Abstract
This study shows that the Bacillus anthracis pXO1 virulence plasmid carries a Rap-Phr system, BXA0205, which regulates sporulation initiation in this organism. The BXA0205Rap protein was shown to dephosphorylate the Spo0F response regulator intermediate of the phosphorelay signal transduction system that regulates the initiation of the developmental pathway in response to environmental, metabolic, and cell cycle signals. The activity of the Rap protein was shown to be inhibited by the carboxy-terminal pentapeptide generated through an export-import processing pathway from the associated BXA0205Phr protein. Deregulation of the Rap activity by either overexpression or lack of the Phr pentapeptide resulted in severe inhibition of sporulation. Five additional Rap-Phr encoding systems were identified on the chromosome of B. anthracis, one of which, BA3790-3791, also affected sporulation initiation. The results suggest that the plasmid-borne Rap-Phr system may provide a selective advantage to the virulence of B. anthracis.
Bacillus anthracis, the etiological agent of anthrax, is a gram-positive spore-forming organism that primarily infects ruminants but can also be highly pathogenic to other mammals, including humans. The intrinsic spore resistance to extreme stresses such as desiccation, solvents, extreme pH, temperature, UV, and ionizing radiation plays a major role in anthrax pathogenesis. Sporulation is essential for survival in the environment, and it evidently contributes to anthrax diffusion, because spores are usually present when the infection is initiated (27).
The process of sporulation has been extensively studied in Bacillus subtilis and shown to be the result of a complex differentiation pathway that has its onset in a signal transduction system called phosphorelay. The phosphorelay is a more complex version of the two-component signal transduction systems, because it is composed of multiple central elements and it is modulated by a variety of ancillary factors (40, 41).
In B. subtilis, five histidine sensor kinases (KinA, -B, -C, -D, and -E) can respond to a multiplicity of inducing signals and activate the pathway by autophosphorylating and transferring the activating phosphoryl group to an intermediate response regulator acceptor called Spo0F. From Spo0F, the phosphoryl group is then transferred to the Spo0A response regulator through the Spo0B phosphotransferase. Spo0A is the critical transcription regulator for sporulation initiation. Accumulation of its activated form, Spo0A∼P, during growth progressively results in the repression of genes not required for sporulation and the activation of genes necessary for spore formation (7, 19, 28, 53).
Negative inputs into the phosphorelay are brought about mainly by aspartyl phosphate phosphatases that specifically dephosphorylate the response regulator components of the system. The three members of the Spo0E family of phosphatases dephosphorylate Spo0A∼P, while three members of the Rap family of phosphatases dephosphorylate the Spo0F∼P intermediate (18, 36, 38). Rap proteins are structurally organized in tetratricopeptide repeats (TPR) which provide the basis for protein-protein or protein-ligand regulatory mechanisms (10, 37). Rap proteins in fact are often inhibited by specific pentapeptide ligands, which results from an export-import pathway of the pre-pro precursor product of the rap-associated phr genes (37).
The Spo0A and Spo0F response regulator proteins are very conserved in amino acid sequence between B. subtilis and B. anthracis (49). The response regulator domain of the Spo0A proteins is highly conserved (79% identical residues) and all of the residues involved in the interaction with the Spo0E phosphatase are identical (50). In the DNA-binding domain, all the residues known to make contact with the target DNA promoters are also conserved, suggesting that the sequence of the “0A box” is invariant in these two organisms (60). The Spo0F proteins (77% identity) also are invariant in all the residues known to affect the interaction with the Rap phosphatase proteins (54). Amino acid conservation is also significantly high in the Spo0B proteins, in particular in the residues known to make interaction with the response regulator, thus maintaining specificity in protein-protein interaction through an identical molecular mechanism in the two organisms (58). Amino acid conservation of the histidine domain has also allowed the identification of nine sensor histidine kinases presumably involved in B. anthracis sporulation initiation (6).
In this communication we report the identification and characterization of Rap proteins of B. anthracis and investigate their role in the sporulation process of this organism.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The B. subtilis strains used in this study are all derivatives of the parental strain JH642 and thus carry the trpC2 and phe1 auxotrophic markers. Strains were grown in Schaeffer's sporulation medium (44) in the presence of the appropriate antibiotic at the following concentrations: kanamycin, 2 μg/ml; erythromycin, 5 μg/ml; lincomycin, 25 μg/ml; chloramphenicol, 5 μg/ml; and spectinomycin, 50 μg/ml. Competent cells were prepared by the method of Anagnostopoulos and Spizizen (1).
B. anthracis strains were grown in Schaeffer's sporulation medium or LB medium in the presence of antibiotics at the same concentrations used for B. subtilis for erythromycin or spectinomycin resistance. Chloramphenicol and kanamycin were used at 7.5 μg/ml. B. anthracis electrocompetent cells were prepared by the method of Koehler et al. (20).
Escherichia coli DH5α was used for plasmid construction and propagation. Transformations were performed by electroporation. Cells were grown in LB medium containing antibiotics at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 30 μg/ml; chloramphenicol, 7.5 μg/ml; and spectinomycin, 100 μg/ml.
Assays for sporulation efficiency were carried out in Schaeffer's sporulation medium. B. subtilis cells were grown at 37°C, and then serial dilutions were plated in duplicate before and after chloroform treatment (10% final concentration). Spores were counted as CFU, and the mean values are shown in the tables. B. anthracis cells were grown for 24 h at 37°C, and serial dilutions were plated for viable cell counts. The cells were sonicated for 30 seconds and treated with chloroform (10% final concentration); serial dilutions were then plated for spore counts as CFU.
Plasmid construction.
The vectors used in this study were the following: pHT315, a multicopy E. coli-Bacillus shuttle vector (2); pJM115, a transcriptional fusion vector derivative of pDH32 carrying the aph3A gene for kanamycin resistance (34); pORI-Cm, a thermosensitive replicative shuttle plasmid (6); and pTCVlac, a replicative plasmid for the construction of transcriptional fusions to the E. coli lacZ gene (42).
The primers used in this work are listed in Table S1 in the supplemental material. The promoter and coding sequence of the rap genes were amplified by PCR of B. anthracis 34F2 chromosomal DNA, using oligonucleotide primers that introduced appropriate restriction sites. Fragments were cloned into pHT315 to generate the derived plasmids, named pHT315-BXA0205, pHT315-3790, pHT315-1582, pHT315-3016, pHT315-3760, and pHT315-4060.
In vivo analysis of the active pentapeptide inhibitor of the BA3790 and BXA0205 proteins was carried out using pHT315 derivatives containing the Rap-encoding gene and its associated phr peptide gene. The fragment cloned in plasmid pHT315-3790phr, containing open reading frames (ORFs) BA3790 and BA3791, was obtained by PCR amplification using oligonucleotides BA37905′Kpn and BA37903′Bam2. The additional plasmids carrying 5-codon serial deletions at the 3′ end of the BA3791 phr gene were obtained using oligonucleotides BA37903′Bam3, -4, -5, and -6.
The fragment cloned in pHT315-BXA0205phr containing the BA0205 gene and its associated phr gene was obtained by PCR amplification using the oligonucleotide primers RappXO15′Kpn and RappXO13′Bam2. The derivative carrying a 5-codon deletion at the 3′ end of the phr gene, named pHT315-BXA0205phr2, was obtained by PCR amplifying a fragment using oligonucleotide primers RappXO15′Kpn and RappXO13′Bam3.
The sequence of BCE3523, the orthologue of BA4060, was amplified from Bacillus cereus ATCC 10987 chromosomal DNA using oligonucleotide primers BCE35235′Eco and BCE35233′Bam1 and cloned into pHT315 vector to generate plasmid pHT315-3523. The pHT315 derivative carrying BCE3523 and its associated phr gene was obtained by cloning a PCR-amplified fragment generated with the oligonucleotide primers BCE35235′Eco and BCE35233′Bam2.
The promoter region of each rap gene was subcloned from the pHT315-derived plasmids using the EcoRI or KpnI site at the 5′ end and an internal restriction site for the 3′ end. The exception was for the promoter region of BA3760 that was obtained by PCR amplification using the oligonucleotide primers BA37605′Eco and BA37603′Bamlac. The fragments were then cloned into the transcriptional lacZ fusion vector pJM115.
