT, corresponding to Erwinia carotovora subsp. atroseptica chromosomal DNA (bp 1306739 to 1306766 [5]) was amplified and cloned between the BamHI and MfeI sites at position 1 in the test plasmid (Fig. 1B). Position 2 contained either the R1162 or the pSC101 oriT, on the DNA fragments described above.
Construction of oriT libraries.
Construction of a plasmid library derived from a cloned, partially degenerate oligonucleotide and enrichment of the nicked oriTs by tailing and PCR were done as outlined in Fig. 2 and as described previously (3). An oligonucleotide degenerate in sequence for the inner arm of the oriT inverted repeat was amplified and cloned by replacement of a HindIII-MfeIlacO fragment in pUT1616 (3). For libraries containing oriTs composed of the pSC101 core and a 10-base degenerate inner arm, the oligonucleotide GCCCAAGCTTATGGATCC(N)10TAAGTGCGCCCTCCCTTTTGGCAA TTGGGCCC was amplified with the primers GGGCCCAATTGCCAAAAG and GCCCAAGCTTATGGATC. The DNA was then used to transform MV10 containing either pUT1309 or pUT1705, which provide the R1162 or pSC101 Mob proteins, respectively. Plasmid DNA was isolated by the cleared-lysate method (15) and tailed at the oriT nick site with terminal transferase as described previously (3). The tailed product was then amplified by PCR, with the primers CCCGAATTCCCCCCCCCCCGCA and GGGAATAAGGGCGACACGGAAATGTTG, and the resulting product was cloned at the HindIII and EcoRI sites of pUC19 (27). As shown in Fig. 2, step 4, the primer hybridizing to the tail was also complementary to the 3′ end of cleaved oriT, so that molecules tailed at positions resulting from random cleavage of DNA were not amplified. In addition, although treatment with terminal transferase results in tails of different lengths, the PCR product was uniform in size, determined by the primer. The cloned DNA was then sequenced and further analyzed. As a control experiment, plasmid DNA from the cleared lysate was sequenced directly. There were equal amounts of each base at each position within the degenerate region, confirming that the original degenerate oligonucleotide had been correctly doped (25% each base) and also that the library was large, without overrepresentation of particular sequences.
FIG. 2.
Outline of method used to obtain population of highly mutated, cleaved oriTs. The degenerate inner arm is indicated by the large horizontal arrow.
Plasmid libraries containing oligonucleotides degenerate in sequence for one half of the inner arm of the inverted repeat (see Fig. 5) were constructed using the oligonucleotides GCCCCAAGCTTATGGATCC(N)7AACGTCTAAGTGCGCCCTCCCTTTTGGCAATTGGGCCC and GCCCCAAGCTTATGGATCCGTGAAGA(N)6TAAGTGCGCCCTCCCTTTTGGCAATTGGGCCC. Each of these was amplified by PCR and ligated between the HindIII and MfeI sites of pUT1616, as described above. Each product was then used to transform DH5α containing either pUT1705 or pUT1841. DH5α was chosen because an EcoK restriction site would be created within oriT for some of the clones, and so a restrictionless recipient was required to avoid skewing the library. Nicked oriTs were then enriched by tailing and PCR amplification, as described before (3). The DNA was cloned into the vector pCRTopo2.1, and the DNA was transformed into E. coli strain TOP10. The vector and strain were purchased from Invitrogen and used according to their instructions. There were between 300 and 2,900 transformants for the four libraries. The plasmid DNA from each was pooled; restriction analysis showed that between 75 and >90% of the plasmid DNA contained the correct insert. We then sequenced the pooled plasmid DNA, with a primer (GCGTATCACGAGGCCCT) complementary to the insert.
FIG. 5.
Dideoxy base sequencing of pooled DNA from plasmid libraries containing the cloned, partially degenerate oligonucleotides, labeled bp(1-7) and bp(8-13) at the top. The patterns of chain termination for the population nicked after propagation in strains containing either the R1162 or the pSC101 Mob proteins are shown in the top panel. At the bottom are the patterns without prior enrichment for nicking.
Other procedures.
Preparation of DNA from cleared lysates, treatment with permanganate, and assay of cleaved positions by primer extension were carried out as described previously (21, 28, 29).
RESULTS
The R1162 Mob genes can efficiently initiate transfer from highly mutated oriTs.