The fragment used to test the presence of a promoter in front of the BXA0205phr gene was obtained as an EcoRI-BamHI 700-bp fragment from pHT315-BXA0205 (the EcoRI site naturally occurring within the gene and the BamHI site generated by the PCR amplification reaction). The fragment was cloned in pJM115 and pTCVlac, giving rise to plasmids pJM115-BXA0205phr-lac and pTCVlac-BXA0205phr-lac, respectively.
The fragment used to test the presence of a promoter in front of the BA3791 phr gene was obtained as a 292-bp EcoRI-BamHI fragment from pHT315-3790 (the EcoRI site naturally occurring within the gene and the BamHI site generated by the PCR amplification reaction). The fragment was cloned in pJM115 and pTCVlac, giving rise to plasmids pJM115-3791lac and pTCVlac-3791lac, respectively.
The promoter regions of the B. anthracis abrB and atxA genes were PCR amplified using the oligonucleotide primers BaAbrB5′Eco and BaAbrB3′Bamlac or AtxA5′promEco and p1185′Eco2, respectively. The fragment carrying the abrB promoter was digested with EcoRI and BamHI and cloned in the similarly digested pTCVlac vector. The fragment carrying the atxA promoter was digested with EcoRI and EcoRV (the latter naturally occurring at the 5′ end of the coding sequence) and cloned in pTCVlac digested with EcoRI and SmaI.
All PCR-generated fragments were subjected to nucleotide sequence verification.
Disruption of B. anthracis BXA0205 or BXA0205phr.
The BXA0205 gene was amplified by PCR using the primers RappXO15′Kpn and RappXO13′Bam2. The KpnI-BamHI fragment was ligated to KpnI- and BamHI-digested pORI-Cm to generate pORI-BXA0205. A BXA0205 disruption was made by insertion of the spectinomycin (spc) cassette from pJM134 (M. Perego, unpublished) into the unique EcoRV site within the BXA0205 sequence to create pORIΔBXA0205.
The disruption of the phr sequence associated with BXA0205 was made by cloning into plasmid pORI-Cm the sequences upstream and downstream of the phr region: the upstream rap sequence was amplified using primers RappXO15′Kpn and RappXO13′Bam and the sequence downstream of the phr gene was amplified by using primers 5′Phr SalI and 3′Phr PstI. A unique HincII site of pORI-Cm was used to insert the spc gene between the two previously cloned fragments and generate the pORI-Δphr derived plasmid. The plasmids pORI-ΔBXA0205 and pORI-ΔBXA0205phr were each transformed in B. anthracis 34F2 with selection for spectinomycin resistance at 28°C. Cultures were then shifted to the nonpermissive temperature (37°C) and screened for the loss of chloramphenicol resistance. Transformants were then screened by PCR amplification to confirm the recombinants which carried the inactivated BXA0205 gene or the inactivated phr gene arising from a double-crossover event. The recombinant strains obtained were labeled Ba34F2Δ0205 and Ba34F2Δ0205phr.
β-Galactosidase assay.
B. subtilis and B. anthracis cultures were grown in Schaeffer's sporulation medium. Samples were taken at hourly intervals and processed as previously described (13) and activity was measured according to Miller (26). Samples were taken and processed in duplicate, and the mean values were used for the calculations.
Protein expression and purification.
The coding sequences of four B. anthracis Rap proteins were amplified by PCR from the chromosomal DNA of strain 34F2, using oligonucleotides that introduced an NdeI site at the 5′ end and a BamHI site at the 3′ end (see Table S1 in the supplemental material). Each fragment was cloned in the NdeI-BamHI sites of plasmid pET28a (BA1582, BA3016), pET30c (BXA0205), or pETblue-2 (BA3790) (Novagen).
Plasmids were transferred to E. coli expression strains BL21(DE3) (BXA0205 and BA3790) or BL21(DE3)pLysS (BA1582 and BA3016) (Novagen). A fresh single-colony transformant was grown overnight at 37°C in LB medium with the appropriate antibiotic. This culture was used to inoculate 2 liters of LB medium containing the appropriate antibiotic and grown at 30°C or 37°C until the optical density at 600 nm reached 0.6 to 0.8. Protein expression was induced with IPTG (isopropyl-β-d-thiogalactopyranoside), 0.2 to 0.5 mM, in the case of BXA0205, BA3790, and BA3016, and growth was continued for 3 h. The expression of BA1582 was not induced and the culture was grown for a total of 6 h. Cells were harvested by centrifugation at 4°C. The pellet was resuspended in lysis buffer (for BXA0205, 20 mM Tris-HCl, pH 8, 100 mM NaCl, 10 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 mM imidazole; for BA3790, 50 mM Tris-HCl, pH 8, 300 mM KCl, 10 mM imidazole, 10 mM β-mercaptoethanol, 1:1,000 dilution of Sigma protease inhibitor cocktail, 1 mM PMSF; for BA1582, 50 mM Tris-HCl, pH 8.5, 300 mM KCl, 10 mM β-mercaptoethanol, 7.5 mM imidazole, 1:1,000 dilution of Sigma protease inhibitor cocktail, 1 mM PMSF; for BA3016, 7 M urea, 50 mM Tris-HCl, pH 8.5, 100 mM KCl, 5 mM imidazole, 1 mM PMSF, 1:1,000 dilution of Sigma protease inhibitor cocktail). Cells were disrupted with a French press and cell debris was removed by ultracentrifugation for 1 h at 4°C at 50,000 rpm, using a Beckman 60 Ti rotor. The supernatant was loaded into a nitrilotriacetic acid agarose column (QIAGEN) and washed with lysis buffer. The protein was eluted with a gradient of imidazole (from 20 mM to 200 mM) in lysis buffer. The fractions containing the protein were pulled, concentrated, and dialyzed against the lysis buffer prepared without the imidazole or the protease inhibitors and with the β-mercaptoethanol replaced by 10 mM dithiothreitol. For BA3016, several dialysis changes were carried out in order to reduce the urea concentration to 1.5 M. Proteins were stored at −80°C with 20% of glycerol.
Native polyacrylamide electrophoresis.
Tris-Tricine native polyacrylamide gel electrophoresis (PAGE) was carried out according to Schägger and von Jagow (45) with some adjustments (17). The gels were prepared with 3 M Tris-HCl (pH 8.45) buffer containing 1 mM EDTA and the running buffer containing 0.1 M Tris-HCl, 0.1 M Tricine, and 1 mM EDTA (pH 8.25).
Purification of Spo0F∼P.
His6-tagged Spo0F (55) (50 μM) was phosphorylated with KinA (0.5 μM) and [γ-32P]ATP (0.3 μM at a specific activity of 6,000 Ci/mmol) in the phosphorelay buffer {50 mM EPPS [4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid; pH 8.5] 20 mM MgCl2, 0.1 mM EDTA, 5% glycerol} for 1 h at room temperature. ATP, 1 mM, was added and the phosphorylation reaction was continued for another hour. Spo0F∼P was purified by using 0.2 ml of nickel-nitrilotriacetic acid resin (QIAGEN) equilibrated with binding buffer (50 mM Tris, pH 8, 300 mM NaCl, 10 mM imidazole, 5 mM β-mercaptoethanol). The column was washed with 10 ml of binding buffer, and protein elution was obtained with binding buffer containing 150 mM EDTA. Fractions containing radioactivity were dialyzed in 20 mM HEPES, pH 7.0, 0.1 mM EDTA, and 10% glycerol. Aliquots were stored at −80°C. The ratio of Spo0F/Spo0F∼P in the purified sample was estimated by analysis on a 10% Tris-glycine native PAGE gel stained with Coomassie blue (59).
The concentration of Spo0F/Spo0F∼P used throughout this study refers to the fraction of Spo0F∼P only.
Purification of KinA∼P.
KinA autophosphorylation was carried out at room temperature for 30 min in a 300-μl volume containing 20 μM KinA, [γ-32P]ATP (0.3 μM at a specific activity of 6,000 Ci/mmol), 50 mM K-EPPS buffer (pH 8.5), 20 mM MgCl2, 0.1 mM EDTA, and 5% glycerol. ATP, 1 mM, was then added and the reaction continued for 1 h. A Microcon-30 concentrator (Amicon) was used to eliminate unincorporated ATP and to concentrate KinA∼P. Aliquots were stored at −80°C.
Biochemical assays.
Two different protocols were used for the dephosphorylation assays. (i) For each Rap protein (5 μM) the dephosphorylation activity was determined in a reaction containing Spo0F (2.5 μM), KinA (0.1 μM), and phosphotransfer buffer (50 mM K-EPPS buffer, pH 8.5, 20 mM MgCl2, 0.1 mM EDTA, 5% glycerol). The reaction was started by adding [γ-32P]ATP, and aliquots were withdrawn at different times.
(ii) The ability of BXA0205 and BA3790 to dephosphorylate Spo0F∼P was assayed in a reaction containing purified Spo0F∼P (2.5 μM) and the Rap phosphatase (5 μM) in the phosphotransfer buffer. Aliquots were taken at the times indicated in the figures and the reaction was stopped by the addition of 5× sodium dodecyl sulfate (SDS) loading dye.
All samples were analyzed by 15% SDS-Tris-glycine PAGE. The gels were dried under vacuum and the radioactivity detected with a PhosphorImager and quantitated by the ImageQuant software (Molecular Dynamics Corp.)
RESULTS
Identification of Rap-encoding genes by bioinformatic analysis.
The availability of the B. anthracis genome sequence allowed us to search for genes encoding proteins with homology to the Rap proteins of B. subtilis (37). The amino acid sequence of the B. subtilis RapA protein involved in the control of the sporulation phosphorelay was used as a query in a BLAST search against the genome sequence of B. anthracis strain A2012. Five genes were identified encoding proteins with approximately the same molecular weight as that of RapA and sharing with it an average of 24% identical residues. The genes were annotated as BA1582, BA3790, BA3016, BA4060, and BXA0205 (NCBI accession number NC_003995) (Fig. 1A and Table 1). An additional gene, annotated as BA3760, was found when the same search was carried out on the B. anthracis Ames genome sequence (NCBI accession number NC_003997). Using the sequence of BA3760 to search the B. anthracis A2012 genome, we noted that an identical gene was present in this strain but that its product seemed to be frameshifted, giving rise to two open reading frames (annotated as BA4239 and BA4240), none of which would have sufficient significant similarity to the B. subtilis RapA protein to be identified by our first BLAST search. The nomenclature BA3760 will be used hereafter to indicate this gene, while for the others the strain A2012 nomenclature will be used. The identified genes and their nomenclature are summarized in Table 1.
FIG.1.
Amino acid sequence alignment of the Rap and Phr proteins of B. anthracis. (A) The Rap proteins of B. anthracis were aligned against the RapA protein of B. subtilis. The six TPR domains in RapA are underlined. The gray box in the BA3760 sequence indicates a duplication in the nucleotide sequence of the 34F2 strain used in this study that is not present in the database at accession number NC_003997. The dash replacing residue 226 in the sequence of BA4060 indicates the position of the frame shift that inactivates this gene product. The alignment was obtained by the ClustalW program. (B) Amino acid sequences of the Phr proteins of B. anthracis and the PhrA protein of B. subtilis. Gray boxes in the BsPhrA and BXA0205phr sequences indicate the sequence of the active pentapeptide inhibitor. The gray box in the BA3791 sequence indicates the region presumably containing the pentapeptide inhibitor. The amino-terminal positively charged domain (N), the hydrophobic domain (H), and the putative signal peptidase cleavage domain (C) of PhrA are indicated. The dashes indicate the putative signal peptidase cleavage site identified by the SignalP program (30).  , identical residue; :, conserved residue.
, identical residue; :, conserved residue.
TABLE 1.
Summary of Rap-Phr ORF annotations in two B. anthracis sequenced genomes
|
B. anthracis A2012
|
B. anthracis Ames
|
||
|---|---|---|---|
| Accession no. | ORF | Accession no. | ORF |
| NC_003995 | BA3790-3791 | NC_003997 | BA3284-3285 |
| NC_003995 | BA4060a/4061-4062 | NC_003997 | BA3569a-3570 |
| NC_003995 | BA1582d | NC_003997 | BA1017-1018 |
| NC_003995 | BA3016c-3015 | NC_003997 | BA2522d |
| NC_003995 | BA4239/4240b,d | NC_003997 | BA3760-3759 |
| NC_003980 | BXA0205d,e | NC_007322 | GBAA_pXO1_0205d |
This ORF is interrupted by a stop codon in both organisms. (This is not a sequencing error.)
This ORF is frameshifted in the A2012 strain only. (This could be a sequencing error.)
This ORF is missing 198 amino acids at the amino-terminal end due to a frameshift. (This could be a sequencing error, since the clone we recovered from strain 34F2 does not have this error.)
In the database, these ORFs do not have an annotated phr gene associated with them.
This gene is called ORF136 by Okinaka et al. (32).
Among themselves, the six B. anthracis Rap proteins share between 37 and 54% identical residues. The gene encoding BXA0205 is located on the pX01 virulence plasmid, while the remaining five genes are all located on the chromosome.
Curiously, the sequence of BA4060 was found to be interrupted by a stop codon at position 225. This stop codon is present in the orthologues of BA4060 identified in the available genome sequences of all B. anthracis strains but one, A1055, in which the stop codon is replaced by a glutamine residue. The B. cereus orthologue of BA4060, BCE3523 (NCBI accession number NC_003909), is also uninterrupted because of a glutamine residue in place of the stop codon. BCE3523 was used instead of BA4060, in order to test the role of this Rap protein on sporulation.
Analysis of the nucleotide sequence at the 3′ end of each gene revealed the presence of open reading frames encoding putative Phr-like proteins. Some of them were annotated in the database as BA3791, BA4061, BA3015, and BA3759. We have named the remaining ORFs BA1582phr, BXA0205phr, and BCE3523phr (Fig. 1B).
Analysis of each Phr protein with the SignalP program (30) indicated structural organizations typical of secreted proteins as previously described for the Phr proteins of B. subtilis (39). The amino-terminal N domain contains positively charged amino acids, the H domain is formed by hydrophobic residues, and the C domain contains the residues presumably recognized by the signal peptidase for the cleavage reaction (57).
Overexpression of B. anthracis Rap proteins in B. subtilis.
The B. subtilis Rap proteins affecting the phosphorelay for sporulation initiation were identified as negative regulators by their ability to inhibit the sporulation process when overexpressed from a multicopy vector. We applied this strategy in order to determine whether any of the Rap proteins of B. anthracis had a role in modulating sporulation in this organism. Since the Spo0F, Spo0B, and Spo0A proteins of the sporulation phosphorelay in B. anthracis are highly homologous to their counterparts in B. subtilis (49), we assumed that if a Rap protein were active on Spo0F of the former it would also be active on the orthologue of the latter organism. Thus, we carried out the initial phenotypic analysis of Rap proteins in B. subtilis.
Each Rap-encoding gene was amplified by PCR as a fragment including approximately 200 to 400 bp at the 5′ end, in order to amplify the promoter region as well. At the 3′ end, each fragment terminated immediately downstream from the stop codon of the rap genes in order not to include the phr gene. The fragments were cloned in the multicopy vector pHT315, which is presumably present at approximately 15 copies per cell (2). The plasmids obtained were transformed in the B. subtilis wild-type strain JH642, and the phenotype of the transformants was analyzed on Schaeffer's sporulation agar plates. Visual analysis on agar plates of the transformants obtained indicated that the expression of BXA0205 and BA3790 inhibited sporulation of B. subtilis JH642. The remaining B. anthracis Rap proteins did not seem to affect the sporulation phenotype. In order to quantitate the effect of the overexpression of the B. anthracis Rap proteins on sporulation, we carried out a liquid sporulation assay. The results shown in Table 2 indicated that BXA0205 severely inhibited sporulation, while BA3790 caused a 2.5-fold reduction in efficiency. The other Rap proteins did not significantly affect the efficiency of sporulation (Table 2 and data not shown). Since BA4060 of B. anthracis contains a premature stop codon that would result in the production of an incomplete protein, we cloned the orthologue gene from B. cereus ATCC 10987, BCE3523, into pHT315 and also analyzed its phenotype upon transformation in B. subtilis JH642. Visual and quantitative analysis indicated that BCE3523 did not affect the sporulation process (Table 2). The overexpression of the Rap protein encoded by BA3760 did not inhibit sporulation as seen on Schaeffer's agar plates, and it was not characterized any further (data not shown).