We showed previously that the R1162 oriT remained active for nicking even when the inner arm was highly mutated (3). We wanted to ask whether the R1162 Mob proteins and the pSC101 Mob proteins were similarly relaxed for sequence specificity with the pSC101 oriT. To obtain a population of highly mutated oriTs, we used the procedure outlined in Fig. 2. An oligonucleotide degenerate in sequence for the inner arm was first amplified by PCR, and the product was cloned. The resulting population of plasmids was then introduced into a strain containing either the R1162 or the pSC101 Mob proteins. The oriTs that were still cleaved by the Mob proteins were identified by first tailing at the nick with terminal transferase and then selectively amplifying and recloning this oriT DNA. Because we wanted to compare the R1162 and pSC101 Mob systems, we used DNA with the pSC101 oriT core, which differs by one base from the core of the R1162 oriT (Fig. 1A). The oriTs of pSC101 and RSF1010, a plasmid almost identical to R1162 (24), have the same core. Therefore, this change is unlikely to affect recognition of oriT by the R1162 proteins.
We first introduced our collection of plasmids with the mutated oriTs into an E. coli strain encoding the R1162 Mob proteins. The plasmid DNA was then isolated by the cleared-lysate procedure (15), which conserves those molecules cleaved at the nick site. We identified the sequences that allow nicking by first tailing at the nick site with terminal transferase and then amplifying the cleaved and tailed oriT with PCR. The amplified DNA was cloned into pUC19 and used to transform E. coli strain TOP10. We blindly selected 18 colonies and determined the sequence of the oriT DNA in each. All of the oriTs in the collection were highly mutated, and there were 17 different sequences, indicating there was a diverse collection of tailed molecules in the library. The distribution of bases for the 18 individual isolates is shown in Fig. 3. As with the library based on the R1162 oriT (3), the distribution of bases was not random; in particular, there was the strong preference for A at positions 4, 5, and 6, as reported previously. These bases are located 4 bases from the core in both the pSC101 and the R1162 oriTs (Fig. 1A). In addition, G was most commonly present at position 10. The base frequency at each position reflected enrichment of nicked molecules: when plasmid DNAs from the cleared lysates were sequenced directly, there were nearly equal amounts of the four bases at each of the degenerate positions (data not shown).
FIG. 3.
Relative frequency of each base at the 10 positions (numbered according to Fig. 1) making up the inner arm of the inverted repeat in the oriT of R1162. The normal base at each position is indicated by the widest portion of each bar in the graph.
Although nicking is required for transfer, it is not the only function of the relaxosome, which must also enter into the transfer system of a type IV secretion apparatus (11). We therefore wanted to test whether the different nicked oriTs were competent for initiation of transfer. We could not simply test the cloned oriTs, derived from the nicked molecules, for ability to promote transfer, since they do not contain the outer arm of the inverted repeat, which is required for efficient termination of a round of transfer (16). Instead, we used plasmids containing two directly repeated copies of oriT (7). Plasmids with the structure shown in Fig. 1B were constructed, with one of the mutated oriTs, lacking the outer arm of the inverted repeat, at position 1, and a normal oriT at position 2. If a round of transfer can be initiated at the oriT at position 1 [oriT(1)], then a fraction of the time this round will be terminated at the oriT at position 2 [oriT(2)], resulting in a recombinant plasmid deleted for lacO. Transconjugants containing these plasmids form white colonies on medium containing X-Gal (7). Initiation of transfer at oriT(2) and termination at the same locus also result in transconjugants. However, these colonies are blue, since the plasmid copies of lacO are retained and these titrate the Lac repressor encoded by the recipient. Thus, the fraction of white transconjugants is proportional to the initiation frequency at oriT(1).
The results of testing selected mutated oriTs for initiation of transfer are shown in Table 1. Not all of the mutated oriTs were active, even though they had been derived by enriching for nicked molecules. Possibly the nicking is too low to detect by measuring the frequency of transfer or, alternatively, the relaxosomes are improperly oriented to engage the secretion machinery effectively. It also appears that a G in the 10th position is important for activity when the rest of the inner arm is highly mutated. However, these data show that in many cases transfer can be initiated at nearly the normal rate, even when the inner arm of the inverted repeat contains many mutations.
TABLE 1.