TABLE 2.
Efficiency of sporulation of B. subtilis JH642 derivative strains expressing the B. anthracis Rap proteins in the pHT315 multicopy plasmid
| Plasmid | Viable cells | Spore count | % Sporulation |
|---|---|---|---|
| pHT315 | 4.7 × 108 | 2.2 × 108 | 46.8 |
| pHT315-BXA0205 | 2.6 × 108 | 1.5 × 102 | 5.7 × 10−4 |
| pHT315-BXA0205phr | 3.4 × 108 | 1.5 × 107 | 4.4 |
| pHT315-3790 | 8.2 × 108 | 1.5 × 108 | 18.3 |
| pHT315-3790phr | 4.0 × 108 | 2.2 × 108 | 55 |
| pHT315-1582 | 3.8 × 108 | 9.3 × 107 | 24.5 |
| pHT315-3016 | 4.3 × 108 | 1.7 × 108 | 39.5 |
| pHT315-4060 | 4.5 × 108 | 1.8 × 108 | 40 |
| pHT315-3523 | 3.7 × 108 | 1.4 × 108 | 37.8 |
| pHT315-3523phr | 3.6 × 108 | 1.1 × 108 | 30.5 |
Growth was carried out in Schaeffer's sporulation medium for 30 hours at 37°C. Data are representative of three independent experiments.
In order to test whether the activity of the two Rap proteins affecting sporulation was inhibited by the associated Phr peptide, plasmids were also generated expressing BXA0205 with the Phr0205 peptide or BA3790 with BA3791 (named pHT315-BXA205phr and pHT315-3790phr, respectively). The liquid sporulation assay showed that both peptides were effective in inhibiting the activity of the corresponding Rap protein (Table 2).
Overexpression of B. anthracis Rap proteins in B. anthracis.
The multicopy plasmids carrying the rap or rap and phr genes were transformed in B. anthracis 34F2 and the effect on sporulation was analyzed by a liquid sporulation assay. As shown in Table 3, overexpression of BXA0205 severely affected the efficiency of sporulation, while the expression of BA3790 caused a consistent threefold reduction. The presence of the corresponding phr gene inhibited the activity of the Rap proteins in both cases, although Phr0205 seemed to be more effective than Phr3790.
TABLE 3.
Efficiency of sporulation of B. anthracis 34F2 derivative strains expressing the Rap proteins in the pHT315 multicopy plasmida
| Plasmid | Insert | Viable cells | Spore count | % Sporulation |
|---|---|---|---|---|
| pHT315 | None | 3.2 × 108 | 3.4 × 107 | 10.6 |
| pHT315-BXA0205 | BXA0205 | 2.4 × 107 | 2.8 × 104 | 1.2 × 10−3 |
| pHT315-BXA0205 phr | BXA0205 phr | 2.4 × 108 | 1.5 × 107 | 5.8 |
| pHT315-3790 | BA3790 | 1.3 × 108 | 4.9 × 106 | 3.7 |
| pHT315-3790 phr | BA3790 phr | 2.2 × 108 | 1.2 × 107 | 5.4 |
| pHT315-1582 | BA 1582 | 2.5 × 108 | 1.5 × 107 | 6 |
| pHT315-3016 | BA 3016 | 2.2 × 108 | 1.1 × 107 | 5 |
| pHT315-4060 | BA 4060 | 3.1 × 108 | 1.3 × 107 | 4.2 |
| pHT315-3523 | BCE3523 | 1.8 × 108 | 1.5 × 107 | 8 |
| pHT315-3523 phr | BCE3523 phr | 2.2 × 108 | 1.9 × 107 | 9 |
Growth was carried out in Schaeffer's sporulation medium for 30 hours at 37°C. Data are representative of three independent experiments.
Microscopic analysis of the strains grown on solid medium revealed that none of the remaining Rap proteins affected sporulation significantly, despite the variability observed by the liquid sporulation assay shown in Table 3.
These results are consistent with the conclusion that only two of the six Rap proteins of B. anthracis, BXA0205 and BA3790, affected sporulation in vivo in B. subtilis and B. anthracis.
In vitro biochemical characterization of B. anthracis Rap proteins.
In order to confirm the results of the in vivo analysis on the role of B. anthracis Rap proteins on sporulation, we overexpressed in E. coli and purified four full-length B. anthracis proteins (BXA0205, BA3016, BA3790, and BA1582) as N-terminal histidine-tagged derivatives. The ability of each protein to modulate the sporulation phosphorelay was tested against the KinA-dependent phosphorylation reaction of Spo0F or specifically against the purified Spo0F∼P intermediate known to be the target of RapA, RapB, and RapE of B. subtilis (18, 38).
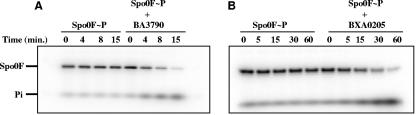
The results shown in Fig. 2 and 3 indicate that the BXA0205 and BA3790 proteins are capable of dephosphorylating the Spo0F∼P protein of the phosphorelay, while BA1582 and BA3016 are not, thus confirming the results obtained by the in vivo analysis. The seemingly more efficient dephosphorylation of Spo0F∼P by BXA0205 and BA3790 observed in the reactions in Fig. 2A and B (compared to the ones in Fig. 3A and B) are most likely the result of the relative concentration of Spo0F, Spo0F∼P, and Rap proteins in the reaction. Alternatively, there may be a stronger affinity of the Rap proteins for the unphosphorylated Spo0F than the affinity previously observed for Spo0F by the B. subtilis RapA (17).
FIG. 2.
In vitro activity assay of B. anthracis Rap proteins against the B. subtilis phosphorelay components. Purified B. anthracis Rap proteins were assayed in a reaction containing KinA (0.1 μM), Spo0F (2.5 μM), and [γ-32P]ATP. Each Rap protein was added at 5 μM final concentration. Time course experiments were carried out and aliquots withdrawn at the indicated time points. The positions of the KinA and Spo0F proteins in each gel are indicated by the bars. The labeled band denoted by the asterisk is a dimer form of Spo0F.
FIG. 3.
Dephosphorylation of B. subtilis Spo0F∼P by B. anthracis Rap proteins. Purified Spo0F∼P (2.5 μM) was incubated in the absence or presence of the BA3790 (A) or BXA0205 (B) proteins (5 μM) and aliquots were withdrawn at the indicated time points. The positions of the Spo0F protein and of the inorganic phosphate (Pi) are indicated by the bars.
Identification of the active Phr pentapeptide inhibitors.
The activity of the B. subtilis Rap proteins is inhibited by a specific pentapeptide that is generated from the precursor Phr protein through an export-import pathway and multiple processing events (35, 39). In most cases analyzed thus far, the Phr pentapeptide inhibitor is in the C-terminal five amino acids of the protein encoded by the phr gene (5, 22, 31). One exception is the PhrE pentapeptide that is generated from within the carboxy-terminal domain of its precursor protein, up nine amino acids from the terminal residue (18).
The phr genes associated with the B. anthracis BXA0205 and BXA3790 rap genes encoded proteins capable of inhibiting the phosphatase activity of their corresponding Rap proteins in vivo. This phenotype allowed us to search in vivo for the specific pentapeptide sequence with inhibitory activity toward BXA0205 and BA3790.
A new derivative of pHT315 was constructed, pHT315-BXA0205phr2, carrying the BXA0205 gene and its phr-associated gene with a premature stop codon that deleted the last five codons (GHTGG). When this plasmid was transformed into JH642, the resulting colonies showed a strong sporulation-deficient phenotype comparable to the phenotype generated by plasmid pHT315-BXA0205 (data not shown). This suggested that the five amino acids at the carboxy-terminal end of BXA0205phr could be the inhibiting pentapeptide. A synthetic GHTGG pentapeptide was then tested in vitro for its ability to inhibit the BXA0205 phosphatase activity against Spo0F∼P. As shown in Fig. 4, the presence of the synthetic pentapeptide inhibited BXA0205, indicating that GHTGG is the inhibitor sequence.
FIG. 4.
Inhibition of BXA0205 phosphatase activity by the BXA0205phr pentapeptide GHTGG in vitro. The B. subtilis KinA (0.1 μM) and Spo0F (2.5 μM) proteins were incubated with or without the B. anthracis BXA0205 Rap protein, at the concentrations indicated, in the presence of [γ-32P]ATP and increasing concentrations of the synthetic BXA0205phr pentapeptide. The reaction was carried out for 15 min prior to analysis by SDS-PAGE.
A similar strategy was applied to the search for the pentapeptide inhibitor of BA3790. Premature stop codons were generated that resulted in the deletion of the last 5, 10, 15, and 20 amino acids of the BA3791 gene product (plasmids pHT315-3790phr 3, -4, -5, and -6, respectively). The pHT315 plasmid derivatives carrying the BA3790 gene with each of these 3′ end deletions in BA3791 were transformed into JH642 for phenotypic analysis. A quantitative analysis of the sporulation efficiency generated by each of these constructs, shown in Table 4, indicated that the active pentapeptide was within the sequence that is located 10 to 20 amino acids from the carboxy-terminal end (GHYPVPTYSV). In fact, deletion of the last 10 amino acids still resulted in inhibition of BA3790 activity, while deletion of the last 20 amino acids completely eliminated the inhibition of phosphatase activity. Surprisingly, a partial loss of inhibition was observed with the construct with the last 15 amino acids deleted, thus not allowing us to clearly outline the sequence of the inhibitor and carry out in vitro studies.
TABLE 4.
Sporulation efficiency of B. subtilis strains carrying carboxy-terminal deletions of the BA3791 Phr proteina
| Plasmid | Viable count | Spore count | % Sporulationb |
|---|---|---|---|
| pHT315 | 1.9 × 108 | 1.3 × 108 | 68.4 |
| pHT315-3790 | 3.9 × 108 | 9.2 × 107 | 23.6 |
| pHT315-3790phr | 3.3 × 108 | 1.0 × 108 | 31 |
| pHT315-3790phr3 | 2.9 × 108 | 1.1 × 108 | 37.9 |
| pHT315-3790phr4 | 2.6 × 108 | 1.0 × 108 | 39.2 |
| pHT315-3790phr5 | 3.5 × 108 | 9.8 × 107 | 28 |
| pHT315-3790phr6 | 5.1 × 108 | 8.2 × 107 | 16 |
JH642 derivative strains were grown for 36 hours in Schaeffer's sporulation medium containing erythromycin at 5 μg/ml.
Representative of two independent experiments.
Deletion analysis of BXA0205 and BXA0205phr in B. anthracis.
In order to confirm in vivo the physiological roles of the BXA0205 and BXA0205phr proteins inferred from the in vitro studies, we carried out a deletion analysis of their coding genes on the genome of B. anthracis. The genes encoding the BXA0205 and BXA0205phr proteins were inactivated with a spectinomycin resistance cassette inserted via a double-crossover recombination event (see Materials and Methods). The strains obtained, Ba34F2Δ0205 and Ba34F2Δ0205phr, were tested for sporulation efficiency in Schaeffer's sporulation medium. The results shown in Table 5 confirmed the roles of these proteins in regulating sporulation. In fact, while the deletion of the BXA0205 Rap protein increased the efficiency of sporulation, the deletion of the gene encoding the inhibitor Phr peptide severely inhibited spore formation. Therefore, the BXA0205 Rap protein dephosphorylates the Spo0F response regulator in vivo in B. anthracis, as well as in vitro.
TABLE 5.
Sporulation efficiency of B. anthracis strains carrying deletions of BXA0205 or BXA0205phr genes encoding the Rap and Phr proteins, respectively, of plasmid pXO1a,b
| Strain | Viable cells | Spore count | % Sporulation |
|---|---|---|---|
| 34F2 | 1.95 × 108 | 3.0 × 107 | 15.4 |
| 34F2ΔBXA0205 | 1.35 × 108 | 3.8 × 107 | 28.1 |
| 34F2ΔBXA0205phr | 1.9 × 106 | 4.25 × 103 | 0.22 |
Cells were grown in Schaeffer's sporulation medium for 24 hours at 37°C.
Data are the averages of two independent experiments.
Transcription analysis of the B. anthracis rap promoters.
In order to determine whether the B. anthracis rap genes were expressed in B. subtilis in the assay conditions used, a promoter fusion to the E. coli lacZ gene was constructed for each of them (see Materials and Methods). Each construct was introduced in the B. subtilis chromosome of strain JH642 at the amyE locus, and promoter activity was measured by means of β-galactosidase activity assays. The results shown in Fig. 5 indicated that the promoter of the pXO1-located rap gene, BXA0205, was the most active of all, reaching the highest level of activity in mid-late exponential phase and then decreasing sharply during stationary phase. The remaining five promoters were significantly less active than BXA0205, with BA3016 seeming to be most active early in exponential phase while BA4060 and BA3790 were transcribed more efficiently in late-exponential phase and during the transition to stationary phase. The transcription of BA3760, on the contrary, seemed to be increasing during growth, although it remained at very low levels throughout. The promoter of BA1582 did not seem to be transcribed at detectable levels, since the β-galactosidase activity never exceeded the background level. Also, transcription activity was not detected in B. subtilis from a 725-bp fragment carrying the 3′ end of the BXA0205 gene and the first 33 bp of the BXA0205phr gene (Fig. 5B). However, a very low level of transcription was detected when the same fragment cloned in pTCVlac was analyzed in B. anthracis. Similar results were obtained for the BA3791phr gene when analyzed in B. subtilis, although a low level of transcription was detected when the analysis was carried out in B. anthracis (data not shown). Differential transcription regulatory mechanisms between the two organisms must exist, and they will be investigated in future studies.
FIG. 5.
Transcription analysis of the B. anthracis rap promoters. β-Galactosidase assays were carried out on B. subtilis strains carrying rap-promoter fusions to the E. coli lacZ gene. Each fusion was integrated at the amyE locus via a double-crossover event. (A) β-Galactosidase activity of the BXA0205 promoter (•). (B) β-galactosidase activity of the BA4060 (▴), BA3016 (▪), BA3790 (♦), BA1582 (▾), BA3760 (•), and BXA0205phr (□) promoters. Cultures were carried out in Schaeffer's sporulation medium. The time zero on the x axis represents the time of transition from exponential to stationary phase. The data are representative of multiple independent experiments.
Thus, the patterns of transcription of the B. anthracis rap genes in general, and of the two involved in sporulation in particular, differ significantly, indicating that different regulatory mechanisms may be governing Rap protein production. Distinctive pathways of gene expression have been previously identified for the rap genes of B. subtilis, each pathway indicative of regulatory mechanisms governing Rap protein production (29, 38).
Effect of BA0205 Rap and Phr on transcription of abrB, atxA, and pagA genes.
Because of the location of the BXA0205 rap and phr genes on the pXO1 virulence plasmid that also carries the genetic determinants for B. anthracis toxin synthesis, we wondered whether their products would affect toxin production or the transcription of the genes encoding the toxin regulators AtxA and AbrB. In B. subtilis, deregulation of RapA due to a deletion of the phrA gene results in reactivation of abrB transcription after the transition to stationary phase (38). In B. anthracis, AbrB was shown to repress atxA transcription during exponential phase when cells were grown in LB medium in the presence of CO2 (43). AtxA is known to be the essential transcriptional activator of the pagA, cya, and lef genes encoding the toxin components (9, 43, 56). Thus, repression of AbrB production by the phosphorelay is thought to be required for activation of AtxA and toxin synthesis.
We constructed abrB- and atxA-lacZ transcriptional fusions in the pTCVlac replicative vector and assayed their transcriptional activities in the parental strain 34F2 and in the BXA0205 rap or phr deletion strains. The results shown in Fig. 6 indicated that the sporulation-deficient phenotype induced by the deletion of the BXA0205phr gene also resulted in extended transcription of the abrB gene during the stationary phase of growth, while in the parental strain the abrB promoter is turned off at around the transition time (time zero). No significant effect was observed on the transcription of atxA (Fig. 6B) or pagA (data not shown) by the deletion of either the BXA0205 rap or phr genes. Both genes were maximally transcribed only until mid-exponential phase in our growth conditions (Schaeffer's sporulation medium), supporting the notion that the initiation of sporulation inhibits toxin production.
FIG. 6.