Effect of multiple mutations in inner arm of R1162 oriT on initiation of transfer
| Type of oriT | Sequence of inner arma | Fraction of recombinantsb |
|---|---|---|
| Nicked oriT genes enriched from the degenerate oligonucleotide | GAGAAACCGG | 0.80 |
| TCTAATGTGG | 0.17 | |
| CTTAATGGCA | 0 | |
| ATTAATCTTA | 0 | |
| TGCAATGAGG | 0.16 | |
| TGAAATAAAG | 0.33 | |
| GTTAAAGATG | 0.45 | |
| oriT genes containing mutations in conserved AAA | GAGCCCCCGG | <0.001 |
| GAGCACCCGG | <0.003 | |
| GAGACACCGG | 0.002 | |
| GAGATACCGG | 0.36 | |
| GAGTTTCCGG | 0.002 |
Mutated bases are underlined.
Average of two independent experiments, with 300 to 1,500 colonies screened in each case.
We also asked whether the highly conserved adenines at positions 4, 5, and 6 found in both the R1162 and the pSC101 oriTs meant that these bases are required for activity, even when there are no other mutations in oriT. These bases were mutated in R1162, and the resulting oriTs were then cloned and tested for initiation of transfer as before (Table 1). Initiation was undetectable from an oriT containing the mutation AAA to CCC (as was AAA to CAC) and occurred at a low rate from an oriT containing the sequence ACA. The analogous set of oriTs with A changed to T instead was more active, with the oriT containing ATA approaching the normal oriT in activity. These results are consistent with those shown in Fig. 3, where a T is frequently substituted for an A within the 3-base region. We tested whether the mutations affected cleavage within the relaxosome (Fig. 4A). Deproteinized, nicked DNA was isolated from cleared lysates of cells and examined for site-specific nicking by primer extension (28). Among the mutated oriTs, only the one with ATA showed detectable nicking (Fig. 4A). In order for nicking to occur, the duplex DNA within the core must be locally disrupted by the relaxosome, and this can be detected by the increased sensitivity of the DNA in this region to oxidation by permanganate (29). A loss of sensitivity to permanganate correlated with a loss of nicking (Fig. 4B).
Strict requirement for base sequence within the inner arm of the oriT inverted repeat for the pSC101 relaxosome.
Since the pSC101 Mob proteins are inactive on the R1162 oriT (19), we asked whether this indicated that these proteins had a stringent requirement for the base sequence of the DNA making up the inner arm of the inverted repeat, in contrast to the relatively relaxed specificity of the R1162 Mob proteins for this region (3). We repeated the tailing procedure to obtain nicked molecules, this time with a strain containing the pSC101 Mob proteins, encoded by the plasmid pUT1705. Despite numerous attempts, either we did not obtain any PCR product or the sequence of the amplified DNA suggested that it was derived from contaminant DNA rather than a cleaved molecule. These failures indicated that the population of cleaved molecules was so small that it was undetectable by the tailing and PCR procedure.
We next reduced the size of the degenerate region to increase the chance of finding mutated oriTs that remained active with the pSC101 Mob proteins. In addition, the inner arm of the pSC101 oriT is longer by 3 bases than that of the R1162 oriT (Fig. 5 top), and this could have affected the results with the first degenerate oligonucleotide. We used two oriT oligonucleotides, having degenerate regions corresponding to bp 1 to 7 [bp(1-7)] or 8 to 13 [bp(8-13)] of the inner arm (Fig. 5, top). In each case, a library of nicked molecules was generated by transforming strains containing either the R1162 or the pSC101 Mob proteins. Rather than examining individual clones of nicked oriTs, we pooled the plasmids from each library of transformants and sequenced the population. As a control, each initial library was also sequenced prior to enrichment for nicking. The results of these procedures are shown in Fig. 5. The autoradiogram at the bottom of the figure shows that the four bases were present in similar amounts at the degenerate positions in each of the libraries. The gel at the top of the figure shows the sequencing results following enrichment of the nicked sequences by tailing and recloning. Base pairs 1 to 7 were significantly degenerate for both the pSC101 and the R1162 libraries, although G and A at positions 6 and 7 are overrepresented in the R1162 library. The conserved A is consistent with the results shown for R1162 in Fig. 3. For the bp(8-13) library, the results for the two plasmids were different. The bases AACCGG, the sequence for the R1162 oriT at these positions, are enriched, but it is clear that other bases are also present in the library. In contrast, the sequence appears much more uniform in the pSC101 bp(8-13) library and corresponds to the normal sequence at these positions for the pSC101 oriT. Moreover, the sequence of bp(1-7) appeared degenerate. This suggested to us that in fact there were no members of the bp(8-13) degenerate library that could be cleaved by the pSC101 proteins and that the tailed and enriched molecules were contaminants from the bp(1-7) library. We attempted enrichment of nicked molecules for the pSC101 Mob proteins four more times, twice from the library initially used and twice with a second, independently derived library of cloned fragments. In the case of the first library, the same, presumably contaminating sequence was obtained both times. With the second library, we did successfully enrich DNA having a single sequence. However, this DNA was not nicked by the pSC101 Mob proteins upon retesting (data not shown).