Transcription analysis of the abrB and atxA promoters in B. anthracis BXA0205 and BXA0205phr mutant strains. (A) β-Galactosidase analysis of an abrB promoter-lacZ fusion construct in the parental strain 34F2 (▴), in the BXA0205 mutant (•), and in the BXA0205phr mutant (♦). (B) β-Galactosidase analysis of the atxA promoter-lacZ fusion construct. Strains and symbols are as for panel A. Strains were grown in Schaeffer's sporulation medium. The time zero on the x axis indicates the time of transition from exponential to stationary phase. The growth curve of one representative strain for each panel is shown by the open triangles. OD525, optical density at 525 nm.
DISCUSSION
The ability of B. anthracis to cause disease is associated with its plasmid content. Plasmid pXO1 carries the genes encoding the toxin components, pagA (protective antigen), cya (edema factor), and lef (lethal factor). Plasmid pXO2 contains the cap genes necessary for capsule production. While the toxin is a required virulence factor that contributes to the pathogenesis of the organism, the capsule provides bacterial resistance to host phagocytosis. Growth and persistence of the microorganism in the host are additional contributing factors in the virulence of B. anthracis. After germination of the infecting spores, rapid growth of capsulated vegetative cells in the bloodstream gives rise to the synergistic effects of bacillemia and toxemia that are characteristic of fatal anthrax infection (for a review, see reference 27).
In this communication we report the identification on the pXO1 plasmid of genes that regulate sporulation initiation in B. anthracis and therefore may contribute to the infectivity of this organism. The BXA0205 gene was found to encode a Rap protein with an aspartyl phosphate phosphatase activity against the Spo0F∼P intermediate response regulator of the phosphorelay for sporulation initiation. An associated gene called BXA0205phr was shown to encode a Phr peptide whose carboxy-terminal five amino acids (GHTGG) acted as an inhibitor of the BXA0205 Rap protein. Deregulation of the Rap activity, either by its overexpression or by deletion of the Phr-encoding gene, was shown to inhibit sporulation of B. anthracis.
The reduced ability of B. anthracis to initiate the sporulation process in the absence of the BXA0205 peptide may have relevance in the pathogenesis of the organism. To establish the disease, germinated spores must be able to rapidly grow and maintain themselves in the vegetative form which is likely to be the most favorable for survival. Host defenses can eliminate the spores, but the encapsulated vegetative cells can avoid ingestion by host phagocytes (15). Physiological conditions that inhibit spore formation but allow toxin production, a prerequisite for effective infection, may then be very advantageous to the bacterial cells. In the absence of the BXA0205phr peptide, these conditions are met, indeed: since the activation of the inhibiting Phr pentapeptide involves an export-import processing pathway (35), any event that reduces the efficiency of pentapeptide reimportation will result in inhibition of sporulation. B. anthracis growth in the bloodstream may result in dilution and reduced concentrations of peptide available for reimportation, thus contributing to maintaining cells in vegetative growth.
The hypothesis that the selective ability to avoid sporulation in the body while retaining the capability to sporulate in other environments (such as laboratory conditions or soil) is advantageous to the cells has been recently proposed by Brunsing et al. (6). The hypothesis was based on the observation that of the nine potential histidine sensor kinase-coding genes for sporulation initiation identified on the B. anthracis genome, some are interrupted, some encode seemingly inactive proteins, and none is necessary or sufficient to induce efficient sporulation in laboratory growth conditions. Thus, while some histidine kinase activation must occur during an infection to reach sufficient production of Spo0A∼P to keep AbrB production in check and allow AtxA and toxin expression, signal transduction for sporulation must be suppressed to maximize growth. Poor activation of histidine kinases, together with lack of inhibition of the BXA0205 Rap protein, may synergistically provide the conditions optimal to establish the infection and allow its progression to its often fatal outcome.
The pXO1 plasmid location of the BXA0205 rap1 and phr system also supports the possibility of a selective advantage provided by its gene products to the bacterial cells. The BXA0205 Rap protein is 100% conserved among B. anthracis strains whose genome sequence is available, and it is 90% identical to a protein identified on plasmid pBC10987 of B. cereus ATCC 10987. The pXO1-like plasmid of B. cereus G9241 (16) also contains a Rap-Phr system 100% identical to the one found on the B. anthracis pXO1 plasmids. The presence of Rap-Phr systems on plasmids of other Bacillus species is not uncommon. Several industrial strains and natural soil isolates of B. subtilis contain plasmids that carry Rap-Phr modules believed to be involved in the regulation of post-exponential-phase processes, and therefore they would be advantageous for the conditions for which the bacterial cells were selected (21, 24). Although the BXA0205 genes of the pXO1 plasmid of B. anthracis are not located within the pathogenicity island (carrying, among others, the toxin genes, atxA, and the gerX operon for germination), the extensive sequence conservation observed within pXO1 ORFs of the Bacillus anthracis/Bacillus cereus/Bacillus thuringiensis group indicates that they are in fact important for the biology of these species (33).
Although the sporulation defect brought about by the lack of the BXA0205 pentapeptide did not seem to affect the production of toxin or toxin regulator, as seen by β-galactosidase transcription analyses of the pagA and atxA promoters, a deregulation of abrB transcription was observed (Fig. 6). AbrB is a transition state transcription regulator known in B. subtilis to generally repress transcription, during the exponential phase of growth, of a number of genes whose products are required during the transition from active growth to stationary phase and/or for sporulation (51). One of the genes repressed by AbrB in B. anthracis is atxA, and a deletion of the abrB gene was shown to result in earlier and higher expression of atxA and therefore pagA, cya, and lef when cells were grown in LB medium in the presence of 5% CO2 (43, 52). Because of this observation, we expected to see a stronger repression of atxA transcription in the BXA0205phr-deleted strain. However, our transcriptional analysis, carried out in Schaeffer's sporulation medium, showed that in the parental strain, atxA and pagA were transcribed only until mid-exponential phase and then their transcription was essentially turned off. In the sporulation-deficient Ba34F2Δ0205phr strain, this transcription pattern was not affected. These results could be interpreted to mean that the Schaeffer's sporulation medium, which causes earlier and faster development of spores than the LB medium, represses atxA and toxin production before the transition phase, while in LB medium with CO2, transcription is still maximal at the beginning of stationary phase. This repression is not increased by the higher level of AbrB observed after the transition phase (T0) in the strain lacking the BXA0205phr peptide gene, suggesting that the regulation of atxA transcription by AbrB may be limited to the exponential phase of growth, while the turnoff at around late-exponential phase occurs independently of the level of AbrB. Clearly, different growth conditions differentially affect transcription regulation of the genes so far known to regulate B. anthracis toxin production, and nothing is known about the regulation of gene transcription when bacteria grow in the bloodstream, in which presumably toxins are favorably expressed and sporulation does not occur. Since environmental growth conditions play a critical role in regulating atxA and toxin gene transcription, a more in-depth analysis is necessary in order to clearly understand the nuances of the regulatory pathway involving the phosphorelay, AbrB, AtxA, and toxin production.
The activity of the BXA0205 Rap protein is inhibited by the carboxy-terminal pentapeptide of the BXA0205Phr protein (GHTGG) both in vivo and in vitro, although with limited efficiency compared to the 1:1 stoichiometry observed with the RapA-PhrA system of B. subtilis (Tables 2 and 3 and Fig. 4) (17). The observation that the phr gene is cotranscribed with the rap gene and thus does not get transcriptionally amplified by an additional specific promoter, as seen in several rap-phr systems of B. subtilis (8, 23), also indicates that an advantage must exist in keeping the Rap protein active by producing little and/or a poorly active pentapeptide.