We conclude that compared to R1162, the pSC101 Mob system has a strict requirement for the base sequence within locations 8 to 13 of the inverted repeat (Fig. 1), next to the core DNA. Since the AAA triplet is present in both oriTs, it is the four base pairs next to the core, CGTC, that are important for recognition of the cognate oriT by the pSC101 Mob proteins.
The high degree of degeneracy for the bp(1-8) libraries, for both the R1162 and the pSC101 Mob systems, suggested that within the inner arm, the six bases (GTGAAG) distal to the core were not required for the specificity of the pSC101 Mob proteins, although they are unique to the pSC101 oriT (Fig. 1A). To test this directly, we constructed a hybrid oriT having the inner arm of R1162 but with the CCGG adjacent to the core replaced by the corresponding CGTC of the pSC101 oriT. Using plasmids of the type shown in Fig. 1B but with a copy of the pSC101 oriT at position 2, we tested the hybrid oriT for initiation of transfer in cells containing the pSC101 Mob proteins. The initiation frequency was 0.84 for the hybrid oriT. An oriT composed of the complete pSC101 inner arm and core showed the same initiation frequency. Thus, the sequence CGTC (plus the core) was sufficient for recognition of oriT and for the full activity of the pSC101 relaxosome at initiation of a round of transfer. The R1162 Mob proteins were active on the hybrid oriT as well (recombination frequency of 0.35). In addition, like the R1162 Mob proteins, the pSC101 Mob proteins were tolerant to an A-to-T base change in the AAA region. When the hybrid oriT had the sequence ATA at this site, the recombination frequency decreased only slightly, to 0.26.
Single mutations in the pSC101 specificity sequence CGTC lowered the transfer frequency and, like the mutations in AAA of the R1162 oriT, prevented strand separation within the core. The transfer frequencies of plasmids with these mutations were 1.5 × 10−4 (CGTG) and 6.4 × 10−6 (CCTC), significantly lower than the normal (CGTC) frequency of 0.03. Part of the lower frequency could be due to a defect in termination, since plasmids containing a single copy of the complete oriT were tested. However, both mutations reduced the sensitivity of core DNA to permanganate (Fig. 6), at a level roughly coordinate with their effect on transfer. All plasmids transferred at the normal high frequency when the R1162 Mob proteins were provided in trans.
FIG. 6.
Sensitivity of pSC101 oriTs containing mutations in the specificity region to oxidation by permanganate. Plasmid DNAs were isolated from treated (+) or untreated (−) cleared lysates. The sequence of the specificity region in each case is shown at the top, with the mutated base underlined.
The R1162 Mob proteins can initiate transfer from a site cloned from the chromosome of Erwinia carotovora.
Our results with the degenerate oriT libraries indicate that although the R1162 and pSC101 Mob systems are closely related, the R1162 relaxosome is much more permissive with respect to the sequence of the oriT DNA adjacent to the core. This raises the possibility that the R1162 Mob proteins could act on DNA not obviously related to the R1162/pSC101 Mob family. We identified a perfectly conserved core at bp 1306766 to 1306777 in the completely sequenced (5) chromosome of Erwinia carotovora subsp. atroseptica. The core is located in an intergenic region but is not simply part of an inserted plasmid: there is no inverted repeat adjacent to the core, and the neighboring genes are not obviously of plasmid origin. We obtained an oligonucleotide containing the core as well as additional Erwinia chromosomal DNA on the side where the inner arm of the inverted repeat is normally located (Fig. 1A), amplified this DNA by PCR, and cloned it at position 1 in the plasmid shown in Fig. 1B. Position 2 contained the oriT from either R1162 or pSC101. We then tested the resulting plasmids for transfer and for initiation from the cloned Erwinia DNA. When the R1162 oriT was at position 2, the plasmid was mobilized from donors containing the R1162 Mob proteins, and 0.05% of the transconjugants were white on medium containing X-Gal (Table 2). These were shown, by restriction analysis and DNA sequencing, to have the structure expected of true recombinant plasmids derived by initiation from oriT(1) and termination at oriT(2). We also obtained colonies when the donor cells contained the pSC101 Mob proteins, possibly because a large number of cells was used in the mating. However, the apparent mating frequency was low (<10−6), and some of the colonies could be donors spontaneously resistant to nalidixic acid. In any case, among the colonies examined there was only one verified recombinant. A very low fraction of recombinants with the pSC101 Mob proteins was also obtained for pUT1884, which has pSC101 oriT at oriT(2), although in this case the transfer frequency was much higher, since transfer could be both initiated and terminated at this site. Surprisingly, the R1162 Mob proteins were also inactive for recombination on this plasmid. Initiation at the Erwinia DNA might be sufficiently infrequent so that the foreign oriT at position 2 has a greater negative effect. However, we conclude that although the Erwinia DNA adjacent to the core bears little resemblance to the inner arm of the R1162 oriT, it can, together with the core, serve as a site for initiation of transfer.
TABLE 2.
Initiation of transfer from Erwinia carotovora DNA
| Source of Mob proteinsa | No. of colonies
|
|||
|---|---|---|---|---|
| R1162 oriT
|
pSC101 oriT
|
|||
| White | Blue | White | Blue | |
| R1162 | 23 | 4,857 | 1 | 7,502 |
| pSC101 | 1 | 2,550 | 1 | 4,542 |
Mob proteins were provided by R1162 and pUT1621 (19).
DISCUSSION
The core and inner arm of the inverted repeat of the R1162 oriT are sufficient for initiation but not termination of transfer (17). The data presented here indicate that it is not the whole inverted repeat but instead the seven base pairs adjacent to the core that are required for initiation and for discrimination between two different oriTs from the same family. The location of these bases is consistent with our model that this group of oriTs arose by duplication and inversion of the core and adjacent DNA (3). The duplication allows formation of a duplex binding site for the relaxase after conjugative transfer of a single DNA strand. The size of the inverted repeat would be determined not only by the size of the DNA binding site for the relaxase but also by the requirement that the hairpin loop is sufficiently large and stable to re-create the duplex binding site for the protein. Thus, additional bases, distal to the binding site, are duplicated and inverted to meet these requirements.
Within the AAA region of the inner arm, A-to-T mutations are much better tolerated by the R1162 relaxase than A-to-C mutations. These results are consistent with those of earlier studies involving relaxase-mediated recombination at oriTs cloned in M13 bacteriophage, where single-stranded oriT DNA was shown to be cleaved and rejoined in vivo by the relaxase in the absence of the other proteins of the relaxosome (1, 18). An A-to-T mutation in one arm of the inverted repeat was suppressed by a second, T-to-A mutation in the other arm that restored base-pairing within the hairpin loop (1). Thus, it is almost certainly the relaxase itself which is binding to the AAA region. The relative tolerance to TA transversions (Fig. 2 and Table 2) is consistent with binding of the relaxase to the minor groove, where the positions of the major potential contacts for the protein are not greatly altered by an AT-to-TA change (25). An AAA-to-ATA change is also tolerated by the pSC101 relaxosome, so the R1162 and pSC101 relaxases probably interact similarly with the DNA at this AT “waist.”
The relaxases of the plasmids R1162 and pSC101 are closely related. For the N-terminal 198 amino acids making up the minimal region required for recognition and processing at oriT by the R1162 relaxase (4), the two proteins contain amino acids that are approximately 45% identical and 16% closely similar. Nevertheless, the R1162 relaxosome shows significantly lower specificity for its substrate than the pSC101 Mob proteins. For the pSC101 relaxase, the identity of each of the four bases (CGTC) adjacent to the core is important for transfer and strand separation in the core, with base changes causing a loss of activity. Replacing the corresponding four bases (CCGG) in the R1162 oriT with CGTC makes this oriT fully active for initiation by the pSC101 Mob proteins. Since the first base is the same in both cases, it is the three base pairs adjacent to the core which are used by the pSC101 Mob proteins to distinguish the pSC101 oriT from a related oriT. The R1162 relaxosome is not completely indifferent to the sequence at these positions: for example, G at the position adjacent to the core appeared important for activity when the inner arm was heavily mutated (Fig. 2 and Table 1). However, the R1162 proteins are able to initiate transfer efficiently on both the pSC101 oriT (3) and, at lower frequency, Erwinia DNA (C and T at this position, respectively). The overall acceptability of the four bases adjacent to the core probably depends on the identity of each, with no base change by itself having a large effect.