The role of BXA0205 Rap and Phr proteins in B. anthracis sporulation is likely to provide a rationale for the observation made by Aronson et al. on regulation of extracellular protease production by these proteins, by them identified as Cot43 and Cot43p (3). If transcription regulation of the proteases analyzed by their work is somehow dependent on the regulation of the transition phase (involving the phosphorelay and AbrB), then changes are expected if the BXA0205rap gene is either deleted or cloned in a multicopy plasmid. Effects on the production of the subtilisin protease of B. subtilis are observed when the rapA gene is either deleted or overexpressed, and an effect on extracellular proteases has been recently reported upon expression of a plasmid-borne Rap-Phr system (M. Perego, unpublished data) (21)
A second Rap-Phr system affecting sporulation initiation, BA3790-3791, is chromosomally located and highly conserved (100% identity) among B. anthracis strains but only 45 to 53% conserved with its orthologues (based on chromosomal mapping) of B. cereus strains. In this system, as previously discussed for the BXA0205 system, the phr gene is cotranscribed with the rap gene but also has a specific promoter. Coexpression of BA3791 with BA3790 resulted in efficient inhibition of the Rap activity in vivo. Unfortunately, we could not clearly define the active pentapeptide sequence in order to test it in vitro. Curiously, this active pentapeptide is not in the sequence GDHGG that strongly resembles the GHTGG of the BXA0205Phr inhibitor (Fig. 1B). This observation suggests caution in attempting to identify Phr pentapeptide inhibitors based solely on sequence homology. As inferred by the sequence analysis of the B. anthracis Rap-Phr systems (Fig. 1A and B), evolution within this species from a common ancestor has resulted in highly homologous proteins with, however, differential specificity in protein-protein and protein-ligand interactions. Avoiding Rap-Phr cross-reactivity among the different systems must be essential in order to avoid physiologically detrimental interferences.
Four additional Rap-Phr systems were identified on the chromosome of B. anthracis. Three of them did not show any involvement in the sporulation process by means of in vivo or and/or in vitro analysis. The fourth one (BA4060) carries a rap gene with a frameshift mutation that generates a truncated protein. However, the orthologue from B. cereus is uninterrupted and, when tested, did not affect sporulation in vivo (Table 3). A physiological function for these systems is unknown at this time. A recent report assigned the function of regulator of an integrative and conjugative element (the mobile genetic element ICEBs1) to RapI and PhrI, the B. subtilis orthologues of BA3760 and BA3759, suggesting that these proteins may have identical functions in B. anthracis (4).
The Rap-Phr systems have so far been identified only in members of the genus Bacillus. Rap proteins seem to have evolved to carry out different regulatory functions based on specific protein-protein interactions and modulation by an inhibitory pentapeptide ligand. The structural basis for these interactions is provided by the Rap organization in TPR (37). TPR are structural modules of 34 amino acids that assume an α-helix-turn-α-helix conformation. The α helices are antiparallel and pack with an angle of approximately 24° between the helix axes. These motifs occur in tandem arrays, the number ranging from 3 to 16, in a variety of different proteins, both prokaryotic and eukaryotic (10, 11, 14). These arrays function as molecular scaffolds known to mediate protein-protein or protein-ligand interactions. Rap proteins contain six TPR motifs whose packing in a typical parallel fashion generates a right-handed superhelical structure with two major surfaces: the outer convex and the inner concave (37). The latter surface has been shown to provide a docking site for linear peptides, and this is most likely the site of interaction of the Phr pentapeptides with the Rap proteins (46).
A related family of TPR-containing proteins includes the members of the PlcR group of transcription regulators characteristic of the B. cereus group of bacteria (48). These proteins carry an additional DNA-binding domain at their amino-terminal ends and, unusually, only two TPR domains (according to PFAM analysis [http://www.sanger.ac.uk]). The work on the B. subtilis Rap-Phr protein has been instrumental in finding that a pentapeptide generated from an export-import processing pathway of an associated gene product is required to activate the transcriptional function of PlcR proteins (47). It has been observed that in B. anthracis, PlcR is inactive because of a nonsense mutation in the plcR gene, and this mutation is ubiquitous among an extensive collection of genetically distinct B. anthracis strains (12, 25). Complementation of the B. anthracis plcR gene with the orthologous B. thuringiensis gene revealed that, although PlcR and its regulon have no influence on virulence, the concurrent expression of the AtxA virulence regulator and/or the presence of the pXO1 plasmid resulted in a sporulation-deficient phenotype (25). Since the inability to sporulate is counterproductive for the survival of the bacteria, a nonfunctional PlcR must have been counterselected in this species. It remains to be established whether the BXA0205 Rap and Phr proteins may have any role in this so-called incompatibility of PlcR and AtxA in B. anthracis by means of their role in regulating the sporulation process.
Supplementary Material
Acknowledgments
This work was supported in part by grant GM55594 from the National Institute of General Medical Sciences and grant AI55860 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Oligonucleotide syntheses and DNA sequencing reactions were supported in part by the Stein Beneficial Trust.
This report is manuscript number 17743-MEM from The Scripps Research Institute.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arantes, O., and D. Lereclus. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115-119. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, A. I., C. Bell, and B. Fulroth. 2005. Plasmid-encoded regulator of extracellular proteases in Bacillus anthracis. J. Bacteriol. 187:3133-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auchtung, J. M., C. A. Lee, R. E. Monson, A. P. Lehman, and A. D. Grossman. 2005. Regulation of a Bacillus subtilis mobile genetic element by intercellular signaling and the global DNA damage response. Proc. Natl. Acad. Sci. USA 102:12554-12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongiorni, C., S. Ishikawa, S. Stephenson, N. Ogasawara, and M. Perego. 2005. Synergistic regulation of competence development in Bacillus subtilis by two Rap-Phr systems. J. Bacteriol. 187:4353-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunsing, R. L., C. La Clair, S. Tang, C. Chiang, L. E. Hancock, M. Perego, and J. A. Hoch. 2005. Characterization of sporulation histidine kinases of Bacillus anthracis. J. Bacteriol. 187:6972-6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbulys, D., K. A. Trach, and J. A. Hoch. 1991. The initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell 64:545-552. [DOI] [PubMed] [Google Scholar]
- 8.Carter, H. L., K. M. Tatti, and C. P. Moran. 1990. Cloning of a promoter used by sigma H RNA polymerase in Bacillus subtilis. Gene 96:101-105. [DOI] [PubMed] [Google Scholar]
- 9.Dai, Z., J. C. Sirard, M. Mock, and T. M. Koehler. 1995. The atxA gene product activates transcription of the anthrax toxin genes and is essential for virulence. Mol. Microbiol. 16:1171-1181. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea, L. D., and L. Regan. 2003. TPR proteins: the versatile helix. Trends Biochem. Sci. 28:655-662. [DOI] [PubMed] [Google Scholar]
- 11.Das, A. K., P. T. W. Cohen, and D. Barford. 1998. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 17:1192-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Easterday, W. R., M. N. Van Ert, T. S. Simonson, D. M. Wagner, L. J. Kenefic, C. J. Allender, and P. Keim. 2005. Use of single nucleotide polymorphisms in the plcR gene for specific identification of Bacillus anthracis. J. Clin. Microbiol. 43:1995-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari, E., S. M. H. Howard, and J. A. Hoch. 1985. Effect of sporulation mutations on subtilisin expression, assayed using a subtilisin-β-galactosidase gene fusion, p. 180-184. In J. A. Hoch and P. Setlow (ed.), Molecular biology of microbial differentiation. American Society for Microbiology, Washington, D.C.