We have suggested earlier that MobC, which assists in strand separation at oriT (28) and is required by the R1162 relaxase for optimal activity on both the pSC101 and the R1162 oriTs (19), might permit a looser fit between relaxase and DNA and thus account for the lower specificity of the R1162 protein (3). The pSC101 Mob system does not encode a homolog to MobC, and the relaxase might need a firmer grip on the DNA in order to separate the DNA strands within oriT. However, we have not ruled out the possibility that the pSC101 relaxase uses a host-encoded protein such as HU to facilitate localized melting of the oriT DNA. As described above, the importance of these bases might lie in providing the protein with additional contacts on the DNA, thus strengthening binding by the relaxase and minimizing the need for an accessory protein to help in strand separation. However, during our screens for nicked molecules (Fig. 2 and 4), we were unable to identify any oriTs nicked by the pSC101 relaxase and containing mutations in the specificity sequence CGTC, even when MobC was provided in the cell (data not shown). One possibility is that CGTC is important for orienting the relaxase, so that it is active on core DNA and so that other combinations of bases are sterically not allowable, even in the presence of MobC. It is also possible that the R1162 relaxase and MobC interact specifically with each other. It will be interesting to see how the R1162 relaxase is oriented properly for activity, sometimes at nearly normal levels (Table 1), without using its own specificity sequence as a guide. Perhaps the R1162 relaxase is oriented by MobC for proper strand separation through a specific protein-protein interaction.
The R1162 Mob proteins can initiate transfer from DNA cloned from the Erwinia chromosome. It has been shown elsewhere that these proteins can also initiate transfer from an oriT in the chromosome of E. coli (16). Taken together, these observations suggest that the R1162 Mob proteins can function at ectopic sites in the chromosome, thus enabling horizontal gene transfer without prior integration of its own DNA. This could be particularly significant in the case of a broad-host-range plasmid like R1162, which can share cytoplasm with many different bacterial chromosomes. Moreover, the core itself does not have to be perfectly conserved: for example, either A or G can occupy the fourth position (Fig. 1). The R1162 Mob system is a member of a large family (3, 12), and remnants of plasmids and other transfer elements integrated previously into chromosomes, as well as sequences occurring by chance, could provide an additional source of core-like DNA.
Acknowledgments
We are grateful for the assistance of Amanda Walker and Molly C. Sutherland.
This work was supported in part by a grant from the National Institutes of Health (GM37462). M.C.S. was supported by NSF Research Experiences for Undergraduates grant DBI-0139881.
REFERENCES
- 1.Barlett, M. M., M. J. Erickson, and R. J. Meyer. 1990. Recombination between directly repeated origins of conjugative transfer cloned in M13 bacteriophage DNA models ligation of the transferred plasmid strand. Nucleic Acids Res. 18:3579-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, E., and R. Meyer. 2000. Recognition of oriT for DNA processing at termination of a round of conjugal transfer. J. Mol. Biol. 300:1067-1077. [DOI] [PubMed] [Google Scholar]
- 3.Becker, E., and R. Meyer. 2003. Relaxed specificity of the R1162 nickase: a model for evolution of a system for conjugative mobilization of plasmids. J. Bacteriol. 185:3538-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, E. C., and R. Meyer. 2002. MobA, the DNA strand transferase of plasmid R1162. The minimal domain required for DNA processing at the origin of transfer. J. Biol. Chem. 277:14575-14580. [DOI] [PubMed] [Google Scholar]