- 14.Groves, M. R., and D. Barford. 1999. Topological characteristics of helical repeat proteins. Curr. Opin. Struct. Biol. 9:383-389. [DOI] [PubMed] [Google Scholar]
- 15.Guidi-Rontani, C., and M. Mock. 2002. Macrophage interactions. Curr. Top. Microbiol. Immunol. 271:115-141. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmaster, A. R., J. Ravel, D. A. Rasko, G. D. Chapman, M. D. Chute, C. K. Marston, B. K. De, C. T. Sacchi, C. Fitzgerald, L. W. Mayer, M. C. J. Maiden, F. G. Priest, M. Barker, L. Jiang, R. Z. Cer, J. Rilstone, S. N. Peterson, R. S. Weyant, D. R. Galloway, T. D. Read, T. Popovic, and C. M. Fraser. 2004. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proc. Natl. Acad. Sci. USA 101:8449-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa, S., L. Core, and M. Perego. 2002. Biochemical characterization of aspartyl phosphate phosphatase interaction with a phosphorylated response regulator and its inhibition by a pentapeptide. J. Biol. Chem. 277:20483-20489. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, M., R. Grau, and M. Perego. 2000. Differential processing of propeptide inhibitors of Rap phosphatases in Bacillus subtilis. J. Bacteriol. 182:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, M., W. Shao, M. Perego, and J. A. Hoch. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 38:535-542. [DOI] [PubMed] [Google Scholar]
- 20.Koehler, T. M., Z. Dai, and M. Kaufman-Yarbray. 1994. Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J. Bacteriol. 176:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koetje, E. J., A. Hajdo-Milasinovic, R. Kiewiet, S. Bron, and H. Tjalsma. 2003. A plasmid-borne Rap-Phr system of Bacillus subtilis can mediate cell-density controlled production of extracellular proteases. Microbiology 149:19-28. [DOI] [PubMed] [Google Scholar]
- 22.Lazazzera, B. A., J. M. Solomon, and A. D. Grossman. 1997. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell 89:917-925. [DOI] [PubMed] [Google Scholar]
- 23.McQuade, R. S., N. Comella, and A. D. Grossman. 2001. Control of a family of phosphatase regulatory genes (phr) by the alternate sigma factor sigma-H of Bacillus subtilis. J. Bacteriol. 183:4905-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meijer, W. J., G. B. Wisman, P. Terpstra, P. B. Thorsted, C. M. Thomas, S. Holsappel, G. Venema, and S. Bron. 1998. Rolling-circle plasmids from Bacillus subtilis: complete nucleotide sequences and analyses of genes of pTA1015, pTA1040, pTA1050 and pTA1060, and comparisons with related plasmids from gram-positive bacteria. FEMS Microbiol. Rev. 21:337-368. [DOI] [PubMed] [Google Scholar]
- 25.Mignot, T., M. Mock, D. Robichon, A. Landier, D. Lereclus, and A. Fouet. 2001. The incompatibility between the PlcR- and AtxA-controlled regulons may have selected a nonsense mutation in Bacillus anthracis. Mol. Microbiol. 42:1189-1198. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 28.Molle, V., M. Fujita, S. T. Jensen, P. Eichenberger, J. E. Gonzalez-Pastor, J. S. Liu, and R. Losick. 2003. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 50:1683-1701. [DOI] [PubMed] [Google Scholar]
- 29.Mueller, J. P., G. Bukusoglu, and A. L. Sonenshein. 1992. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J. Bacteriol. 174:4361-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 31.Ogura, M., K. Shimane, K. Asai, N. Ogasawara, and T. Tanaka. 2003. Binding of response regulator DegU to the aprE promoter is inhibited by RapG, which is counteracted by extracellular PhrG in Bacillus subtilis. Mol. Microbiol. 49:1685-1697. [DOI] [PubMed] [Google Scholar]
- 32.Okinaka, R. T., K. Cloud, O. Hampton, A. R. Hoffmaster, K. K. Hill, P. Keim, T. M. Koehler, G. Lamke, S. Kumano, J. Mahillon, D. Manter, Y. Martinez, D. Ricke, R. Svensson, and P. J. Jackson. 1999. Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J. Bacteriol. 181:6509-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pannucci, J., R. T. Okinaka, R. Sabin, and C. R. Kuske. 2002. Bacillus anthracis pXO1 plasmid sequence conservation among closely related bacterial species. J. Bacteriol. 184:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perego, M. 1993. Integrational vectors for genetic manipulation in Bacillus subtilis, p. 615-624. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 35.Perego, M. 1997. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc. Natl. Acad. Sci. USA 94:8612-8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perego, M. 2001. A new family of aspartyl-phosphate phosphatases targeting the sporulation transcription factor Spo0A of Bacillus subtilis. Mol. Microbiol. 42:133-144. [DOI] [PubMed] [Google Scholar]
- 37.Perego, M., and J. A. Brannigan. 2001. Pentapeptide regulation of aspartyl-phosphate phosphatases. Peptides 22:1541-1547. [DOI] [PubMed] [Google Scholar]
- 38.Perego, M., C. G. Hanstein, K. M. Welsh, T. Djavakhishvili, P. Glaser, and J. A. Hoch. 1994. Multiple protein aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in Bacillus subtilis. Cell 79:1047-1055. [DOI] [PubMed] [Google Scholar]
- 39.Perego, M., and J. A. Hoch. 1996. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:1549-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perego, M., and J. A. Hoch. 2002. Two-component systems, phosphorelays and regulation of their activities by phosphatases, p. 473-481. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 41.Piggot, P. J., and D. W. Hilbert. 2004. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 7:579-586. [DOI] [PubMed] [Google Scholar]
- 42.Poyart, C., and P. Trieu-Cuot. 1997. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to β-galactosidase in Gram-positive bacteria. FEMS Microbiol. Lett. 156:193-198. [DOI] [PubMed] [Google Scholar]
- 43.Saile, E., and T. M. Koehler. 2002. Control of anthrax toxin gene expression by the transition state regulator abrB. J. Bacteriol. 184:370-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaeffer, P., J. Millet, and J. P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 46.Scheufler, C., A. Brinker, G. Bourenkov, S. Pegoraro, L. Moroder, H. Bartunik, F. U. Hartl, and I. Moarefi. 2000. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101:199-210. [DOI] [PubMed] [Google Scholar]
- 47.Slamti, L., and D. Lereclus. 2002. A cell-cell signaling peptide activates the PlcR virulence regulon in bacteria of the Bacillus cereus group. EMBO J. 21:4550-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slamti, L., and D. Lereclus. 2005. Specificity and polymorphism of the PlcR-PapR quorum-sensing system in the Bacillus cereus group. J. Bacteriol. 187:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephenson, K., and J. A. Hoch. 2002. Evolution of signaling in the sporulation phosphorelay. Mol. Microbiol. 46:297-304. [DOI] [PubMed] [Google Scholar]
- 50.Stephenson, S., and M. Perego. 2002. Interaction surface of the Spo0A response regulator with the Spo0E phosphatase. Mol. Microbiol. 44:1455-1467. [DOI] [PubMed] [Google Scholar]
- 51.Strauch, M. A. 1993. AbrB, a transition state regulator, p. 757-764. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology and molecular genetics. American Society for Microbiology, Washington, D.C.
- 52.Strauch, M. A., P. Ballar, A. J. Rowshan, and K. L. Zoller. 2005. The DNA-binding specificity of the Bacillus anthracis AbrB protein. Microbiology 151:1751-1759. [DOI] [PubMed] [Google Scholar]
- 53.Strauch, M. A., V. Webb, G. Spiegelman, and J. A. Hoch. 1990. The Spo0A protein of Bacillus subtilis is a repressor of the abrB gene. Proc. Natl. Acad. Sci. USA 87:1801-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzeng, Y.-L., V. A. Feher, J. Cavanagh, M. Perego, and J. A. Hoch. 1998. Characterization of interactions between a two-component response regulator, Spo0F, and its phosphatase, RapB. Biochemistry 37:16538-16545. [DOI] [PubMed] [Google Scholar]
- 55.Tzeng, Y.-L., and J. A. Hoch. 1997. Molecular recognition in signal transduction: the interaction surfaces of the Spo0F response regulator with its cognate phosphorelay proteins revealed by alanine scanning mutagenesis. J. Mol. Biol. 272:200-212. [DOI] [PubMed] [Google Scholar]
- 56.Uchida, I., J. M. Hornung, C. B. Thorne, K. R. Klimpel, and S. H. Leppla. 1993. Cloning and characterization of a gene whose product is a trans-activator of anthrax toxin synthesis. J. Bacteriol. 175:5329-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Dijl, J. M., A. Bolhuis, H. Tjalsma, J. D. H. Jongbloed, A. de Jong, and S. Bron. 2002. Protein transport pathways in Bacillus subtilis: a genome-based road map, p. 337-355. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 58.Zapf, J., U. Sen, Madhusudan, J. A. Hoch, and K. I. Varughese. 2000. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Structure (Cambridge) 8:851-862. [DOI] [PubMed] [Google Scholar]
- 59.Zapf, J. W., J. A. Hoch, and J. M. Whiteley. 1996. A phosphotransferase activity of the Bacillus subtilis sporulation protein Spo0F that employs phosphoramidate substrates. Biochemistry 35:2926-2933. [DOI] [PubMed] [Google Scholar]
- 60.Zhao, H., T. Msadek, J. Zapf, Madhusudan, J. A. Hoch, and K. I. Varughese. 2002. DNA complexed structure of the key transcription factor initiating development in sporulating bacteria. Structure (Cambridge) 10:1041-1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.