- 5.Bell, K. S., M. Sebaihia, L. Pritchard, M. T. G. Holden, L. J. Hyman, M. C. Holeva, N. R. Thomson, S. D. Bentley, L. J. C. Churcher, K. Mungall, R. Atkin, N. Bason, K. Brooks, T. Chillingworth, K. Clark, J. Doggett, A. Fraser, Z. Hance, H. Hauser, K. Jagels, S. Moule, H. Norbertczak, D. Ormond, C. Price, M. A. Quail, M. Sanders, D. Walker, S. Whitehead, G. P. C. Salmond, P. R. J. Birch, J. Parkhill, and I. K. Toth. 2004. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl. Acad. Sci. USA 101:11105-11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernardi, A., and F. Bernardi. 1984. Complete sequence of pSC101. Nucleic Acids Res. 12:9415-9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharjee, M., X. M. Rao, and R. J. Meyer. 1992. Role of the origin of transfer in termination of strand transfer during bacterial conjugation. J. Bacteriol. 174:6659-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharjee, M. K., and R. J. Meyer. 1991. A segment of a plasmid gene required for conjugal transfer encodes a site-specific, single-strand DNA endonuclease and ligase. Nucleic Acids Res. 19:1129-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasch, M. A., and R. J. Meyer. 1987. A 38 base-pair segment of DNA is required in cis for conjugative mobilization of broad host-range plasmid R1162. J. Mol. Biol. 198:361-369. [DOI] [PubMed] [Google Scholar]
- 10.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christie, P., and J. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francia, M., A. Varsaki, M. Garcillan-Barcia, A. Latorre, C. Drainas, and F. de la Cruz. 2004. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 28:79-100. [DOI] [PubMed] [Google Scholar]
- 13.Hershfield, V., H. W. Boyer, C. Yanofsky, M. A. Lovett, and D. R. Helinski. 1974. Plasmid ColE1 as a molecular vehicle for cloning and amplification of DNA. Proc. Natl. Acad. Sci. USA 71:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton, R., Z. Cai, S. Ho, and L. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 15.Katz, L., D. T. Kingsbury, and D. R. Helinski. 1973. Stimulation of cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J. Bacteriol. 114:577-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, K., and R. J. Meyer. 1989. Unidirectional transfer of broad host-range plasmid R1162 during conjugative mobilization. Evidence for genetically distinct events at oriT. J. Mol. Biol. 208:501-505. [DOI] [PubMed] [Google Scholar]
- 17.Kim, Y.-J., L.-S. Lin, and R. J. Meyer. 1987. Two domains at the origin are required for replication and maintenance of broad-host-range plasmid R1162. J. Bacteriol. 169:5870-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer, R. 1989. Site-specific recombination at oriT of plasmid R1162 in the absence of conjugative transfer. J. Bacteriol. 171:799-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer, R. 2000. Identification of the mob genes of plasmid pSC101 and characterization of a hybrid pSC101-R1162 system for conjugal mobilization. J. Bacteriol. 182:4875-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perwez, T., and R. Meyer. 1996. MobB protein stimulates nicking at the R1162 origin of transfer by increasing the proportion of complexed plasmid DNA. J. Bacteriol. 178:5762-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perwez, T., and R. J. Meyer. 1999. Stabilization of the relaxosome and stimulation of conjugal transfer are genetically distinct functions of the R1162 protein MobB. J. Bacteriol. 181:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherzinger, E., V. Kruft, and S. Otto. 1993. Purification of the large mobilization protein of plasmid RSF1010 and characterization of its site-specific DNA-cleaving/DNA-joining activity. Eur. J. Biochem. 217:929-938. [DOI] [PubMed] [Google Scholar]
- 23.Scherzinger, E., R. Lurz, S. Otto, and B. Dobrinski. 1992. In vitro cleavage of double- and single-stranded DNA by plasmid RSF1010-encoded mobilization proteins. Nucleic Acids Res. 20:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholz, P., V. Haring, B. Wittmann-Liebold, K. Ashman, M. Bagdasarian, and E. Scherzinger. 1989. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 75:271-288. [DOI] [PubMed] [Google Scholar]
- 25.Seeman, N., J. Rosenberg, and A. Rich. 1976. Sequence-specific recognition of double helical nucleic acids by proteins. Proc. Natl. Acad. Sci. USA 73:804-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willetts, N., and C. Crowther. 1981. Mobilization of the nonconjugative IncQ plasmid RSF1010. Genet. Res. 37:311-316. [DOI] [PubMed] [Google Scholar]
- 27.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, S., and R. Meyer. 1997. The relaxosome protein MobC promotes conjugal plasmid mobilization by extending DNA strand separation to the nick site at the origin of transfer. Mol. Microbiol. 25:509-516. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, S., and R. J. Meyer. 1995. Localized denaturation of oriT DNA within relaxosomes of the broad-host-range plasmid R1162. Mol. Microbiol. 17:727-735. [DOI] [PubMed] [Google Scholar]